Abstract
Type 2 inflammation related diseases, such as atopic dermatitis, asthma, and allergic rhinitis, are diverse and affect multiple systems in the human body. It is common for individuals to have multiple co-existing type 2 inflammation related diseases, which can impose a significant financial and living burden on patients. However, the exact pathogenesis of these diseases is still unclear. The NLRP3 inflammasome is a protein complex composed of the NLRP3 protein, ASC, and Caspase-1, and is activated through various mechanisms, including the NF-κB pathway, ion channels, and lysosomal damage. The NLRP3 inflammasome plays a role in the immune response to pathogens and cellular damage. Recent studies have indicated a strong correlation between the abnormal activation of NLRP3 inflammasome and the onset of type 2 inflammation. Additionally, it has been demonstrated that suppressing NLRP3 expression effectively diminishes the inflammatory response, highlighting its promising therapeutic applications. Therefore, this article reviews the role of NLRP3 inflammasome in the development and therapy of multiple type 2 inflammation related diseases.
1. Introduction
Type 2 inflammation related diseases is a broad term used to describe a group of diseases that are caused by type 2 inflammation. Clinically, these diseases include asthma, atopic dermatitis (AD), and allergic rhinitis, and affect the respiratory, gastrointestinal, and dermatological systems. They have emerged as chronic conditions that significantly impact human well-being [Citation1–3]. However, the underlying mechanisms of Type 2 inflammation related diseases remain unclear.
An essential member of the NOD-like receptor (NLR) family, NOD-like receptor thermal protein domain associated protein 3 (NLRP3) binds to pro-caspase-1 and an apoptosis-associated speck-like protein (ASC) in the cytoplasm to form an inflammasome [Citation4,Citation5]. Previous studies have shown that NLRP3 plays a role in the development of diseases like atherosclerosis, obesity, and type 2 diabetes. In the context of type 2 inflammation related diseases, NLRP3 has also been found to play a crucial role in disease progression and is a target for many drugs. Despite the increasing attention given to the NLRP3 inflammasome in recent years, there is still a lack of comprehensive review on its role in type 2 inflammatory related diseases [Citation6–10]. Therefore, the article aims to review the significance of NLRP3 inflammasome in type 2 inflammation related diseases and its potential as a therapeutic target.
2. NLRP3 inflammasome activation
NLRP3, encoded by cold induced autoinflammatory syndrome 1 (CIAS1), is a cytoplasmic nod-like receptor (NLR). The NLRP3 inflammasome is formed when the cell is activated along with apoptosis-associated speck-like protein (ASC) and pro-caspase-1 precursor [Citation11–13]. The generation of the NLRP3 inflammasome involves two signals: priming and activation. In the priming signal, cells recognise danger signals from pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) through TLRs, leading to activation of the NF-κB pathway. This results in the up-regulation of various inflammasome-associated components (pro IL-1β, and pro IL-18), including NLRP3 [Citation11,Citation13–18].
The second signal of activation involves the assembly of NLRP3 inflammasomes and the secretion of IL-1β and IL-18 [Citation19–21]. Current studies indicate that agonists do not directly affect the assembly of inflammasomes, but rather they affect NLRP3 through the activation of common pathways. There are three main mechanisms that regulates the activation of NLRP3 inflammasome ().
Figure 1. The composition and activation of the NLRP3 inflammasome involve two main signals. In the priming signal, Toll-like receptors (TLRs) recognise pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), leading to the activation of the NF-κB pathway. This activation results in the increased expression of various components associated with the inflammasome. In the activation signal, the NLRP3 inflammasome is triggered by factors such as lysosomal damage, reactive oxygen species (ROS), and potassium efflux. Once activated, the inflammasome activates caspase 1, which then cleaves pro-IL-1β and pro-IL-18, generating active IL-1β and IL-18. Additionally, caspase 1 cleaves gasdermin D (GSDMD), and its N-terminal fragment (GSDMD-N) can create pores in the cell membrane, and induce pyroptosis. PAMPs,pathogen-associated molecular patterns; DAMPs,damage-associated molecular patterns; TLR, Toll-like receptor; IL-1β, interleukin-1β; IL-18, interleukin-18; ASC, associated speck-like protein; P2X7, P2X purinoceptor 7; ROS, reactive oxygen species.
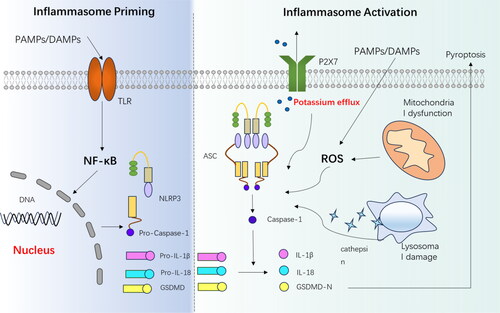
2.1. Mechanisms associated with ion channels and calcium signalling
In studies involving NLRP3 agonists such as ATP, Nigerian bacteriocins, particulate matter, perfringolysin O, and streptolysin O, it has been observed that a decrease in intracellular potassium ion concentration is a critical requirement for NLRP3 inflammasome activation [Citation22,Citation23]. Extracellular ATP can activate the P2X7 receptor (P2X7R), which is an ATP-ligand-gated ion channel located on the cell membrane. This activation allows intracellular potassium ions to enter the extracellular compartment, thereby reducing the intracellular potassium ion concentration [Citation24,Citation25]; Bacterial toxins such as perfringolysin O and streptolysin O can form membrane pores on the cell membrane surface, resulting in potassium ion efflux and inflammasome activation [Citation23,Citation26].
In addition to potassium channels, chloride channels, and calcium signalling may also be involved. In the absence of exogenous ATP, CASR agonists can activate the NLRP3 inflammasome, leading to the release of calcium ions from stores and the production of the inflammasome. At the same time, CASR can also decrease intracellular cyclic AMP (cAMP) levels, which promotes inflammasome production. This is because cAMP can bind to NLRP3 and inhibit inflammasome assembly [Citation27,Citation28]. Furthermore, previous studies have shown that chloride intracellular channel proteins (CLICS) 1-4 are involved in NLRP3 inflammasome activation. These proteins facilitate chloride efflux, which is a process downstream of ROS activation and is associated with the production of IL-1β [Citation29,Citation30].
2.2. Elevated reactive oxygen species (ROS) and mitochondrial damage
The mechanism of ROS action is not fully understood, but it is clear that thioredoxin interacting protein (TXNIP), a ligand for NLRP3, is sensitive to ROS. Under normal physiological conditions, the oxidoreductase thioredoxin (TRX) binds to TXNIP and inhibits its activity. When ROS levels are elevated, the TRX-TXNIP complex dissociates, allowing TXNIP to bind to NLRP3 (mainly the structural domain of LRRs), which in turn activates NLRP3 inflammasome. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, as a common activator of ROS, promotes the activation of inflammasomes in this form [Citation31]. Paraquat and silymarin both act through a similar mechanism to NADPH, with paraquat upregulating the production of ROS and TXINP, inducing the formation of NLRP3 inflammasome and leading to cell death, while silymarin inhibits the toxicity of paraquat by increasing the production of TRX and antioxidant enzymes [Citation32].
Apart from ROS agonists, Rongbin Zhou et al. demonstrated that inhibition of mitosis/autophagy leads to the production and accumulation of ROS. This, in turn, activates the formation of the NLRP3 inflammasome. Conversely, mitochondria are an important source of ROS, dysregulation of mitochondrial activity, achieved by inhibiting voltage-gated anion channels, prevents ROS activation and NLRP3 inflammasome production [Citation33,Citation34]. All of this highlights the importance of ROS production in NLRP3 inflammasome activation.
2.3. Lysosomal damage
Lysosomes, which are organelles found in eukaryotic cells, contain a diverse range of hydrolytic enzymes including protease, nuclease, phosphatase, and lipase. These enzymes are responsible for breaking down biological macromolecules such as proteins, nucleic acids, and polysaccharides. When exogenous substances like silica and asbestos enter the human body, macrophages engulf them and convert them into crystals or particles. These substances can cause damage to lysosomes, leading to the release of cathepsin and activation of NLRP3 inflammasomes. This mechanism of lysosomal damage is also influenced by the efflux of potassium ions and is associated with various particulate matter like uric acid, silica, and cholesterol [Citation35–37].
3. The role of NLRP3 inflammasome in type 2 inflammatory-related diseases
Type 2 inflammation is an adaptive immune response triggered by antigenic stimuli, such as bacteria, viruses, parasites, etc. This response occurs through antigen presentation by dendritic cells (DCs) and activation of ICL2 cells, which induce Th2 lymphocytes to secrete type 2 cytokines like IL-4, IL-5, IL-13, etc. IL-4 prompts B cells to differentiate into plasma cells that produce specific IgE antibodies and bind to high-affinity receptors (FcRI) on the surface of mast cells and basophils, which sensitises the body [Citation2]. Upon exposure to allergens again, IgE recognises antigenic molecules, leading to the activation and degranulation of mast cells and basophils. This process results in the release of enzymes, lipids, various cytokines, and other agents, leading to pathophysiological processes like pruritus, exudation, apoptosis, and allergic diseases such as atopic dermatitis, asthma, allergic rhinitis, and food allergy [Citation38–42]. Type 2 inflammation related diseases including atopic dermatitis, contact allergic dermatitis (ACD), asthma, and allergic rhinitis, is a general term for a range of diseases that are due to type 2 inflammation. Multiple studies have found that NLRP3 inflammasome plays a vital role in type 2 inflammation related diseases. Therefore, we will summarise the important role of NLRP3 inflammasome in the pathogenesis and treatment of atopic dermatitis, contact allergic dermatitis (ACD), asthma, and allergic rhinitis in the following work.
3.1. Atopic dermatitis (AD)
Atopic dermatitis (AD) is an inflammatory skin disease that arises from a combination of genetic and environmental factors, as well as epidermal barrier dysfunction and skin flora disorders. The activation of Th2 cells and the subsequent release of cytokines like IL-4, IL-13, and IL-31 play a crucial role in inducing an inflammatory response. This response involves various processes such as the activation of keratinocytes, the induction of pruritus, and the chemotaxis of inflammatory cells [Citation43–45]. As early as 2011, it was shown that house dust mites (HDM) stimulate keratinocytes to recruit NLRP3, ASC, and caspase-1 to the perinuclear region, leading to the formation of inflammasomes. This process results in the secretion of IL-1β and IL-18 in an inflammasome-dependent manner [Citation46–48]. Furthermore, it has been observed that UVB exposure to the eyes of mice increases plasma levels of ACTH and the inflammasome NLRP3, thereby exacerbating AD. These findings collectively support a connection between increased NLRP3 expression and AD [Citation49]. The current study revealed that the activation of NLRP3 inflammasomes in keratinocytes was linked to the production of reactive oxygen species (ROS) and the activation of the NF-κB signalling pathway [Citation50]. Subsequent investigations demonstrated that the phosphorylation of p38, ERK, and JNK in keratinocytes triggered the activation of the MAPK pathway. This, in turn, stimulated the intranuclear translocation of NF-κB through the activation of AP-1, resulting in the activation of inflammasomes via the MAPK/AP-1/NF-κB pathway [Citation51,Citation52]. Apart from skin lesions, the onset of AD is often accompanied by mood disorders. NLRP3 inflammasomes serve as a crucial target for psychiatric conditions like anxiety and depression. A recent study observed a significant increase in NLRP3 inflammasomes and caspase-1 levels in the hippocampus of mice in a DNFB-induced animal model of AD. These levels were found to be correlated with the duration of psychiatric disordered behaviour in mice [Citation53,Citation54]. Interestingly, however, the NLRP3 inflammasome does not play an entirely negative role: M Niebuhr et al. showed that Th2 cytokines (IL-4, IL-13, etc.) downregulated NLRP3 inflammasome production in primary keratinocytes, whereas Th1-type cytokines (IFN-γ) upregulated its expression, a phenomenon associated with a propensity for co-infection with Staphylococcus aureus in AD patients [Citation55]. In addition to the inflammasome, NLRP3 can also function independently in AD. In this case, NLRP3 interacts with the transcription factor IPF4 to bind to the IL-33-specific promoter in the nucleus of the keratinocyte, resulting in increased secretion of IL-33 and worsening of AD [Citation56].
Targeting the NLRP3 inflammasome has been shown to have a therapeutic effect on AD in several studies. Additionally, non-targeted drugs or herbal extracts have also demonstrated benefits by inhibiting NLRP3 inflammasome. For instance, Mdivi-1 inhibits mitochondrial fission in keratinocytes, blocking the NF-κB pathway and consequently inhibiting inflammasome activation and cytokine release. Another traditional herbal ingredient, Cicadidae Periostracum (CP), also exhibits therapeutic effects by inhibiting inflammasome activation [Citation51,Citation52 ,Citation57–61]. It is important to note that apart from directly inhibiting the NLRP3 inflammasome, heparinoids have been found to reduce IL-1β expression in keratinocytes by inhibiting the activation of extracellular signal-regulated kinases and the p38 pathway. This reduction in expression of inflammasome-related components, without affecting caspase-1 and inflammasome activation, leads to a therapeutic effect [Citation62]. Recent years have witnessed a gradual increase in therapeutic research, further highlighting the therapeutic potential of targeting the NLRP3 inflammasome.
3.2. Contact allergic dermatitis (ACD)
Contact Allergic Dermatitis (ACD) is a commonly occurring occupational disease that is classified as a type IV hypersensitivity reaction. This reaction consists of two phases: the sensitisation phase and the activation phase. During the sensitisation phase, semi-antigen activation triggers the release of DAMPs from epidermal cells. These DAMPs are then recognised by pattern recognition receptors (PRRs) like Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in dendritic cells. Subsequently, the dendritic cells migrate to the lymph nodes, where they activate naive T cells to differentiate into cytotoxic T lymphocytes (CTLs) and helper cells (THs), which then migrate to various peripheral sites. The activation phase occurs when the body is re-exposed to the sensitiser, leading to T-cell recruitment and an immune response [Citation63,Citation64]. In ACD, it is well-known that allergens trigger the release of DAMPs (such as mtROS, ATP, cardiolipin, etc.), which in turn activate the NF-κB inflammatory pathway in keratinocytes. This activation induces the NLRP3 inflammasome to release cytokines like IL-1β, IL-18, and caspase-1 and promotes dendritic cell migration, recruitment, and maturation in living organisms. However, it should be noted that the DAMPs produced by the same allergen are not always isolated. For example, TNCB can cause the release of ATP, uric acid, and cathepsins. The interaction of multiple DAMPs further enhances the expression of the NLRP3 inflammasome, leading to the onset of ACD [Citation65–67].
In terms of therapeutic approaches, Mohamed F Balaha et al. discovered that epimedine A inhibited keratinocyte pyroptosis and improved DNFB-induced ACD in mice by targeting the NF-κB/NLRP3 pathway [Citation67]. Additionally, pterostilbene has been reported to inhibit NLRP3 inflammasome-related IL-1β, providing a potential treatment for hexavalent chromium-induced dermatitis [Citation68]. Although many experiments have proved the therapeutic effect of inhibiting NLRP3 on ACD, specific drug research is still limited.In addition, due to the variety of allergens in ACD, the common pathway of different allergens just proves its importance. In the future, it is worth further exploring drugs.
3.3. Asthma
Asthma is a common allergic respiratory disease that typically begins in childhood. This chronic inflammatory disease is characterised by reversible airflow limitation, leading to recurrent symptoms such as wheezing, shortness of breath, chest tightness, and cough. These symptoms often worsen at night and/or early in the morning. Pathologically, asthma is characterised by eosinophilic inflammation and T-lymphocyte inflammation with CD4 aggregates [Citation69]. A 2009 study identified an association between rs4612666, one of 15 NLRP3 single nucleotide polymorphisms (SNPs), and aspirin-induced asthma [Citation70,Citation71]. Subsequent research found that the expression of the NLRP3 inflammasome and its downstream products, caspase-1 and IL-1β, was increased in bronchoalveolar lavage fluid in a mouse model of asthma. The activation of the NLRP3 inflammasome and IL-1β was closely linked to Th2 cell activation, type 2 cytokine secretion, chemokine secretion, and steroid resistance, but the effects may vary between allergens [Citation72–78]; additionally, IL-1β and caspase-1 can worsen the disease by increasing the secretion of IL-17A in (TCR)β(+) Th17 cells, as well as inducing IL-1β production and raising the amount of ICL3 in the lungs [Citation79–81]. In DCs, High mobility protein 1 (HMGB1) expression and secretion are induced by the NLRP3 inflammasome, which mediates airway inflammation through the ATP/P2X7-NLRP3 axis and increases Th2 and Th17 inflammatory responses in a mouse model of asthma [Citation82]. Interestingly, in contrast to atopic dermatitis, NLRP3 inflammasome-activated caspase 1 reduces IL-33 expression, which inhibits lung inflammation induced by house dust mites [Citation83]. Furthermore, NLRP3 can bind to the IL-4 promoter and co-activate it with the IL-4 transcription factor IRF4 in CD4+ T cells, promoting Th2 polarisation and contributing to the onset of asthma [Citation4]. NLRP3 also promotes M2 cell differentiation in monocytes through a similar mechanism, further promoting inflammation [Citation84].
Therapeutically, Sheng-Jie Yu et al. discovered that in addition to NLRP3 inhibitors, the cell-permeable peptide of human eosinophil cationic protein (CPPecp) inhibited house dust mite-induced pneumonia by suppressing inflammasome activation [Citation85–87]. Similarly, Xue Liu et al. found that Yupingfeng San (YPFS) had beneficial therapeutic effects by inhibiting the activation of the NLRP3 inflammasome and its components. Treatment of asthmatic mice with YPFS resulted in reduced clinical symptoms and inflammatory cell infiltration in lung tissue [Citation88]. Lixia Wang et al. demonstrated that sevoflurane alone can improve asthma by inhibiting NLRP3 expression without involving the inflammasome [Citation89]. Furthermore, suhuang antitussive capsule, schisandrin B, haem oxygenase-1 (HO-1), and certain ethnic traditional remedies have also shown promising efficacy in inhibiting the NLRP3 inflammasome and its related components [Citation90–96]. Based on current research on mechanisms and treatments, although the activation mechanism of NLRP3 in asthma is not fully understood, it is certain that the NLRP3 inflammasome and related pathways play an important role in the pathogenesis of asthma. In addition, recent research on asthma has mainly focused on drug treatment, whether it is the exploration of the mechanism of new drugs or the supplement of the mechanism of old drugs, which can all show the important role of NLRP3 in the pathogenesis of asthma. This also indirectly proves the results of mechanism research. It is believed that future targeted drugs that precisely target NLRP3 will bring good therapeutic effects to patients with asthma.
3.4. Allergic rhinitis
In recent decades, there has been a significant increase in the prevalence of allergic rhinitis, making it a global health concern. This condition occurs when Th2 cells are stimulated to release cytokines like IL-4 and IL-5, leading to the development and expression of IgE antibodies by B cells, and subsequent activation of mast cells [Citation97]. The activation of mast cells triggers a series of events, including vasodilation, increased permeability, itching, nasal discharge, and mucus secretion [Citation98,Citation99]. A Månsson et al. discovered that patients with diseases such as chronic sinusitis exhibit elevated expression of NLR receptors, including NLRP3, which can be reduced through topical steroid treatment [Citation100]; similar findings were reported by J Bogefors, who confirmed that NLRP3 Inflammasome expression is increased in allergic rhinitis and correlates with disease severity [Citation101]. Previous studies have primarily focused on patient biopsies, but subsequent research has made significant progress by exploring cellular and animal models. These studies have revealed that reactive oxygen species (ROS) in macrophages play a crucial role in activating the NLRP3 inflammasome. Activation of the NLRP3 inflammasome leads to increased expression of various cytokines including IL-1β, IL-18, caspase-1, IL-6, IL-10, IL-12, IL-13, IL-17, and inhibits RBCK1. This, in turn, results in macrophage scorching, disease exacerbation, and tissue damage [Citation102–106]. In addition, recent studies have found that dendritic cell pyroptosis induced by allergens promotes the development of allergic rhinitis through GSDMD-N-mediated cell pyroptosis, further supplementing the role of the NLRP3 inflammasome in allergic rhinitis [Citation107]. However, allergic rhinitis is a disease process involving multiple cell types, and the mechanisms involved in other cell types still need to be further explored.
In recent years, there has been a growing interest in the treatment of allergic rhinitis through the inhibition of NLRP3 inflammasome expression. Wo et al. found that human placenta extract can modulate macrophage polarisation by reducing NLRP3 inflammasome and immunity-related GTPase M (IRGM) expression. This leads to the suppression of M1 macrophages and the enhancement of M2 macrophages, providing mucosal protection [Citation108]. Another study showed that the pneumococcal mutant Δ pep27 can reduce inflammation by inhibiting the activation of the NLRP3 inflammasome through Toll-like receptor expression [Citation109]. MicroRNA research is also of great importance in this field, as previous studies have demonstrated that reducing miR-205-5p or overexpressing miR-224-5p can suppress NLRP3 inflammasome expression for therapeutic purposes [Citation110,Citation111]. We can observe that inhibitors targeting NLRP3 exhibit promising therapeutic effects, and numerous drugs also demonstrate good therapeutic potential in the NLRP3-related pathway. However, this is not primarily a study of the drug’s primary mechanism, but rather a complement to the mechanism of drug treatment. Its specific therapeutic role still needs to be further explored in disease models, and in the future, deeper research on marketable drugs will also be required.
3.5. Parasitic infections
Type 2 immune responses are important for protecting against parasites and promoting tissue repair, controlling inflammation, and expelling parasites [Citation112,Citation113]. In the context of parasitic infections, NLRP3 inflammasomes have a dual role. In Trypanosoma cruzi infections, NLRP3 inflammasomes, dependent on caspase-1, produce NO to control the disease [Citation114]. This is similar to Toxoplasma gondii infections [Citation115]. However, in mice infected with the intestinal parasite Heligmosomoides polygyrus, IL-1β secreted by the NLRP3 inflammasome inhibits ILC2 and suppresses IL-25 expression, leading to an incomplete expression of type 2 inflammatory response. This creates an environment in the intestinal mucosa that is suitable for parasite growth, reduces parasite clearance, and induces chronic infection [Citation116]. Similarly, the release of IL-18 by the NLRP3 inflammasome in Trichuris-infected mice reduces the type 2 inflammatory response, resulting in reduced production of Th2 cytokines and exacerbating the infection [Citation117]. NLRP3 can function independently of the inflammasome in mice infected with Ancylostoma braziliense. It promotes exacerbation by suppressing the type 2 inflammatory response, pulmonary neutrophils, and parasite death [Citation118].
On the other hand, the role of NLRP3 may vary in infections with the same parasite. In Trichinella infection, the NLRP3 inflammasome can have a defensive role in reducing larval burden by activating DCs to produce higher levels of IL-4, IL-10, and TGF-β [Citation119]; however, it has also been observed that in Trichinella infection, the NLRP3 inflammasome in macrophages reduces the expression of Th2 cytokines while increasing the expression of the Th1 cytokine INFγ, resulting in an increased burden of adult and larval parasites in muscle [Citation120]. NLRP3 inflammasomes act as insecticides in Leishmania infections by stimulating macrophages to produce IL-1β and reactive oxygen species (ROS) [Citation121], however, the induced secretion of IL-18 by NLRP3 inflammasomes can disrupt the balance of the Th1/Th2 immune response, reducing Th2 cytokines and the resistance of mice to Leishmania protozoa [Citation122]. Given the variability of consequences across different pathogens and sites, it is important to consider the role of NLRP3 in parasitic infections on a case-by-case basis.
Several studies have reported therapeutic effects by inhibiting NLRP3 inflammasome expression through infection-related mechanisms. For instance, Xin Liu et al. found that taurine can reduce NLRP3-dependent liver damage in schistosome infection [Citation123]; praziquantel has also been shown to attenuate M1 macrophage activity by inhibiting NLRP3 inflammasome [Citation124]. Additionally, Xuemin Jin et al. demonstrated that shiitake polysaccharide can reduce parasite burden in mice in an NLRP3-dependent manner [Citation125]. These findings highlight the potential of NLRP3 inflammasome as a target for the treatment of parasitic diseases.
4. Conclusion
In this article, we summarise the process of NLRP3 inflammasome assembly and activation. It aggravates type 2 inflammation related diseases such as AD, asthma, and allergic rhinitis by promoting the release of inflammatory factors, pyroptosis, and inhibiting mucosal immunity, etc. On the therapeutic front, several drugs, both previously used and novel agents, have demonstrated effectiveness in animal models, highlighting the potential for targeting the NLRP3 inflammasome in type 2 inflammation related diseases. Although there is currently no targeted drug available for NLRP3, the therapeutic effects of current drugs are enough to highlight the clinical significance of studying targeted drugs. Therefore, it is important to continue our research on the NLRP3 inflammasome. Future research should focus on investigating the mechanism of the NLRP3 inflammasome in different cell types and developing new drugs that specifically target the human NLRP3 inflammasome for the benefit of patients.
Author contributions
All authors contributed to the conception, writing and revision of this article.
Acknowledgements
I would like to thank my supervisor for his guidance through each stage of the process.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gandhi NA, Bennett BL, Graham NMH, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):1–9.
- Busse WW, Kraft M, Rabe KF, et al. Understanding the key issues in the treatment of uncontrolled persistent asthma with type 2 inflammation. Eur Respir J. 2021;58(2):2003393.
- Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135(3):626–635.
- Bruchard M, Rebé C, Derangère V, et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16(8):859–870.
- Sharma BR, Kanneganti T-D. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22(5):550–559.
- Hoseini Z, Sepahvand F, Rashidi B, et al. NLRP3 inflammasome: its regulation and involvement in atherosclerosis. J Cell Physiol. 2018;233(3):2116–2132.
- Luo B, Huang F, Liu Y, et al. NLRP3 inflammasome as a molecular marker in diabetic cardiomyopathy. Front Physiol. 2017;8:519.
- Vandanmagsar B, Youm Y-H, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188.
- Wu Y, Bian Y, Fei J, et al. Isorhynchophylline attenuates proliferation and migration of synovial fibroblasts via the FOXC1/β-catenin axis. Autoimmunity. 2023;56(1):2289868.
- Chen X, Li W, Chang C. NR3C2 mediates oxidised low-density lipoprotein-induced human coronary endothelial cells dysfunction via modulation of NLRP3 inflammasome activation. Autoimmunity. 2023;56(1):2189135.
- Shao B-Z, Xu Z-Q, Han B-Z, et al. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol. 2015;6:262.
- Akbal A, Dernst A, Lovotti M, et al. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell Mol Immunol. 2022;19(11):1201–1214.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–2127.
- Hoffman HM, Mueller JL, Broide DH, et al. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29(3):301–305.
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426.
- Agostini L, Martinon F, Burns K, et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20(3):319–325.
- He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41(12):1012–1021.
- Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13(4):325–332.
- Lamkanfi M, Kanneganti T-D. Nlrp3: an immune sensor of cellular stress and infection. Int J Biochem Cell Biol. 2010;42(6):792–795.
- Zhang Z, Zhu T, Zhang L, et al. Critical influence of cytokines and immune cells in autoimmune gastritis. Autoimmunity. 2023;56(1):2174531.
- Muñoz-Planillo R, Kuffa P, Martínez-Colón G, et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–1153.
- Greaney AJ, Leppla SH, Moayeri M. Bacterial exotoxins and the inflammasome. Front Immunol. 2015;6:570.
- Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232.
- Kahlenberg JM, Dubyak GR. Differing caspase-1 activation states in monocyte versus macrophage models of IL-1beta processing and release. J Leukoc Biol. 2004;76(3):676–684.
- Yan R, Liang X, Hu J. miR-141-3p alleviates ulcerative colitis by targeting SUGT1 to inhibit colonic epithelial cell pyroptosis. Autoimmunity. 2023;56(1):2220988.
- Rossol M, Pierer M, Raulien N, et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun. 2012;3(1):1329.
- Lee G-S, Subramanian N, Kim AI, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492(7427):123–127.
- Tang T, Lang X, Xu C, et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun. 2017;8(1):202.
- Domingo-Fernández R, Coll RC, Kearney J, et al. The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL-1β transcription and activate the NLRP3 inflammasome. J Biol Chem. 2017;292(29):12077–12087.
- van Bruggen R, Köker MY, Jansen M, et al. Human NLRP3 inflammasome activation is Nox1-4 independent. Blood. 2010;115(26):5398–5400.
- Liu Z, Sun M, Wang Y, et al. Silymarin attenuated paraquat-induced cytotoxicity in macrophage by regulating trx/TXNIP complex, inhibiting NLRP3 inflammasome activation and apoptosis. Toxicol in Vitro. 2018;46:265–272.
- Zhou R, Yazdi AS, Menu P, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225.
- Bae JY, Park HH. Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J Biol Chem. 2011;286(45):39528–39536.
- Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847–856.
- Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241.
- Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361.
- Gause WC, Rothlin C, Loke P. N Heterogeneity in the initiation, development and function of type 2 immunity. Nat Rev Immunol. 2020;20(10):603–614.
- Lloyd CM, Snelgrove RJ. Type 2 immunity: expanding our view. Sci Immunol. 2018;3(25):eaat1604.
- Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337(6093):431–435.
- Spits H, Mjösberg J. Heterogeneity of type 2 innate lymphoid cells. Nat Rev Immunol. 2022;22(11):701–712.
- von Moltke J, Locksley RM. I-L-C-2 it: type 2 immunity and group 2 innate lymphoid cells in homeostasis. Curr Opin Immunol. 2014;31:58–65.
- Ständer S. Atopic dermatitis. N Engl J Med. 2021;384(12):1136–1143.
- Schuler CF, Billi AC, Maverakis E, et al. Novel insights into atopic dermatitis. J Allergy Clin Immunol. 2023;151(5):1145–1154.
- Silverberg JI. Atopic dermatitis. JAMA Dermatol. 2014;150(12):1380.
- Dai X, Sayama K, Tohyama M, et al. Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. J Allergy Clin Immunol. 2011;127(3):806–814.e4.
- Sroka-Tomaszewska J, Trzeciak M. Molecular mechanisms of atopic dermatitis pathogenesis. Int J Mol Sci. 2021;22:4130.
- Zhang Q, Fan HW, Zhang JZ, et al. NLRP3 rs35829419 polymorphism is associated with increased susceptibility to multiple diseases in humans. Genet Mol Res. 2015;14(4):13968–13980.
- Hiramoto K, Yamate Y, Yokoyama S. Ultraviolet B eye irradiation aggravates atopic dermatitis via adrenocorticotropic hormone and NLRP3 inflammasome in NC/nga mice. Photodermatol Photoimmunol Photomed. 2018;34(3):200–210.
- Chang Q-X, Lyu J-L, Wu P-Y, et al. Coffea arabica extract attenuates atopic dermatitis-like skin lesions by regulating NLRP3 inflammasome expression and skin barrier functions. Int J Mol Sci. 2023;24:12367.
- Kim K-M, Kim S-Y, Mony TJ, et al. Moringa concanensis L. alleviates DNCB-induced atopic dermatitis-like symptoms by inhibiting NLRP3 inflammasome-mediated IL-1β in BALB/c mice. Pharmaceuticals (Basel). 2022;15(10):1217.
- Liu W, Song W, Luo Y, et al. Angelica yinzi alleviates 1-chloro-2,4-dinitrobenzene-induced atopic dermatitis by inhibiting activation of NLRP3 inflammasome and down-regulating the MAPKs/NF-kB signaling pathway. Saudi Pharm J. 2022;30(10):1426–1434.
- Yuan H, Sun Y, Zhang S, et al. NLRP3 neuroinflammatory factors may be involved in atopic dermatitis mental disorders: an animal study. Front Pharmacol. 2022;13:966279.
- Yuan H, Tang Y, Zhang S, et al. NLRP3 neuroinflammatory intervention of Mahuang-Lianqiao-Chixiaodou decoction for mental disorders in atopic dermatitis mice. J Ethnopharmacol. 2024;319(Pt 2):117263.
- Niebuhr M, Baumert K, Heratizadeh A, et al. Impaired NLRP3 inflammasome expression and function in atopic dermatitis due to Th2 milieu. Allergy. 2014;69(8):1058–1067.
- Zheng J, Yao L, Zhou Y, et al. A novel function of NLRP3 independent of inflammasome as a key transcription factor of IL-33 in epithelial cells of atopic dermatitis. Cell Death Dis. 2021;12(10):871.
- Li L, Mu Z, Liu P, et al. Mdivi-1 alleviates atopic dermatitis through the inhibition of NLRP3 inflammasome. Exp Dermatol. 2021;30(12):1734–1744.
- Park G, Moon BC, Ryu SM, et al. Cicadidae periostracum attenuates atopic dermatitis symptoms and pathology via the regulation of NLRP3 inflammasome activation. Oxid Med Cell Longev. 2021;2021:8878153–8878116.
- Lin C-Y, Hsieh Y-T, Chan LY, et al. Dictamnine delivered by PLGA nanocarriers ameliorated inflammation in an oxazolone-induced dermatitis mouse model. J Control Release. 2021;329:731–742.
- Ghaderpour A, Jeong J-Y, Kim Y-H, et al. Taurodeoxycholate, a GPCR19 agonist, ameliorates atopic dermatitis in balb/c mice. Eur J Immunol. 2023;53(5):e2250048.
- Bai X, Rao X, Wang Y, et al. A homogeneous lonicera japonica polysaccharide alleviates atopic dermatitis by promoting Nrf2 activation and NLRP3 inflammasome degradation via p62. J Ethnopharmacol. 2023;309:116344.
- Utsunomiya R, Dai X, Murakami M, et al. Heparinoid suppresses der p-induced IL-1β production by inhibiting ERK and p38 MAPK pathways in keratinocytes. Exp Dermatol. 2018;27(9):981–988.
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7(1):38.
- Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149(4):1162–1171.
- Sebastião AI, Ferreira I, Brites G, et al. NLRP3 inflammasome and allergic contact dermatitis: a connection to demystify. Pharmaceutics. 2020;12(9):867.
- Corsini E, Galbiati V, Nikitovic D, et al. Role of oxidative stress in chemical allergens induced skin cells activation. Food Chem Toxicol. 2013;61:74–81.
- Balaha MF, Ahmed NJ, Almalki ZS, et al. Epimedin a ameliorates DNFB-induced allergic contact dermatitis in mice: role of NF-κB/NLRP3-driven pyroptosis, Nrf2/HO-1 pathway, and inflammation modulation. Life Sci. 2022;302:120653.
- Wang B, Jr., Chiu H-W, Lee Y-L, et al. Pterostilbene attenuates hexavalent Chromium-Induced allergic contact dermatitis by preventing cell apoptosis and inhibiting IL-1β-related NLRP3 inflammasome activation. J Clin Med. 2018;7:489.
- Boulet L-P, Reddel HK, Bateman E, et al. The global initiative for asthma (GINA): 25 years later. Eur Respir J. 2019;54(2):1900598.
- Hitomi Y, Ebisawa M, Tomikawa M, et al. Associations of functional NLRP3 polymorphisms with susceptibility to food-induced anaphylaxis and aspirin-induced asthma. J Allergy Clin Immunol. 2009;124(4):779–785.e6.
- Mangan MSJ, Olhava EJ, Roush WR, et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17(8):588–606.
- Cheng C, Wu H, Wang M, et al. Estrogen ameliorates allergic airway inflammation by regulating activation of NLRP3 in mice. Biosci Rep. 2019;39:BSR20181117.
- Besnard AG, Guillou N, Tschopp J, et al. NLRP3 inflammasome is required in murine asthma in the absence of aluminum adjuvant. Allergy. 2011;66(8):1047–1057.
- Allen IC, Jania CM, Wilson JE, et al. Analysis of NLRP3 in the development of allergic airway disease in mice. J Immunol. 2012;188(6):2884–2893.
- Kim RY, Pinkerton JW, Essilfie AT, et al. Role for NLRP3 inflammasome-mediated, IL-1β-dependent responses in severe, steroid-resistant asthma. Am J Respir Crit Care Med. 2017;196(3):283–297.
- Kim BG, Lee PH, Lee SH, et al. Effect of TiO2 nanoparticles on inflammasome-mediated airway inflammation and responsiveness. Allergy Asthma Immunol Res. 2017;9(3):257–264.
- Huang C, Wang J, Zheng X, et al. Commensal bacteria aggravate allergic asthma via NLRP3/IL-1β signaling in post-weaning mice. J Autoimmun. 2018;93:104–113.
- Rodríguez-Alcázar JF, Ataide MA, Engels G, et al. Charcot-Leyden crystals activate the NLRP3 inflammasome and cause IL-1β inflammation in human macrophages. J Immunol. 2019;202(2):550–558.
- Ather JL, Ckless K, Martin R, et al. Serum amyloid a activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol. 2011;187(1):64–73.
- Kim HY, Lee HJ, Chang Y-J, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20(1):54–61.
- Martin RA, Ather JL, Lundblad LKA, et al. Interleukin-1 receptor and caspase-1 are required for the Th17 response in nitrogen dioxide-promoted allergic airway disease. Am J Respir Cell Mol Biol. 2013;48(5):655–664.
- Li R, Wang J, Li R, et al. ATP/P2X7-NLRP3 axis of dendritic cells participates in the regulation of airway inflammation and hyper-responsiveness in asthma by mediating HMGB1 expression and secretion. Exp Cell Res. 2018;366(1):1–15.
- Madouri F, Guillou N, Fauconnier L, et al. Caspase-1 activation by NLRP3 inflammasome dampens IL-33-dependent house dust mite-induced allergic lung inflammation. J Mol Cell Biol. 2015;7(4):351–365.
- Liu Y, Gao X, Miao Y, et al. NLRP3 regulates macrophage M2 polarization through up-regulation of IL-4 in asthma. Biochem J. 2018;475(12):1995–2008.
- Yu S-J, Liao E-C, Sheu M-L, et al. Cell-penetrating peptide derived from human eosinophil cationic protein inhibits mite allergen der p 2 induced inflammasome activation. PLoS One. 2015;10(3):e0121393.
- Ma M, Li G, Qi M, et al. Inhibition of the inflammasome activity of NLRP3 attenuates HDM-Induced allergic asthma. Front Immunol. 2021;12:718779.
- Chen L, Hou W, Liu F, et al. Blockade of NLRP3/caspase-1/IL-1β regulated Th17/treg immune imbalance and attenuated the neutrophilic airway inflammation in an Ovalbumin-Induced murine model of asthma. J Immunol Res. 2022;2022:9444227–9444211.
- Liu X, Shen J, Fan D, et al. Yupingfeng san inhibits NLRP3 inflammasome to attenuate the inflammatory response in asthma mice. Front Pharmacol. 2017;8:944.
- Wang L, Zha B, Shen Q, et al. Sevoflurane inhibits the Th2 response and NLRP3 expression in murine allergic airway inflammation. J Immunol Res. 2018;2018:9021037–9021038.
- Tomani JCD, Gainkam LOT, Nshutiyayesu S, et al. An ethnobotanical survey and inhibitory effects on NLRP3 inflammasomes/caspase-1 of herbal recipes’ extracts traditionally used in Rwanda for asthma treatment. J Ethnopharmacol. 2018;227:29–40.
- Lv J, Su W, Yu Q, et al. Heme oxygenase-1 protects airway epithelium against apoptosis by targeting the proinflammatory NLRP3-RXR axis in asthma. J Biol Chem. 2018;293(48):18454–18465.
- Qin W, Wu X, Jia Y, et al. Suhuang antitussive capsule inhibits NLRP3 inflammasome activation and ameliorates pulmonary dysfunction via suppression of endoplasmic reticulum stress in cough variant asthma. Biomed Pharmacother. 2019;118:109188.
- Zhu C, Zhang L, Liu Z, et al. Atractylenolide III reduces NLRP3 inflammasome activation and Th1/Th2 imbalances in both in vitro and in vivo models of asthma. Clin Exp Pharmacol Physiol. 2020;47(8):1360–1367.
- Chen X, Xiao Z, Jiang Z, et al. Schisandrin B attenuates airway inflammation and airway remodeling in asthma by inhibiting NLRP3 inflammasome activation and reducing pyroptosis. Inflammation. 2021;44(6):2217–2231.
- Xu L-T, Wang T, Fang K-L, et al. The ethanol extract of flower buds of tussilago farfara L. attenuates cigarette smoke-induced lung inflammation through regulating NLRP3 inflammasome, Nrf2, and NF-κB. J Ethnopharmacol. 2022;283:114694.
- Jiang H, Bai Z, Ou Y, et al. β-Hydroxybutyric acid upregulated by suhuang antitussive capsule ameliorates cough variant asthma through GSK3β/AMPK-Nrf2 signal axis. J Ethnopharmacol. 2023;307:116013.
- Ke X, Chen Z, Wang X, et al. Quercetin improves the imbalance of Th1/Th2 cells and treg/Th17 cells to attenuate allergic rhinitis. Autoimmunity. 2023;56(1):2189133.
- Small P, Keith PK, Kim H. Allergic rhinitis. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):51.
- Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S103–S115.
- Månsson A, Bogefors J, Cervin A, et al. NOD-like receptors in the human upper airways: a potential role in nasal polyposis. Allergy. 2011;66(5):621–628.
- Bogefors J, Rydberg C, Uddman R, et al. Nod1, Nod2 and Nalp3 receptors, new potential targets in treatment of allergic rhinitis? Allergy. 2010;65(10):1222–1226.
- Zhou H, Zhang W, Qin D, et al. Activation of NLRP3 inflammasome contributes to the inflammatory response to allergic rhinitis via macrophage pyroptosis. Int Immunopharmacol. 2022;110:109012.
- Li J, Zhang Y, Zhang L, et al. Fine particulate matter exposure exacerbated nasal mucosal damage in allergic rhinitis mice via NLRP3 mediated pyroptosis. Ecotoxicol Environ Saf. 2021;228:112998.
- Zhang W, Ba G, Tang R, et al. Ameliorative effect of selective NLRP3 inflammasome inhibitor MCC950 in an ovalbumin-induced allergic rhinitis murine model. Int Immunopharmacol. 2020;83:106394.
- Shi Q, Lei Z, Cheng G, et al. Mitochondrial ROS activate interleukin-1β expression in allergic rhinitis. Oncol Lett. 2018;16(3):3193–3200.
- Du J, Peng L, Feng J, et al. RBCK1 overexpression attenuates inflammation and mobility of Derp1-Induced nasal mucosal cells by downregulating NLRP3. Int Arch Allergy Immunol. 2023;184(5):471–480.
- Qiao Y-L, Zhu M-W, Xu S, et al. Allergen-induced CD11c + dendritic cell pyroptosis aggravates allergic rhinitis. Cell Commun Signal. 2023;21(1):281.
- Wo B, Du C, Yang Y, et al. Human placental extract regulates polarization of macrophages via IRGM/NLRP3 in allergic rhinitis. Biomed Pharmacother. 2023;160:114363.
- Yu JI, Kim J-H, Nam K-E, et al. Pneumococcal Δpep27 immunization attenuates TLRs and NLRP3 expression and relieves murine Ovalbumin-Induced allergic rhinitis. J Microbiol Biotechnol. 2022;32(6):709–717.
- Wu J, Wu L, Zhang L, et al. Overexpression of miR-224-5p alleviates allergic rhinitis in mice via the TLR4/MyD88/NF-κB pathway. Exp Anim. 2021;70(4):440–449.
- Zhang S, Lin S, Tang Q, et al. Knockdown of miR‑205‑5p alleviates the inflammatory response in allergic rhinitis by targeting B‑cell lymphoma 6. Mol Med Rep. 2021;24(5):1–11.
- Zhu J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine. 2015;75(1):14–24.
- Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13(8):607–614.
- Gonçalves VM, Matteucci KC, Buzzo CL, et al. NLRP3 controls Trypanosoma cruzi infection through a caspase-1-dependent IL-1R-independent NO production. PLoS Negl Trop Dis. 2013;7(10):e2469.
- Gorfu G, Cirelli KM, Melo MB, et al. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. mBio. 2014;5(1):10–1128.
- Zaiss MM, Maslowski KM, Mosconi I, et al. IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PLoS Pathog. 2013;9(8):e1003531.
- Alhallaf R, Agha Z, Miller CM, et al. The NLRP3 inflammasome suppresses protective immunity to gastrointestinal helminth infection. Cell Rep. 2018;23(4):1085–1098.
- Chenery AL, Alhallaf R, Agha Z, et al. Inflammasome-Independent role for NLRP3 in controlling innate antihelminth immunity and tissue repair in the lung. J Immunol. 2019;203(10):2724–2734.
- Jin X, Bai X, Yang Y, et al. NLRP3 played a role in Trichinella spiralis-triggered Th2 and regulatory T cells response. Vet Res. 2020;51(1):107.
- Pan T-X, Huang H-B, Lu H-N, et al. NLRP3 plays a key role in antihelminth immunity in the enteral and parenteral stages of Trichinella spiralis-infected mice. Infect Immun. 2023;91(4):e0038222.
- Shio MT, Christian JG, Jung JY, et al. PKC/ROS-Mediated NLRP3 inflammasome activation is attenuated by leishmania Zinc-Metalloprotease during infection. PLoS Negl Trop Dis. 2015;9(6):e0003868.
- Gurung P, Karki R, Vogel P, et al. An NLRP3 inflammasome-triggered Th2-biased adaptive immune response promotes leishmaniasis. J Clin Invest. 2015;125(3):1329–1338.
- Liu X, Zhang Y-R, Cai C, et al. Taurine alleviates Schistosoma-Induced liver injury by inhibiting the TXNIP/NLRP3 inflammasome signal pathway and pyroptosis. Infect Immun. 2019;87(12):10–1128.
- Kong D, Zhou C, Guo H, et al. Praziquantel targets M1 macrophages and ameliorates splenomegaly in chronic schistosomiasis. Antimicrob Agents Chemother. 2018;62(1):10–1128.
- Jin X, Liu X, Ding J, et al. Lentinan improved the efficacy of vaccine against Trichinella spiralis in an NLRP3 dependent manner. PLoS Negl Trop Dis. 2020;14(9):e0008632.
