Abstract
Background
Chronic periodontitis (CP) and allergic rhinitis (AR) have attracted wide attention as global public health problems with high incidence. Recent studies have shown that circulating interleukin-27 (IL-27) is associated with the risk of CP and AR. The aim of this study is to analyze the causal effect between them using Mendelian randomization (MR)
Methods
Bidirectional MR analyses were performed with the use of publicly available genome-wide association study (GWAS) data. Summary data on circulating IL-27, CP, and AR published in genome-wide association studies were collected. Instrumental variables (IV) were extracted using assumptions of correlation, independence and exclusivity as criteria. Inverse variance weighting (IVW) was used as the main method, combined with weighted median method (WM) and MR-Egger and other MR Analysis methods for causal inference of exposure and outcome. Cochran’s Q and MR-Egger intercept were used for sensitivity analysis
Results
The IVW study showed a causal effect between increased circulating IL-27 levels and increased risk of CP (OR = 1.14, 95%CI = 1.02–1.26, p = .020). Similarly, the increase of circulating IL-27 level had a causal effect on the decreased risk of AR (OR = 0.88, 95%CI = 0.80–0.97, p = .012). In addition, IVW study found that there was a causal between the increased risk of CP and circulating IL-27 level (OR = 1.05, 95%CI = 1.01–1.10, p = .016). However, there was no significant causal relationship between the risk of AR and circulating IL-27 levels (OR = 0.97, 95%CI = 0.91–1.02, p = .209). no significant heterogeneity or horizontal pleiotropy was found in sensitivity analysis.
Conclusions
There is a causal effect between circulating IL-27 level and CP, AR, which will help to find new ideas and methods for the diagnosis and treatment of CP and AR.
Introduction
Introduction to IL-27
Interleukin-27(IL-27) is a relatively new cytokine, which was first reported by StefaPflanz and his team in 2002. It belongs to the IL-12 family and is a heterodimeric cytokine composed of Epstein–Barr virus-induced gene 3 (EBI 3) and p28 subunit [Citation1]. IL-27 is mainly produced by dendritic cells, monocytes and macrophages. In addition, IL-27 induces the proliferation of CD4+ T cells, promotes the differentiation of Th1 and Tr1 cells, and inhibits the differentiation of Th2, Th17 and Treg cell [Citation2–4]. Thus IL-27 plays an important role in inflammatory and autoimmune diseases.
Chronic periodontitis (CP) is a chronic inflammatory disease with the main clinical manifestations of gingival bleeding, periodontal pocket formation, alveolar bone loss, and tooth loosening [Citation5]. According to the 2010 Global Burden of Disease Study, periodontitis has been identified as the sixth most prevalent disease in the world [Citation6], and the prevalence of mild periodontitis in adults is estimated to be 45–50%. The prevalence rate of patients over 70 years old increased to more than 65% [Citation7]. Oral microbial flora infection is the main cause of periodontitis. In addition, the chronic inflammatory response caused by inflammatory mediators such as IL and TNF is also an important cause of periodontal tissue destruction [Citation8,Citation9]. There is no uniform conclusion on the role of IL-27 in CP. Some studies have found that IL-27 can promote the absorption of alveolar bone in periodontitis [Citation10], but clinical studies have found that the plasma level of IL-27 in patients with CP is significantly reduced [Citation11].
Allergic rhinitis (AR) is one of the most common allergic diseases, which is caused by contact with allergens and mediated by allergen-specific immunoglobulin E (sIgE). The main symptoms are recurrent sneezing, runny nose, nasal congestion and itching, accompanied by red eyes and tears [Citation12]. About 10%–40% of people in the world suffer from varying degrees of AR. Moreover, the incidence continues to rise, which seriously affects the health of patients and increases the economic burden [Citation13–15]. Clinical studies have found that the level of IL-27 is also negatively correlated with the clinical severity of AR patients [Citation16].
As a global public health problem [Citation17,Citation18], CP and AR have high clinical incidence and seriously affect the quality of life of patients, and they are closely related, because nasal obstruction caused by AR can lead to mouth breathing [Citation19], which leads to the imbalance of normal oral flora. In turn, it causes various oral-related diseases, especially periodontitis [Citation20]. Therefore, studying whether IL-27 has a causal effect on CP and AR will help to develop effective intervention strategies for the treatment of CP and AR.
Introduction to Mendelian randomization studies
At present, there is no evidence to prove the causal effect between IL-27 and CP, AR. so we plan to use Mendelian randomization study to confirm. Mendelian randomization (MR) is a commonly used method in genetic epidemiology [Citation21]. It obtains exposure and outcome data files of different cohorts from GWAS databases, and uses single nucleotide polymorphisms(SNPs)as instrumental variables to observe whether there is a causal relationship between exposure and outcome. Compared with traditional observational studies, MR studies are not easily affected by confounding factors, which makes up for the limitations of basic experiments and avoids the influence of reverse causality [Citation22]. Therefore, this study used bidirectional MR Analysis to verify whether there is a causal effect of IL-27 with CP and AR at the genetic level.
Methods
Data source and study design
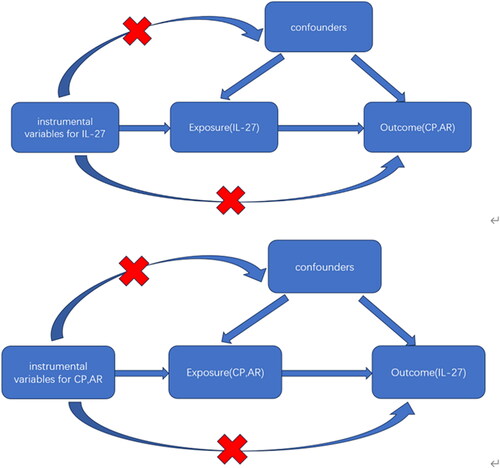
Summary statistics for IL-27, CP, and AR were downloaded from the ‘IEU Open GWAS project’ (number ID: ebi-a-GCST90012017, finn-b-K11_PERIODON_CHRON, ebi-a-GCST90086038). The OpenGWAS database, which consists of more than 200,000,000,000 genetic associations from more than 40,000 genome-wide association study (GWAS) abstract data sets, covers a wide range of human diseases and traits, and can be explored with the use of a search function. Summary statistics on circulating IL-27 levels were obtained from EBI GWAS database of 21,758 individuals of European ancestry [Citation23]. Summary statistics for CP were obtained from the FinnGen Consortium genome-wide association meta-analysis of 195,395 individuals of European ancestry, including 3,046 cases, 195,395 controls, with a number of SNPS of 16,380,378, Criteria for the diagnosis of periodontitis cases were provided by the Centres for Disease Control and Prevention/American Academy of Periodontology (CDC/AAP) [Citation24]. Pooled data on AR were obtained from EBI GWAS database of 56,637 individuals of European ancestry, including 13936 cases and 42701 controls [Citation25]. Case inclusion criteria were diseases caused by IgE-mediated inflammation of the nasal mucosa following inhalation of allergens. Symptoms include a runny nose (anterior or posterior nasal drainage), nasal congestion, itching, and sneezing [Citation26]. A bidirectional MR analysis was performed with the following flow chart ().
Extraction of instrumental variables
This study will be related to the extraction of single nucleotide polymorphisms (SNPs) strongly associated with exposure, namely instrumental variables (IV), which must simultaneously satisfy three core assumptions: Correlation hypothesis, independence hypothesis and exclusivity hypothesis [Citation27], specifically, SNPs(p < 5e − 6)with significant associations with exposure were selected, the value of F statistic is greater than 10, SNPs with linkage disequilibrium are removed, and then confounding factors related to outcome are excluded through PhenoScanner.
Data analysis
We primarily employ three methods for Mendelian analysis: Inverse variance weighting (IVW) [Citation28], weighted median (WM) [Citation29], and MR-Egge [Citation30], in which IVW assumes all instrumental variables to be valid, WM assumes half of them to be valid, and MR-Egger assumes all instrumental variables to be invalid. In practice, the IVW method is commonly used as the primary MR analysis approach to evaluate the causal effect of exposure and outcome by integrating the Wald ratio estimate of each SNP on the outcome, while WM and MR-Egger are employed as supplementary analyses.
For the sensitivity analyses, first, Cochran’s Q test was used to detect heterogeneity, p < .05 indicates the presence of heterogeneity. Second, MR-Egger regression and MR-Pleiotropy Residual Sum and Outlier method (MR-PRESSO) were used to test horizontal pleiotropy and to correct horizontal pleiotropy by removing outliers [Citation31]. Finally, the leave-one-out method was used to test whether each SNP had a significant effect on the overall results.
Results
Causal analysis of circulating IL-27 and CP, AR
By data analysis and use Phenoscanner database (http://www.phenoscanner.medschl.cam.ac.uk) to remove confounding factors, 24 SNPs were found to be strongly associated with IL-27 when CP was the outcome after excluding confounding factors, and 21 SNPs were found to be strongly associated with IL-27 when AR was the outcome after excluding confounding factors. The F (21.47–1468.5) statistics of SNPs in the above two groups were all greater than 10, excluding the possibility of weak instrumental variables. MR Analysis mainly included IVW, MR-Egger and WM. IVW was used as our main analysis method, and the results showed that there was a causal effect between the increased circulating IL-27 level and the increased risk of CP (OR =1.14, 95%CI = 1.02–1.26, p = .020). The OR values of MR-eggr (OR =1.16, 95%CI = 0.96–1.39, p = .143) and WM (OR = 1.08, 95%CI = 0.97–1.21, p = .176) had the same trend as IVW. There was a causal effect between the increased level of circulating IL-27 and the reduced risk of AR (OR = 0.88, 95%CI = 0.80–0.97, p = .012). MR-eggr (OR =0.88, 95%CI = 0.67–1.17, p = .394) and WM (OR = 0.91, 95%CI = 0.79–1.05, p = .175) showed the same trend as IVW ( and and ).
Figure 2. Scatter plot of the causal relationship between circulating interleukin-27 and chronic periodontitis
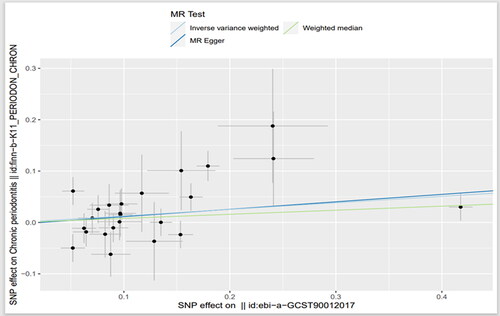
Figure 3. Scatter plot of the causal relationship between circulating interleukin-27 and allergic rhinitis
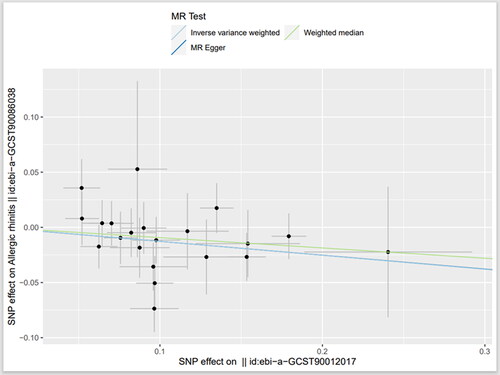
Table 1. Results of Mendelian randomization analysis of the association between circulating interleukin-27 and chronic periodontitis, allergic rhinitis.
Sensitivity analysis
No significant heterogeneity was found in the causal relationship between IL-27 and CP or AR (CP, ivw p = .057, AR, IVW p = .43). No horizontal pleiotropy was found (CP:intercept = −0.003, SE = 0.014, p = .882; AR:intercept = 0, SE = 0.0155, p = .992), and MR-PRESSO also did not detect outliers. Furthermore, no individual SNP demonstrated a significant bias on the outcomes in the leave-one-out test ( and ).
Causal relationship of chronic periodontitis and allergic rhinitis to circulating IL-27
Using the same reverse analysis as described above, we screened 16 SNPS from CP with IL-27 level as the outcome. A total of 19 SNPS were selected from AR, and both of them had F statistics greater than 10. By IVW method, a causal effect was found between CP and IL-27 (OR = 1.05, 95%CI = 1.01–1.10, p = .016) ( and ). However, we did not find a causal effect between AR and IL-27 level (OR = 0.97, 95%CI = 0.91–1.02, p = .209). No significant heterogeneity was found by Cochran’s Q test, and no pleiotropy was found by MR-egger intercept and MR-PRESSO.
Figure 6. Scatter plot of the causal relationship between chronic periodontitis and circulating interleukin-27
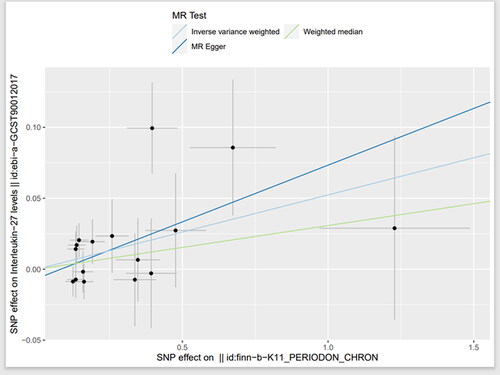
Table 2. Results of Mendelian randomization analysis of the association between chronic periodontitis, allergic rhinitis and circulating interleukin-27
Discussion
As a relatively new immune factor, some MR Studies have found that circulating IL-27 levels has a causal effect in the occurrence of COVID-19, cerebral atherosclerosis, and sleep apnoea syndrome [Citation32,Citation33]. Therefore, The relationship between circulating IL-27 and two prevalent diseases, CP and AR, was assessed using MR. This study represents the pioneering utilization of a comprehensive GWAS database to conduct an analysis on the association between IL-27 and CP, AR. The findings obtained are characterized by their high accuracy and credibility, revealing a reciprocal causal relationship between elevated levels of circulating IL-27 and an increased susceptibility to CP. Furthermore, it has been established that there exists a causal link between augmented levels of circulating IL-27 and a reduced risk of developing AR, while no causality has been observed between an increased risk of AR and circulating IL-27 levels.
It has been reported that the destruction of periodontal tissue is related to a variety of cytokines and immune responses. Studies have found that IL-27 can reduce the migration of th17 in neutrophils in periodontal tissue [Citation10], while the high level of lysosomal enzymes, superoxide and reactive oxygen species derivatives in neutrophils can promote the destruction of periodontal tissue, and th17 can also aggravates the destruction of alveolar bone [Citation34,Citation35]. Similarly, IL-27 increases the number of Th1 cells in periodontal lesions, and Th1 immune response promotes severe alveolar bone loss caused by Porphyromonas gingivalis [Citation36]. All these evidence indicates that IL-27 levels may aggravate CP. Although the role of IL-27 in periodontitis has been relatively poorly studied, our study provides a genetic basis for a causal effect between IL-27 and CP.
Previous studies have demonstrated that the pathogenesis of AR involves an imbalance between Th1 and Th2 cells, characterized by Th2 cell-mediated inflammation [Citation37]. IL-27 has been shown to inhibit the Th2 response in AR [Citation38]. Furthermore, related research has indicated a significant increase in the proportion of Th17 cells and a decrease in Treg cells in the peripheral blood of AR patients [Citation39]. IL-27 can reverse the down-regulation of regulatory T cells (Treg), promoting their role in immune activation while inhibiting the differentiation and function of Th17 cells [Citation3,Citation40]. These findings suggest that IL-27 may play a crucial role in maintaining immune inflammation balance within vivo. Clinical studies have also revealed a negative correlation between IL-27 levels and clinical severity among AR patients [Citation16]. Animal experimental studies have further demonstrated that intranasal administration of IL-27 improves allergic reactions and symptoms in mice with allergic rhinitis [Citation41]. The aforementioned studies align with our MR Results analysis.
Interestingly, little is known about the relationship between CP and AR. Although the nasal cavity and oral cavity are anatomically adjacent and connected organs, relevant clinical studies have found that CP is negatively correlated with the incidence of AR [Citation17,Citation42,Citation43]. The possible reason is that the destruction of periodontal tissue in periodontitis is mainly due to cellular immune response and related inflammatory factors (th1, th17) [Citation44], while we mentioned earlier that the pathogenesis of AR is the imbalance of Th1/Th2 caused by the activation of Th2 cells. Therefore, under the above mechanism, AR in Th2 dominant state may lead to the down-regulation of Th1 pathway, thereby inhibiting the destruction of periodontal tissue by proinflammatory cytokines [Citation17]. The above-related studies also confirm the reliability of the results in this article from the side.
This study possesses several notable strengths. First, we employed a two-way validation approach in Mendelian randomization genetic analysis to explore the association between IL-27 an CP, AR. By doing so, we effectively mitigated confounding factors and the potential influence of reverse causality, thereby enhancing the accuracy of our findings. Secondly, we conducted sensitivity analyses using diverse methodologies to bolster the robustness of our results. Thirdly, we utilised updated GWAS data to ensure the prospective nature of our discoveries. Nonetheless, it is important to acknowledge certain limitations within this study. Primarily, IL-27 represents only one among numerous interleukin factors; thus, our findings may present a relatively narrow perspective on this subject matter. To gain further insights into the impact of circulating interleukin factors on CP and AR, more extensive research is warranted. Second, given that our sample population solely consisted of individuals with European ancestry, caution should be exercised when generalising these results to other ethnic groups. Lastly, while adopting p < 5e − 6 as a screening criterion enriched the number of SNPs analysed in this study, it also introduced an inherent risk for bias in the data.
Conclusions
CP an AR are among the most prevalent diseases in the Stomatology and otolaryngology departments. Although they are not life-threatening, they have a significant impact on quality of life, emotional state and financial stress, and are continuing to be solved in clinical practice. This study demonstrated that circulating IL-27 levels play an important role in the pathogenesis of CP and AR, which may help to find new ideas and methods for the diagnosis and treatment of CP and AR.
Acknowledgement
We are grateful to the research teams from EBI and FinnGen consortium for providing us with publicly available GWAS data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16(6):1–8. Jun
- Huber M, Steinwald V, Guralnik A, et al. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20(2):223–234.
- Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7(9):929–936.
- Artis D, Villarino A, Silverman M, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173(9):5626–5634.
- Albandar JM. Epidemiology and risk factors of periodontal diseases. Dent Clin North Am. 2005;49(3):517–532.
- Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045–1053.
- Trindade D, Carvalho R, Machado V, et al. Prevalence of periodontitis in dentate people between 2011 and 2020: a systematic review and meta-analysis of epidemiological studies. J Clin Periodontol. 2023;50(5):604–626.
- Liu YC, Lerner UH, Teng YT. Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 2000. 2010;52(1):163–206.
- Cardoso EM, Reis C, Manzanares-Céspedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med. 2018; 130(1):98–104.
- Hosokawa Y, Hosokawa I, Ozaki K, et al. IL-27 modulates chemokine production in TNF-α -stimulated human oral epithelial cells. Cell Physiol Biochem. 2017;43(3):1198–1206.
- Ho JY, Yeo BS, Yang XL, et al. Local and systemic expression profile of IL-10, IL-17, IL-27, IL-35, and IL-37 in periodontal diseases: a cross-sectional study. J Contemp Dent Pract. 2021;22(1):73–79.
- Jia Y, Zou J, Wang Y, et al. Mechanism of allergic rhinitis treated by centipeda minima from different geographic areas. Pharm Biol. 2021;59(1):606–618.
- Papapostolou G, Kiotseridis H, Romberg K, et al. Cognitive dysfunction and quality of life during pollen season in children with seasonal allergic rhinitis. Pediatr Allergy Immunol. 2021;32(1):67–76.
- Muñoz-Cano R, Ribó P, Araujo G, et al. Severity of allergic rhinitis impacts sleep and anxiety: results from a large Spanish cohort. Clin Transl Allergy. 2018;8(1):23.
- Meng Y, Wang C, Zhang L. Advances and novel developments in allergic rhinitis. Allergy. 2020;75(12):3069–3076.
- Xu T, Yang Y. Clinical value of serum IL-27 in allergic rhinitis patients and its relationship with treg associated cytokine levels. Hum Immunol. 2023;84(2):130–135.
- Kim DY, Lee JK, Pang EK, et al. Unique inverse association between allergic rhinitis and periodontitis: a nationwide population-based study. Sci Rep. 2023;13(1):7444.
- Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J. 2014;7(1):12.
- Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(1 Suppl):S2–S8.
- Jacobson L. Mouthbreathing and gingivitis. 1. Gingival conditions in children with epipharyngeal adenoids. J Periodontal Res. 1973;8(5):269–277.
- Lawlor DA, Harbord RM, Sterne JA, et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–1163.
- Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology U sing mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–1621.
- Folkersen L, Gustafsson S, Wang Q, et al. Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat Metab. 2020;2(10):1135–1148.
- Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7 Suppl):1387–1399.
- Guindo-Martínez M, Amela R, Bonàs-Guarch S, et al. The impact of non-additive genetic associations on age-related complex diseases. Nat Commun. 2021;12(1):2436.
- Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(2):197–206.
- Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–1926.
- Birney E. Mendelian randomization. Cold Spring Harb Perspect Med. 2022;12(4):a041302
- Bowden J, Davey Smith G, Haycock PC, et al. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314.
- Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. 2015;44(2):512–525.
- Verbanck M, Chen C-Y, Neale B, et al. Publisher correction: detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between co mplex traits and diseases. Nat Genet. 2018;50(8):1196–1196.
- Wang C, Wang X, Zhang S, et al. Causal relationships between interleukins, interferons and COVID-19 risk: a mendelian randomization study. Int J Environ Health Res. 2023;2:1–10.
- Sun Y, Wang F, Li S. Bidirectional relationship between 56 peripheral inflammatory regulators and sleep apnea syndrome: a Mendelian randomization study. Heart Lung. 2023;62:116–121.
- Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673–2682.
- Guentsch A, Puklo M, Preshaw PM, et al. Neutrophils in chronic and aggressive periodontitis in interaction with Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J Periodontal Res. 2009;44(3):368–377.
- Stashenko P, Gonçalves RB, Lipkin B, et al. Th1 immune response promotes severe bone resorption caused by Porphyromonas gingivalis. Am J Pathol. 2007;170(1):203–213.
- Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the world health organization, GA(2)LEN and AllerGen). Allergy. 2008;63 Suppl 86(s86):8–160.
- Gan H, Du J, Ouyang H, et al. Interleukin-27 inhibits helper T cell type-2 response in allergic rhinitis. Auris Nasus Larynx. 2020;47(1):84–89.
- Huang X, Chen Y, Zhang F, et al. Peripheral Th17/treg cell-mediated immunity imbalance in allergic rhinitis patients. Braz J Otorhinolaryngol. 2014;80(2):152–155.
- Sekar D, Hahn C, Brüne B, et al. Apoptotic tumor cells induce IL-27 release from human DCs to activate treg cells that express CD69 and attenuate cytotoxicity. Eur J Immunol. 2012;42(6):1585–1598.
- Suzuki M, Yokota M, Ozaki S, et al. Intranasal administration of IL-27 ameliorates nasal allergic responses and symptoms. Int Arch Allergy Immunol. 2019;178(2):101–105.
- Friedrich N, Völzke H, Schwahn C, et al. Inverse association between periodontitis and respiratory allergies. Clin Exp Allergy. 2006;36(4):495–502.
- Hung SH, Tsai MC, Lin HC, et al. Allergic rhinitis is associated with periodontitis: a population-based study. J Periodontol. 2016;87(7):749–755.
- Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35(1):3–11.