Abstract
Sepsis-induced acute respiratory distress syndrome (ARDS) remains a major complication of death from bacterial infection. Regulatory T cells (Tregs) are important regulators in addressing lung injury. Considering the extensive research of circular RNAs (circRNAs), the role of circRNA in Treg modulation during ARDS remains unclear. In this study, patients with sepsis-induced ARDS along with non-ARDS controls were obtained, and bronchoalveolar lavage fluid (BALF) was collected as clinical samples. Additionally, cecal ligation and puncture (CLP) was performed to construct a septic ARDS model, and lung tissues as well as peripheral blood were collected. mRNA expressions were measured by RT-qPCR. ELISA was carried out to measure the concentration of inflammatory factors. A combination of online bioinformatics, dual-luciferase reporter, and RND pull-down assays was performed to verify interactions between microRNA (miRNA) and circRNA/mRNA. Tregs were measured by flow cytometry. Our data suggested that circFLNA was aberrantly elevated in ARDS, and depletion of circFLNA upregulated CD4+CD25+Foxp3+ Tregs and decreased inflammatory response. Additionally, miR-214-5p which binds with circFLNA, reversed circFLNA-induced effects in ARDS. Programmed cell death protein 1 (PD-1) is a downstream target gene of miR-214-5p, and abrogated the effects of miR-214-5p on regulating CD4+CD25+Foxp3+ Tregs and inflammatory response. In a word, circFLNA/miR-214-5p/PD-1 signaling is a novel pathway that modulates Tregs in ARDS.
Keywords:
Introduction
Sepsis is an organ dysfunction caused by the host’s malfunctioning response to infection [Citation1]. The lung is the earliest and most frequently failing organ in the pathological process of sepsis [Citation2]. Patients with sepsis can develop lung damage at an early stage and develop acute respiratory distress syndrome (ARDS) [Citation3, Citation4].
Recently, abnormal immune responses have been found to be involved in the pathological process of ARDS, and the immune function status of the host largely determines the outcome of the inflammatory response [Citation1, Citation5]. The latest research shows that the body’s immune function decline and excessive inflammatory damage are the leading causes of sepsis and its complications ARDS, especially in the acquired immune T regulatory cells (Tregs), in alleviating the important function of lung injury has been gradually recognized and paid attention to refs. [Citation6–8]. A decrease in the proportion of Tregs is likely to cause autoimmune diseases [Citation9, Citation10]. Among them, CD4+CD25+FoxP3+ Tregs are a group of cells with immunosuppressive function that can inhibit the excessive activation of effector T cells and play an extremely important role in maintaining the body’s immune self-stability and preventing the occurrence of autoimmune diseases [Citation11–13].
Circular RNA (circRNA) is a kind of endogenous non-coding RNA that exists stably in eukaryotes and is mainly enriched in the nucleus [Citation14, Citation15]. CircRNAs have been proposed as good biomarkers for various diseases, including sepsis [Citation16]. For instance, a combination of circRNA_104484 and circRNA_104670 has been reported to be a promising diagnostic method for sepsis [Citation17]. What’s more, Wan et al. identified five unusually expressed circRNAs in lung tissues from LPS-induced ARDS rats [Citation18]. Likewise, newfound aberrantly expressed circRNAs have also been identified by Ye et al. [Citation19] in acute lung injury rat lungs. Currently, prominently abnormally expressed circFLNA (hsa_circ_0092012) has been found in diverse tumors, including lung cancer [Citation20]. We speculated that circFLNA may participate in the development of ARDS induced by sepsis.
In this study, circFLNA was measured in the BALF of patients with the ARDS model. CD4+CD25+Foxp3+ Tregs and inflammatory responses were evaluated to justify the role of circFLNA in ARDS. Furthermore, this study mainly explored the function of circFLNA and miR-214-5p on Tregs and inflammatory factors by regulating programmed cell death protein 1 (PD-1).
Methods and materials
Clinical research objects
A total of 10 adult patients hospitalized in the Intensive Care Department of Dongguan Tungwah Hospital from June 2018 to January 2020 were selected. All patients were consistent with ARDS caused by severe sepsis or septic shock. Ten healthy blood donors from volunteers whose lung function had been examined to rule out any lung disease served as the normal control group. Ethical certification prior to the clinical study was ratified and supported by the Ethics Committee of Dongguan Tungwah Hospital. The informed consent of all subjects included in this study was signed by their immediate family members. The time of ARDS diagnosis was less than 24 h for all sepsis patients at the time of inclusion [Citation21]. Patients with autoimmune or allergic diseases and serious underlying diseases were not eligible for inclusion.
Bronchoalveolar lavage fluid (BALF) collection
BALF was collected by bronchoalveolar lavage performance on patients with ARDS along with control participants as previously reported [Citation22].
Animal
Male SPF C57BL/6 wild-type mice were grouped into the Sham group, cecal ligation and puncture (CLP) group, the CLP + LV-sh-circFLNA group, the CLP + LV-sh-NC group, the CLP + sh-circFLNA + antagomiR-214-5p group, the CLP + sh-circFLNA + antagomiR-NC group, the CLP + agomiR-214-5p + LV-PD-1 group, and the CLP + agomiR-214-5p + LV-NC group, six in each group, according to the random number table method.
Establishment of ARDS model in mice
CLP was performed to construct a septic acute lung injury model [Citation23]. Mice were anesthetized by intraperitoneal injection of 3.5% chloral hydrate in 0.1 mL. The mice were fixed, the abdominal skin was disinfected, and the cecum was found and exposed through a midline incision into the abdominal cavity. In the CLP group, the proximal cecum was ligated with silk thread, the distal cecum was perforated with a 20-mL syringe (21-gauge needle), and a few intestinal contents were extruded. The abdominal cavity was then restored, and the abdomen was closed with sutures. In the Sham group, only the cecum was turned after laparotomy without any other treatment, and the abdomen was closed by suturing. All mice were harvested 16 h later, and the peripheral blood, bronchoalveolar lavage fluid (BALF), as well as lung tissues, were collected for subsequent experiments.
Construction of lentivirus vectors
The sequences of the short hairpin RNA (shRNA) targeting circFLNA (1#: GACTTCAAGGTGTACACAAAG and 2#: GCGTGTATGGCTTCGAGTATT) were designed by ThermoFisher (USA). Then HEK293T cells transfected with the shRNA vectors were packaged with recombinant lentiviruses using pLenti6.2/C-Lumio/V5-DEST (Invitrogen, USA). To generate PD-1 expression vectors, PD-1 was amplified from HEK293T cDNA by PCR and cloned into a pLenti6.2-/C-Lumio/V5-DEST lentiviral vector [Citation24]. Meanwhile, antagomiR-214-5p, antagomiR-NC, agomiR-214-5p, and agomiR-NC were obtained from Shanghai GenePharma Co., Ltd. After modeling, the above plasmids were injected into the tail veins of the corresponding groups of mice at a concentration of 30 mg/kg every seven days, and the sham and CLP groups were injected with normal saline. After 8 weeks of intervention, mice were sacrificed to obtain spleen, lung, and peripheral blood samples.
Histopathology
The upper lobe of the right lungs was imbedded in paraffin [Citation25]. The paraffin sections were observed with hematoxylin and eosin under microscope.
Cell isolation
Mice from all groups were anesthetized, 1 mL of 2% Bio-Gel P-100 (BioRad, Hercules, CA, USA) was injected into the peritoneal cavity of mice to generate peritoneal macrophages [Citation26]. Afterwards, spleen T cells as well as peripheral blood were purified using a T Cell Isolation Kit (Catalog #19851 19851; RFSTEMCELL Technologies, Shanghai, China). T cells were stimulated with anti-CD3 (1/1000, ab16669, Abcam, California, USA) and anti-CD28 (1/500, ab246517, Abcam).
RT-qPCR
Total RNA was extracted from BALF or lung tissues by the TRIzol method, 1.0 μg total RNA was obtained, and cDNA was reverse transcribed by SuperScript IV CellsDirect cDNA kit (Thermo Fisher). β-actin was used as an internal reference to detect circFLNA, and PD-1, while U6 was for miR-214-5p. PCR reaction and data analysis were performed in an ABI StepOnePlus Real-Time PCR System/Applied Biosystems 7500 Real-Time PCR system (Thermo Fisher), and the relative expression levels of mRNA and miRNA were calculated by a 2−ΔΔCt method. The sequences of all primers were listed as follows (5′→3′):
circFLNA: F: ACCACCATGACAACACCTACA;
R: GCCAGCAGCTTTGGCATTTAC;
miR-214-5p: F: GCCTGGCTGGACAGAGTTG;
R: GGCTGGGTTGTCATGTGACT;
PD-1: F: ATCTGGAACTGTGGCCATGG;
R: GGGGAGGGAGAGAGAGACAG;
β-actin: F: ATCACTGCCACCCAGAAGAC;
R: TTTCTAGACGGCAGGTCAGG;
U6: F: CTCGCTTCGGCAGCACA;
R: AACGCTTCACGAATTTGCGT.
gDNA extraction
The gDNA extraction were performed according to the previous study [Citation27].
RNase R digestion
RNase R was used to decompose linear RNAs instead of circRNAs. Briefly, 1 μL RNase R was added to 5 μg RNA, followed by 1 μL RNase R buffer and 7 μL ddH2O. Then the mixture was bathed in water at 37 °C for 15 min. Actinomycin D (2 mg/mL) was applied to degrade RNA, and RT-qPCR was used to detect linear RNA and circRNA levels.
Flow cytometry analysis
T cells were stained with anti-CD4 (2 µg/mL, ab269349), anti-CD25 (1/1000, ab283576), and anti-FOXP3 (ab210230) under the flow cytometry analysis [Citation7].
ELISA
Concentrations of TNF-α, IL-1β, and IL-6 in BALF and lung tissues were accessed by using ELISA kits followed instructions (MyBioSource, USA). The absorbance at 450 nm was determined via a spectrophotometer (Bio-TEK, USA).
Binding interaction between mRNA and miRNA
Human embryonic kidney cells HEK293T (ATCC, USA) were cultivated as previously described [Citation28]. Dual luciferase reporter assay along with RNA pull-down assay was carried out to verify the predicted binding relationship between circFLNA, miR-214-5p, and PD-1 as previously reported [Citation29].
Statistical analysis
miRDB and TargetScan were used to predict binding relationship between miRNA and mRNA. SPSS 25.0 was used to analyze the statistical data shown as the mean ± SD. Student’s t-test and one-way ANOVA were used to assess the statistical significance of differences between two or multiple groups. Potential diagnostic value was presented by receiver operating characteristics (ROC) curve analysis. In addition, the power analysis was performed to examine the sample size using PASS software. p < 0.05 was deemed as significant.
Results
circFLNA was elevated in patients with ARDS and mice following CLP operation
First of all, we measured the relative mRNA expression of circFLNA in the BALF, and our data revealed that circFLNA was obviously up-regulated in BALF of patients with sepsis-induced ARDS compared with that of non-ARDS controls (). Next, ROC curve analysis was performed to evaluate the sensitivity and specificity of circFLNA for the diagnosis of ARDS. Our data illustrated that the AUC was 0.9600 (), which indicated that highly expressed circFLNA may be an indicator of ARDS. Then demonstrated that the specific circRNA amplification bands only appeared in the cDNA samples of circFLNA, suggesting that the same circular DNA molecules as circFLNA did not exist in the genome; that is, circFLNA is derived from transcripts rather than genomes (). First, we found that circFLNA is composed of exons 9 to 15 of FLNA. The circRNA is formed by exon cleavage and is located in chrX: 153592389–153594592 (). Afterward, the cyclization stability of circFLNA was detected. After RNase R digestion, linear FLNA was significantly decreased while circFLNA was not significantly affected (). The mRNA level of the linear form of circFLNA was significantly decreased, while the decrease of circFLNA was not obvious under the treatment of actinomycin D (). Then, we established a sepsis-induced ARDS mouse model. The alveolar cavity of rats in the sham group was smooth, without exudation, and with a clear and complete structure, and the alveolar cavity of mice in the CLP group showed a large number of red blood cells, inflammatory cells, and plasma-like material exudation (). Furthermore, the circFLNA mRNA levels of lung tissues obtained from mice in CLP groups were dramatically elevated compared with that in the sham group, and the trend was consistent with that in ARDS patients ().
Figure 1. circFLNA was elevated in patients with ARDS and mice following CLP operation. (A) circFLNA expression analyses accessed by RT-qPCR in BALF of patients with ARDS and non-ARDS controls (n = 10). (B) ROC curve of circFLNA in sepsis-induced ARDS (n = 10). (C) Comparison of convergent and divergent primer amplifications in cDNA and gDNA of circFLNA (n = 3). (D). circFLNA schematic diagram of shear. (E) mRNA expression of circFLNA and its linear form before and after digestion by rnase R (n = 3). (F) RT-qPCR analysis of circFLNA and its linear form mRNA expression by actinomycin D treatment (n = 3). (G) pathological changes of lung tissues from ARDS mice and sham group were observed in HE staining (n = 6). RT-qPCR analyses of circFLNA levels in lung tissues (H) and BALF (I) of ARDS mice model (n = 3). ***p < 0.001. circ: cyclic RNA; ARDS: acute respiratory distress syndrome; CLP: cecal ligation and puncture; BALF: bronchoalveolar lavage fluid; ROC: receiver operator characteristic; BALF, bronchoalveolar lavage fluid.
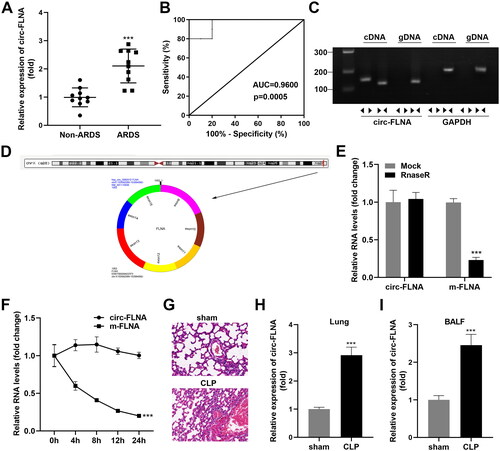
Depletion circFLNA ameliorated inflammatory response of sepsis-induced ARDS mice
Then, we knockdown circFLNA in ARDS mice, and the results illustrated that LV-sh-circFLNA 1# decreased the circFLNA levels more obviously (). CD4+CD25+Foxp3+ Tregs obtained from the CLP model were obviously lower than that in the sham group. After the inhibition of circFLNA, we found that the proportion of CD4+CD25+Foxp3+ Tregs was dramatically elevated (). Meanwhile, concentration and protein levels of TNF-α, IL-1β, and IL-6 in lung tissues induced in the sepsis-induced ARDS group were both dramatically down-regulated by deficiency of circFLNA ().
Figure 2. Depletion circFLNA ameliorated inflammatory response of sepsis-induced ARDS mice. (A) the RT-qPCR analyze of circFLNA expression in HEK293T cells transfected with LV-sh-circFLNA plasmids (n = 3). (B) Two-parameter frequency profile of flow cytometry (n = 6). (C) the percentages of CD4+CD25+Foxp3+ Tregs in different groups (n = 6). (D–F) TNF-α, IL-1β, and IL-6 concentrations of lung tissues obtained from mice were accessed by ELISA (n = 6).**p < 0.01, ***p < 0.001, vs. sham group. ##p < 0.01, vs. CLP group. LV: lentivirus; circ: cyclic RNA; TNF-α: tumor necrosis factor-α; IL-1β: interleukin 1 beta; IL-6: interleukin 6; CLP: cecal ligation and puncture; NC: negative control; sh: short hairpin RNA.
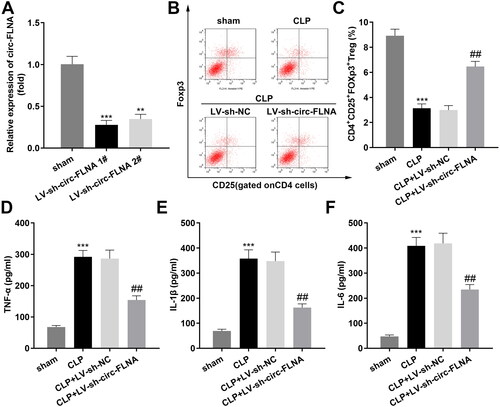
miR-214-5p served as a target of circFLNA
Then we found the binding domain between circFLNA and miR-214-5p (). Luciferase assay results demonstrated that over-expression of miR-214-5p to reduced luciferase activity of wild-type circFLNA (); likewise, circFLNA was enriched in biotin-miR-214-5p (). Additionally, miR-214-5p in the BALF of patients with sepsis-induced ARDS was prominently lower than that in the non-ARDS group (). Meanwhile, miR-214-5p was also remarkably lowly expressed in sepsis-induced ARDS mice (), and suppression of circFLNA led to upregulation of miR-214-5p expression ().
Figure 3. miR-214-5p Serves as a target of circFLNA. (A) Binding sites between miR-214-5p and circFLNA. (B) Luciferase activity, and (C) RNA pull-down assays were performed to verify the binding relationship (n = 3). (D) RT-qPCR analyses of miR-214-5p expression in BALF of sepsis-induced ARDS patients (n = 10). RT-qPCR analyses of miR-214-5p expression in lung tissues (E) and BALF (F) (n = 6). (G) RT-qPCR analyses of miR-214-5p expression in HEK293T cells transfected with inhibited circFLNA (n = 3). ***p < 0.001, vs. mimic NC, biotin-NC, non-ARDS, sham, and LV-sh-NC groups. circ: cyclic RNA; WT, wild type; MUT, wild type; NC: negative control; ARDS: acute respiratory distress syndrome; CLP: cecal ligation and puncture; BALF, bronchoalveolar lavage fluid.
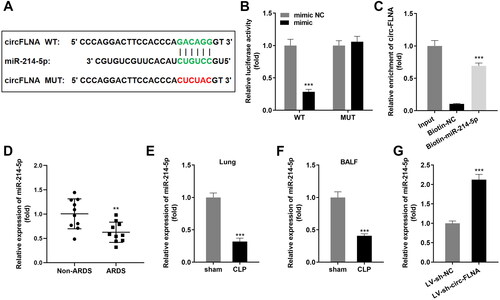
Inhibition of miR-214-5p abrogated the functions of inhibited circFLNA on the inflammatory response of sepsis-induced ARDS mice
Then, we successfully up-regulated or down-regulated miR-214-5p in sepsis-induced ARDS mice, and the regulatory efficiency was indicated in . AntagomiR-214-5p prominently upregulated CD4+CD25+Foxp3+ Tregs which suppressed by inhibited circFLNA (). Meanwhile, concentration and protein levels of TNF-α, IL-1β, and IL-6 in lung tissues induced in the ARDS group dramatically decreased by deficiency of circFLNA were both reversed by down-regulated miR-214-5p ().
Figure 4. Inhibition of miR-214-5p abrogated the functions of inhibited circFLNA on inflammatory response of sepsis-induced ARDS mice. (A) the RT-qPCR analyze of miR-214-5p expression in HEK293T cells before and after transfection (n = 3). (B) Two-parameter frequency profile of flow cytometry (n = 6). (C) The percentages of CD4+CD25+Foxp3+ Tregs in different groups (n = 6). (D–F). TNF-α, IL-1β, and IL-6 concentrations of lung tissues obtained from mice were accessed by ELISA (n = 6). ***p < 0.001, vs. control group. ##p < 0.01, vs. CLP group. &&p < 0.01, vs. CLP + LV-sh-circFLNA + AntagomiR-NC group. LV: lentivirus; circ: cyclic RNA; TNF-α: tumor necrosis factor-α; IL-1β: interleukin 1 beta; IL-6: interleukin 6; CLP: cecal ligation and puncture; NC: negative control; sh: short hairpin RNA.
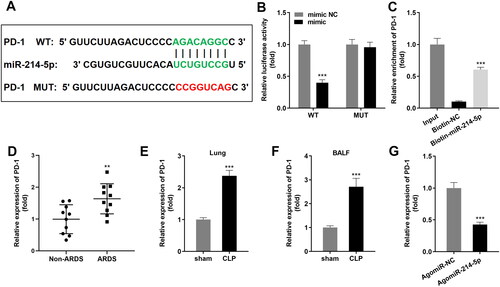
PD-1 is a target gene of miR-214-5p
The seeding domain of miR-214-5p and PD-1 were indicated in . Dual luciferase reporter along with RNA pull-down assays were constructed to validate the prediction (). Besides, PD-1 in BALF of patients with sepsis-induced ARDS was notably higher than that in a non-ARDS group (). Meanwhile, PD-1 was also notably highly expressed in sepsis-induced ARDS mice (), and upregulation of miR-214-5p led to suppression of PD-1 on mRNA expression ().
Figure 5. PD-1 is a target gene of miR-214-5p. (A) The binding sites of miR-214-5p and PD-1 were predicted. (B) Luciferase activity and (C) RNA pull-down assays were performed to verify the binding relationship (n = 3). (D) RT-qPCR analyses of PD-1 expression in BALF of sepsis-induced ARDS patients (n = 10). RT-qPCR analyses of PD-1 expression in lung tissues (E) and BALF (F) (n = 6). (G) RT-qPCR analyses of PD-1 expression in HEK293T cells transfected with upregulated miR-214-5p (n = 3). ***p < 0.001, vs. mimic NC, Biotin-NC, Non-ARDS, sham, and AgomiR-NC groups. circ: cyclic RNA; WT, wild type; MUT, wild type; NC: negative control; ARDS: acute respiratory distress syndrome; CLP: cecal ligation and puncture; BALF, bronchoalveolar lavage fluid.
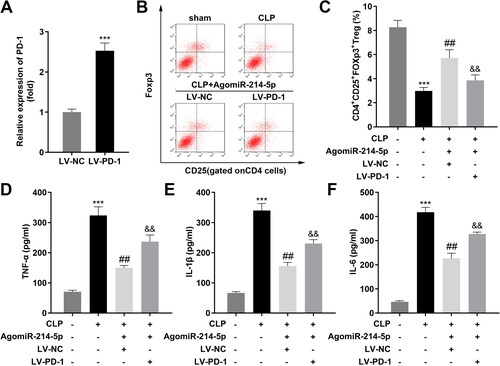
Over-expression of PD-1 inhibited the effects of upregulated miR-214-5p
PD-1 expression was prominently up-regulated in after transfected with PD-1 over-expression plasmids (). LV-PD-1 prominently downregulated CD4+CD25+Foxp3+ Tregs which are upregulated by agomiR-214-5p (). Meanwhile, concentration and protein levels of TNF-α, IL-1β, and IL-6 in the ARDS group dramatically reduced by agomiR-214-5p were both reversed by upregulated PD-1 ().
Figure 6. Over-expression of PD-1 inhibited the effects of upregulated miR-214-5p. (A) The RT-qPCR analyze of PD-1 expression in HEK293T cells before and after transfection (n = 3). (B) Two-parameter frequency profile of flow cytometry (n = 6). (C) The percentages of CD4 + CD25 + Foxp3+ Tregs in different groups (n = 6). (D–F) TNF-α, IL-1β, and IL-6 concentrations of lung tissues obtained from mice were accessed by ELISA (n = 6). ***p < 0.001, vs. control group. ##p < 0.01, vs. CLP group. &&p < 0.01, vs. CLP + AgomiR-214-5p + LV-NC group. LV: lentivirus; TNF-α: tumor necrosis factor-α; IL-1β: interleukin 1 beta; IL-6: interleukin 6; CLP: cecal ligation and puncture; NC: negative control; sh: short hairpin RNA.
Discussion
Sepsis is now considered to be a systemic inflammatory response secondary to infection by microorganisms and some microbial components, and is an important inducing factor of multiple organ dysfunction syndrome [Citation1]. Lung is the most vulnerable target organ in sepsis, and the incidence of ARDS is the highest and early in sepsis patients [Citation2].
CircRNA is widely present in eukaryotes and can bind miRNA and then modulate the miRNA target genes, thus playing a biological modulating role [Citation14, Citation15]. CircRNA contributes to the regulation of ARDS, providing molecular targets for reducing septic ARDS [Citation30]. For example, Cao et al. [Citation31] found that targeting circNCLN with its antisense oligonucleotide effectively alleviated LPS-induced ARDS. Li et al. [Citation32] found that circHECTD1 was decreased in LPS-treated alveolar epithelial cells, and circHECTD1 overexpression inhibited the apoptotic rates of the cells and relieved the ARDS progression. Additionally, circFLNA, as a circRNA, is abnormally expressed in many diseases and participates in regulating the development of diseases [Citation20, Citation33]. However, whether circFLNA plays a role in ARDS remains unknown. In current work, our data illustrated that circFLNA was aberrantly upregulated not only in BALF samples collected from patients with ARDS but also in lung tissues and the BALF of ARDS mice.
Recent studies have shown that abnormal immune responses, which determine the outcome of inflammatory responses, are involved in the pathological process of ARDS [Citation34]. Previous studies have shown that decreased immune defense function and excessive inflammatory damage are the main causes of sepsis-induced ARDS and its complications [Citation35]. In particular, CD4+CD25+FoxP3+ Tregs have become a current research hotspot because of their important role in maintaining immune homeostasis and suppressing excessive immune responses [Citation11–13]. Animal experiments have proved that the reduction of CD4+CD25+FoxP3+ Tregs will aggravate the occurrence of autoimmune diseases, while exogenous supplementation of Tregs can alleviate the disease [Citation36]. Although the animal model of septic ARDS has been established, it has been proven that CD4+CD25+FoxP3+ Tregs can alleviate ARDS [Citation7, Citation37]. However, no clinical study has been reported on the relationship between circFLNA levels and the number of Tregs. Therefore, we first proposed a hypothesis: whether there is a correlation between circFLNA and Tregs in patients with ARDS induced by sepsis.
In this work, CD4+CD25+FoxP3+ Tregs number was prominently decreased in ARDS mice; after inhibition of circFLNA, CD4+CD25+FoxP3+ Tregs were obviously upregulated. Meanwhile, the knockdown of circFLNA suppressed the release of TNF-α, Il-1β, and IL-6. It is believed that the release of TNF-α, Il-1β, and IL-6 will increase after the immune cells are stimulated or the lung epithelial cells are damaged. Therefore, the level of inflammatory factors reflects the severity of immune stimulation or tissue injury. Taken together, the knockdown of circFLNA functioned to relieve ARDS.miR-214 is located in the DNM3 gene of q24.3 on human chromosome 1, within the 14th intron of the DNM3 gene [Citation38]. miR-214 is highly conserved in different species, indicating its diversity of physiological functions [Citation39, Citation40]. miR-214 is aberrantly expressed to promote or inhibit cancer [Citation41, Citation42]. miR-214-5p could bind with circFLNA, inhibited miR-214-5p alleviated the function of inhibited circFLNA by elevating CD4+CD25+FoxP3+ Tregs and suppressing TNF-α, Il-1β, and IL-6. Similarly, He et al. [Citation43] found that miR-214 inhibition relieved lung injury and inflammation in ventilator-induced lung injury mice via increasing FGFR1 expression. The PD-1 can be expressed in T cells and has a broad role in immune regulation [Citation44]. PD-1 signaling pathway is linked to sepsis [Citation45, Citation46]. In our current work, our data revealed that PD-1 was a target gene of miR-214-5p and reversed the miR-214-5p-induced effects on CD4+CD25+FoxP3+ Tregs and TNF-α, Il-1β, and IL-6 concentrations. These results indicated that miR-214, as a sponge of circFLNA, was the key to ARDS treatment by targeting PD-1.
Conclusion
These findings indicated a potential mechanism by which disruption of the circFLNA/miR-214-5p/PD-1 axis affected inflammatory factors, which contributed to CD4+CD25+Foxp3+ Tregs development during ARDS. This study provided a theoretical basis for the treatment of ARDS in the future and broadened the understanding of the ceRNA mechanism of circRNA.
Ethical approval
This study was approved by the Ethics Committee of Dongguan Tungwah Hospital.
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. JZ drafted the work and revised it critically for important intellectual content; WZ, LZ, JL and LK were responsible for the acquisition, analysis, or interpretation of data for the work; XL made substantial contributions to the conception or design of the work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Kumar V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front Immunol. 2020;11:1.
- Hwang J, Kim K, Park J, et al. Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis-induced lung injury and inflammation. J Biol Chem. 2019;294(2):608–9. 0101
- Root-Bernstein R. Innate receptor activation patterns involving TLR and NLR synergisms in COVID-19, ALI/ARDS and sepsis cytokine storms: a review and model making novel predictions and therapeutic suggestions. Int J Mol Sci. 2021;22(4):2108.
- Xu Z, Huang Y, Mao P, et al. Sepsis and ARDS: the dark side of histones. Mediators Inflamm. 2015;2015:205054.
- Rostam HM, Singh S, Vrana NE, et al. Impact of surface chemistry and topography on the function of antigen presenting cells. Biomater Sci. 2015;3(3):424–441.
- Chen J, Zhang X, Xie J, et al. Overexpression of TGFbeta1 in murine mesenchymal stem cells improves lung inflammation by impacting the Th17/treg balance in LPS-induced ARDS mice. Stem Cell Res Ther. 2020;11(1):311.
- Zhou M, Fang H, Du M, et al. The modulation of regulatory T cells via HMGB1/PTEN/beta-catenin axis in LPS induced acute lung injury. Front Immunol. 2019;10:1612. 2019-0120
- Yu ZX, Ji MS, Yan J, et al. The ratio of Th17/treg cells as a risk indicator in early acute respiratory distress syndrome. Crit Care. 2015;19(1):82.
- Sakaguchi S, Mikami N, Wing JB, et al. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541–566.
- Eggenhuizen PJ, Ng BH, Ooi JD. Treg enhancing therapies to treat autoimmune diseases. Int J Mol Sci. 2020;21(19):7015.
- Wu T, Zhang L, Xu K, et al. Immunosuppressive drugs on inducing Ag-specific CD4(+)CD25(+)Foxp3(+) treg cells during immune response in vivo. Transpl Immunol. 2012;27(1):30–38. 0801
- Shankar EM, Vignesh R, Velu V, et al. Does CD4 + CD25 + foxp3+ cell (treg) and IL-10 profile determine susceptibility to immune reconstitution inflammatory syndrome (IRIS) in HIV disease? J Inflamm (Lond). 2008;5:2.
- Luz-Crawford P, Kurte M, Bravo-Alegria J, et al. Mesenchymal stem cells generate a CD4 + CD25 + Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4(3):65.
- Ashwal-Fluss R, Meyer M, Pamudurti NR, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56(1):55–66.
- Zhang H, Jiang L, Sun D, et al. CircRNA: a novel type of biomarker for cancer. Breast Cancer. 2018;25(1):1–7.
- Beltran-Garcia J, Osca-Verdegal R, Nacher-Sendra E, et al. Circular RNAs in sepsis: biogenesis, function, and clinical significance. Cells. 2020;9(6):1544.
- Tian C, Liu J, Di X, et al. Exosomal hsa_circRNA_104484 and hsa_circRNA_104670 may serve as potential novel biomarkers and therapeutic targets for sepsis. Sci Rep. 2021;11(1):14141.
- Wan QQ, Wu D, Ye QF. The expression profiles of circRNAs in lung tissues from rats with lipopolysaccharide-induced acute respiratory distress syndrome: a microarray study. Biochem Biophys Res Commun. 2017;493(1):684–689.
- Ye Z, Liu X, Yang Y, et al. The differential expression of novel circular RNAs in an acute lung injury rat model caused by smoke inhalation. J Physiol Biochem. 2018;74(1):25–33.
- Pan J, Huang G, Yin Z, et al. Circular RNA FLNA acts as a sponge of miR-486-3p in promoting lung cancer progression via regulating XRCC1 and CYP1A1. Cancer Gene Ther. 2022;29(1):101–121.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. 0301
- Darlington P, Kullberg S, Eklund A, et al. Subpopulations of cells from bronchoalveolar lavage can predict prognosis in sarcoidosis. Eur Respir J. 2020;55(1):1901450. 0101
- Zhang H, Liu J, Zhou Y, et al. Neutrophil extracellular traps mediate m6A modification and regulates sepsis-associated acute lung injury by activating ferroptosis in alveolar epithelial cells. Int J Biol Sci. 2022;18(8):3337–3357.
- Joung J, Konermann S, Gootenberg JS, et al. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat Protoc. 2017;12(4):828–863.
- Gao Z, Sui J, Fan R, et al. Emodin protects against acute Pancreatitis-Associated lung injury by inhibiting NLPR3 inflammasome activation via Nrf2/HO-1 signaling. Drug Des Devel Ther. 2020;14:1971–1982.
- Yue S, Rao J, Zhu J, et al. Myeloid PTEN deficiency protects livers from ischemia reperfusion injury by facilitating M2 macrophage differentiation. J Immunol. 2014;192(11):5343–5353. 2014-0601
- Zhang Q, Sun Y, Wang C, et al. Circular RNA-microRNA-mRNA network identified circ_0007618 and circ_0029426 as new valuable biomarkers for lung adenocarcinoma. Bioengineered. 2022;13(3):6257–6270.
- Costa J, Gatermann M, Nimtz M, et al. N-glycosylation of extracellular vesicles from HEK-293 and glioma cell lines. Anal Chem. 2018;90(13):7871–7879.
- Qiu N, Xu X, He Y. LncRNA TUG1 alleviates sepsis-induced acute lung injury by targeting miR-34b-5p/GAB1. BMC Pulm Med. 2020;20(1):49.
- Wang Z, Chang P, Ye J, et al. Genome-wide landscape of mRNAs, microRNAs, lncRNAs, and circRNAs in hemorrhagic shock-induced ALI/ARDS in rats. J Trauma Acute Care Surg. 2021;90(5):827–837.
- Cao J, Kuang D, Luo M, et al. Targeting circNCLN/miR-291a-3p/TSLP signaling axis alleviates lipopolysaccharide-induced acute lung injury. Biochem Biophys Res Commun. 2022;617(Pt 1):60–67.
- Li H, Niu X, Shi H, et al. circHECTD1 attenuates apoptosis of alveolar epithelial cells in acute lung injury. Lab Invest. 2022;102(9):945–956.
- Lin S, Wang L, Shi Z, et al. Circular RNA circFLNA inhibits the development of bladder carcinoma through microRNA miR-216a-3p/BTG2 axis. Bioengineered. 2021;12(2):11376–11389.
- Boyd DF, Allen EK, Randolph AG, et al. Exuberant fibroblast activity compromises lung function via ADAMTS4. Nature. 2020;587(7834):466–471.
- Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18.
- Riazifar M, Mohammadi MR, Pone EJ, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13(6):6670–6688.
- D'Alessio FR, Tsushima K, Aggarwal NR, et al. CD4 + CD25 + Foxp3+ tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119(10):2898–2913.
- Tanaka M, Homme M, Yamazaki Y, et al. Cooperation between SS18-SSX1 and miR-214 in synovial sarcoma development and progression. Cancers (Basel). 2020;12(2):324.
- Wang X, Guo B, Li Q, et al. miR-214 targets ATF4 to inhibit bone formation. Nat Med. 2013;19(1):93–100.
- Sun Y, Kuek V, Liu Y, et al. MiR-214 is an important regulator of the musculoskeletal metabolism and disease. J Cell Physiol. 2018;234(1):231–245.
- Xu M, Chen X, Lin K, et al. lncRNA SNHG6 regulates EZH2 expression by sponging miR-26a/b and miR-214 in colorectal cancer. J Hematol Oncol. 2019;12(1):3.
- Wang X, Zhang H, Bai M, et al. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26(3):774–783.
- He K, Han S, An L, et al. Inhibition of MicroRNA-214 alleviates lung injury and inflammation via increasing FGFR1 expression in ventilator-induced lung injury. Lung. 2021;199(1):63–72.
- Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727–742.
- Chen R, Zhou L. PD-1 signaling pathway in sepsis: does it have a future? Clin Immunol. 2021;229:108742.
- Nakamori Y, Park EJ, Shimaoka M. Immune deregulation in sepsis and septic shock: reversing immune paralysis by targeting PD-1/PD-L1 pathway. Front Immunol. 2020;11:624279.
