Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disorder characterized by immune system dysfunction that can lead to serious health issues and mortality. Recent investigations highlight the role of gut microbiota alterations in modulating inflammation and disease severity in SLE. This review specifically summaries the variations in gut microbiota composition across various murine models of lupus. By focusing on these differences, we aim to elucidate the intricate relationship between gut microbiota dysbiosis and the development and progression of SLE in preclinical settings.
Introduction
Systemic lupus erythematosus (SLE) is a well-known systemic autoimmune disease with varying incidence rates based on sex, race, and age. SLE presents a wide range of clinical manifestations, affecting nearly every organ in the body [Citation1]. Despite advancements in treatment methods, SLE continues to pose significant challenges due to its prevalence, diverse manifestations, and the associated rates of mortality, and it has a notable impact on the productivity and quality of life of the patients [Citation2,Citation3].
The pathogenesis of SLE involves multifaceted interplays between genetic, epigenetic, and environmental elements, resulting in the mismanagement of both innate and adaptive immune reactions [Citation4]. Progresses in next-generation sequencing technologies have enabled a better understanding of how the gut microbiota contributes to both wellness and illnesses [Citation5]. This microbial ecosystem comprises a diverse consortium of organisms such as bacteria, viruses, and fungi [Citation6]. Among them, bacteria such as Firmicutes and Bacteroidetes dominate, while Actinobacteria, Spirochetes, and Proteobacteria represent smaller proportions [Citation7]. Changes in the composition and functionality of the gut microbiota, termed dysbiosis, can arise from various environmental and/or host-related factors [Citation8]. Dysbiosis has been linked to several diseases, including inflammatory bowel disease [Citation5], cancers [Citation9], and SLE [Citation8]. This review provides a summary of the animal models utilized for studying the pathophysiology of SLE, emphasizing the gut microbiota’s role in the disease. Notably, alterations in gut microbiota differ drastically depending on the mouse model. It is thus important to understand these differences and extract the gut microbiota signatures that are translatable from murine lupus to human SLE. The long-term goal of studying gut microbiota in the context of SLE is to identify gut microbial biomarkers that predict disease manifestations for newly diagnosed patients, which would provide the basis for personalized medicine and thus benefit drug selection and patient prognosis.
Different mouse models play a pivotal role in advancing our comprehension of SLE's development and in the investigation of innovative therapies [Citation10]. Nevertheless, due to the anatomical and immunological distinctions between mouse and human, along with the diverse manifestations of SLE, no single mouse model can fully mimic the intricacies of human SLE [Citation9–12]. Despite the existence of numerous models, each comes with its unique set of advantages and limitations. Appropriate mouse models can be chosen based on specific preclinical goals.
Common murine models of lupus
For the past five decades, researchers have used mouse models of lupus to delve into the pathophysiology of the disease and explore potential new therapeutic targets. Typically, these murine models can be classified into the following categories:
Spontaneous murine models of lupus
There are four main strains of mice exhibiting spontaneous lupus, which are genetically and immunologically diverse.
NZB/NZW F1
The NZB/NZW F1 mouse, established by crossbreeding of a New Zealand Black (NZB) mouse with a New Zealand White (NZW) mouse [Citation13,Citation14], serves as a prominent example. This model reliably develops a lupus-like autoimmune syndrome, including the generation of autoantibodies against nuclear antigens, the accumulation of immune complexes in tissues (especially the kidneys), glomerulonephritis (kidney inflammation), arthritis, and other lupus manifestations. Mirroring human SLE, the NZB/NZW F1 model demonstrates a sex bias, with female mice displaying increased susceptibility to severe autoimmune disease compared to males [Citation14]. While the NZB/NZW F1 model primarily affects females, aligning with human populations, mice in this model tend to develop clinical symptoms later in life, leading to prolonged and costly SLE studies [Citation14]. However, recent observations indicate that this time delay can be mitigated by administering adenoviruses expressing interferon-α or by injecting agonists of toll-like receptor 7 (TLR7), as demonstrated in recent studies [Citation15,Citation16]. This makes NZB/NZW F1 mice more conducive to SLE research.
C57BL/6.Sle1.Sle2.Sle3
The NZM strains are closely linked to the NZB/NZW F1 mouse, developed through successive generations of breeding these mice. This process has yielded over 20 NZM strains, each characterized for specific research purposes [Citation17]. Two strains commonly employed in research laboratories for studying SLE include the NZM2410 mouse. Much like the parental NZB/NZW F1 mice, NZM2410 mice exhibit autoantibody production and manifest lupus clinical signs. However, this strain presents immune complex glomerulonephritis without vasculitis and lacks a sex bias, setting them apart from NZB/NZW F1 mice [Citation18–21]. The NZM2410 mouse strain has been extensively utilized in delineating the genetics of lupus. Morel and colleagues developed a series of congenic mice carrying the SLE susceptibility loci of NZM2410 mice by backcrossing them to C57BL/6 (B6) mice. They have identified and characterized three SLE susceptibility loci (Sle1,2,3) in mice, commonly known as the triple congenic (TC) mice [Citation18–21]. Severe systemic autoimmune and fatal glomerulonephritis have been observed in TC or B6.Sle1.Sle2.Sle3 mice, with 100% of cases occurring by 9 months of age [Citation22,Citation23]. Furthermore, the researchers performed a thorough examination of the immune abnormalities phenotype and disease manifestation linked to each of the three loci. They also conducted detailed mapping of the genes involved, placing particular emphasis on the Sle1 locus [Citation24]. Another NZM strain known as NZM2328 also exhibits characteristics of renal disease and produces anti-dsDNA antibodies. However, the disease onset in NZM2328 is slower compared to other strains, with a 50% mortality rate occurring at 9 months of age [Citation17]. This strain offers the advantage of a significantly higher incidence in females compared to males, mirroring the disease pattern observed in NZB/NZW F1 mice and humans. Furthermore, this strain demonstrates a two-stage renal disease progression, starting with acute glomerulonephritis and transitioning into a more chronic nephritis phase [Citation25].
MRL/lpr
The Murphy-Roths-Large/lymphoproliferation (MRL/lpr) model is a unique strain in SLE research, resulting from the interbreeding of LG, B6, AKR, and C3H mouse strains [Citation26]. This model contains a spontaneous lpr mutation, which was subsequently identified as a retrotransposon that affects the Fas gene, which plays a crucial role in regulating the apoptosis of immune cells [Citation27,Citation28]. The MRL/lpr model is recognized for displaying a variety of autoantibodies detected in human SLE, such as anti-double stranded DNA (dsDNA) antibodies, anti-nuclear antibodies (ANA), anti-Ro antibodies, anti-La antibodies, and anti-Sm antibodies. Moreover, it exhibits a broad spectrum of clinical symptoms, and the lpr gene substantially exacerbates the severity of the disease in this model. The MRL+/+ mouse model, in contrast to the MRL/lpr model, does not have the Fas mutation, which leads to the development of a milder form of lupus that usually appears at a later stage [Citation26]. Researchers primarily use this model to study factors that hasten disease progression.
BXSB
The BXSB mouse model is unique in that it predominantly affects males as a result of a genetic risk factor on the Y chromosome referred to as Yaa [Citation29,Citation30]. The primary manifestation of the disease in this model is glomerulonephritis. Despite its limitations, the BXSB model offers an opportunity to evaluate TLR7 driven mechanisms, which play a crucial role in the pathogenesis of SLE [Citation31].
There are additional mouse models that have been utilized for lupus research. An example of such a model is SWR × NZB F1 (SNF1), which displays detectable anti-dsDNA autoantibodies at 16 weeks and severe glomerulonephritis at 22 weeks. It is worth noting that this model exhibits a sex bias [Citation12,Citation32]. Similarly, BXD2 mice have been observed to develop autoantibodies, arthritis, glomerulonephritis, and splenomegaly [Citation33].
Many of these spontaneous strains serve as preclinical models for evaluating potential treatments for lupus. Due to their genetic similarity and the controllable experimental conditions, mouse studies are a vital initial phase in drug development. In contrast, human SLE is heterogeneous and presents confounding factors that are not present in mouse studies. Therefore, it is crucial to recognize that success in mouse models does not always equate to efficacy in human SLE.
Induced murine models of lupus
Spontaneous lupus models have yielded invaluable insights into the intricate genetics of the disease. However, in humans, SLE is thought to stem from an environmental trigger that activates the condition in genetically susceptible individuals. Pinpointing this environmental trigger has proven to be as challenging as defining the susceptibility genes.
Induced murine lupus models are experimental setups where lupus-like symptoms are intentionally induced in mice. Unlike spontaneous lupus models, induced models serve as valuable instruments for scrutinizing particular facets of lupus pathogenesis, evaluating potential therapeutic interventions, and exploring the impacts of environmental triggers on disease progression. Specific chemicals or compounds, such as pristane [Citation34,Citation35], mercury [Citation36] or silica [Citation37], are commonly utilized in these inducible models.
The pristane-induced model may be the most extensively utilized mouse model of induced lupus [Citation34,Citation35,Citation38,Citation39]. Pristane, a type of mineral oil, is administered intraperitoneally in BALB/c or B6 mice to induce inflammation, modulate immune function, or inflict tissue damage. This induction process leads to the onset of lupus-like symptoms characterized by immune complex glomerulonephritis, erosive arthritis, skin rash, pulmonary vasculitis, and potentially even hemorrhage [Citation40,Citation41]. Autoantibodies associated with SLE, such as anti-ANA antibodies, anti-dsDNA antibodies, and anti-ribonucleoprotein (RNP) antibodies, are commonly detected [Citation34]. Numerous studies have elucidated crucial pathways in lupus development through this model. Studies have indicated that pristane-induced lupus is dependent on type I and type II interferons (IFNs), mirroring the situation in over half of patients with SLE [Citation42–44].
Resiquimod creams, which contain TLR7 ligands such as Imiquomod, have been topically applied to the ears of specific mouse strains to induce lupus-like clinical signs [Citation45]. Within 2–4 weeks post-administration, these mice develop a lupus-like disease without a sex bias. Nonetheless, the disease does not completely resemble the systemic nature of human SLE because it remains confined to a few organs. Moreover, disease induction in B6 mice is less consistent, limiting the effectiveness of this model in uncovering disease mechanisms and essential pathways through the use of transgenic or genetically modified mice, which are usually generated on the B6 background.
Transgenic murine models of lupus
Transgenic lupus models have been developed using transgenic techniques to express or overexpress gene products or proteins in order to understand their roles in immunity and lupus. Overexpression of a gene has been observed to lead to lupus disease, as seen in BXSB mice with TLR7 overexpression [Citation46]. The overexpression of immune modulatory genes has provided insight into their roles in immune tolerance [Citation47]. The development of knock-in technology allows for the targeted insertion of a gene directly into the locus of a native gene, and this advancement facilitates a more thorough exploration of immunological function defects and the progression of autoimmunity in lupus [Citation48].
The gene knockout technique has been extensively utilized to explore pathogenic mechanisms in lupus. Several knockout studies have been conducted on the MRL/lpr background, yielding significant insights into the impact of particular genes and cells on the disease. The Shlomchik group conducted experiments on B cells and provided new insights from MRL/lpr knockout studies [Citation49]. Collectively, these studies underscore the significance of B cells in lupus development. The findings suggest that B cells are central to lupus pathogenesis, regardless of autoantibody generation. Complete elimination of B cells led to reduced disease severity, and mice with B cells but unable to produce antibodies showed a decrease in disease burden [Citation50,Citation51]. Another notable instance involves breeding TLR7 and TLR9 gene knockouts onto the MRL/lpr background. Investigations into this model yielded noteworthy disparities. TLR7-deficient MRL/lpr mice displayed diminished disease severity, lacked the production of antibodies against RNP or Sm, and demonstrated prolonged survival. Conversely, MRL/lpr mice lacking TLR9 unexpectedly showed accelerated disease progression, even though they did not produce anti-dsDNA antibodies [Citation52]. Moreover, mice with double knockout of TLR7 and TLR9 were shielded from developing the disease [Citation53]. These findings suggest that TLR7 and TLR9 functions oppositely in the pathogenesis of lupus, and that the impact of TLR9 on lupus depends on TLR7.
Utilizing Cre recombinase and floxed technology, it is possible to delete a specific gene in a chosen cell type while ensuring its expression remains intact in all other cell types. This methodology facilitates the precise elucidation of a gene’s function within a specific cell type. Furthermore, conditional knockout technology permits the induction of deletion at a specific time point, ensuring that the immune system undergoes full development and maturation before the targeted gene is eliminated. The Shlomchik group has extensively investigated the role of TLR9 in SLE by employing cell-specific TLR9 knockout models [Citation54]. Their research revealed that B cell-specific TLR9 expression confers protection against murine lupus, as its absence worsens nephritis and diminishes anti-nucleosome antibodies, whereas its overexpression alleviates nephritis [Citation54]. Conversely, deficiency of TLR9 in dendritic cells (DCs) or neutrophils showed no effect [Citation54]. In their recent study, TLR7 was selectively deleted in CD19+ B cells, resulting in a modest alleviation of lupus symptoms, whereas its absence in CD11c + cells showed no effect [Citation55]. Intriguingly, the absence of TLR7 in B cells of TLR9-deficient mice notably improved disease outcomes. These findings underscore a complex interplay between TLR7 and TLR9 within the B cell compartment [Citation55]. Knocking out several genes, including C1q, C4, DNase1L3, and TGF-β, led to autoimmunity [Citation56]. Research has provided valuable insights into the roles of different factors in immunity and autoimmunity by specifically knocking out Fc receptors, apoptosis-related genes, and genes involved in cellular debris clearance. Furthermore, knocking out gene products like complement factor B and IFN-α has led to reduced disease expression, indicating their pivotal involvement in disease development [Citation57–59].
The CRISPR/Cas9 technique stands as the most advanced and efficient method for investigating gene function. This represents a considerable improvement over previous knockout approaches, which were cumbersome unless conducted in B6 mice. CRISPR/Cas9 technology enables experimentation in any mouse strain, including MRL/lpr and NZM2410, allowing for rapid determination of the effects of genetic alterations without the need for generations of backcrosses. Recently, our group utilized CRISPR/Cas9 technology to construct MRL/lpr mice with a global deletion of Tlr5 and observed an exacerbation of lupus symptoms in female MRL/lpr mice with Tlr5 deficiency (unpublished data).
Mouse models have significantly enhanced our comprehension of SLE. Yet, replicating human SLE traits in mice presents challenges. Alongside traditional mouse models, efforts are underway to develop humanized immune system mice, aiming to partially mimic the human immune system and simulate in vivo conditions of SLE [Citation60–62]. While these models hold promise for understanding SLE mechanisms and treatments, several challenges persist. Enhancing the efficiency of immune response reconstruction and effectively translating the mechanisms elucidated in mice to human SLE remain key areas requiring further investigations.
Gut microbiota alterations in SLE
Alterations of gut microbiota in SLE mouse models
Recent studies have highlighted the association between gut microbiota dysbiosis and SLE, with remarkable changes in the gut microbiota across various mouse models of lupus [Citation23,Citation63–68].
The gut microbiota typically comprises two primary phyla: Firmicutes and Bacteroidetes. Firmicutes, predominant in number, encompass numerous beneficial bacteria crucial for metabolic functions, including the digestion of dietary fibers and the synthesis of short-chain fatty acids (SCFAs). Similarly, Bacteroidetes, another major phylum, also excel in breaking down indigestable carbohydrates to synthesize metabolites like SCFAs [Citation69,Citation70]. Furthermore, Actinobacteria and Proteobacteria are two diverse groups of bacteria that play important roles in the gut microbiota. Actinobacteria, such as Bifidobacterium, are known for their beneficial effects on gut health and immune function. Proteobacteria, on the other hand, include many pathogenic bacteria but also some beneficial species found in the gut microbiota. It is important to maintain a balance between Actinobacteria and Proteobacteria for optimal gut health, as an imbalance in their numbers can contribute to gut inflammation [Citation71]. Finally, the phylum Verrucomicrobia is less abundant in the gut microbiota compared to Firmicutes and Bacteroidetes but are still significant. One well-known genus in this phylum is Akkermansia, which is associated with gut health and mucin degradation [Citation69].
Recently, sequencing-based methods have elucidated that the composition of the gut microbiota in patients with extraintestinal autoimmune diseases, such as SLE, multiple sclerosis, type 1 diabetes, and rheumatoid arthritis, is markedly different from that of healthy individuals [Citation72]. While murine models of lupus have inherent limitations, they continue to serve as invaluable tools for unraveling the pathophysiology of SLE. Mice offer a compelling platform for investigating host-microbiota interactions relevant to humans, owing to their high genetic resemblance and certain parallels in gut microbial taxonomy [Citation73,Citation74]. The changes in gut microbiota in murine lupus models are evident through various measurements including microbial dysbiosis, alpha- and beta-diversity, the Firmicutes/Bacteroidetes ratio, and changes in microbial metabolism.
Dysbiosis
Dysbiosis denotes the disruption or imbalance of the function and composition of the gut microbiota, leading to an unhealthy state [Citation8]. This imbalance can involve changes in the diversity, abundance, and distribution of microbial species within the gut ecosystem, potentially resulting in adverse effects on host health and homeostasis. Dysbiosis has been associated with multiple metabolic disorders and autoimmune diseases. Both patients with SLE and lupus mouse models have shown evidence of dysbiosis in their gut microbiota [Citation66,Citation75], which may suggest a potential involvement of dysbiosis in lupus pathogenesis.
Alpha- and beta-diversity
Alpha-diversity assesses species richness and evenness of species abundance within a microbial community [Citation76]. It has been shown that this parameter correlates with several diseases [Citation77]. In SLE, numerous studies have evaluated alpha-diversity, yet the results have shown heterogeneity, likely stemming from variations in murine models, duration of SLE characteristics, and responses to external factors such as diet and environment. For example, Wang et al. observed that six-week-old MRL/lpr mice exhibited lower alpha-diversity compared to mice after the onset of SLE, whereas this decrease was not evident in MRL+/+ mice [Citation78]. Chen et al. [Citation79] reported that in the NZB/NZW F1 mice, diversity was noted to increase following the onset of SLE induced by a viral peptide of human cytomegalovirus (HCMVpp65).
Beta-diversity reflects variances in microbial community composition. Our research group has investigated beta-diversity and observed its increase in both NZB/NZW F1 mice and MRL/lpr mice as SLE progresses [Citation66,Citation80]. Johnson et al. observed that beta-diversity was altered only in the 16-week-old SNF1 mice, while no changes were observed in 4-week-old mice when they were housed as littermates [Citation32]. These findings suggest that disease progression may have an impact on the gut microbiota.
Firmicutes/bacteroidetes (F/B) ratio
The F/B ratio serves as an indicator of gut homeostasis, with deviations from normal linked to various diseases. However, its interpretation should be complemented with additional assessments of gut microbiota composition and diversity. The discrepancies in results across studies highlight the varied nature of gut microbiota in lupus mouse models. For instance, some researchers observed an elevated F/B ratio in lupus mice [Citation79], while others observed the opposite trend [Citation78]. Valiente et al. found a tendency toward reduction of F/B ratio in 30-week-old NZM2410 mice positive for segmented filamentous bacteria (SFB), although there was no statistical significance [Citation81].
Changes in microbial metabolism
Dietary interventions can effectively modulate microbial metabolism in the microecological systems of the gut microbiota. Recent studies have shown that dietary resistant starch promotes SCFA-producing bacteria while inhibiting the growth of Lactobacillus reuteri, whose colonization exacerbates lupus-like autoimmunity in a TLR7-dependent mouse model of SLE [Citation82]. In addition, microbial metabolism of dietary tryptophan has been recently shown to affect the gut microbiota composition and disease severity in B6.Sle1.Sle2.Sle3 mice [Citation23]. These findings suggest that changes in microbial metabolism can impact SLE pathogenesis.
Factors contributing to gut microbiota alterations
In this section, we summarize several factors that contribute to gut microbiota alterations in SLE:
Sex-dependent differences
Alterations in microbiota might be due to sex differences. Johnson et al. observed a sex-specific influence of gut microbiota in SNF1 mice, as the depletion of microbiota in male mice exerted minimal effects on pathogenetic characteristics [Citation32]. Additionally, only adult male and female littermates showed significant difference in gut microbiota composition but not in juveniles [Citation32]. Castrated male SNF1 mice exhibited unique microbial communities at the genus level compared to non-castrated mice, potentially leading to more pronounced lupus symptoms and an earlier onset of the disease [Citation32]. Our group also observed differences in microbial communities between male and female lupus mice. Lupus-prone females exhibited increased levels of S24-7, Lachnospiraceae, and Bacteroidetes, as well as reduced levels of Erysipelotrichaceae and Bifidobacterium. In contrast, males showed higher levels of Bifidobacterium and Erysipelotrichaceae [Citation80]. Furthermore, Lactobacillus treatment improved renal function and survival only in female mice and castrated male mice, but not in non-castrated males [Citation67].
Differences among mouse models
Apart from sex differences, variations in gut microbiota may also originated from different murine lupus models that were applied. summaries the roles of gut microbiota in various murine lupus models.
Table 1. Changes and roles of gut microbiota in various murine lupus models.
Our group found that Lactobacillaceae exhibited a decrease, while Lachnospiraceae showed an increase in lupus-prone MRL/lpr mice [Citation80]. Increased presence of Lactobacillaceae in the intestines correlated with an amelioration of lupus clinical signs, whereas increased abundance of Lachnospiraceae was linked to the progression of the disease [Citation80]. Reduced abundance of Lactobacillaceae in adult MRL/lpr mice was also observed by other researchers [Citation78,Citation84]. Consistently, we also found that increasing Lactobacillales in the gut led to enhanced kidney function and prolonged the lifespan in MRL/lpr mice, accompanied by elevated serum level of IL-10 and restored balance of T helper 17 (Th17) cells and regulatory T (Treg) in the kidney [Citation67]. In this study, we noted that Lactobacillus reuteri and an uncultured Lactobacillus sp. might have contributed to most of the observed improvements. Although subsequent studies excluded L. reuteri as a beneficial bacterium in MRL/lpr mice, a mixture of multiple Lactobacillus spp. were shown to be protective [Citation86]. However, in a different lupus mouse model, NZB/NZW F1, we noticed significant alterations in gut microbiota before and after lupus onset, and interestingly, a specific group of Lactobacilli appeared to be associated with more pronounced clinical signs [Citation66]. Using TLR7.1 Tg mouse model, Kiegel and colleagues found that the abundance of L. reuteri increased in the gut microbiota, and colonization of L. reuteri aggravated systemic autoimmunity under both gnotobiotic and pathogen-free conditions [Citation82]. Administration of L. reuteri via gavage resulted in splenomegaly, increased accumulation of pDCs in the spleen and Peyer’s patches, as well as robust leukocyte recruitment to the kidney [Citation82]. Moreover, SCFA-producing starchy diets exert beneficial effects in lupus-prone hosts by inhibiting L. reuteri [Citation82]. Based on these discussions, the species L. reuteri and genus Lactobacillus may affect the progression of SLE, but further comprehensive studies are necessary to determine whether this effect is beneficial or detrimental in the context of lupus.
Changes in other bacterial taxa were also found in studies using the MRL/lpr model. Six-week-old MRL/lpr mice showed decreased abundance of Anaeroplasmataceae and Muribaculaceae as compared with age-matched MRL+/+ mice [Citation78]. Conversely, 18-week-old MRL+/+ mice had heightened levels of Akkermansiaceae which can promote immune-mediated responses prior to disease progression through compromising intestinal permeability and activating mucosal immunity. Meanwhile, Akkermansiaceae exhibited significant lower abundance in adult MRL/lpr mice [Citation78]. In a recent study, treatment with Akkermansia muciniphila was found to alleviate systemic inflammation and enhance renal function [Citation95]. A. muciniphila facilitated an anti-inflammatory milieu by remodeling the gut microbiome, reinstating intestinal barrier integrity, as well as modulating cytokine levels in the circulation [Citation95]. In addition, an elevation in Bacteroidetes and a decrease in Firmicutes within the gut microbiota of MRL/lpr mice were reported [Citation83]. NZB/NZW F1 mice also exhibited highly abundant Bacteroidetes and reduced levels of Firmicutes, similar to MRL/lpr mice [Citation88]. Moreover, the heightened proportions of Bacteroidetes may correlate with elevated blood pressure in these mice [Citation88]. We also reported that in a pristane-induced lupus model, glomerular pathological scores were positively correlated with the abundance of Bacteroidetes [Citation91].
Furthermore, increased levels of Prevotellaceae and Rikenellaceae, along with decreased abundance of Lachnospiraceae, were associated with severe glomerulonephritis in 30-week-old NZM2410 mice upon inoculation with SFB [Citation81]. In the HCMVpp65 peptide-induced lupus model, Chen et al. found increased abundances of Saccharimonas, Candidatus, Odoribacter, Roseburia, and Desulfovibrio is potentially associated with lupus progression [Citation79].
The direct link between microbiota and lupus pathogenesis has been explored through Fecal Microbiota Transplantation (FMT) experiments. In these experiments, the gut microbiota of lupus mice were administrated to germ-free mice, and they can induce autoantibody production and promote the expression of lupus-related genes in the recipient mice [Citation90]. Furthermore, administration of feces from SLE patients to germ-free mice led to various lupus-like phenotypic features as well as elevated expression of SLE-related genes in recipient mice [Citation96]. In another mouse model, dysbiotic gut microbiota was transferred from TC mice into germ-free congenic C57BL/6 mice, and the recipient mice exhibited enhanced autoantibody production and immune cell activation [Citation23]. These findings demonstrate that gut microbiota alterations may serve as a driving factor in the progression of SLE.
Other contributing factors
Microbiota composition and modulations could also be impacted by genetic alterations. In a human cohort study, Bifidobacterium abundance was found to be associated with the LCT (lactase gene) locus, and levels of Faecalicatena lactaris was found to be associated with the ABO (ABO blood group system) locus [Citation97]. In lupus mice, compromised T-cell receptor signaling disrupts the gut microbiota, leading to systemic autoimmunity through the encouragement of Th17 cell differentiation [Citation98]. Additionally, increased levels of proinflammatory cytokines were found to facilitate the changes in the gut microbiota [Citation79].
Underlying mechanisms linking gut microbiota and SLE
Compromised intestinal barrier and translocation of pathobionts
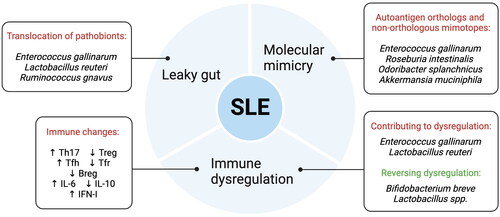
Dysbiosis of the gut microbiota disrupts intestinal barrier function, resulting in increased permeability of the intestinal lining to pathogens, viruses, and pathogenic antigens, which can infiltrate internal organs. Studies have indicated a correlation between gut microbiota dysbiosis and/or leaky gut, and autoimmune diseases such as type 1 diabetes [Citation99] and multiple sclerosis [Citation100]. Numerous studies have revealed that lupus mice and patients diagnosed with SLE develop intestinal barrier dysfunction and translocation of potential pathobionts.
Our study unveiled significantly higher endotoxin levels in the blood of MRL/lpr mice [Citation67]. Intestinally derived FITC-dextran was also much higher in the blood, indicating more severe leaky gut in MRL/lpr mice [Citation67]. In another study, NZB/NZW F1 mice showed significantly higher endotoxin level in the plasma compared to the control mice [Citation101]. Similarly, Enterococcus gallinarum can be detected in the spleen, mesenteric lymph nodes (MLNs), mesenteric veins, and liver of NZW × BXSB F1 hybrid mice, indicating gut bacteria translocation [Citation65]. Treatment with imiquimod led to impaired gut barrier function in both TLR7.1 Tg and B6 mice, as indicated by the leakage of FITC-dextran into the bloodstream [Citation82]. The translocation of L. reuteri was also observed in TLR7.1 Tg mice [Citation82].
Thim-Uam et al. discovered that inducing gut leakage with dextran sodium sulfate (DSS) led to elevated levels of anti-dsDNA antibodies, exacerbated lupus symptoms, promoted the translocation of gut bacterial components, as well as increased apoptosis in the spleen and MLNs of FcγRIIb-/- mice [Citation102]. Silverman et al. introduced specific strains of Ruminococcus gnavus (RG) isolated from lupus patients into B6 mice and noted that RG colonization heightened intestinal permeability and raised the level of zonulin, a protein that loosens tight junctions, in the serum. The patient-derived RG was detected in the mouse MLN, suggesting bacterial translocation [Citation103]. Additionally, the extent of intestinal permeability promoted by RG correlated significantly with the levels of antibodies against an RG cell-wall component, lipoglycan [Citation103].
It has been extensively documented that the impaired intestinal barrier can be restored and the translocation of gut bacteria can be inhibited. Antibiotic treatment reduces leaky gut and suppresses microbial translocation [Citation65,Citation68]. Intramuscular injection of a vaccine against E. gallinarum inhibited its translocation into internal organs in NZW × BXSB F1 lupus-prone mice [Citation65]. Resistant starch enhanced the integrity of the gut epithelial barrier and inhibited the migration of L. reuteri across the gut epithelium [Citation82]. Supplementation with Lactobacillus strengthened the barrier function of intestinal epithelial cells [Citation101]. Treatment with all-trans-retinoic acid reversed gut leakage caused by pristane [Citation91]. Administering larazotide acetate, a specific zonulin antagonist, improved gut barrier function [Citation103]. Importantly, all of these studies with reversal of leaky gut showed attenuation in lupus-like disease.
In summary, compromised intestinal barrier induces the tranlocation of gut commensal bacteria to the systemic circulation and internal organs. This heightened exposure to autoantigens can trigger or exacerbate apoptosis in cells like splenocytes, ultimately contributing to increased immune complex deposition in organs and worsening lupus. Fortunately, certain interventions such as antibiotics and probiotics have the potential to reduce or even reverse intestinal leakage, offering avenues for alleviating lupus symptoms.
Molecular mimicry
Autoantibodies targeting the 60-kDa Ro protein are frequently found in SLE patients [Citation104]. For individuals with high-risk human leukocyte antigen (HLA) genes, bacteria that harbor similar structures to these genes may continuously trigger self-reactive T cells in the gut, especially when the intestinal barrier is compromised [Citation105]. Early findings revealed that Ro60 cross-reacted with the Ebstein-Barr virus nuclear antigen-1 (EBNA-1) and suggested that humoral autoimmunity of lupus might be triggered through molecular mimicry between Ro60 and EBNA-1 antigens [Citation106]. Bacteria containing Ro60 orthologs were found in the skin, oral cavity, and gut of both patients with SLE and healthy individuals [Citation107]. Serum samples collected from SLE patients with positive anti-Ro60 antibodies were able to immunoprecipitate commensal Ro60 RNPs. Additionally, Ro60-containing bacteria extracted from the skin were capable of stimulating human CD4 memory T cells that are specific for the Ro60 autoantigen [Citation107]. Furthermore, when germ-free mice were colonized with Ro60-containing Bacteroides thetaiotaomicron, it triggered T and B cell reactions against human Ro60 resulting in the deposition of immune complexes in the glomeruli [Citation107].
Patients with autoimmune diseases like SLE and antiphospholipid syndrome (APS) often have anti-beta 2 glycoprotein 1 (anti-β2GP1) antibodies [Citation108]. It has been discovered that β2GP1 ortholog expressed by Enterococcus gallinarum triggers the production of anti-β2GP1 antibodies [Citation65]. It was found that APS-derived β2GPI autoantibodies and β2GPI-specific CD4 memory T cells were cross-reactive with mimotopes presented by the gut commensal Roseburia intestinalis [Citation109]. Moreover, oral administration of R. intestinalis to NZW × BXSB F1 mice resulted in higher production of anti-human β2GPI IgG autoantibodies and increased the occurrence of thrombotic events [Citation109].
In addition, the antigen present in RG strain CC55_001C was shown to have cross-reactivity with anti-dsDNA antibodies [Citation64]. A peptide resembling the human Sm antigen epitope derived from Odoribacter splanchnicus was found to enhance IL-17A and IFN-γ secretion [Citation64]. Moreover, a peptide from A. muciniphila, "DGQFCM", mimicking the human FAS antigen, exhibited specific binding to IgG antibodies produced by memory B cells in individuals with SLE [Citation63].
In summary, the gut microbiota plays a crucial role in activating autoreactive T and B cells, thereby initiating autoimmunity. This process involves the encoding of both autoantigen orthologs and non-orthologous mimotopes, which ultimately contribute to the initiation and progression of SLE.
Immune dysregulation associated with gut dysbiosis in SLE
Inflammatory Th17 cells and anti-inflammatory Treg cells play important roles in the pathogenesis of SLE. Their imbalance was reported in patients with SLE [Citation110]. The intestinal microbiota from SLE patients or lupus mice can induce disrupted balance of Th17/Treg in germ-free mice [Citation90]. Administration of feces from lupus mice to germ-free mice significantly increased B cells but reduced Treg cells in the intestinal mucosa of recipient mice [Citation90]. Ma et al. discovered increased levels of Th17 cells and reduced Treg cells in the spleens of germ-free mice that received fecal material from individuals with SLE [Citation96]. Morel and colleagues found increased frequency of T-follicular-helper (Tfh) cells and a reduced T-follicular-regulatory (Tfr) to Tfh cell ratio in germ-free mice receiving feces from TC lupus-susceptible mice [Citation23]. Furthermore, it has been reported that specific pathobionts contribute to Th17/Treg imbalance in lupus mouse models. NZM2410 mice colonized with SFB had an elevated level of Th17 cells in the small intestinal lamina propria [Citation81]. Vieira et al. discovered that E. gallinarum increased Th17 cells in the MLNs and small intestine lamina propria of C57BL/6 mice [Citation65]. Importantly, E. gallinarum-induced autoantibody production and Th17 expansion could be attenuated by administrating a selective aryl hydrocarbon receptor (AhR) antagonist, indicating that AhR signaling is involved in E. gallinarum mediated autoimmunity [Citation65].
The imbalance between Th17 and Treg cells can be restored by administration of probiotics. Lactobacillus is a typical probiotic which has been studied the most. It has been reported that supplementation of Lactobacillus led to increased level of Treg cells and reduced level of Th17 cells, restoring the disrupted Th17/Treg balance [Citation66,Citation101,Citation111]. Administering Bifidobacterium breve CECT7263 and Lactobacillus fermentum CECT5716 helped rebalance the ratio of Th17 to Treg cells in the MLNs. This treatment also decreased the vascular infiltration of Th1 and Th17 cells and reinstated endothelial function in a lupus mouse model induced by TLR7 activation [Citation112]. Lactobacillus acidophilus was found to regulate the balance between Th17 and Treg cells in MRL/lpr mice through the SIGNR3 pathway [Citation84]. In patients with SLE, Bifidobacterium can restrain the overactivation of CD4+ T cells to sustain Th1/Th17/Treg homeostasis [Citation113].
In addition, a subpopulation of B cells known as regulatory B (Breg) functions as an immunosuppressor and supports immune tolerance [Citation114]. Our group reported that oral administration of bacterial DNA induced expansion of the Breg population and alleviated the pathogenesis of lupus [Citation67].
Besides immune cell dysregulation, cytokine dysregulation is also associated with gut dysbiosis in SLE. Patients with SLE exhibit abnormally high levels of a series of cytokines/cytokine receptors as compared with healthy individuals [Citation115]. Bacteroides, Parabateroides, Bilophila, and Succinivibrio were positively associated with IFN-γ, IL-10, IL-17, IL-21, IL-35, TWEAK, and IL-2R, while Gemmiger and Dialister were negatively correlated with IL-17, IL-35, and IL-2R [Citation115]. Yao et al. reported elevated levels of IL-6 and IL-2 in the serum of patients with SLE. They observed an inverse correlation between the abundance of Roseburia and Faecalibacterium and IL-6 levels. Additionally, Roseburia abundance exhibited a negative association with the level of IL-2, while the presence of Bacteroides showed a positive correlation with IL-2 [Citation116].
In a murine model of lupus, our group observed that treatment with Lactobacillus reduced IL-6 level while increasing IL-10 production in the gut, fostering an anti-inflammatory environment [Citation67]. The Kriegel group discovered that the presence of E. gallinarum induced the elevation of Enpp3, which can subsequently lead to the expansion of pDCs [Citation65]. Both human and mouse hepatocytes showed increased type I IFNs when stimulated by E. gallinarum [Citation65]. Furthermore, Zegarra-Ruiz et al. discovered that L. reuteri colonization led to the expansion of pDCs and activation of IFN signaling pathways under various housing conditions [Citation82].
In summary, dysbiosis of the gut microbiota contributes to leaky gut, molecular mimicry, and immune dysregulation in SLE (). This dysregulation extends beyond the immune cells to affect cytokine levels as well.
Strategies for modulating gut microbiota in SLE
Dietary changes have been demonstrated to influence the presentation of SLE, representing one of the primary environmental factors known to impact gut microbiota. Resistant starch, which is a type of non-digestible fiber that can be fermented by gut bacteria, stands out as one such dietary component. In a TLR7-dependent lupus mouse model, an increase in dietary resistant starch resulted in reduced abundance and translocation of L. reuteri and enhanced production of SCFAs, which are accompanied by increased survival and reduced organ damage [Citation82]. It has been demonstrated that supplementing with vitamins, phytoestrogens, and polyunsaturated fatty acids led to reduced proteinuria in lupus mice [Citation117]. Vitamin D is well known for its impact on immunity. It is recognized for its ability to inhibit Th17 and Th1 responses, promote Treg cell production, and reduce B cell activation [Citation118]. Furthermore, the effect of vitamin D on gut microbiota composition has been documented [Citation119]. It can improve intestinal epithelial integrity by modulating mucus production or tight junction proteins, especially under stress [Citation119]. Supplementation with vitamin C may protect against cardiovascular complications and reduce the degree of inflammation and antibody production [Citation120]. Furthermore, fiber intake can control hyperlipidemia, lowering blood pressure and the level of C-reactive protein [Citation120]. Our group found that treatment with retinoic acid revived the downregulated Lactobacilli in lupus-prone mice and improved lupus symptoms [Citation80]. In a pristane-induced mouse model of lupus, administration of all-trans-retinoic acid resulted in diminished deposition of autoantibodies in the kidney and circulation, as well as reduced expression of proinflammatory mediators in the kidney [Citation91]. Johnson et al. found that SNF1 mice treated with acidic and neutral pH water exhibited significant differences in their gut microbiota [Citation93]. The mice drinking acidic water developed nephritis more slowly than the mice drinking neutral water [Citation93]. Low dietary tryptophan levels have been shown to prevent pathology in mice prone to develop lupus [Citation23]. Moreover, supplementation with butyrate not only alleviated gut microbiota dysbiosis but also attenuated renal injury in MRL/lpr mice [Citation83].
Another microbial therapeutic approach involves the application of probiotics [Citation121]. Administration of the probiotic L. fermentum CECT5716 (LC40) to NZB/NZW F1 mice resulted in changes to the gut microbiota composition, including reductions in Blautia and Lachnospira [Citation101]. The mice showed less endotoxemia and improved gut barrier function. Moreover, treatment with LC40 reduced the degree of splenomegaly, anti-dsDNA antibody production, the pro-inflammatory cytokine secretion, but increased IL-18 expression. These data suggest that LC40 may serve as a promising agent to intervene SLE [Citation101]. Our group found that the supplementation with Lactobacillus spp. in MRL/lpr mice significantly reduced lupus nephritis, lowered the level of anti-dsDNA antibodies, and prolonged the survival of these mice [Citation67]. Manirarora et al. noted that administering Lactobacillus could potentially stall lupus advancement in NZB/NZW F1 mice [Citation111]. Specifically, L. acidophilus was found to ameliorate gut dysbiosis, reduce renal inflammation, and augment the therapeutic efficacy of tacrolimus [Citation111]. Bifidobacterium has been shown to prevent overactivation of CD4_ T cells, thereby preserving the normal Th1/Th17/Treg balance in patients with SLE [Citation113]. Furthermore, supplementation with a common probiotic bacterium B. bifidum in patients with SLE potently prevented overactivation of CD4+ T cells [Citation113]. A very recent study showed that patients with new-onset lupus nephritis treated with a synbiotic resulted in decreased relative abundances of pathobionts such as Prevotella, Bacteroides, and Enterobacteriaceae_unclassified, while the levels of Actinobacteria and Firmicutes increased [Citation122].
Other interventions, including antibiotic treatment, vaccination, and FMT, have also demonstrated the ability to modulate gut microbiota and elicit beneficial effects in lupus mice. We found that in MRL/lpr mice, treatment with antibiotics reshaped the composition of gut microbiota, with potentially beneficial bacteria expanded while harmful bacteria reduced [Citation68]. Importantly, systemic autoimmunity and kidney histopathology were dramatically alleviated in the lupus mice after antibiotic administration [Citation68]. In NZB/NZW F1 mice, treatment with antibiotics also led to an altered composition of the gut microbiota, which was accompanied by alleviated renal injury, decreased blood pressure, and reduced disease activity [Citation112]. Furthermore, administration of the antioxidant N-acetylcysteine affected the gut microbiota composition and attenuated autoimmunity in MRL/lpr mice [Citation78]. NZW × BXSB F1 mice inoculated with the E. gallinarum vaccine led to restoration of intestinal barrier function and attenuation of lupus symptoms [Citation65]. Olia et al. discovered that infection with Hymenolepis microstoma prevented NZB/NZW F1 mice from developing lupus clinical signs [Citation123]. Wang et al. treated MRL/lpr mice with prednisone and then transferred their gut microbiota into naïve MRL/lpr mice, and found that the gut microbiota modulated by prednisone can mitigate many clinical signs of lupus without showing side effects [Citation124]. It was hypothesized that prednisone-regulated gut microbiota might improve lupus pathogenesis through maintaining the level of Lactobacillus while reducing Ruminococcus and Alistipes [Citation124]. Elshikha et al. discovered that inhibiting glycolysis with 2-deoxy-D-glucose (2DG) in lupus-prone mice (both NZB/NZW F1 and NZW × BXSB F1 mice) altered the gut microbiota, reduced autoantibodies, and ameliorated disease [Citation89]. Importantly, FMT from 2DG-treated mice was able to reduce lupus development in the recipient mice [Citation89].
In summary, strategies capable of modulating gut microbiota dysbiosis, including dietary adjustments, probiotic supplementation, antibiotic treatment, vaccination, and FMT, hold promise as potential treatments for individuals with lupus.
Conclusions
Lupus mouse models have been invaluable tools for lupus research over the past four decades. They have provided crucial insights into the cellular and molecular mechanisms underlying lupus pathogenesis. They have also served as indispensable preclinical models for assessing the therapeutic potential of pharmacological interventions. With advancements like CRISPR/Cas9 and humanized mice, our ability to dissect lupus pathogenesis continues to expand. Despite the disparities between mouse and human immune systems and the challenges in translating treatment efficacy, studies in lupus mouse models remain pivotal for advancing our understanding of lupus pathogenesis and for testing potential treatments.
In both lupus mice and patients with SLE, the gut microbiota undergoes alterations characterized by an increased level of harmful bacteria and a decreased level of beneficial bacteria, often evidenced by a decreased F/B ratio. This dysbiosis is implicated in initiating and perpetuating autoimmunity through mechanisms such as bacterial translocation and molecular mimicry. These processes lead to dysregulation of immune cells, including a skewed Th17/Treg balance and altered cytokine expression, such as heightened levels of type I IFNs. Collectively, these changes that are associated with gut microbiota dysbiosis contribute to the onset and progression of SLE.
Various strategies aim to modulate the gut microbiota in SLE, including dietary interventions, such as the incorporation of resistant starch to promote the growth of beneficial bacteria. Additionally, drug therapy, vaccination, and FMT have been explored as potential interventions. Probiotics, such as Lactobacillus and Bifidobacterium strains, have shown promise in lupus mouse models and patients with SLE by exerting anti-inflammatory effects, restoring gut barrier integrity, and regulating immune responses. While further research is needed to fully understand probiotic treatment efficacy in human SLE, preliminary findings suggest its potential as a complementary approach for managing gut dysbiotic alterations associated with the disease. Overall, these modulation strategies offer promising avenues for managing SLE by targeting dysbiotic alterations of the gut microbiota.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol. 2020;21(6):605–614. doi: 10.1038/s41590-020-0677-6
- Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18(6):871–882. doi: 10.1038/nm.2752
- Yen EY, Singh RR. Lupus-an unrecognized leading cause of death in young females: a population-based study using Nationwide Death Certificates, 2000-2015. Arthritis Rheumatol. 2018;70(8):1251–1255. doi: 10.1002/art.40512
- Gupta S, Kaplan MJ. Bite of the wolf: innate immune responses propagate autoimmunity in lupus. J Clin Invest. 2021;131(3): e144918 doi: 10.1172/JCI144918
- Malla MA, Dubey A, Kumar A, et al. Exploring the human microbiome: the potential future role of next-generation sequencing in disease diagnosis and treatment. Front Immunol. 2018;9:2868. doi: 10.3389/fimmu.2018.02868
- Ramirez J, Guarner F, Bustos Fernandez L, et al. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020;10:572912. doi: 10.3389/fcimb.2020.572912
- King CH, Desai H, Sylvetsky AC, et al. Baseline human gut microbiota profile in healthy people and standard reporting template. PLoS One. 2019;14(9):e0206484. doi: 10.1371/journal.pone.0206484
- Levy M, Kolodziejczyk AA, Thaiss CA, et al. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–232. doi: 10.1038/nri.2017.7
- Helmink BA, Khan MAW, Hermann A, et al. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377–388. doi: 10.1038/s41591-019-0377-7
- Perry D, Sang A, Yin YM, et al. Murine models of systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:271694. doi: 10.1155/2011/271694
- Li W, Titov AA, Morel L. An update on lupus animal models. Curr Opin Rheumatol. 2017;29(5):434–441. doi: 10.1097/BOR.0000000000000412
- Richard ML, Gilkeson G. Mouse models of lupus: what they tell us and what they don’t. Lupus Sci Med. 2018;5(1):e000199. doi: 10.1136/lupus-2016-000199
- Andrews BS, Eisenberg RA, Theofilopoulos AN, et al. Spontaneous murine lupus-like syndromes – clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148(5):1198–1215. doi: 10.1084/jem.148.5.1198
- Dixon FJ, Andrews BS, Eisenberg RA, et al. Etiology and pathogenesis of a spontaneous lupus-like syndrome in mice. Arthritis Rheum. 1978;21(5 Suppl):S64–S67. doi: 10.1002/art.1780210909
- Ramanujam M, Bethunaickan R, Huang WQ, et al. Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis Rheum. 2010;62(5):1457–1468. doi: 10.1002/art.27368
- Boneparth A, Woods M, Huang WQ, et al. The effect of BAFF inhibition on autoreactive B-cell selection in murine systemic lupus erythematosus. Mol Med. 2016;22(1):173–182. doi: 10.2119/molmed.2016.00022
- Rudofsky UH, Lawrence DA. New Zealand mixed mice: a genetic systemic lupus erythematosus model for assessing environmental effects. Environ Health Perspect. 1999;107 Suppl 5(Suppl 5):713–721. doi: 10.1289/ehp.99107s5713
- Morel L, Croker BP, Blenman KR, et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci USA. 2000;97(12):6670–6675. doi: 10.1073/pnas.97.12.6670
- Mohan C, Morel L, Yang P, et al. Genetic dissection of systemic lupus erythematosus pathogenesis: sle2 on murine chromosome 4 leads to B cell hyperactivity. J. Immunol. 1997;159(1):454–465. doi: 10.4049/jimmunol.159.1.454
- Wakui M, Kim J, Butfiloski EJ, et al. Genetic dissection of lupus pathogenesis: sle3/5 impacts IgH CDR3 sequences, somatic mutations, and receptor editing. J Immunol. 2004;173(12):7368–7376. doi: 10.4049/jimmunol.173.12.7368
- Wakui M, Morel L, Butfiloski EJ, et al. Genetic dissection of systemic lupus erythematosus pathogenesis: partial functional complementation between Sle1 and Sle3/5 demonstrates requirement for intracellular coexpression for full phenotypic expression of lupus. J Immunol. 2005;175(2):1337–1345. doi: 10.4049/jimmunol.175.2.1337
- Wakeland EK, Liu K, Graham RR, et al. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15(3):397–408. doi: 10.1016/s1074-7613(01)00201-1
- Choi SC, Brown J, Gong MH, et al. Gut microbiota dysbiosis and altered tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice. Sci Transl Med. 2020;12(551): eaax2220 doi: 10.1126/scitranslmed.aax2220
- Sobel ES, Mohan C, Morel L, et al. Genetic dissection of SLE pathogenesis: adoptive transfer of Sle1 mediates the loss of tolerance by bone marrow-derived B cells. J Immunol. 1999;162(4):2415–2421. doi: 10.4049/jimmunol.162.4.2415
- Waters ST, Fu SM, Gaskin F, et al. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin Immunol. 2001;100(3):372–383. doi: 10.1006/clim.2001.5079
- Izui S, Kelley VE, Masuda K, et al. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1984;133(1):227–233. doi: 10.4049/jimmunol.133.1.227
- Cohen PL, Eisenberg RA. The Lpr and Gld genes in systemic autoimmunity – life and death in the Fas lane. Immunol Today. 1992;13(11):427–428. doi: 10.1016/0167-5699(92)90066-G
- Adachi M, Watanabe-Fukunaga R, Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of Lpr mice. Proc Natl Acad Sci USA. 1993;90(5):1756–1760. doi: 10.1073/pnas.90.5.1756
- Merino R, Fossati L, Izui S. The lupus-prone BXSB strain: the Yaa gene model of systemic lupus erythematosus. Springer Semin Immunopathol. 1992;14(2):141–157. doi: 10.1007/BF00195291
- Izui S, Higaki M, Morrow D, et al. The Y chromosome from autoimmune BXSB/MpJ mice induces a lupus-like syndrome in (NZW x C57BL/6)F1 male mice, but not in C57BL/6 male mice. Eur J Immunol. 1988;18(6):911–915. doi: 10.1002/eji.1830180612
- Deane JA, Pisitkun P, Barrett RS, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27(5):801–810. doi: 10.1016/j.immuni.2007.09.009
- Johnson BM, Gaudreau MC, Gudi R, et al. Gut microbiota differently contributes to intestinal immune phenotype and systemic autoimmune progression in female and male lupus-prone mice. J Autoimmun. 2020;108:102420. doi: 10.1016/j.jaut.2020.102420
- Hong H, Alduraibi F, Ponder D, 3rd, et al. Host genetics but not commensal microbiota determines the initial development of systemic autoimmune disease in BXD2 mice. Arthritis Rheumatol. 2022;74(4):634–640. doi: 10.1002/art.42008
- Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in Balb/C mice by intraperitoneal injection of pristane. J Exp Med. 1994;180(6):2341–2346. doi: 10.1084/jem.180.6.2341
- Freitas E, de Oliveira MS, Monticielo OA. Pristane-induced lupus: considerations on this experimental model. Clin Rheumatol. 2017;36(11):2403–2414. doi: 10.1007/s10067-017-3811-6
- Pollard KM, Pearson DL, Hultman P, et al. Lupus-prone mice as models to study xenobiotic-induced acceleration of systemic autoimmunity. Environ Health Perspect. 1999;107 Suppl 5(Suppl 5):729–735. doi: 10.2307/3434334
- Brown JM, Archer AJ, Pfau JC, et al. Silica accelerated systemic autoimmune disease in lupus-prone New Zealand mixed mice. Clin Exp Immunol. 2003;131(3):415–421. doi: 10.1046/j.1365-2249.2003.02094.x
- Yun Y, Wang X, Xu J, et al. Pristane induced lupus mice as a model for neuropsychiatric lupus (NPSLE). Behav Brain Funct. 2023;19(1):3. doi: 10.1186/s12993-023-00205-y
- Yi X, Huang C, Huang C, et al. Fecal microbiota from MRL/lpr mice exacerbates pristane-induced lupus. Arthritis Res Ther. 2023;25(1):42. doi: 10.1186/s13075-023-03022-w
- Reeves WH, Lee PY, Weinstein JS, et al. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30(9):455–464. doi: 10.1016/j.it.2009.06.003
- Zhuang H, Han S, Lee PY, et al. Pathogenesis of diffuse alveolar hemorrhage in murine lupus. Arthritis Rheumatol. 2017;69(6):1280–1293. doi: 10.1002/art.40077
- Lee PY, Kumagai Y, Li Y, et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J Exp Med. 2008;205(13):2995–3006. doi: 10.1084/jem.20080462
- Lee PY, Weinstein JS, Nacionales DC, et al. A novel type I IFN-producing cell subset in murine lupus. J Immunol. 2008;180(7):5101–5108. doi: 10.4049/jimmunol.180.7.5101
- Nacionales DC, Kelly KM, Lee PY, et al. Type I interferon production by tertiary lymphoid tissue developing in response to 2,6,10,14-tetramethyl-pentadecane (pristane). Am J Pathol. 2006;168(4):1227–1240. doi: 10.2353/ajpath.2006.050125
- Yokogawa M, Takaishi M, Nakajima K, et al. Epicutaneous application of toll-like receptor 7 agonists leads to systemic autoimmunity in wild-type mice: a new model of systemic Lupus erythematosus. Arthritis Rheumatol. 2014;66(3):694–706. doi: 10.1002/art.38298
- Giltiay NV, Chappell CP, Sun X, et al. Overexpression of TLR7 promotes cell-intrinsic expansion and autoantibody production by transitional T1 B cells. J Exp Med. 2013;210(12):2773–2789. doi: 10.1084/jem.20122798
- Blenman KR, Duan B, Xu Z, et al. IL-10 regulation of lupus in the NZM2410 murine model. Lab Invest. 2006;86(11):1136–1148. doi: 10.1038/labinvest.3700468
- Friedmann D, Yachimovich N, Mostoslavsky G, et al. Production of high affinity autoantibodies in autoimmune New Zealand Black/New Zealand white F1 mice targeted with an anti-DNA heavy chain. J Immunol. 1999;162(8):4406–4416. doi: 10.4049/jimmunol.162.8.4406
- Chan O, J. Shlomchik M. A new role for B cells in systemic autoimmunity: b cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160(1):51–59. doi: 10.4049/jimmunol.160.1.51
- Chan OT, Hannum LG, Haberman AM, et al. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189(10):1639–1648. doi: 10.1084/jem.189.10.1639
- Chan OT, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999;169(1):107–121. doi: 10.1111/j.1600-065x.1999.tb01310.x
- Nickerson KM, Christensen SR, Cullen JL, et al. TLR9 promotes tolerance by restricting survival of anergic anti-DNA B cells, yet is also required for their activation. J Immunol. 2013;190(4):1447–1456. doi: 10.4049/jimmunol.1202115
- Nickerson KM, Wang Y, Bastacky S, et al. Toll-like receptor 9 suppresses lupus disease in Fas-sufficient MRL mice. PLoS One. 2017;12(3):e0173471. doi: 10.1371/journal.pone.0173471
- Tilstra JS, John S, Gordon RA, et al. B cell-intrinsic TLR9 expression is protective in murine lupus. J Clin Invest. 2020;130(6):3172–3187. doi: 10.1172/JCI132328
- Cosgrove HA, Gingras S, Kim M, et al. B cell-intrinsic TLR7 expression drives severe lupus in TLR9-deficient mice. JCI Insight. 2023;8(16):e172219. doi: 10.1172/jci.insight.172219
- Wang X. Cre transgenic mouse lines. Methods Mol Biol. 2009;561:265–273. doi: 10.1007/978-1-60327-019-9_17
- Watanabe H, Garnier G, Circolo A, et al. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol. 2000;164(2):786–794. doi: 10.4049/jimmunol.164.2.786
- Suzuki E, Karam E, Williams S, et al. Fli-1 transcription factor affects glomerulonephritis development by regulating expression of monocyte chemoattractant protein-1 in endothelial cells in the kidney. Clin Immunol. 2012;145(3):201–208. doi: 10.1016/j.clim.2012.09.006
- Reilly CM, Gilkeson GS. Use of genetic knockouts to modulate disease expression in a murine model of lupus, MRL/lpr mice. Immunol Res. 2002;25(2):143–153. doi: 10.1385/ir:25:2:143
- Gunawan M, Her ZS, Liu M, et al. A novel human systemic lupus erythematosus model in humanised mice. Sci Rep. 2017;7(1):16642. doi: 10.1038/s41598-017-16999-7
- Haotian Z, Jingjing S, Xue L, et al. Establishment of a humanized lupus nephritis mouse model. Eur. J. Immunol. 2019;49:856–857.
- Chen JX, Liao SZ, Zhou HM, et al. Humanized mouse models of systemic lupus erythematosus: opportunities and challenges. Front Immunol. 2021;12:816956. doi: 10.3389/fimmu.2021.816956
- Chen BD, Jia XM, Xu JY, et al. An autoimmunogenic and proinflammatory profile defined by the gut microbiota of patients with untreated systemic lupus erythematosus. Arthritis Rheumatol. 2021;73(2):232–243. doi: 10.1002/art.41511
- Azzouz D, Omarbekova A, Heguy A, et al. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann Rheum Dis. 2019;78(7):947–956. doi: 10.1136/annrheumdis-2018-214856
- Manfredo Vieira S, Hiltensperger M, Kumar V, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359(6380):1156–1161. doi: 10.1126/science.aar7201
- Luo XM, Edwards MR, Mu Q, et al. Gut microbiota in human systemic lupus erythematosus and a mouse model of lupus. Appl Environ Microbiol. 2018;84(4):e172219. doi: 10.1128/AEM.02288-17
- Mu Q, Zhang H, Liao X, et al. Control of lupus nephritis by changes of gut microbiota. Microbiome. 2017;5(1):73. doi: 10.1186/s40168-017-0300-8
- Mu Q, Tavella VJ, Kirby JL, et al. Antibiotics ameliorate lupus-like symptoms in mice. Sci Rep. 2017;7(1):13675. doi: 10.1038/s41598-017-14223-0
- Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550
- Yoshida N, Yamashita T, Kishino S, et al. A possible beneficial effect of bacteroides on faecal lipopolysaccharide activity and cardiovascular diseases. Sci Rep. 2020;10(1):13009. doi: 10.1038/s41598-020-69983-z
- Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944
- Miyauchi E, Shimokawa C, Steimle A, et al. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat Rev Immunol. 2023;23(1):9–23. doi: 10.1038/s41577-022-00727-y
- Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9(4):279–290. doi: 10.1038/nrmicro2540
- Kostic AD, Howitt MR, Garrett WS. Exploring host-microbiota interactions in animal models and humans. Genes Dev. 2013;27(7):701–718. doi: 10.1101/gad.212522.112
- Liu FP, Ren TL, Li XD, et al. Distinct microbiomes of gut and saliva in patients with systemic lupus erythematous and clinical associations. Front Immunol. 2021;12:626217. doi: 10.3389/fimmu.2021.626217
- Hagerty SL, Hutchison KE, Lowry CA, et al. An empirically derived method for measuring human gut microbiome alpha diversity: demonstrated utility in predicting health-related outcomes among a human clinical sample. PLoS One. 2020;15(3):e0229204. doi: 10.1371/journal.pone.0229204
- Manor O, Dai CL, Kornilov SA, et al. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat Commun. 2020;11(1):5206. doi: 10.1038/s41467-020-18871-1
- Wang H, Wang G, Banerjee N, et al. Aberrant gut microbiome contributes to intestinal oxidative stress, barrier dysfunction, inflammation and systemic autoimmune responses in MRL/lpr Mice. Front Immunol. 2021;12:651191. doi: 10.3389/fimmu.2021.651191
- Chen YF, Hsieh AH, Wang LC, et al. Fecal microbiota changes in NZB/W F1 mice after induction of lupus disease. Sci Rep. 2021;11(1):22953. doi: 10.1038/s41598-021-02422-9
- Zhang H, Liao X, Sparks JB, et al. Dynamics of gut microbiota in autoimmune lupus. Appl Environ Microbiol. 2014;80(24):7551–7560. doi: 10.1128/AEM.02676-14
- Valiente GR, Munir A, Hart ML, et al. Gut dysbiosis is associated with acceleration of lupus nephritis. Sci Rep. 2022;12(1):152. doi: 10.1038/s41598-021-03886-5
- Zegarra-Ruiz DF, El Beidaq A, Iniguez AJ, et al. A diet-sensitive commensal lactobacillus strain mediates TLR7-dependent systemic autoimmunity. Cell Host Microbe. 2019;25(1):113–127 e116. doi: 10.1016/j.chom.2018.11.009
- He H, Xu H, Xu J, et al. Sodium Butyrate ameliorates gut microbiota dysbiosis in lupus-like mice. Front Nutr. 2020;7:604283. doi: 10.3389/fnut.2020.604283
- Kim DS, Park Y, Choi JW, et al. Lactobacillus acidophilus supplementation exerts a synergistic effect on tacrolimus efficacy by modulating Th17/Treg balance in lupus-prone mice via the SIGNR3 pathway. Front Immunol. 2021;12:696074. doi: 10.3389/fimmu.2021.696074
- Cabana-Puig X, Bond JM, Wang Z, et al. Phenotypic drift in lupus-prone MRL/lpr mice: potential roles of microRNAs and gut microbiota. Immunohorizons. 2022;6(1):36–46. doi: 10.4049/immunohorizons.2100082
- Cabana-Puig X, Mu Q, Lu R, et al. Lactobacillus spp. act in synergy to attenuate splenomegaly and lymphadenopathy in lupus-prone MRL/lpr mice. Front Immunol. 2022;13:923754. doi: 10.3389/fimmu.2022.923754
- Wang C, Lin Y, Chen L, et al. Gut microbiota mediated the effects of high relative humidity on lupus in female MRL/lpr mice. Adv Rheumatol. 2023;63(1):24. doi: 10.1186/s42358-023-00306-2
- de la Visitación N, Robles-Vera I, Toral M, et al. Gut microbiota contributes to the development of hypertension in a genetic mouse model of systemic lupus erythematosus. Br J Pharmacol. 2021;178(18):3708–3729. doi: 10.1111/bph.15512
- Elshikha AS, Ge Y, Brown J, et al. Pharmacologic inhibition of glycolysis prevents the development of lupus by altering the gut microbiome in mice. iScience. 2023;26(7):107122. doi: 10.1016/j.isci.2023.107122
- Ma Y, Xu X, Li M, et al. Gut microbiota promote the inflammatory response in the pathogenesis of systemic lupus erythematosus. Mol Med. 2019;25(1):35. doi: 10.1186/s10020-019-0102-5
- Abdelhamid L, Cabana-Puig X, Swartwout B, et al. Retinoic acid exerts disease stage-dependent effects on pristane-induced lupus. Front Immunol. 2020;11:408. doi: 10.3389/fimmu.2020.00408
- Toumi E, Goutorbe B, Plauzolles A, et al. Gut microbiota in systemic lupus erythematosus patients and lupus mouse model: a cross species comparative analysis for biomarker discovery. Front Immunol. 2022;13:943241. doi: 10.3389/fimmu.2022.943241
- Johnson BM, Gaudreau MC, Al-Gadban MM, et al. Impact of dietary deviation on disease progression and gut microbiome composition in lupus-prone SNF1 mice. Clin Exp Immunol. 2015;181(2):323–337. doi: 10.1111/cei.12609
- Cabana-Puig X, Lu R, Geng S, et al. CX(3)CR1 modulates SLE-associated glomerulonephritis and cardiovascular disease in MRL/lpr mice. Inflamm Res. 2023;72(5):1083–1097. doi: 10.1007/s00011-023-01731-1
- Guo M, Lu M, Chen K, et al. Akkermansia muciniphila and Lactobacillus plantarum ameliorate systemic lupus erythematosus by possibly regulating immune response and remodeling gut microbiota. mSphere. 2023;8(4):e0007023. doi: 10.1128/msphere.00070-23
- Ma Y, Guo R, Sun Y, et al. Lupus gut microbiota transplants cause autoimmunity and inflammation. Clin Immunol. 2021;233:108892. doi: 10.1016/j.clim.2021.108892
- Qin Y, Havulinna AS, Liu Y, et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54(2):134–142. doi: 10.1038/s41588-021-00991-z
- Shirakashi M, Maruya M, Hirota K, et al. Effect of impaired T cell receptor signaling on the gut microbiota in a mouse model of systemic autoimmunity. Arthritis Rheumatol. 2022;74(4):641–653. doi: 10.1002/art.42016
- Sorini C, Cosorich I, Lo Conte M, et al. Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes. Proc Natl Acad Sci USA. 2019;116(30):15140–15149. doi: 10.1073/pnas.1814558116
- Secher T, Kassem S, Benamar M, et al. Oral administration of the probiotic strain escherichia coli Nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front Immunol. 2017;8:1096. doi: 10.3389/fimmu.2017.01096
- Toral M, Robles-Vera I, Romero M, et al. Lactobacillus fermentum CECT5716: a novel alternative for the prevention of vascular disorders in a mouse model of systemic lupus erythematosus. Faseb J. 2019;33(9):10005–10018. doi: 10.1096/fj.201900545RR
- Thim-Uam A, Surawut S, Issara-Amphorn J, et al. Leaky-gut enhanced lupus progression in the Fc gamma receptor-IIb deficient and pristane-induced mouse models of lupus. Sci Rep. 2020;10(1):777. doi: 10.1038/s41598-019-57275-0
- Silverman GJ, Deng J, Azzouz DF. Sex-dependent Lupus Blautia (Ruminococcus) gnavus strain induction of zonulin-mediated intestinal permeability and autoimmunity. Front Immunol. 2022;13:897971. doi: 10.3389/fimmu.2022.897971
- Foulquier N, Le Dantec C, Bettacchioli E, et al. Machine learning for the identification of a common signature for anti-SSA/Ro 60 antibody expression across autoimmune diseases. Arthritis Rheumatol. 2022;74(10):1706–1719. doi: 10.1002/art.42243
- Garabatos N, Santamaria P. Gut microbial antigenic mimicry in autoimmunity. Front Immunol. 2022;13:873607. doi: 10.3389/fimmu.2022.873607
- McClain MT, Heinlen LD, Dennis GJ, et al. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11(1):85–89. doi: 10.1038/nm1167
- Greiling TM, Dehner C, Chen X, et al. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci Transl Med. 2018;10(434):eaan2306. doi: 10.1126/scitranslmed.aan2306
- Ho YC, Ahuja KDK, Körner H, et al. Beta(2)GP1, anti-beta(2)GP1 antibodies and platelets: key players in the antiphospholipid syndrome. Antibodies (Basel). 2016;5(2):5. doi: 10.3390/antib5020012
- Ruff WE, Dehner C, Kim WJ, et al. Pathogenic autoreactive T and B cells cross-react with mimotopes expressed by a common human gut commensal to trigger autoimmunity. Cell Host Microbe. 2019;26(1):100–113.e8. +. doi: 10.1016/j.chom.2019.05.003
- Chen M, Chen X, Wan Q. Altered frequency of Th17 and Treg cells in new-onset systemic lupus erythematosus patients. Eur J Clin Invest. 2018;48(11):e13012. doi: 10.1111/eci.13012
- Manirarora JN, Kosiewicz MM, Alard P. Feeding lactobacilli impacts lupus progression in (NZBxNZW)F1 lupus-prone mice by enhancing immunoregulation. Autoimmunity. 2020;53(6):323–332. doi: 10.1080/08916934.2020.1777282
- de la Visitación N, Robles-Vera I, Moleón-Moya J, et al. Probiotics prevent hypertension in a murine model of systemic lupus erythematosus induced by toll-like receptor 7 activation. Nutrients. 2021;13(8):2669. doi: 10.3390/nu13082669
- López P, de Paz B, Rodríguez-Carrio J, et al. Th17 responses and natural IgM antibodies are related to gut microbiota composition in systemic lupus erythematosus patients. Sci Rep. 2016;6(1):24072. doi: 10.1038/srep24072
- Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–612. doi: 10.1016/j.immuni.2015.04.005
- Guo MC, Wang HX, Xu SX, et al. Alteration in gut microbiota is associated with dysregulation of cytokines and glucocorticoid therapy in systemic lupus erythematosus. Gut Microbes. 2020;11(6):1758–1773. doi: 10.1080/19490976.2020.1768644
- Yao H, Yang H, Wang YY, et al. Gut microbiome and fecal metabolic alteration in systemic lupus erythematosus patients with depression. Front Cell Infect Microbiol. 2022;12:1040211. doi: 10.3389/fcimb.2022.1040211
- Vieira SM, Pagovich OE, Kriegel MA. Diet, microbiota and autoimmune diseases. Lupus. 2014;23(6):518–526. doi: 10.1177/0961203313501401
- Yamamoto EA, Jørgensen TN. Relationships between vitamin D, gut microbiome, and systemic autoimmunity. Front Immunol. 2019;10:3141. doi: 10.3389/fimmu.2019.03141
- Fakhoury HMA, Kvietys PR, AlKattan W, et al. Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol. 2020;200:105663. doi: 10.1016/j.jsbmb.2020.105663
- Klack K, Bonfa E, Borba Neto EF. Diet and nutritional aspects in systemic lupus erythematosus. Rev Bras Reumatol. 2012;52(3):384–408.
- Guo X, Yang X, Li Q, et al. The microbiota in systemic lupus erythematosus: an update on the potential function of probiotics. Front Pharmacol. 2021;12:759095. doi: 10.3389/fphar.2021.759095
- Zhu Q, Cui J, Liu S, et al. Synbiotic regulates gut microbiota in patients with lupus nephritis: an analysis using metagenomic and metabolome sequencing. Front Microbiol. 2024;15:1295378. doi: 10.3389/fmicb.2024.1295378
- Olia A, Shimokawa C, Imai T, et al. Suppression of systemic lupus erythematosus in NZBWF1 mice infected with Hymenolepis microstoma. Parasitol Int. 2020;76:102057. doi: 10.1016/j.parint.2020.102057
- Wang M, Zhu Z, Lin X, et al. Gut microbiota mediated the therapeutic efficacies and the side effects of prednisone in the treatment of MRL/lpr mice. Arthritis Res Ther. 2021;23(1):240. doi: 10.1186/s13075-021-02620-w