Abstract
Systemic lupus erythematosus (SLE) poses formidable challenges due to its multifaceted etiology while impacting multiple tissues and organs and displaying diverse clinical manifestations. Genetic and environmental factors contribute to SLE complexity, with relatively limited approved therapeutic options. Murine models offer insights into SLE pathogenesis but do not always replicate the nuances of human disease. This review critically evaluates spontaneous and induced animal models, emphasizing their validity and relevance to neuropsychiatric SLE (NPSLE). While these models undoubtedly contribute to understanding disease pathophysiology, discrepancies persist in mimicking some NPSLE intricacies. The lack of literature addressing this issue impedes therapeutic progress. We underscore the urgent need for refining models that truly reflect NPSLE complexities to enhance translational fidelity. We encourage a comprehensive, creative translational approach for targeted SLE interventions, balancing scientific progress with ethical considerations to eventually improve the management of NPSLE patients. A thorough grasp of these issues informs researchers in designing experiments, interpreting results, and exploring alternatives to advance NPSLE research.
Introduction
Systemic lupus erythematosus (SLE) is a multifaceted autoimmune disease affecting various organs, with a higher prevalence in women of childbearing age and specific racial and ethnic groups [Citation1]. Both genetic and environmental factors are thought to contribute to the development of the disease, with triggers such as UV light exposure, infection, and hormonal changes affecting genetically susceptible individuals [Citation2]. Disease development involves chronic immune activation, immune complex deposition, and tissue infiltration by activated T and B cells, as well as monocytes, leading to tissue damage [Citation3]. The pathogenesis entails complex interactions among B cells, T cells, myeloid cells, and cytokines, eventually leading to diverse clinical manifestations [Citation4].
The intricate nature of SLE makes it extremely challenging to identify underlying, specific causes directly in patients. Classification criteria have been developed to aid in clinical trials, but therapeutic options remain limited [Citation5]. Despite the urgent need, only two targeted biologic drugs have been FDA-approved for SLE treatment. To overcome these challenges, diverse models have been designed capable of dissecting some of the cellular and genetic aspects of SLE, thereby facilitating the identification of potential therapeutic targets and biomarkers, as well as the development of possible interventions.
Animal models have been traditionally utilized in medical research to study human diseases, and SLE is no exception. However, the translation of findings from animal models to human applications poses substantial challenges and raises important issues [Citation6–9]. In pharmaceutical research, specifically, animal models are suboptimal predictors of human toxicity, contributing to high costs and potential harm to subjects in clinical trials [Citation10,Citation11]. Moreover, all animal models introduce fundamental differences in anatomy, physiology, and immune system responses [Citation12]. In the context of SLE, the disease’s manifestations and progression in animal models may not always precisely replicate those in humans. Recognizing these limitations is essential for accurately interpreting research outcomes.
Neuropsychiatric SLE (NPSLE) represents a particularly complex and challenging manifestation wherein the autoimmune response extends to the nervous system, leading to a wide spectrum of neurological and psychiatric disturbances [Citation13]. NPSLE symptoms include cognitive dysfunction, mood disorders, seizures, psychosis, and even life-threatening conditions such as cerebrovascular events [Citation14]. The heterogeneity of NPSLE symptoms often makes the diagnosis and treatment remarkably difficult, requiring a complex multidisciplinary approach [Citation15].
The existing literature falls short of thoroughly examining the complex relationship between existing models and various NPSLE aspects in the human context. This lack of analysis impedes our understanding of the translational potential of these models and, therefore, hinders the elucidation of crucial underlying mechanisms required for advancing therapeutic strategies in NPSLE research. Addressing this gap is imperative for enhancing the translational relevance and fidelity critical for developing more efficacious interventions for individuals affected by NPSLE.
Here, we provide a panoramic depiction of both spontaneous and induced mouse models of SLE, with a specific emphasis on their relevance to neuropsychiatric manifestations. We critically examine the noteworthy contributions made by animal models, illuminating their pivotal role in advancing our understanding of the pathophysiological underpinnings of the disease. While these models have provided valuable insights into the disease pathogenesis, room for improvement still exists in accurately reproducing some of the complex NPSLE features, which represents an ambitious goal. In navigating these challenges, we encourage a comprehensive and creative translational approach that will ultimately facilitate the development of more effective and targeted interventions.
Neuropsychiatric systemic lupus erythematosus
NPSLE represents a complex manifestation of SLE, wherein the autoimmune pathology extends to the nervous system, leading to a spectrum of neurological and psychiatric disturbances [Citation16]. In 1999, the American College of Rheumatology (ACR) outlined criteria for 19 distinct neuropsychiatric syndromes, classifying them into two overarching categories: central and peripheral (Supplementary Table S1) [Citation17]. Notably, NPSLE presents clinically with focal or diffuse pathology, spanning a broad range from subtle cognitive dysfunction to acute confusional states, seizures, and psychosis. Its most prevalent manifestations include headaches, anxiety, mood disorders, and cognitive impairment [Citation18]. Several neuropsychological testing batteries assessing attention, learning, memory, and other relevant endpoints have been proposed to facilitate the diagnosis of NPSLE [Citation19,Citation20]. In addition, magnetic resonance imaging (MRI) [Citation21] and cerebrospinal fluid (CSF) analysis [Citation22] are helpful in the differential diagnosis of NPSLE. Of note, peripheral nervous system (PNS) disease is also encompassed under the NPSLE umbrella, exerting a considerable negative impact on both health and quality of life [Citation23,Citation24].
The ACR report highlighted a considerable difficulty in diagnosing NPSLE. In many cases, it can be challenging to determine whether neuropsychiatric signs and symptoms in a given patient are caused by lupus or other factors such as pre-existing conditions or medications. Whether non-specific symptoms (e.g. headache) should be included as a defining NPSLE manifestation is also a matter of debate [Citation13]. This ambiguity poses a challenge for physicians diagnosing the disease and researchers modeling its nuances. Significantly, within the group of outlined syndromes, certain conditions defy modeling in animal systems. For instance, mood disorders and psychosis have historically been difficult to mimic in the laboratory for study in preclinical settings [Citation25,Citation26]. Of note, comorbidities, which are common in lupus patients, introduce additional diagnostic challenges [Citation27,Citation28], while potentially masking the symptoms of NPSLE [Citation29], and impacting the severity and prognosis of the disease.
Despite decades of effort, our mechanistic understanding of NPSLE is still incomplete. Multiple pathogenic pathways have been identified and purportedly linked to definite clinical manifestations. These include antibody-mediated neurotoxicity, vasculopathy due to anti-phospholipid antibodies, cytokine-induced neurotoxicity, and the loss of neuroplasticity [Citation20]. The blood-brain barrier (BBB), which serves as a structural and functional interface protecting the brain from the unregulated passage of immune mediators and cells from the bloodstream into the central nervous system (CNS), is believed to be significantly compromised in SLE. A breach in the BBB was postulated to trigger the development of neuropsychiatric manifestations [Citation30–32]. Several animal NPSLE models have indeed proven the critical role of heightened BBB permeability in facilitating the entry of autoantibodies into the brain, where they subsequently bind to neurons, triggering apoptosis [Citation33–35]. Nevertheless, human evidence regarding spontaneous or sustained BBB dysfunction in NPSLE remains less than definitive [Citation36].
Apart from the BBB, the blood–cerebrospinal fluid barrier (BCSFB) formed by choroid plexus (CP) epithelial cells structurally and functionally separates the systemic circulation and CSF [Citation37]. The CP is one of the least investigated structures within the CNS, despite its central significance in neurobiology. An increasing body of research, particularly in animal models, underlines the role of the BCSFB; recent studies reveal that the choroid plexus epithelium serves as a pathway for the entry of pathogenic autoantibodies and leukocytes into the CSF, emerging as a primary site of neuropathology. Notably, certain studies suggest that even in the absence of BBB dysfunction, the BCSFB could experience disruption, implicating BCSFB dysfunction as a potential causative factor for the infiltration of immune mediators into the brain [Citation38,Citation39].
Current landscape of lupus animal models
Animal models are commonly relied upon to predict human outcomes in biomedical research. Murine models, in particular, have been instrumental in uncovering complex SLE mechanisms. Exploiting a range of mouse models, each boasting unique merits and limitations, allowed researchers to delve deeply into many of the disease mechanisms and discover some of the involved biological pathways. While these models primarily mirror the diffuse symptoms rather than the focal ones, they exhibit variable SLE phenotypes [Citation13]. Thus, the overarching assumption that lupus animal models can consistently predict human physiology requires scrutiny [Citation40].
Spontaneous rodent models
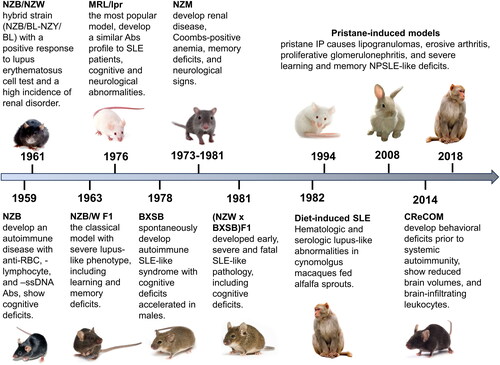
Decades ago, researchers noted that certain inbred mouse strains and their crosses exhibited spontaneous autoimmune manifestations akin to those seen in SLE patients. Bielschowsky et al. [Citation41] documented the first reported spontaneously occurring autoimmune disease in the New Zealand black (NZB) strain (). Two years later, the first SLE model was created by crossing NZB with the New Zealand White (NZW) mouse. Helyer and Howie [Citation42] delineated a strain of hybrid mice (NZB/BL-NZY/BL) with a positive lupus erythematosus cell test. Subsequent reports revealed that these mice manifest hemolytic anemia as well as renal disease, mirroring human lupus nephropathy [Citation43]. NZB/NZW inbred hybrid strains gradually gained popularity as an SLE model after the original report by Dubois et al. [Citation44]. Through selective inbreeding, this strain has become commercially available, along with numerous other autoimmune disease-prone strains that exhibit clinical and serological features mimicking human disease. Variations in disease manifestations exist among strains and sexes, along with distinctive differences in the type and severity of symptoms [Citation45].
NZB/W F1
NZB/WF1 mice were created as a hybrid cross between NZB females and NZW males. Notably, animals under three months of age are unaffected, but the severity of the disease steadily rises thereafter. Significantly, parallel to human patterns, lupus primarily develops in female mice, which feature elevated antinuclear antibodies, hemolytic anemia, and progressive glomerulonephritis. However, NZB/WF1 mice do not demonstrate other canonical lupus manifestations, such as rash or arthritis [Citation46]. As for brain involvement, NZB/WF1 mice were recently shown to demonstrate significant hippocampus-related behavioral deficits (), endorsing the utility of this model for probing the underlying mechanisms of diffuse NPSLE [Citation35]. The deficits are detectable in novel object location (NOL) and recognition (NOR) behavioral tests. Other groups reported that NZB/WF1 mice exhibit anxiety- and depressive-like behaviors and impaired motor coordination [Citation47,Citation48]. Assessment of righting and postural responses, as well as grip strength testing, detects gradually developing neurological abnormalities in older animals [Citation49]. While no significant differences were noted in the social novelty test, NZB/WF1 mice showed altered social recognition in the social preference test [Citation35,Citation48].
Graïc et al. [Citation50] recently applied an advanced morphometric technique to compare neuron morphology, layer by layer, in NZB/W F1 with wild-type mice. The authors found increased neuronal density across all hippocampal regions except for the pyramidal layer in NZB/WF1 mice. Additionally, hippocampal neurons were larger and more uniform compared to those in the control group across all subregions. Of note, hippocampal pyramidal neurons are responsive to both spatial and temporal stimuli [Citation51,Citation52], which makes this observation particularly important considering the specific deficits in working memory and attention observed in SLE patients [Citation53]. Of note, the NZB/WF1 mouse strain is a widely used model to test therapeutic modalities [Citation54–56].
(NZW x BXSB) F1
(NZW x BXSB)F1 mice were created by Hang et al. [Citation57] by mating NZW females with male BXSB mice. (NZW x BXSB)F1 mice develop impairments in learning and memory that correlate with the severity of lupus-like disease. Of note, only relatively old males (20-week-old) demonstrate deficits in spatial orientation abilities in the Morris water maze [Citation58]. Significantly, this strain spontaneously displays severe and progressive disease leading to death at 13-18 weeks of age. Accordingly, concerns have been raised that the severity of disease in (NZW x BXSB)F1 mice may hinder the utility of this hybrid strain in investigating SLE and testing potential treatment modalities [Citation59].
NZM
NZM mice were generated by mating of NZB/NZWF1 pairs for multiple generations. In 1981, Olsen et al. [Citation60] initiated the inbreeding process from a single pair. Subsequently, at the F4-F5 generations, Gabrielsen et al. [Citation61] selected lines with severe nephritis for further development. Over 20 NZM strains were created and characterized for manifestations of lupus-like disease. Notably, male NZM88 mice develop spatial memory deficiency and show increased anxiety levels before nephritis onset (). Within the NZM strains, NZM88 mice also show the poorest performance compared to the 391, 2328, and 2758 sub-strains in the Morris water maze and open field paradigms [Citation62].
MRL/lpr
The MRL/lpr strain exhibits the hallmark serological markers and systemic pathologies closely resembling human SLE. Moreover, at a relatively young age, they develop cognitive dysfunction, anxiety, and depression-like symptoms characteristic of NPSLE () [Citation63]. This model is advantageous for elucidating early NPSLE mechanisms and distinguishing between CNS-specific and non-specific illness factors. Lupus severity is worse in females, which resembles human disease. Of note, pre-diseased (6-week-old) MRL/lpr mice are indistinguishable from congenic MRL/+ controls in most behavioral measures, except for variations in locomotion speed and novelty-induced hyperactivity [Citation64].
Severe cognitive and sensorimotor deficits are pronounced in MRL/lpr mice by 16 weeks of age, with abnormal Morris water maze performances detectable by 8 weeks compared to their congenic counterparts [Citation65–67]. Depressive-like behavior is a common phenomenon observed in MRL/lpr mice that appears early in the absence of substantial pathology involving other target organs [Citation68]. It is easily detectable via the Porsolt swim and anhedonia tests [Citation69–73]. Conflicting reports, however, concern the anxiety-related phenotype in this strain. While some studies show heightened anxiety in MRL/lpr mice detectable in the elevated plus maze [Citation74,Citation75], others report normal or even reduced anxiety levels [Citation68,Citation69,Citation76]. In addition, 6-week-old and older female MRL-lpr mice demonstrate significantly reduced preference for sucrose [Citation77,Citation78], interpreted as anhedonia. Thus, MRL/lpr mice demonstrate a complex behavioral phenotype consisting of spatial memory deficits and depressive-like behavior. Notably, this phenotype exhibits a degree of independence from systemic autoimmunity [Citation79].
BXSB/MpJ
BXSB mice develop a spontaneous autoimmune lupus-like syndrome, which is accelerated in males (due to Y chromosome-linked autoimmune accelerator locus Yaa) [Citation80]. Roopenian et al. from the Jackson Laboratory generated a collection of BXSB congenic mice and proved their value as potent tools in elucidating the roles of specific cytokines and immune cell populations in the SLE progression [Citation81,Citation82].
Of note, BXSB/MpJ-Yaa mice develop neocortical ectopias, characterized by mislocalized clusters of neurons within layer I of the cortex. Typically situated in the prefrontal and motor regions, these ectopias bear a striking resemblance to those observed in post-mortem analyses of the brains of individuals with dyslexia [Citation83,Citation84]. Dyslexia is relatively common in women with SLE [Citation85]. Moreover, dyslexia is surprisingly frequent in the male offspring of female SLE patients [Citation86]. Further investigations are necessary to establish causal relationships between these structural and functional phenomena and their association with lupus. Old BXSB males are greatly deficient in their working memory and show diminished spatial memory, while the young male and the female mice are cognitively preserved (). The deficits are easily detectable via the Morris water maze and radial arm maze tests [Citation87–89].
CReCOM
Several studies associated dendritic cells (DCs) with the initiation and persistence of SLE [Citation90,Citation91]. Specifically, loss of caspase 8 in DCs was shown to induce an SLE-like disease in mice [Citation92], and a critical role for caspase-8 in the pathogenesis of SLE was suggested. Subsequently, the Caspase-8 Removed CD11c-specific Overactive MyD88 (CReCOM) mouse was created to test this hypothesis in vivo. CReCOM mice gradually develop aggressive systemic inflammatory diseases reminiscent of SLE, including splenomegaly, lymphadenopathy, hypergammaglobulinemia, auto-antibody induction, and glomerulonephritis [Citation92]. Makinde et al. [Citation93] demonstrated that CReCOM mice also develop NPSLE-like disease with behavioral features, including spatial memory, contextual associative learning, startle response and motor coordination defects (), corresponding to hippocampal and cerebellar defects, similar to SLE patients. Of note, SLE-related cerebellar pathology has been reported by several groups [Citation94–96]. Moreover, cerebellar ataxia has been described as the presenting sign of SLE [Citation97]. Accordingly, the CReCOM model seems highly appealing for researching specific aspects of NPSLE pathogenesis, in particular those that are not apparent in other models.
Induced models
Traditionally, induced models excel at providing a constrained framework for replicating disease complexity, which facilitates targeted exploration of disease mechanisms. In the context of NPSLE research, deliberately creating a reproducible environment allows for testing therapeutic interventions in a highly controlled experimental setting. While induced models may not fully capture the spontaneous and systemic nature of lupus, their controlled nature can enhance experimental reproducibility, contributing to scientific consistency.
Pristane-induced mouse models (PIM)
Exposing wild-type mice to chemical agents allows for the investigation of non-genetic factors in the breakdown of immune tolerance and the evaluation of therapeutic drugs for SLE. Pristane, a natural alkane from shark liver oil, serves as a tool to induce SLE-like pathology in laboratory animals through intraperitoneal injection [Citation98]. While the exact mechanism remains incompletely understood, pristane is thought to initiate inflammation, activating autoreactive B and T cells [Citation99,Citation100]. Additionally, the decreased clearance of apoptotic cells, characteristic of pristane-treated mice, helps to maintain high levels of autoantibodies [Citation101]. Notably, the pristane-induced models operate effectively on diverse non-autoimmune backgrounds, including Balb/c and C57BL6 [Citation102].
The pristane-induced models serve as useful tools for studying environmentally related SLE [Citation100]. Recently, these models have been extensively examined in the context of neuropsychiatric manifestations, offering valuable insights [Citation103]. Notably, the disease progression closely mirrors human SLE, exhibiting female predominance and eliciting specific neuropsychiatric symptoms easily detectable by common behavioral tests. Mice injected with pristane display abnormalities in olfactory sensitivity, as well as anxiety- and depression-like behaviors () [Citation103]. Additionally, pristane-induced models in Balb/c mice show downregulation of NR2A/2B subunits in the hippocampus associated with spatial learning and memory impairment [Citation104].
Table 1. Behavioral phenotypes in key spontaneous and pristane-induced mouse SLE models.
Pristane-induced models are widely utilized to test potential therapeutic interventions and foster the development of promising treatments [Citation105]. However, these models, though valuable, may not faithfully replicate natural disease triggers, significantly limiting their ability to capture the complete interplay of genetic, environmental, and immunological factors in the condition’s onset. Moreover, induced SLE animal models tend to be less severe than those observed in the strains described above, in which disease arises spontaneously. Therefore, one should be cautious when considering the generalization of findings from induced models, keeping in mind their limitations to ensure accurate interpretation.
Immunization and antibody induction of lupus-like neuropsychiatric manifestations
Autoantibodies are pivotal in NPSLE development, triggering neuropsychiatric deficits by reacting with neuronal tissues. Rabbits or mice can be immunized with lupus autoantigenic proteins or oligopeptides, eliciting through epitope spreading [Citation106] an immune response directed not only to the immunogen but also to various self-components, which include SLE-associated autoantigens [Citation107]. Immunization studies have reported manifestations resembling human SLE [Citation108,Citation109], though only a few demonstrated NPSLE-related pathology. Subsequently, Diamond et al. developed an original NPSLE murine model. They introduced a novel method of triggering anti-DNA antibody production in BALB/c mice by immunization with the DWEYS peptide (a dsDNA mime type) multimerized on a branched polylysine backbone [Citation110,Citation111]. In this model, antibody-mediated brain pathology proceeds through an acute phase characterized by excitotoxic neuron loss, followed by persistent alterations in neuronal integrity and impairment of spatial memory [Citation112].
Peripheral nervous system disease in SLE
While the animal models described above replicate some of the key features of diffuse CNS lupus, involvement of the peripheral nervous system is also included in the ACR criteria for NPSLE discussed above. Peripheral neuropathy is common in individuals with SLE, wherein sensorimotor polyneuropathy stands out as the most frequent subtype, giving rise to widespread pain attributed to nerve damage [Citation113]. This clinical syndrome is substantiated by objective nerve conduction studies revealing an asymmetric axonal-demyelination neuropathy pattern [Citation114]. The prevalence of peripheral neuropathy in SLE, a manifestation that can be associated with intense suffering and diminished quality of life, emphasizes the critical need for identifying effective therapeutic interventions [Citation115].
Assessing peripheral neuropathy in animal models is generally challenging due to the complex nature of neuropathic symptoms and the obvious limitations in animals’ ability to communicate pain. Current methods involve a mix of behavioral assays, electrophysiological measurements, and histological analyses; however, modeling lupus-associated peripheral neuropathy in mice presents a serious challenge.
Of note, female MRL/lpr mice develop thermal hyperalgesia and mechanical allodynia [Citation116]. These phenomena can be readily measured using an original method for assessing nociception [Citation117]. In addition, animals (non-lupus) with chronic pain demonstrate heightened activation of microglia and astrocytes, accompanied by upregulation of CSF-1 and IL-1β, along with diminished glial glutamate transport in the spinal cord [Citation116]. Remarkably, intrathecal administration of CSF-1 receptor and IL-1 receptor inhibitors enhanced glutamate transporter activities in the spinal dorsal horn and ameliorated hyperalgesia. These findings underscore the significance of CSF-1 in pain signaling and highlight the involvement of IL-1β in the development of pathological pain, although these have not been explored in murine NPSLE.
Rabbits
Rabbits have been traditionally considered an important model organism. Their size, genomic [Citation118], and brain development [Citation119] similarities to humans substantially exceed those of rodents, positioning them as a key preclinical animal model. Moreover, rabbits play a crucial role in immunological research. Their extensive antibody repertoire continues to be a significant source for the development of versatile diagnostic and therapeutic antibodies [Citation120]. In pursuit of developing rabbit SLE models, the National Institute of Allergy and Infectious Diseases provided rabbits with a non-inbred pedigree, closely mirroring human population structure. These animals, defined by immunoglobulin allotypes in their pedigrees, possess a distinctive potential for selection and breeding as SLE models [Citation121]. In the initial study, rabbits immunized with a multimerized peptide sequence from the Smith antigen on a branched poly-lysine backbone exhibited autoantibody responses closely resembling those observed in human SLE patients [Citation122]. Subsequent investigations substantiated the model’s validity [Citation123], enhanced our understanding of SLE pathogenesis [Citation124], and demonstrated its utility in NPSLE studies [Citation125]. Specifically, microarray-based expression profiling demonstrated that rabbit RNA can be used in parallel with human expression arrays to yield valuable information on molecular pathways involved in the clinical phenotype [Citation123].
The trace eyeblink conditioning (EBC) paradigm, involving the cerebellum, forebrain, and stimulus awareness, is valuable for studying cerebro–cerebellar interactions. Various neuropsychiatric conditions including schizophrenia, Alzheimer’s disease, and progressive supranuclear palsy exhibit impaired EBC acquisition [Citation126]. While several laboratory species have been employed for studying EBC, the rabbit stands out due to its high tolerance for restraint and the relative ease of neuroimaging. Additionally, its sizable skull simplifies chronic neuronal recordings; and unlike rodents, it possesses a differentiated striatum with distinct caudate and putamen, facilitating the investigation and comparative analysis of striatum-dependent behaviors [Citation127].
Clinical data indicate that in SLE patients, brainstem reflexes (blink reflex, masseter inhibitory reflex) are affected even in the absence of peripheral nervous system involvement and clinical neurological findings. Abnormalities in brainstem reflexes suggest a disruption in the functional integrity of inhibitory or excitatory interneurons in the lateral caudal pons and lateral medulla of SLE patients [Citation128]. Since EBC is readily testable in rabbits, this model can facilitate the exploration of neural mechanisms associated with maladaptive behaviors and cognitive impairments underlying NPSLE [Citation126].
Nevertheless, despite obvious advantages as a model, rabbits present significant drawbacks. Notably, the cost of individual adult rabbits is tenfold higher than that of mice, and rabbit breeding and maintenance is much more costly since individual housing requires more space [Citation129]. Moreover, rabbits lack a sufficient range of standardized cognitive and other neurobehavioral tests.
Non-human primates
Non-human primates are critical in scientific research due to their genetic and physiological resemblance to humans. Their human-like behavior and cognitive abilities make them invaluable for exploring neurological disorders and the effects of interventions. Additionally, their immune system parallels humans, and shared drug metabolism similarities enable the assessment of the safety and efficacy of new drugs. Therefore, this model is potentially well-suited for preclinical studies on NPSLE.
Macaque monkeys, favored in preclinical settings, are extensively used to investigate human brain mechanisms due to their size and an extensive existing database [Citation130]. Pristane-induced lupus was established in the cynomolgus monkey, demonstrating a notable decline in renal function [Citation128]. Although cognitive functions were not evaluated, this primate model has been suggested for lupus translational studies on pathogenesis, symptomatology, and therapeutic candidates [Citation131].
Other models
Dogs
Canine SLE was first characterized by Lewis et al. [Citation132] as a naturally occurring disease in dogs [Citation132]. As reported in the initial study, the principal features of this condition include hemolytic anemia, thrombocytopenia, glomerulonephritis, facial eruptions, polyarthritis, splenomegaly, and vascular changes.
Also similar to human disease, canine lupus is characterized by a chronic course featuring alternating periods of remission and relapse. This cyclic evolution contrasts with the steady progression to the terminal stage observed in mice [Citation133]. A review of cases in dogs suggests no sex predilection, although the number of cases was too limited to reach firm conclusions [Citation134,Citation135]. Further, the presence of neurological manifestations has yet to be addressed.
Cats
Feline lupus is a rare pathology. The first suspicion of SLE in a cat was documented in 1971 [Citation136]. Since then, reports on feline lupus have been limited. Feline models serve in numerous preclinical studies, leveraging their susceptibility to certain human-like pathologies to offer unique insights into genetics and pathophysiology. There were even suggestions that cats would provide invaluable clues to the etiology of SLE [Citation137]. However, to date, no established feline model available for preclinical studies exists.
Validity considerations
To ensure accurate predictions from an animal model, assessing its validity is critical. This involves evaluating the model’s fidelity in representing and measuring the targeted disorder or condition, thus enhancing confidence in its utility and insights. McKinney and Bunney [Citation138] pioneered animal model research by establishing criteria for evaluating the external validity of these models, particularly in the context of neuropsychiatric disorders. They stated that the optimal model should mirror the targeted condition in terms of etiology, biochemistry, symptoms, and treatment. Wilner [Citation139] introduced a simplified framework with three external validation criteria: construct validity, face validity, and predictive validity, as depicted in . Construct validity evaluates how well biological processes are replicated; face validity ensures accuracy in representing human conditions; and predictive validity helps predict outcomes () [Citation138]. Geyer and Markou [Citation140] suggested additional criteria regarded as essential for validating an animal model. The authors stressed the importance of evaluating predictive validity for model assessment. They also advised considering etiological, convergent, and discriminant validities to improve the validation process. In the context of NPSLE research, etiological validity is determined by the similarity of mechanisms underlying the observed behavior in animal models with the associated human behavior. Convergent validity evaluates how well an animal model agrees with other models that aim to replicate the same neuropsychiatric condition [Citation141], while discriminant validity refers to the degree to which a particular test measures distinct aspects of a phenomenon [Citation140]. These criteria are currently used as benchmarks to measure how precise and reliable particular animal models are for human disease.
Table 2. Validating criteria and evaluating challenges in animal models of NPSLE.
The elusive etiology and pathogenesis of SLE pose challenges to achieving high construct validity. Dissimilarities in both brain structures and functions between animals and humans raise concerns about translational applicability, particularly in the context of NPSLE, which has intricate behavioral and cognitive features that animals cannot fully replicate. This prompts questions about accurately translating observed animal behaviors to nuanced human symptoms. Significantly, the genetic mutations observed in current murine spontaneous SLE models do not align with those seen most often in patients [Citation13]. Instead, these models mimic several common clinical manifestations, facilitating the investigation of possible biological mechanisms and the identification of potential therapeutic targets for further exploration.
In addition, human genetic diversity profoundly impacts drug responses and neuropsychiatric disorder development [Citation142]. However, mouse models, often genetically homogenous, fall short in mirroring human genetic variability. Accordingly, caution is needed in interpreting and applying animal study findings to human contexts, recognizing inherent limitations in capturing the complexity of neuropsychiatric conditions.
The environment constitutes a pivotal factor in the genesis and expression of neuropsychiatric disorders [Citation143]. The profound impact of stress, trauma, and social determinants on human mental health is well-established [Citation144]. However, accurately replicating these intricate environmental influences in animal models poses a formidable challenge. The multifaceted nature of human experiences, shaped by diverse social, psychological, and environmental factors, complicates the translation of such complexities into controlled experimental settings with animals. Consequently, the fidelity of animal models in faithfully capturing the environmental dimensions of neuropsychiatric disorders raises important questions, emphasizing the need for comprehensive methodologies that incorporate the intricate interplay between environmental factors and neural processes in both animals and humans.
Mouse SLE models possess strong face and convergent validity, demonstrating alignment between phenotypic measures and disease-related mechanisms. However, the lack of a curative disease-modifying therapy challenges their predictive validity, which underscores the limitations in translating preclinical results and the urgent need for better models [Citation141]. Moreover, no universally agreed upon definition of disease modification for SLE exists, which makes the task of modeling the disease even more complicated [Citation145].
Predictive validity challenges
The intricate nature of NPSLE poses a challenge for preclinical development. It is fundamentally difficult to predict the impact of drugs on human brain function based on animal data. Disappointingly, none of the >1000 drugs found showing efficacy in animal models of neurologic disease have been neuroprotective in humans. Accordingly, some even assert that animal models have failed as a modality for predicting neuroprotection [Citation40].
The dearth of active clinical trials for NPSLE is unfortunate but predictable, given the current lack of consensus surrounding this condition. This scientific uncertainty has also impeded clinical development. Significantly, more than 400 SLE clinical trials (generally excluding patients with neuropsychiatric disease) have been completed with only two biological agents granted FDA approval (i.e. anifrolumab, belimumab). Of note, many of these clinical trials were initiated following very promising preclinical findings. In our perspective, these results might have been anticipated given the less than optimal construct validity of SLE animal models. While some models truly exhibit lupus-like symptoms, none comprehensively replicate the entire syndrome. Additionally, researchers do not always provide the rationale for selecting one model over another, nor do they consider the methods to evaluate the treatment’s effects on behavioral paradigms to assess the improvement in brain function. We suggest that a nuanced and context-aware approach is required to bridge the translational gap in the quest for breakthroughs. This approach should consider human-based studies as the basis for selecting animal models with specific pathologies and behavioral phenotypes related to the subject of investigation.
Despite major advances in treating psoriatic arthritis, rheumatoid arthritis, and other autoimmune rheumatic diseases, effective targeted lupus therapies are notably delayed. Recent successes with anifrolumab and belimumab are inspiring, yet the persisting frustration lies in a long list of drugs excelling in phase II but consistently failing in phase III trials [Citation146].
Nevertheless, we strongly believe that animal models offer an evident and robust level of predictiveness. In fact, while corticosteroids, cyclophosphamide, and mycophenolate have been part of the standard care of lupus patients for years, they also have a long history of being tested successfully in animal models. Cyclophosphamide demonstrated its efficacy in 1968 in NZB/WF1 mice [Citation147]. Consequently, its value was proven in MRL/lpr mice and other models [Citation148,Citation149]. Dexamethasone was also very effective in NZB/WF1 [Citation150] and MRL/lpr mice [Citation151]. Mycophenolate was tested by several groups in the same models [Citation152–154]. It suppressed the development of autoimmunity and prolonged the animals’ lifespan. Moreover, suppression of autoimmunity was achieved without obvious side effects or altered CD4/CD8 T cell ratios [Citation153]. Remarkably, mycophenolate was no less effective than cyclophosphamide in inducing remission in lupus patients and demonstrated a more favorable safety profile [Citation155,Citation156].
Certain agents showing potential efficacy against NPSLE in preclinical studies appear highly promising and are currently undergoing clinical evaluation. Mike et al. [Citation154] demonstrated that fingolimod (selective sphingosine-1-phosphate (S1P) receptor modulator) significantly attenuated spatial memory deficits and depression-like behavior in MRL/lpr mice. Notably, the treatment leads to reduction of total IgG and T cell infiltrates in the brain parenchyma and reduces lymphocyte infiltrate in the CP. Strasser et al. [Citation157] demonstrated the positive effects of a novel, potent and selective S1P1 receptor modulator, cenerimod, in MRL/lpr mice and SLE patients. Cenerimod significantly ameliorates systemic and organ-specific pathology and inflammation in mice [Citation158]. In SLE patients, 12-week-long cenerimod treatment (phase II) led to a dose-dependent reduction in blood lymphocytes, antibody-secreting cells, and plasma IFN-α [Citation159]. Moreover, cenerimod induced S1P1 receptor internalization in lymphocytes. Subsequently, Idorsia started a phase III clinical trial aiming to assess the efficacy of this drug. Considering the lack of efficient treatments and importance of the discovery, FDA granted the therapy fast-track status, expediting development and regulatory review. The development of an inaugural drug specifically effective in treating NPSLE patients is highly anticipated.
Discussion
Animal models have faced challenges in predicting human responses to drugs and diseases, with methodological issues consistently affecting animal-based research. In an influential study, Pablo Perel et al. conducted a comparative analysis of treatment outcomes between animal experiments and clinical trials, revealing significant disparities attributed to biases or limitations in the ability of animal models to faithfully replicate clinical diseases [Citation160]. Another notable issue is the chronic absence of clear and transparent methodology [Citation161]. A suggested remedy for these challenges is the standardization of protocols, aiming to enhance the accuracy of prediction for human responses to drugs. Furthermore, species-specific differences in drug metabolism and pharmacokinetics can lead to unexpected side effects during clinical trials. Therefore, some scientists argue that even with standardized methodologies in place, animal models may still be inadequate as predictive tools [Citation40].
Obvious differences exist in evolutionary paths and the inherent complexity between humans and animals. However, undoubtedly, laboratory animals are essential for evaluating the safety, effectiveness, and overall potential of new interventions. Thus, it is indeed important to carefully evaluate the value of animal models in each experiment. Factors such as how closely the animal model resembles human pathology, alignment with research goals, predictive accuracy, ethical considerations, and relevance compared to alternative methods should all be taken into account.
Generally, species disparities and deviations from natural habitats in preclinical studies may impact outcomes and introduce biases. In contemporary NPSLE research, animal models are less than ideal in accurately representing human environmental and social factors, resulting in discounting the impact of the latter upon the disease progression. Additionally, the highly heterogeneous nature of SLE within the human population, coupled with considerable genetic diversity, does differ with that of popular inbred murine models, potentially at least impacting the generalizability of animal data and reducing the degree of predictability in human context.
While SLE is fundamentally an autoimmune disorder, accurately replicating the immune system’s intricacies in animals is challenging due to significant species disparities. This results in marked discrepancies in disease manifestations and the rate of disease progression, theoretically impinging upon the reliability of existing animal models. In this context, creating a humanized SLE model using immune-deficient mice would be a top priority. This model is expected to exhibit responses to treatments that are more similar to those seen in humans [Citation162].
The emergence of innovative techniques in animal research and clinical diagnostics marks a promising stride forward. Integration and parallel use of comparable technology with fresh perspectives in immunology and psychiatry will enhance our ability to establish more accurate animal models. Identification of reliable NPSLE biomarkers, optimally shared between animal models and patients, is particularly crucial for differential diagnosis, disease activity monitoring, and preclinical development [Citation163].
The parallel application of advanced methods in preclinical and clinical studies has facilitated the precise alignment of particular findings while advancing their translational value. The use of high-resolution volumetric magnetic resonance imaging revealed damage to the amygdala and the hippocampus in SLE patients [Citation164,Citation165]. Moreover, the rate of damage correlates with the titres of anti-NMDAR antibodies [Citation166]. These findings suggest a potential memory deficit that can be readily identified through the contextual and cued fear conditioning tests in mice. Regrettably, only a limited number of studies have evaluated the memory in SLE model animals using this paradigm (), despite its demonstrated efficacy [Citation67] and documented damage to the neurons in the lateral amygdala in SLE mice [Citation167]. Furthermore, NPSLE patients exhibit lower activation in prefrontal brain regions, predicting notable functional abnormalities in prefrontal neural networks involved in decision-making. These abnormalities can be easily detected through traditional tests [Citation168]. In SLE mice, various paradigms can be employed to assess prefrontal cortex-dependent decision-making behaviors. The Barnes maze, developed by Carol Barnes in 1979 [Citation169], is a dry-land behavioral paradigm for assessing spatial learning and memory that offers an advantage over the Morris water maze by eliminating the confounding influence of swimming behavior [Citation170]. The Barnes maze, particularly its reversal learning phase, which heavily involves the hippocampus, amygdala, and prefrontal cortex, can provide valuable insights on the functionality of these brain regions [Citation171]. Unfortunately, this informative paradigm is also not widely adopted among lupus researchers ().
Of note, both the Barnes maze and the Morris water maze are widely-used tests in rodents that provide valuable information. However, the Morris water maze paradigm elicits higher elevations in plasma corticosterone compared to the Barnes maze [Citation172]. Significantly, spatial learning exhibits an inverse correlation with corticosterone levels in the Morris water maze, but not in the Barnes maze. This implies that performance in the Morris water maze is exceptionally susceptible to test-induced stress, even among wild-type subjects of identical age and gender [Citation173]. Given the prevalence of anxiety in some of the inbred strains used as SLE models (), we posit that the Barnes maze represents a more appropriate choice for behavioral tests. Hence, we similarly propose a deliberate selection of behavioral paradigms for preclinical studies, considering both available clinical data and ethical considerations.
In order to comprehensively evaluate animal behavior, it is of utmost importance to carefully select different sets of paradigms (i.e. standardized behavioral tests). These paradigms should be chosen based on the specific goals of the experiment, as well as the unique characteristics of the animal strain being studied. The behavioral experiments’ specific goals must align with clinical data to better detect correlations between human symptoms and observed animal phenotypes. Furthermore, we encourage the publication of neutral and negative results, including behavioral experiments, to advance the pace of preclinical studies, prevent unnecessary waste of public funds, improve reliability and credibility, and ultimately increase productivity.
In NPSLE research and drug development, unresolved questions persist, including the correlation between peripheral and central immune responses, the role of autoantibodies, and the precise mechanisms by which cytokines and chemokines impact CNS function. While genetic factors undeniably contribute to NPSLE pathogenesis, the complex interplay between genetic predisposition, environmental triggers, and immune dysregulation remains incompletely understood.
Some recent studies have attempted to creatively uncover these multifactorial interactions. As an example, Hayakawa et al. [Citation174] investigated the response of lupus-prone NZB/WF1 mice to specific environmental factors, particularly imiquimod. Imiquimod is an immunomodulator acting as a toll-like receptor 7 (TLR7) agonist [Citation175]. TLR7 is expressed on dendritic cells in mice and humans [Citation176], and their increased expression has been conclusively linked to the pathogenesis of SLE [Citation177]. Remarkably, the imiquimod-induced lupus-like phenotype appeared to have some distinct features that more closely resembled human pathology. Particularly, interferon-related gene expression and pathways are characteristic of SLE pathogenesis [Citation178]. The adoption of this approach holds significant promise in terms of improving our understanding of the underlying causes and mechanisms that give rise to SLE. By leveraging this insight, we can develop more innovative and effective therapeutic modalities to address this challenging condition.
Within this context, we stress the importance of employing thoughtfully selected spontaneous and induced models, complemented by meticulously designed experiments, to enhance the representativeness of chosen models and the validity of experimental outcomes. This strategic approach will best address some of the current challenges in NPSLE research.
Of importance, the use of animals in research raises ethical concerns as well, necessitating a balance between scientific advancement and the well-being of research subjects. This issue has prompted increased scrutiny of in vivo studies, leading researchers to explore alternative methodologies that reduce reliance on animal testing. Non-animal models obviously avoid the ethical dilemmas associated with the traditional preclinical approach, yet they will hopefully offer the potential to provide insights into the complex interactions underlying NPSLE in the not-so-distant future.
Conclusions and future directions
Cognitive dysfunction is prevalent in SLE, significantly impacting patients’ quality of life. Still, no specific therapeutic agent for NPSLE exists, and reliably assessing cognitive impairment remains challenging. NPSLE presentations are non-specific, encompassing deficits in attention, working memory, executive function, and processing speed. Consequently, evaluating these aspects in animal models is fundamentally challenging. The lack of a comprehensive and unified understanding of disease pathogenesis impedes therapeutic development. Thus, intensified research with novel approaches is crucial for bridging this knowledge gap and developing effective interventions.
While it is currently challenging to identify an animal model that accurately reflects the complexities of NPSLE in humans, it is important to recognize that animal models still provide valuable insights and data. By considering the findings from various models, we can gain a more thorough understanding of the disease and develop more effective treatment strategies. Accordingly, and especially when the goal is preclinical development, careful consideration (when practical) should be given to testing two distinct animal models (e.g. one being spontaneous and the other being induced), considering specific behaviors and treatment responses.
With the latest advancements in genetic engineering and sophisticated modeling techniques, it is now possible to study the complex genetic makeup of lupus in much greater detail [Citation179]. This presents an exciting opportunity to better understand the pathogenesis of NPSLE. By improving lupus representation, we can ultimately improve the lives of those who suffer from this devastating condition.
Supplemental Material
Download MS Word (14.5 KB)Additional information
Funding
References
- Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2(1):1.
- Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384(9957):1878–18.
- Putterman C, Caricchio R, Davidson A, et al. Systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:437282–437282.
- Mohan C, Zhang T, Putterman C. Pathogenic cellular and molecular mediators in lupus nephritis. Nat Rev Nephrol. 2023;19(8):491–508.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1400–1412.
- Hackam DG. Translating animal research into clinical benefit. BMJ. 2007;334(7586):163–164.
- van der Worp HB, Howells DW, Sena ES, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7(3):e1000245.
- Moore E, Reynolds JA, Davidson A, et al. Promise and complexity of lupus mouse models. Nat Immunol. 2021;22(6):683–686.
- Moore E, Putterman C. Are lupus animal models useful for understanding and developing new therapies for human SLE? J Autoimmun. 2020;112:102490.
- Van Norman GA. Limitations of animal studies for predicting toxicity in clinical trials: is it time to rethink our current approach? JACC Basic Transl Sci. 2019;4(7):845–854.
- Van Norman GA. Limitations of animal studies for predicting toxicity in clinical trials: part 2: potential alternatives to the use of animals in preclinical trials. JACC Basic Transl Sci. 2020;5(4):387–397.
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–2738.
- Schwartz N, Stock AD, Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol. 2019;15(3):137–152.
- Sarwar S, Mohamed AS, Rogers S, et al. Neuropsychiatric systemic lupus erythematosus: a 2021 update on diagnosis, management, and current challenges. Cureus. 2021;13(9):e17969.
- Emerson JS, Gruenewald SM, Gomes L, et al. The conundrum of neuropsychiatric systemic lupus erythematosus: current and novel approaches to diagnosis. Front Neurol. 2023;14:1111769.
- Seet D, Allameen NA, Tay SH, et al. Cognitive dysfunction in systemic lupus erythematosus: immunopathology, clinical manifestations, neuroimaging and management. Rheumatol Ther. 2021;8(2):651–679.
- The American college of rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608.
- Gulinello M, Wen J, Putterman C. Neuropsychiatric symptoms in lupus. Psychiatr Ann. 2012;42(9):322–328.
- Kozora E, Ellison MC, West S. Reliability and validity of the proposed American college of rheumatology neuropsychological battery for systemic lupus erythematosus. Arthritis Rheum. 2004;51(5):810–818.
- Kivity S, Agmon-Levin N, Zandman-Goddard G, et al. Neuropsychiatric lupus: a mosaic of clinical presentations. BMC Med. 2015;13(1):43.
- Petri M, Naqibuddin M, Carson KA, et al. Brain magnetic resonance imaging in newly diagnosed systemic lupus erythematosus. J Rheumatol. 2008;35(12):2348–2354.
- Kitagori K, Yoshifuji H, Oku T, et al. Utility of osteopontin in cerebrospinal fluid as a diagnostic marker for neuropsychiatric systemic lupus erythematosus. Lupus. 2019;28(3):414–422.
- Hanly JG, Li Q, Su L, et al. Peripheral nervous system disease in systemic lupus erythematosus: results from an international inception cohort study. Arthritis Rheumatol. 2020;72(1):67–77.
- Bortoluzzi A, Piga M, Silvagni E, et al. Peripheral nervous system involvement in systemic lupus erythematosus: a retrospective study on prevalence, associated factors and outcome. Lupus. 2019;28(4):465–474.
- Cryan JF, Slattery DA. Animal models of mood disorders: recent developments. Curr Opin Psychiatry. 2007;20(1):1–7.
- Beyer DKE, Freund N. Animal models for bipolar disorder: from bedside to the cage. Int J Bipolar Disord. 2017;5(1):35.
- Yu C-Y, Kuo C-F, Chou I-J, et al. Comorbidities of systemic lupus erythematosus prior to and following diagnosis in different age-at-onset groups. Lupus. 2022;31(8):963–973.
- Samuels H, et al. Autoimmune disease classification based on PubMed text mining. J Clin Med. 2022;11(15):4345.
- Rees F, Doherty M, Grainge M, et al. Burden of comorbidity in systemic lupus erythematosus in the UK, 1999-2012. Arthritis Care Res (Hoboken). 2016;68(6):819–827.
- Abbott NJ, Mendonça LLF, Dolman DEM. The blood-brain barrier in systemic lupus erythematosus. Lupus. 2003;12(12):908–915.
- Chi JM. Alterations in blood-brain barrier permeability in patients with systemic lupus erythematosus. AJNR Am J Neuroradiol. 2019;40(3):470–477.
- Wang X, Ma L, Luo Y, et al. Increasing of blood brain barrier permeability and the association with depression and anxiety in systemic lupus erythematosus patients. Front Med (Lausanne). 2022;9:852835.
- Sidor MM, Sakic B, Malinowski PM, et al. Elevated immunoglobulin levels in the cerebrospinal fluid from lupus-prone mice. J Neuroimmunol. 2005;165(1-2):104–113.
- Jacob A, Hack B, Chiang E, et al. C5a alters blood-brain barrier integrity in experimental lupus. FASEB J. 2010;24(6):1682–1688.
- Nikolopoulos D, Manolakou T, Polissidis A, et al. Microglia activation in the presence of intact blood-brain barrier and disruption of hippocampal neurogenesis via IL-6 and IL-18 mediate early diffuse neuropsychiatric lupus. Ann Rheum Dis. 2023;82(5):646–657.
- Stock AD, Gelb S, Pasternak O, et al. The blood brain barrier and neuropsychiatric lupus: new perspectives in light of advances in understanding the neuroimmune interface. Autoimmun Rev. 2017;16(6):612–619.
- Solár P, Zamani A, Kubíčková L, et al. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers CNS. 2020;17(1):35.
- Gueye M, Preziosa P, Ramirez GA, et al. Choroid plexus and perivascular space enlargement in neuropsychiatric systemic lupus erythematosus. Mol Psychiatry. 2023.
- Gelb S, Stock AD, Anzi S, et al. Mechanisms of neuropsychiatric lupus: the relative roles of the blood-cerebrospinal fluid barrier versus blood-brain barrier. J Autoimmun. 2018;91:34–44.
- Greek R, Menache A. Systematic reviews of animal models: methodology versus epistemology. Int J Med Sci. 2013;10(3):206–221.
- Bielschowsky M, Helyer BJ, Howie JB. Spontaneous hemolytic anemia in mice of the NZB/BL strain. Proceedings of the University of Otago Medical School; 1959. p. 9–11.
- Helyer BJ, Howie JB. Positive lupus erythematosus tests in a Cross-Bred strain of mice NZB/BL-NZY/BL. Proceedings of the University of Otago Medical School; 1961. p. 3–4.
- Helyer BJ, Howie JB. Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature. 1963;197(4863):197–197.
- Dubois EL. NZB/NZW mice as a model of systemic lupus erythematosus. JAMA. 1966;195(4):285–289.
- Putterman C, Naparstek Y. Murine models of spontaneous systemic lupus erythematosus. In: Cohen IR, editor. Autoimmune disease models. Cambridge: Academic Press; 1994. p. 217–243.
- Richard ML, Gilkeson G. Mouse models of lupus: what they tell us and what they don’t. Lupus Sci Med. 2018;5(1):e000199.
- Schrott LM, Crnic LS. Increased anxiety behaviors in autoimmune mice. Behav Neurosci. 1996;110(3):492–502.
- Zeng J, Meng X, Zhou P, et al. Interferon-alpha exacerbates neuropsychiatric phenotypes in lupus-prone mice. Arthritis Res Ther. 2019;21(1):205.
- Kier AB. Clinical neurology and brain histopathology in NZB/NZW F1 lupus mice. J Comp Pathol. 1990;102(2):165–177.
- Graïc J-M, Finos L, Vadori V, et al. Cytoarchitectureal changes in hippocampal subregions of the NZB/W F1 mouse model of lupus. Brain Behav Immun Health. 2023;32:100662.
- Howard MW, Eichenbaum H. Time and space in the hippocampus. Brain Res. 2015;1621:345–354.
- Umbach G, Kantak P, Jacobs J, et al. Time cells in the human hippocampus and entorhinal cortex support episodic memory. Proc Natl Acad Sci U S A. 2020;117(45):28463–28474.
- Barraclough M, McKie S, Parker B, et al. Altered cognitive function in systemic lupus erythematosus and associations with inflammation and functional and structural brain changes. Ann Rheum Dis. 2019;78(7):934–940.
- Jacob CO, van der Meide PH, McDevitt HO. In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J Exp Med. 1987;166(3):798–803.
- Wang Y, Hu Q, Madri JA, et al. Amelioration of lupus-like autoimmune disease in NZB/WF1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc Natl Acad Sci U S A. 1996;93(16):8563–8568.
- Murakami Y, Fukui R, Tanaka R, et al. Anti-TLR7 antibody protects against lupus nephritis in NZBWF1 mice by targeting B cells and patrolling monocytes. Front Immunol. 2021;12:777197.
- Hang LM, Izui S, Dixon FJ. (NZW x BXSB)F1 hybrid. A model of acute lupus and coronary vascular disease with myocardial infarction. J Exp Med. 1981;154(1):216–221.
- Arabo A, Costa O, Tron F, et al. Spatial and motor abilities during the course of autoimmune disease in (NZW x BXSB)F1 lupus-prone mice. Behav Brain Res. 2005;165(1):126–137.
- Almizraq RJ, Frias Boligan K, Loriamini M, et al. NZW x BXSB) F1 male mice: an unusual, severe and fatal mouse model of lupus erythematosus. Front Immunol. 2022;13:977698.
- Olsen CT, Gabrielsen AE. Early complement components in NZB/NZW mice. I. The first component. J Immunol. 1979;122(1):133–135.
- Rudofsky UH, Lawrence DA. New Zealand mixed mice: a genetic systemic lupus erythematosus model for assessing environmental effects. Environ Health Perspect. 1999;107(Suppl 5):713–721.
- Lawrence DA, Bolivar VJ, Hudson CA, et al. Antibody induction of lupus-like neuropsychiatric manifestations. J Neuroimmunol. 2007;182(1-2):185–194.
- Gulinello M, Putterman C. The MRL/lpr mouse strain as a model for neuropsychiatric systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:1–15.
- Sakic B, Szechtman H, Talangbayan H, et al. Behavior and immune status of MRL mice in the postweaning period. Brain Behav Immun. 1994;8(1):1–13.
- Hess DC, et al. Cognitive and neurologic deficits in the MRL/lpr mouse: a clinicopathologic study. J Rheumatol. 1993;20(4):610–617.
- Guan X, Wang J. Cognitive impairment of MRL mice is related to NMDA receptor-mediated inflammatory response and production of adhesion molecules in MRL/lpr mice-derived micro-vascular endothelial cells. Folia Neuropathol. 2023;61(1):25–36.
- Ni J, Liu X, Zhang R, et al. Systemic administration of shikonin ameliorates cognitive impairment and neuron damage in NPSLE mice. J Neuroimmunol. 2023;382:578166.
- Gao H-X, Campbell SR, Cui M-H, et al. Depression is an early disease manifestation in lupus-prone MRL/lpr mice. J Neuroimmunol. 2009;207(1-2):45–56.
- Gao H-X, Sanders E, Tieng AT, et al. Sex and autoantibody titers determine the development of neuropsychiatric manifestations in lupus-prone mice. J Neuroimmunol. 2010;229(1-2):112–122.
- Li Y, Eskelund AR, Zhou H, et al. Behavioral deficits are accompanied by immunological and neurochemical changes in a mouse model for neuropsychiatric lupus (NP-SLE). Int J Mol Sci. 2015;16(7):15150–15171.
- Sakic B, Lacosta S, Denburg JA, et al. Altered neurotransmission in brains of autoimmune mice: pharmacological and neurochemical evidence. J Neuroimmunol. 2002;129(1-2):84–96.
- Lu F, Lu H, Xie M, et al. Limited preventive effect of prednisone on neuropsychiatric symptoms in murine systemic lupus erythematosus. Inflammopharmacology. 2019;27(3):511–520.
- Wang Y, Ren Y, Hong T, et al. Lipidomics changes in a murine model of neuropsychiatric lupus. J Inflamm Res. 2022;15:6569–6580.
- Sakic B, Szechtman H, Talangbayan H, et al. Disturbed emotionality in autoimmune MRL-lpr mice. Physiol Behav. 1994;56(3):609–617.
- Han X, Xu T, Ding C, et al. Neuronal NR4A1 deficiency drives complement-coordinated synaptic stripping by microglia in a mouse model of lupus. Signal Transduct Target Ther. 2022;7(1):50.
- Nielsen DM, Crnic LS. Elevated plus maze behavior, auditory startle response, and shock sensitivity in predisease and in early stage autoimmune disease MRL/lpr mice. Brain Behav Immun. 2002;16(1):46–61.
- Sakic B, Szechtman H, Braciak T, et al. Reduced preference for sucrose in autoimmune mice: a possible role of interleukin-6. Brain Res Bull. 1997;44(2):155–165.
- Sakic B, Denburg JA, Denburg SD, et al. Blunted sensitivity to sucrose in autoimmune MRL-lpr mice: a curve-shift study. Brain Res Bull. 1996;41(5):305–311.
- Stock AD, Wen J, Doerner J, et al. Neuropsychiatric systemic lupus erythematosus persists despite attenuation of systemic disease in MRL/lpr mice. J Neuroinflamm. 2015;12(1):205.
- Murphy ED, Roths JB. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum. 1979;22(11):1188–1194.
- McPhee CG, Bubier JA, Sproule TJ, et al. IL-21 is a double-edged sword in the systemic lupus erythematosus-like disease of BXSB.Yaa mice. J Immunol. 2013;191(9):4581–4588.
- Bubier JA, Sproule TJ, Foreman O, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A. 2009;106(5):1518–1523.
- Hyde LA, Hoplight BJ, Harding S, et al. Effects of ectopias and their cortical location on several measures of learning in BXSB mice. Dev Psychobiol. 2001;39(4):286–300.
- Galaburda AM, Sherman GF, Rosen GD, et al. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol. 1985;18(2):222–233.
- Lahita RG. Systemic lupus erythematosus: learning disability in the male offspring of female patients and relationship to laterality. Psychoneuroendocrinology. 1988;13(5):385–396.
- Yousef Yengej FA, van Royen-Kerkhof A, Derksen RHWM, et al. The development of offspring from mothers with systemic lupus erythematosus. A systematic review. Autoimmun Rev. 2017;16(7):701–711.
- Boehm GW, Sherman GF, Hoplight BJ, et al. Learning and memory in the autoimmune BXSB mouse: effects of neocortical ectopias and environmental enrichment. Brain Res. 1996;726(1-2):11–22.
- Arabo A, Costa O, Dubois M, et al. Effects of systemic lupus erythematosus on spatial cognition and cerebral regional metabolic reactivity in BxSB lupus-prone mice. Neuroscience. 2005;135(3):691–702.
- Hyde LA, Sherman GF, Hoplight BJ, et al. Working memory deficits in BXSB mice with neocortical ectopias. Physiol Behav. 2000;70(1-2):1–5.
- Crispín JC, Vargas-Rojas MI, Monsiváis-Urenda A, et al. Phenotype and function of dendritic cells of patients with systemic lupus erythematosus. Clin Immunol. 2012;143(1):45–50.
- Blanco P, Palucka AK, Gill M, et al. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294(5546):1540–1543.
- Cuda CM, Misharin AV, Gierut AK, et al. Caspase-8 acts as a molecular rheostat to limit RIPK1- and MyD88-mediated dendritic cell activation. J Immunol. 2014;192(12):5548–5560.
- Makinde HM, Winter DR, Procissi D, et al. A novel Microglia-Specific transcriptional signature correlates with behaviora l deficits in neuropsychiatric lupus. Front Immunol. 2020;11:230.
- Appenzeller S, Cendes F, Costallat LT. Cerebellar ataxia in systemic lupus erythematosus. Lupus. 2008;17(12):1122–1126.
- Smith RW, Ellison DW, Jenkins EA, et al. Cerebellum and brainstem vasculopathy in systemic lupus erythematosus: two clinico-pathological cases. Ann Rheum Dis. 1994;53(5):327–330.
- Al-Arfaj HF, Naddaf HO. Cerebellar atrophy in systemic lupus erythematosus. Lupus. 1995;4(5):412–414.
- Kutlubaev MA, Idrisova RF, Zakirova EN, et al. Cerebellar ataxia as a first manifestation of systemic lupus erythematosus. Acta Neurol Belg. 2020;120(5):1241–1243.
- Correa MA, Borrego A, Jensen JR, et al. Mice selected for acute inflammation present altered immune response during pristane-induced arthritis progression. Biomed Res Int. 2018;2018:1267038–1267010.
- Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994;180(6):2341–2346.
- Reeves WH, Lee PY, Weinstein JS, et al. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30(9):455–464.
- Han S, Zhuang H, Xu Y, et al. Maintenance of autoantibody production in pristane-induced murine lupus. Arthritis Res Ther. 2015;17(1):384.
- Devarapu SK, et al. Cellular and molecular mechanisms of autoimmunity and lupus nephritis. Int Rev Cell Mol Biol. 2017;332:43–154.
- Yun Y, Wang X, Xu J, et al. Pristane induced lupus mice as a model for neuropsychiatric lupus (NPSLE). Behav Brain Funct. 2023;19(1):3.
- Luciano-Jaramillo J, Sandoval-García F, Vázquez-Del Mercado M, et al. Downregulation of hippocampal NR2A/2B subunits related to cognitive impairment in a pristane-induced lupus BALB/c mice. PLoS One. 2019;14(9):e0217190.
- Guo Q, Zhang L, Yaron JR, et al. Preclinical testing of viral therapeutic efficacy in pristane-induced lupus nephritis and diffuse alveolar hemorrhage mouse models. Methods Mol Biol. 2021;2225:241–255.
- Monneaux F, Muller S. Epitope spreading in systemic lupus erythematosus: identification of triggering peptide sequences. Arthritis Rheum. 2002;46(6):1430–1438.
- Scofield RH, James JA. Immunization as a model for systemic lupus erythematosus. Semin Arthritis Rheum. 1999;29(3):140–147.
- Qiao B, Wu J, Chu YW, et al. Induction of systemic lupus erythematosus-like syndrome in syngeneic mice by immunization with activated lymphocyte-derived DNA. Rheumatology (Oxford). 2005;44(9):1108–1114.
- Mendlovic S, Brocke S, Shoenfeld Y, et al. Induction of a systemic lupus erythematosus-like disease in mice by a common human anti-DNA idiotype. Proc Natl Acad Sci U S A. 1988;85(7):2260–2264.
- Nestor J, et al. Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors. J Exp Med. 2018;215(10):2554–2566.
- Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188(1):29–38.
- Chang EH, Volpe BT, Mackay M, et al. Selective impairment of spatial cognition caused by autoantibodies to the N-Methyl-D-Aspartate receptor. EBioMedicine. 2015;2(7):755–764.
- Bortoluzzi A, Silvagni E, Furini F, et al. Peripheral nervous system involvement in systemic lupus erythematosus: a review of the evidence. Clin Exp Rheumatol. 2019;37(1):146–155.
- Jasmin R, Sockalingam S, Ramanaidu LP, et al. Clinical and electrophysiological characteristics of symmetric polyneuropathy in a cohort of systemic lupus erythematosus patients. Lupus. 2015;24(3):248–255.
- Xianbin W, Mingyu W, Dong X, et al. Peripheral neuropathies due to systemic lupus erythematosus in China. Medicine (Baltimore). 2015;94(11):e625.),
- Yan X, Maixner DW, Li F, et al. Chronic pain and impaired glial glutamate transporter function in lupus-prone mice are ameliorated by blocking macrophage colony-stimulating factor-1 receptors. J Neurochem. 2017;140(6):963–976.
- Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88.
- Graur D, Duret L, Gouy M. Phylogenetic position of the order lagomorpha (rabbits, hares and allies). Nature. 1996;379(6563):333–335.
- de Almeida da Anunciação AR, Favaron PO, de Morais-Pinto L, et al. Central nervous system development in rabbits (oryctolagus cuniculus L. 1758). Anat Rec (Hoboken). 2021;304(6):1313–1328.
- Mage RG, Esteves PJ, Rader C. Rabbit models of human diseases for diagnostics and therapeutics development. Dev Comp Immunol. 2019;92:99–104.
- Puliyath N, Ray S, Milton J, et al. Genetic contributions to the autoantibody profile in a rabbit model of systemic lupus erythematosus (SLE). Vet Immunol Immunopathol. 2008;125(3-4):251–267.
- James JA, Gross T, Scofield RH, et al. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: sm B/B’-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181(2):453–461.
- Rai G, Ray S, Milton J, et al. Gene expression profiles in a rabbit model of systemic lupus erythematosus autoantibody production. J Immunol. 2010;185(7):4446–4456.
- Yang J, Pospisil R, Ray S, et al. Investigations of a rabbit (oryctolagus cuniculus) model of systemic lupus erythematosus (SLE), BAFF and its receptors. PLoS One. 2009;4(12):e8494.
- Mage RG, Rai G. A rabbit model of systemic lupus erythematosus, useful for studies of neuropsychiatric SLE. In: Almoallim H, editor. Systemic lupus erythematosus. London: IntechOpen Limited; 2012. p. 201–2016.
- Weiss C, Disterhoft JF. Eyeblink conditioning and novel object recognition in the rabbit: behavioral paradigms for assaying psychiatric diseases. Front Psychiatry. 2015;6:142.
- Weiss C, Procissi D, Power JM, et al. The rabbit as a behavioral model system for magnetic resonance imaging. J Neurosci Methods. 2018;300:196–205.
- Salcini C, Baştan B, Sunter G, et al. Brainstem reflexes in systemic lupus erythematosus patients without clinical neurological manifestations. Noro Psikiyatr Ars. 2017;54(1):78–81.
- Fan J, Chen Y, Yan H, et al. Principles and applications of rabbit models for atherosclerosis research. J Atheroscler Thromb. 2018;25(3):213–220.
- Shen H, Yang Z, Rodrigues AD. Cynomolgus monkey as an emerging animal model to study drug transporters: in vitro, in vivo, in vitro-to-in vivo translation. Drug Metab Dispos. 2022;50(3):299–319.
- Wang J, et al. Characterization of a PRISTANE-induced lupus-associated model in the non-human primate cynomolgus monkey. J Med Primatol. 2018;47(1):18–28.
- Lewis RM, Schwartz R, Henry WB.Jr. Canine systemic lupus erythematosus. Blood. 1965;25(2):143–160.
- Choi E, Shin I, Youn H, et al. Development of canine systemic lupus erythematosus model. J Vet Med A Physiol Pathol Clin Med. 2004;51(7-8):375–383.
- Halliwell RE. Autoimmune diseases in domestic animals. J Am Vet Med Assoc. 1982;181(10):1088–1096.
- Shanley KJ. Lupus erythematosus in small animals. Clin Dermatol. 1985;3(3):131–138.
- Slauson DO, Russell SW, Schechter RD. Naturally occurring immune-complex glomerulonephritis in the cat. J Pathol. 1971;103(2):131–133.
- Powell RJ, Jones DR. Can man’s best friend provide clues to the aetiology of systemic lupus erythematosus? Ann Rheum Dis. 1992;51(7):833–834.
- McKinney WT, Jr., Bunney WE.Jr. Animal model of depression. I. Review of evidence: implications for research. Arch Gen Psychiatry. 1969;21(2):240–248.
- Willner P. The validity of animal models of depression. Psychopharmacology (Berl). 1984;83(1):1–16.
- Geyer MA, Markou A. Animal models of psychiatric disorders, in psychophamacology: the fourth generation of progress. In: Bloom FE and Kupfer DJ, editors. New York: Raven Press; 2000. p. 787–798.
- Hoffman KL. Modeling neuropsychiatric disorders in laboratory animals. Sawston, Cambridge: Woodhead Publishing; 2015.
- Belzung C, Lemoine M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol Mood Anxiety Disord. 2011;1(1):9.
- Hollander JA, Cory-Slechta DA, Jacka FN, et al. Beyond the looking glass: recent advances in understanding the impact of environmental exposures on neuropsychiatric disease. Neuropsychopharmacology. 2020;45(7):1086–1096.
- Schmitt A, Malchow B, Hasan A, et al. The impact of environmental factors in severe psychiatric disorders. Front Neurosci. 2014;8:19.
- van Vollenhoven R, Askanase AD, Bomback AS, et al. Conceptual framework for defining disease modification in systemic lupus erythematosus: a call for formal criteria. Lupus Sci Med. 2022;9(1):e000634.
- Lorenzo-Vizcaya A, Isenberg DA. Clinical trials in systemic lupus erythematosus: the dilemma-Why have phase III trials failed to confirm the promising results of phase II trials? Ann Rheum Dis. 2023;82(2):169–174.
- Casey TP. Immunosuppression by cyclophosphamide in NZB X NZW mice with lupus nephritis. Blood. 1968;32(3):436–444.
- Shiraki M, Fujiwara M, Tomura S. Long term administration of cyclophosphamide in MRL/1 mice. I. The effects on the development of immunological abnormalities and lupus nephritis. Clin Exp Immunol. 1984;55(2):333–339.
- Horowitz RE, et al. Cyclophosphamide treatment of mouse systemic lupus erythematosus. Lab Invest. 1969;21(3):199–206.
- Macanovic M, Sinicropi D, Shak S, et al. The treatment of systemic lupus erythematosus (SLE) in NZB/W F1 hybrid mice; studies with recombinant murine DNase and with dexamethasone. Clin Exp Immunol. 1996;106(2):243–252.
- Shen C, Xue X, Zhang X, et al. Dexamethasone reduces autoantibody levels in MRL/lpr mice by inhibiting TFH cell responses. J Cell Mol Med. 2021;25(17):8329–8337.
- Ramos MA, Piñera C, Setién MA, et al. Modulation of autoantibody production by mycophenolate mofetil: effects on the development of SLE in (NZB x NZW)F1 mice. Nephrol Dial Transplant. 2003;18(5):878–883.
- McMurray RW, Elbourne KB, Lagoo A, et al. Mycophenolate mofetil suppresses autoimmunity and mortality in the female NZB x NZW F1 mouse model of systemic lupus erythematosus. J Rheumatol. 1998;25(12):2364–2370.
- Lui SL, Tsang R, Wong D, et al. Effect of mycophenolate mofetil on severity of nephritis and nitric oxide production in lupus-prone MRL/lpr mice. Lupus. 2002;11(7):411–418.
- Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353(21):2219–2228.
- Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103–1112.
- Piali L, et al. Cenerimod, a novel selective S1P(1) receptor modulator with unique signaling properties. Pharmacol Res Perspect. 2017;5(6):e00370.
- Strasser DS, Froidevaux S, Sippel V, et al. Preclinical to clinical translation of cenerimod, a novel S1P(1) receptor modulator, in systemic lupus erythematosus. RMD Open. 2020;6(2):e001261.
- Hermann V, Batalov A, Smakotina S, et al. First use of cenerimod, a selective S1P(1) receptor modulator, for the treatment of SLE: a double-blind, randomised, placebo-controlled, proof-of-concept study. Lupus Sci Med. 2019;6(1):e000354.
- Perel P, Roberts I, Sena E, et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ. 2007;334(7586):197.
- Macleod M. Why animal research needs to improve. Nature. 2011;477(7366):511–511.
- Andrade D, Redecha PB, Vukelic M, et al. Engraftment of peripheral blood mononuclear cells from systemic lupus erythematosus and antiphospholipid syndrome patient donors into BALB-RAG-2-/- IL-2Rgamma-/- mice: a promising model for studying human disease. Arthritis Rheum. 2011;63(9):2764–2773.
- Moore RE, Kirwan J, Doherty MK, et al. Biomarker discovery in animal health and disease: the application of post-genomic technologies. Biomark Insights. 2007;2:117727190700200.
- Liu S, Cheng Y, Zhao Y, et al. Hippocampal atrophy in systemic lupus erythematosus patients without major neuropsychiatric manifestations. J Immunol Res. 2020;2020:2943848–2943847.
- Appenzeller S, Carnevalle AD, Li LM, et al. Hippocampal atrophy in systemic lupus erythematosus. Ann Rheum Dis. 2006;65(12):1585–1589.
- Emmer BJ, van der Grond J, Steup-Beekman GM, et al. Selective involvement of the amygdala in systemic lupus erythematosus. PLoS Med. 2006;3(12):e499.
- Huerta PT, Kowal C, DeGiorgio LA, et al. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci U S A. 2006;103(3):678–683.
- Wu, Bei-Bei, Ma, Ye, Xie, Lei, et al. Impaired decision-making and functional neuronal network activity in systemic lupus erythematosus. J Magn Reson Imaging, 2018;48(6):1508–1517.
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104.
- Pitts MW. Barnes maze procedure for spatial learning and memory in mice. Bio Protoc. 2018;8(5):e2744.
- Negrón-Oyarzo I, Espinosa N, Aguilar-Rivera M, et al. Coordinated prefrontal-hippocampal activity and navigation strategy-related prefrontal firing during spatial memory formation. Proc Natl Acad Sci USA. 2018;115(27):7123–7128.
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198(1):247–251.
- Rodríguez Peris L, Scheuber MI, Shan H, et al. Barnes maze test for spatial memory: a new, sensitive scoring system for mouse search strategies. Behav Brain Res. 2024;458:114730.
- Hayakawa K, Fujishiro M, Yoshida Y, et al. Exposure of female NZBWF1 mice to imiquimod-induced lupus nephritis at an early age via a unique mechanism that differed from spontaneous onset. Clin Exp Immunol. 2022;208(1):33–46.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200.
- Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194(6):863–869.
- Souyris M, Cenac C, Azar P, et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3(19):eaap8855.
- Liu W, Li M, Wang Z, et al. IFN-gamma mediates the development of systemic lupus erythematosus. Biomed Res Int. 2020;2020:7176515.
- Hein WR, Griebel PJ. A road less travelled: large animal models in immunological research. Nat Rev Immunol. 2003;3(1):79–84.
- Ballok DA, Woulfe J, Sur M, et al. Hippocampal damage in mouse and human forms of systemic autoimmune disease. Hippocampus. 2004;14(5):649–661.
- Sakic B, et al. Progressive atrophy of pyramidal neuron dendrites in autoimmune MRL-lpr mice. J Neuroimmunol. 1998;87(1-2):162–170.
- Sherman GF, Galaburda AM, Behan PO, et al. Neuroanatomical anomalies in autoimmune mice. Acta Neuropathol. 1987;74(3):239–242.
- Sakic B, Kolb B, Whishaw IQ, et al. Immunosuppression prevents neuronal atrophy in lupus-prone mice: evidence for brain damage induced by autoimmune disease? J Neuroimmunol. 2000;111(1-2):93–101.
- Spencer DG, Humphries K, Mathis D, Jr., et al. Behavioral impairments related to cognitive dysfunction in the autoimmune New Zealand black mouse. Behav Neurosci. 1986;100(3):353–358.,
- Wright JW, Alt JA, Turner GD, et al. Differences in spatial learning comparing transgenic p75 knockout, New Zealand black, C57BL/6, and Swiss webster mice. Behav Brain Res. 2004;153(2):453–458.
- Vogelweid CM, Wright DC, Johnson JC, et al. Evaluation of memory, learning ability, and clinical neurologic function in pathogen-free mice with systemic lupus erythematosus. Arthritis Rheum. 1994;37(6):889–897.
- Jaramillo JL, et al. Alterations in memory and visio-spatial learning in a pristane-induced lupus balb/c mice. Ann Rheum Dis. 2018;77(Suppl 2):677–677.
- Nagata W, Koizumi A, Nakagawa K, et al. Treatment with lysophosphatidic acid prevents microglial activation and depression-like behaviours in a murine model of neuropsychiatric systemic lupus erythematosus. Clin Exp Immunol. 2023;212(2):81–92.
- Aki B, Szechtman H, Denburg S, et al. Immunosuppressive treatment prevents behavioral deficit in autoimmune MRL-lpr mice. Physiol Behav. 1995;58(4):797–802.
- Wang S, Zhao X, Qiao Z, et al. Paeoniflorin attenuates depressive behaviors in systemic lupus erythematosus mice. Biomed Pharmacother. 2018;103:248–252.