ABSTRACT
The ballast water from ships carries marine organisms that have invasive potential. The International Maritime Organization Ballast Water Management Convention (2004) requires ballast water exchange or ballast water management (BWM) systems either onboard or ashore. Ships can be exempted on the basis of risk assessment, when exclusively sailing between specific ports or in an enclosed area. In reply to our questionnaire, the shipping sector argues that the North Sea is ecologically homogeneous and exemptions could therefore be granted. This paper proposes that the North Sea area is, in fact, not homogeneous in terms of hydrographical and biological conditions; therefore, ballast water is a relevant transport mechanism for organisms. Within the North Sea, the short shipping routes indicate a high risk for survival. We examined actual simulation models for ballast water risk assessment in the North Sea, and we have identified the major parameters that need to be included in such models. These models provided a basis; they further need to be combined and adapted for the purpose of evaluating the rationale for an exemption. We concluded that exemptions from BWM are not recommended for the North Sea area. Anticipating the Ballast Water Management Convention, ship owners might do well to study possibilities for installing BWM systems onboard.
Introduction
Marine invasions
Merchant shipping is a major source of unintentional introduction of species in marine ecosystems (Carlton and Geller Citation1993). Ships translocate organisms around the world by means of hull fouling and onboard ballast water (Gollasch Citation2002). Ballast water is required in order to enhance stability and hydrodynamic sailing characteristics (Clark Citation2002; Van Dokkum Citation2003). The estimation of ballast water discharges worldwide is 3.1 billion tons for 2013 (David Citation2015). As many as 7000 species (Carlton Citation1999) are transferred this way around the globe. A variety of species can survive in ballast water tanks for several days (Gollasch et al. Citation2000; Flagella et al. Citation2007) and so will be discharged in the port of destination.
Once established, populations of non-indigenous species are at risk of becoming invasive in marine and estuarine habitats, with adverse impacts on ecology, the economy, and human health (Anil et al. Citation2002; Lovell, Stone, and Fernandez Citation2006). Invasions of non-indigenous species have been shown to affect biodiversity and ecosystem functions (Stachowicz, Bruno, and Emmett Duffy Citation2007). The costs to fisheries and industries are significant (Kideys Citation1994). The introduction of pathogenic viruses and bacteria by means of ballast water poses a threat to human health (Dahlstrom, Hewitt, and Campbell Citation2011).
Ballast Water Management Convention
The International Maritime Organization (IMO) adopted the Ballast Water Management Convention (BWMC) to prevent, minimize, and ultimately eliminate the transfer of harmful aquatic organisms and pathogens through the control and management of ships' ballast water and sediments (IMO Citation2004).
The BWMC requires BWM in the recipient port or onboard ships in order to reduce the number of viable organisms in the ballast water discharge, according to the Regulation D-2 Ballast Water Performance Standard (IMO Citation2004). Management can be performed by ballast water exchange (BWE) during the voyage, and by chemical or physical disinfection by BWM systems to remove or kill the organisms (IMO Citation2004).
The BWMC will come into force 12 months after ratification by a minimum of 30 IMO member states, covering 35% of world gross tonnage (IMO Citation2004). To date, the total number of contracting states has reached 44 states and 32.86% of the gross tonnage of the world's merchant shipping (IMO Citation2015a). It is expected that the convention will come into force in 2016, or soon thereafter (BIMCO Citation2014).
BWE is an intermediate option, which will be phased out after the BWMC has come into force. According to the Regulation D-1 Ballast Water Exchange Standard, BWE is only allowed in water at least 200 m in depth and at least 200 nautical miles from the nearest land or, in case of a coastal voyage, at least 50 nautical miles offshore (IMO Citation2004). For vessels on a coastal voyage in areas that do not meet these requirements, the port state may designate locations for BWE (Regulation B-4 2, IMO Citation2004).
The Ballast Water Performance Standard of Regulation D-2 can be achieved by means of BWM systems. BWM systems are designed to remove, kill, or inactivate organisms in ballast water prior to discharge. Several systems have been developed as BWM options (Tsolaki and Diamadopoulos Citation2010). The dominant systems combine removal by filtering, or hydrocyclonage, with physical or chemical disinfection. Physical disinfection includes UV irradiation. Oxidizing agents for chemical disinfection include chlorine, ozone, and hydrogen peroxide (Balaji and Yaakob Citation2011). All BWM systems require approval by the authorities of the certifying state. Through to October 2014, 51 systems have been approved by these authorities, and 50 BWM systems have received Basic and 36 Final Approval from IMO (Citation2014).
Only a few ships out of more than 50,000 ships that need to be in compliance in the near future have actually planned or installed BWM systems. There is hardly any data available, however, concerning the onboard performance of BWM systems in practice. Experience is restricted to the findings of shipboard certification tests. Retrofitting of older ships, especially, has several drawbacks: cost and availability of equipment, cost of installation, lack of space onboard, maintenance requirements, and availability of docking capacity (Gregg, Rigby, and Hallegraeff Citation2009). It is generally considered that there is insufficient time to retrofit the fleet before the BWMC deadline.
Ballast water management exemptions
The BWMC offers an opportunity for exemption from the BWE/BWM requirements, by providing ships with a waiver from installing BWM systems onboard. Exemptions are granted solely to those ships sailing exclusively between specified ports within a biogeographical region with similar environmental conditions. The exemptions are granted by the IMO member states, in whose waters the ships operate. Duration of an exemption is limited to a maximum of five years subject to intermediate review (IMO Citation2004). The foundation for exemptions is Regulation A-4 of the BWMC; the required risk assessment must be performed in accordance with the G-7 Guidelines for Risk Assessment (IMO Citation2007).
The present study will focus on the center of shipping routes in Western Europe, the North Sea. Is an exemption from the BWE/BWM requirements appropriate and permissible in this area?
North Sea exemptions
Several vessels operate exclusively within the North Sea area, such as ferries, vessels engaged in short-sea trading, and fishing vessels (European Commission Citation2014). The North Sea is a semi-enclosed and shallow sea ( and ). Water depth and distances to shore are below the BWMC minimum limits for BWE. Therefore, BWE requirements cannot be met, except for a limited area in the northern North Sea, part of the Norwegian Trench (; Gollasch, David and Leppäkoski Citation2011). Moreover, sailing distances between North Sea ports are often too short to perform a proper BWE during the voyage (). As a compromise, the nations bordering the North Sea are proposing exchange areas for intra North Sea voyages throughout the entire North Sea area, except for coastal and protected areas (IMO Citation2015b).
Table 1. Location and salinity of the major North Sea ports (AAPA World Port Rankings Citation2010).
Figure 1. An overview of the North Sea bathymetry, northwestern Europe (from ICONA Citation1992). The dark dots are the major North Sea ports. The numbers correspond to the numbers in Table 1.
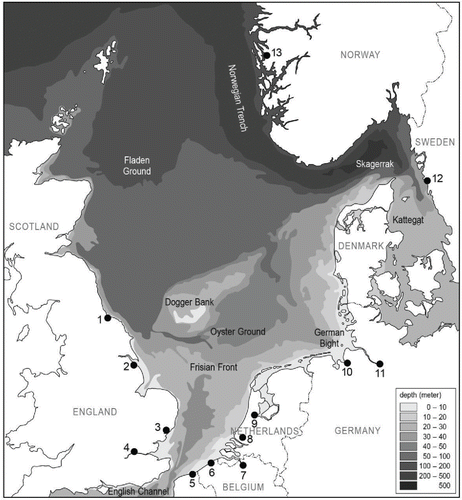
Table 2. Sailing times in hours between the major ports in the North Sea, based on a speed-over-ground of 10 knots.
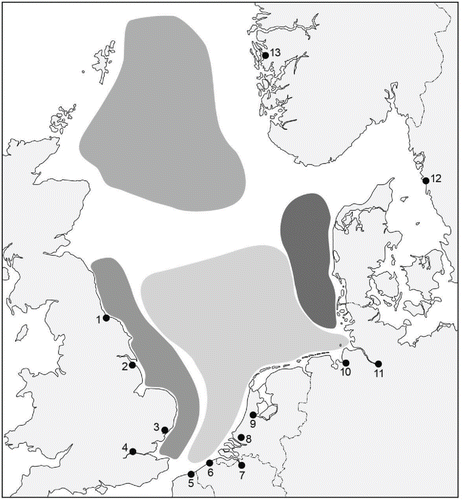
The North Sea is regarded as an area in which the impact from the introduction of non-indigenous species is high (Vila et al. Citation2010). Over 150 non-indigenous species have been identified in the North Sea area (Gollasch et al. Citation2009). Most of the ports where ballast water is taken in or released are located in North Sea estuaries and river mouths ( and ; Nehring Citation2006; Reise, Gollasch, and Wolff Citation1999). North Sea estuaries are considered to be highly susceptible to the introduction of new species, due to a combination of intensive international shipping traffic and ports situated in areas with a wide range of salinity ( and ; Nehring Citation2006). Their ecological value is recognized in Natura 2000's international conservation regulations (EEA Citation2012), Marine Protective Areas (OSPAR Citation2013), and Particularly Sensitive Sea Areas (IMO Citation2013).
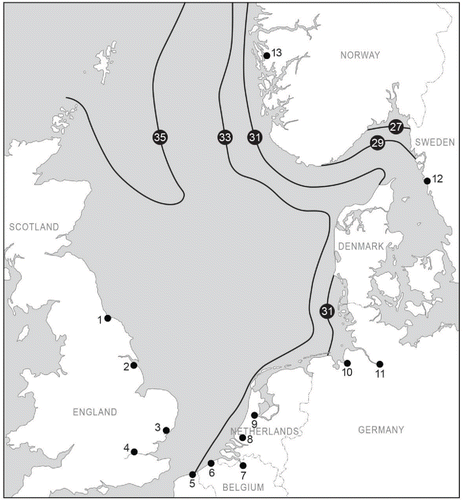
Figure 2. The salinity of the North Sea during August (from Van Aaken Citation1990). The numbered dots with lines show the boundary of the salinity (psu). The dark dots are the major North Sea ports. The numbers correspond to the numbers in Table 1.
The chance of acquiring a BWM exemption is of interest to the shipping industry in order to circumvent the cost of installing, operating, and maintaining a BWM system. We distributed a questionnaire among operators in the North Sea shipping industry. The questionnaire investigated the level of awareness of the shipping sector vis-à-vis mitigation measures featured in the BWMC, as well as their expectations as to the likelihood of obtaining BWM exemptions for ships in the North Sea area. Thirteen companies, mainly Dutch ones, responded to the questionnaire (Appendix A). These companies represent many different types of vessels (ferries, cruise vessels, oil carriers, containers, etc.). Twelve companies have vessels that frequently take in ballast water, and four of the companies have vessels that operate exclusively within the North Sea (from 30% to 100% of their fleet).
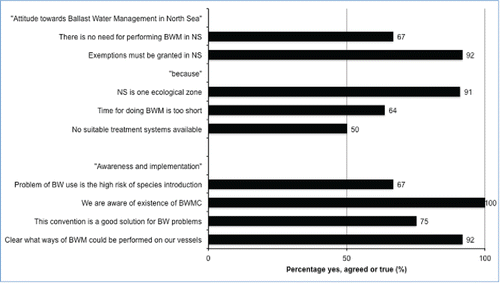
The response showed that all companies are aware of the BWMC regulations, and a majority of them have prepared a BWM strategy. As for ships in the North Sea area, nearly all companies expect that exemptions will be granted (92%), based on the assumption that the entire region can be considered as an ecologically homogeneous zone (91%; ). This assumption is based on the view that organisms can be transported throughout the area by means of natural processes, such as currents, without encountering any environmental barrier to their ability to establish. Ballast water is not unique as a transport mechanism in the North Sea area, in other words. As a consequence, species are therefore able to be distributed evenly across the area.
Figure 3. Attitude of ship companies toward ballast water management (BWM) exemptions in the North Sea area (NS). Thirteen companies responded to the questionnaire; twelve of these companies have ships that frequently take in ballast water (Appendix A). BW, ballast water; BWMC, Ballast Water Management Convention.
Strategies for issuing exemptions from the BWM requirements have been investigated for the Baltic and North Sea areas (Behrens, Leppäkoski, and Olenin Citation2005; Gollasch and Leppäkoski Citation2007). Implementation of the strategy in the North Sea area is in the process of preparation. The member countries of Oslo Paris convention (OSPAR) and Helsinki commission (HELCOM) (North Sea and Baltic states) are preparing a harmonized procedure for risk assessment (Helsinki and OSPAR Commission Citation2014). Under the HELCOM/OSPAR Harmonized Procedure, an online risk assessment tool is available for administrations and ship owners to identify routes that may qualify for exemptions (HELCOM/OSPAR Harmonized Procedure Citation2014). The key risk criteria are limited to the difference in water salinity between the ports that are visited and the presence of target species in the donor port (Helsinki and OSPAR Commissions Citation2014).
With reference to the questionnaire, we evaluated the permissibility of BWM exemptions for ships operating in the North Sea area only, based on the key factors for introducing an aquatic species. We will then go on to discuss models to assess the risk of the translocation of ballast water on various shipping routes in the North Sea area.
Risk assessment in the Ballast Water Management Convention
The BWMC distinguishes three approaches for assessing an ecological risk from ballast water: 1) the environmental similarity approach; 2) the species biogeographic approach; and 3) the species-specific approach (IMO Citation2007). The environmental similarity approach is the simplest, being based on the comparison of water salinity and temperature between the ballast water donor and the recipient area. Similar conditions in the donor and recipient area indicate the risk for a species to establish.
The biogeographic approach compares the distribution of species in the donor area and in the recipient area. Risk is based on the occurrence of similar species in both areas.
The species-specific approach is a combination of the previous approaches. This approach includes species-specific data on the minimum requirements of a species to survive, to reproduce, and therefore to spread in both donor and recipient areas. The species-specific approach requires detailed data, since the risk of single species is being assessed (Barry et al. Citation2008). A detailed approach to risk assessment requires more data, time, and effort. Only relevant aspects and details should therefore be included in a risk assessment (Campbell Citation2009).
Key factors of ballast water introduction in the North Sea
The key factors in the introduction of aquatic species are derived from the risk approaches according to the BWMC. Preconditions for risk are: 1) species are not transported between the donor and recipient areas by natural pathways, as they are influenced by the hydrography of the sea area; 2) the areas are separated by a barrier of environmental dissimilarity (e.g., a body of water of a different salinity), with conditions in donor and recipient area similar; 3) the areas vary as to species distribution; and 4) shipping acts as a vector of forced transport.
Hydrography of the North Sea
The North Sea is an Atlantic margin continental shelf sea (; Van Weering and Kramer Citation1984). The Dogger Bank separates the North Sea into two hydrologically different parts: 1) in the deep areas north of the Dogger Bank (including the Skagerrak), the water column is vertically stratified by a thermocline during summer; and 2) in the shallow areas of the Dogger Bank, and south and east of the Dogger Bank, the water column is mixed year-round due to wind and tides (Rees et al. Citation2007; Van Beusekom and Diel-Christiansen Citation2009). In the northern section, the water inflow from the Atlantic Ocean creates stratification of the water column; this phenomenon causes stable water temperatures (; Otto et al. Citation1990). The southern part is more susceptible to hydrometeorological influences, resulting in turbulent distribution of suspended matter throughout the entire water column and less stable water temperatures. Due to the outflow of Baltic surface water, the water column of the Skagerrak and the surrounding areas deeper than 50 m can be vertically stratified by a halocline (Ten Hallers-Tjabbes et al. Citation2003). The differences between the two sections lead to differences in species composition and distribution in both sections (; Rees et al. Citation2007). Some species are adapted to the more stable environment in the north, while others are adapted to the more turbulent conditions in the southern part (Rees et al. Citation2007). We note that this is a primary indication of non-homogeneity in the North Sea.
Figure 4. The counterclockwise residual current in the North Sea (black) and the deep water flow from the Atlantic Ocean (gray) (Turrell Citation1992). The arrow width indicates the magnitude of volume transport. The dark dots are the major North Sea ports. The numbers correspond to the numbers in Table 1.
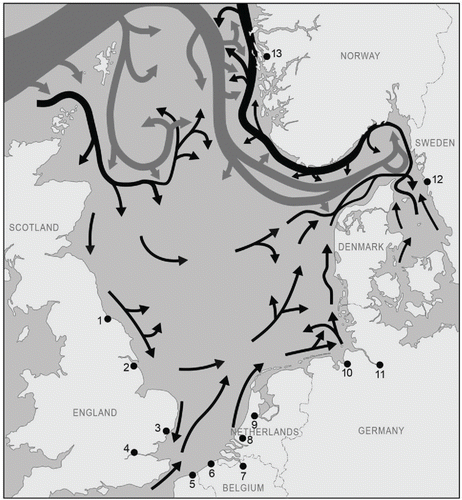
Figure 5. The different benthic habitats in the North Sea. The benthic habitats are determined by the bathymetry, the residual current (see and ), and the sediment particle size of the North Sea (Eisma 1991; Paramor et al. Citation2009). The habitats are checked vis-à-vis the distribution patterns of different benthic organisms (Rees et al. Citation2007; ICES Citation2008). The variation in gray scale indicates the proposed benthic zones. The dark dots are the major North Sea ports. The numbers correspond to the numbers in Table 1.
The very great inflow of water in the northern part, the tides, and meteorological influences together create a counterclockwise current circulation in the North Sea (; Otto Citation1983). The southward current passes along the British east coast, changes direction in the south, due to the inflow of Channel water north of Dover Strait, and then flows northward along continental Europe. The water that first passed along Scotland eventually reaches the Danish and Norwegian coasts, but the current will not turn to the west again; it continues north and northeastward, and will not flow back in the direction of Scotland. No natural water transport from east to west occurs, so there is no transport of organisms by natural pathways from east to west.
Conditions of salinity and temperature
Water temperature and salinity are the major factors determining the ability for a species to survive. When the water temperatures and the salinities in the donor and recipient areas show a high degree of similarity, a transferred species is more likely to survive and establish.
Due to the hydrological conditions of the North Sea, the surface and seabed waters of the northern North Sea (I) show a smaller range in seasonal temperature and salinity than the southern North Sea (II) and the coastal zones (; Paramor et al. Citation2009; UKMMASS Citation2010). Based on temperature, the biogeographical region of the entire North Sea is a cold temperate region (Lüning Citation1990; Spalding et al. Citation2007). Cold temperate species survive through the whole North Sea area, and nonindigenous cold temperate species have a chance to survive and establish.
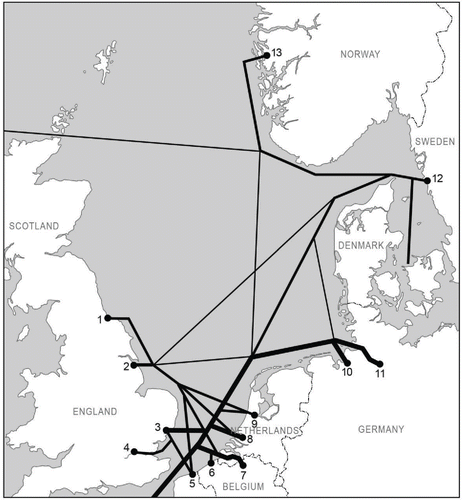
The salinity of the North Sea's central water body is stable and mainly determined by influx of Atlantic waters in the western part of the North Sea ( and ; ICES Citation2008). The coastal waters in the North Sea area have a higher variation in salinity because of freshwater runoff from rivers (Otto et al. Citation1990), which lowers the salinities near the coast. Several major rivers discharge into the southern North Sea. Many of the major North Sea ports (as selected on the basis of cargo transfer) are located in the southern North Sea along rivers or estuaries ( and ).
Figure 6. Major shipping links of the Global Network of Shipping projected on the North Sea area (from ESA Citation2009). Line thickness indicates the shipping density between the different nodes of the network. The dark dots are the major North Sea ports. The numbers correspond to the numbers in Table 1.
The waters of the fully marine water body of the North Sea act as a barrier to the dispersal of coastal or estuarine species toward other parts of the North Sea, since these species are adapted to lower salinities (). Due to the tidal conditions, estuarine species are rather well adapted to a wide range of salinities and are able to survive in other areas with a wide range of salinities (Nehring Citation2006). The salinity conditions in the North Sea indicate a risk of establishment of species transferred by ballast water between port areas of similar salinity ranges; such transport circumvents the salinity barrier of the marine water body.
Table 3. The range of water salinities in which various groups of organisms are able to survive and to establish (Boesch Citation1977).
Biota of the North Sea area
Variations in both hydrography and salinity are determinants for species' distribution in the North Sea area, as are the sediment particle size, the nutrient supply (Kaiser et al. Citation2005; Rees et al. Citation2007), and the food web structure. The current patterns are a dominant factor determining the passive transport of pelagic planktonic species that follow the residual current ().
Currents, however, have a limited influence on the distribution of aquatic benthic species. The boundaries of the main benthic communities in the North Sea are broadly defined by the 50-, 100-, and 200-m depth contours (), with the local community structure further modified by sediment type (; Callaway et al. Citation2002; ICES Citation2008; Kunnitzer et al. Citation1992). Many of the successful introductions in the North Sea area are those of aquatic benthic species, for example, the zebra mussel (Dreissena polymorpha), the Chinese mitten crab (Eriocheir sinensis), the American jackknife clam (Ensis americanus), and the Australian tubeworm (Ficopomatus enigmaticus; DAISIE Citation2015). Benthic species especially have particular spatial habitats, which occur in some locations in the North Sea but not others (Van Beusekom and Diel-Christiansen Citation2009). As a consequence, such species can be invasive within the North Sea area. Euryhaline benthic species that tolerate a wide range of salinities (; Kaiser et al. Citation2005; Ketchum Citation1983), which only occur in specific areas in the region, are expected to be able to survive in other estuarine areas around the North Sea.
The introduction of freshwater species also represents high risk vis-à-vis freshwater ports (). The Thames and other rivers and estuaries in the southeast of England are areas with the highest invasion rates of freshwater species introduced by ballast water from continental ports (Gallardo and Aldridge Citation2014; Jackson and Grey Citation2013).
In addition to the environmental match of species donor and recipient areas, propagule pressure is another key factor in the success of species introductions (Haydar and Wolff Citation2011; Lockwood, Cassey, and Blackburn Citation2005; Verling et al. Citation2005). Propagule pressure is a measurement of the number of viable individuals (adults, juveniles, larvae, eggs, cysts, etc.) of a nonindigenous species released into the introduction area (Carlton Citation1996). It represents the potential for introduction rather than an existent introduction (Johnston, Piola, and Clark Citation2009).
Successful establishment in a new area requires habitat suitability in terms of the availability of sufficient food, including nutrients, compared to the donor area. In the water column, turbulence is an important factor for nutrient distribution. Turbulent waters create a high organic load of suspended bottom deposits through the water column, which are a primary food source. Estuaries are turbulent areas as a result of the mixing of marine water and freshwater (Wolff Citation1983). Benthic species in turbulent estuaries can be distributed over the full water column. While loading ballast water, moored vessels easily take in such species, in addition to pelagic species and pelagic stages of benthic species.
Ballast water as vector for nonindigenous species
Propagule pressure may increase due to an increased number of arrival events or an increased intensity of exposure during any one event (Johnston, Piola, and Clark Citation2009). Ships act as vectors for nonindigenous species. Ballast water is usually loaded in ports located alongside riverbanks or in areas with coastal water conditions. The vessels circumvent the natural salinity barrier for species living in such areas. For water taken in along the eastern shores of the North Sea by ships destined for the Great Britain, the residual current barrier is circumvented as well.
Most ports have a limited water depth, a high turbulence, and a high concentration of suspended sediments as a result of passing ships, and the tidal mixing of marine water and freshwater (Wolff Citation1983). Consequently, ships are likely to load sediments, and benthic and pelagic species, even when a high-positioned seawater inlet chest is used.
Transfer potential is a function of the transit time of the ship. Many organisms will die in the dark ballast water tanks after a minimum of three days; only a few individuals still live after 10 days (Gollasch et al. Citation2000). Sailing times between the major North Sea ports are often less than three days (), indicating a high survival rate of species in the tanks. Some species are highly resistant to in-tank conditions. Benthic species, microalgae, and bacteria are especially able to survive a voyage; the first group does so by settling in the sediment in the tank (Radziejewska, Gruszka, and Rokicka-Praxmajer Citation2006).
The transfer potential is also determined by the released volume, the release frequency, and the concentration of individual species (Dunstan and Bax Citation2008). The frequency of ballast water release is recorded by the global shipping network. The global shipping network describes the links between ports worldwide as a function of the frequency of visits by ships (Kaluza et al. Citation2010; Keller et al. Citation2011).
An indication of the volume can be derived from the specific types of vessels sailing between specific ports. Different ship types have different ballast water demands. Highly frequently sailed routes with short duration times may present an indication of higher risk of the introduction of species.
Available risk models
Modeling the risk of species introduction is the proper tool for evaluating the risk associated with granting BWM exemptions. Models are limited to modeling the likelihood of an introduction, of course, since the impact of a species in a new area is hard to predict.
The risk of an introduction due to ballast water transport is mainly described by those key factors that have already been discussed in the previous sections. As an approach to modeling for the assessment of risk in the North Sea, we propose including the hydrographical and abiotic conditions, the biota, and the transport vector.
We will evaluate two actual models for the North Sea area, DUE Innovator II (Stelzer Citation2010) and GETM_ERSEM (NIOZ Citation2015).
Abiotic modeling
The gradients of salinity and temperature across the area are the key factors for abiotic modeling. The risk is based on similarities or differences in the physical conditions between the donor and recipient areas for the ballast water. The model has to include the water currents in the area in order to explain the natural transport of species across that area.
The DUE Innovator II model calculates the risk of BWE in the North Sea, based on abiotic conditions in the North Sea (Stelzer Citation2010). This model includes water currents, water salinity, and temperature in a recipient area for BWE (in open seas) that determine the risk for waters downstream to the ballast water release position while performing BWE. The basic principle of the DUE Innovator II model is suitable for the abiotic part of a ballast water risk assessment for the region.
The approach of DUE Innovator II is related to the risk associated with BWE; the model is not intended to describe the risk in terms of acting as a tool for selecting strategies for BWM exemption. The location of ports and the conditions in estuaries are not included in the model.
Biotic modeling
The most challenging aspect in risk modeling is the inclusion of biological determinants and data: the location of a species population, the interaction between species, and the nutrient availability in the different areas in the region. Models like GETM_ERSEM (NIOZ Citation2015) can be supportive in this respect. GETM_ERSEM is a 3D hydrodynamical ecosystem model of the North Sea. In addition to the hydrography of the area, the model analyzes population dynamics (species growth, decline, and food web structure) of species, which are crucial aspects for predicting the establishment of a new species.
To overcome the problem of lack of data, a list of indicator species can be made according to the consensus method of horizon scanning (Roy et al. Citation2014). Target groups are distinguished according to planktonic lifetime (short or long planktonic phase) and habitat suitability (habitat specialist or generalist; Forrest, Gardner, and Taylor Citation2009).
The GETM_ERSEM can model turbulence in the seawater column and can be used to study the effect of turbulence in the water column. Modeling the turbulence in port areas is a requirement for a risk assessment model. The GETM_ERSEM model is not suitable for calculating turbulence in port and river areas.
Vector modeling
None of the models that have been discussed in this study consider the ballast water vector. Some studies indicate that there is no quantitative relationship between the quantity and frequency of ballast water discharges and the number of species introduced (Ruiz et al. Citation2013). However, the risk of invasive species introductions from ballast water discharge varies between ports, which might well be related to the transit time and therefore the viability of the propagules (NRC Citation2011; Ruiz et al. Citation2013).
The intended model aims to calculate the amount of viable individuals of each species that will be released in the recipient area. The yearly released volume per ship in a recipient port is a function of the visiting frequency and the volume of ballast water transferred per voyage. The concentration of viable individuals in the ballast water released can either be calculated by a stochastic analysis of the decline of a species population during the voyage or by using species-specific information about its ability to survive in ballast tanks.
Discussion
Attitude in the shipping sector
Two-thirds of the respondents to our questionnaire are aware of the risk of ballast water transport concerning species introduction. The majority of them say that it is clear what the ways are for performing BWM on their vessels (). For the North Sea area, however, ship owners argue that exemptions from the BWE/BWM requirements should be granted because they consider the North Sea to be one ecological zone (). They also argue that sailing time is too short to perform BWM. Although ship owners believe that exemptions for the North Sea area should be granted, some already anticipate BWMC. Two-thirds of the questioned ship owners have prepared their vessels for performing BWM (Appendix A).
We do not accept the proposition that the North Sea is one ecological zone. The North Sea is not homogeneous. Salinity is an abiotic key factor that differs between ports () as well as within ports (). This salinity difference determines the survival of transported species (). Ballast water discharge in the ports and out in the North Sea could well affect the benthic communities living there ().
Modeling ballast water risk indications for the North Sea
This study was performed in order to investigate the feasibility for BWM exemptions in the North Sea area. We discussed how actual models of the North Sea could be helpful in the procedures for granting BWM exemptions. Exemptions are solely granted to those ships exclusively sailing between specified ports within a biogeographical region with similar environmental conditions. The models that are proposed are not specifically designed for use in terms of ballast water risk assessment methods. Specific contents of the models, however, can be used in a new model. Both proposed models, DUE Innovator II and GETM_ERSEM, focus on the hydrography of the North Sea. DUE Innovator II is designed for risk calculation of BWE, based on environmental similarity. The GETM_ERSEM includes biology for the region. The vector ballast water is not included in any of these models, but it can easily be included in a new model in terms of transfer potential. Duration of the voyage is a key factor in modeling the transfer potential.
Performing a ballast water risk assessment with the tools presently in use is complex, and all these assessments need to be executed by specialists. The methods for ballast water risk assessment need to be made more easy to use in order to eliminate the uncertainty involved in the exemptions faced by the shipping industry.
Validity of exemptions
The industry would do well to study the feasibility of installing BWM systems onboard, since the validity of an exemption is limited to up to five years. Exemptions apply to specific routes between permitted ports only. The routes of most ships, however, are not limited to specific ports, except for ferries and ships with long-term contracts. Exemptions are also not as flexible as the availability of cargo in the market. Ships often have to divert to load available cargo at another port that may not be included in the exemption. In that case the vessel would have to apply BWM.
An exemption expires once a vessel is sold and serves other ports. The new owner of a formerly exempted vessel would have to install a BWM system or apply for a new exemption. It is probably more attractive to buy a vessel that is already mounted with a BWM system. If the risk situation in a port changes, an exemption may be withdrawn. An exemption not only requires initial port research for the risk assessment but also regular monitoring to identify risk species turning up during the exemption period. The port sampling protocol described in the joint HELCOM/OSPAR procedure for ballast water exemptions (HELCOM/OSPAR Citation2013) was tested in the Rotterdam scoping project (GiMaRIS Citation2014). In the port of Rotterdam, 32 nonnative species were found that were suitable as indicator species (GiMaRIS Citation2014). The scoping study shows that the monitoring protocol does work effectively (GiMaRIS Citation2014); however, it is labor-intensive and costly. The study raises the question of the implementation of monitoring results in current exemptions.
Monitoring intensity is also questionable in terms of the scale of the same location concept raised in regulation A3 of BWMC (IMO Citation2004). We agree with Gollasch and David (Citation2012) that an entire sea cannot be seen as being the same location. Should the same location concept be applied to the smallest practical units, that is, the same harbor or anchorage (David, Gollasch and Pavliha Citation2013; Gollasch and David Citation2012), it would still need intensive monitoring as required for port exemptions. Do the monitoring costs and effort outweigh the costs of BWM?
Risk based on North Sea routes
In this study, we found that the risk of the introduction of species, based on the temperature and salinity ranges in the North Sea area, can be high. In most cases, coastal ballast water is transferred to coastal waters in another part of the North Sea area, circumventing the salinity barrier. Passive migration patterns by the residual current indicate that a pelagic species naturally occurring in the waters on the Dutch and German North Sea coasts can pose a high risk of introduction in areas along the British eastern shores. The rate of colonization of freshwater species into the Great Britain, especially the southeast of England, routing from the Netherlands, has accelerated in recent years (Gallardo and Aldridge Citation2014).
The most high-risk donor ports ranging toward the Baltic Sea are the major hub ports in Europe: Rotterdam (GiMaRIS Citation2014), Antwerp, Hamburg, and Bremerhaven (; Gollasch and Leppäkoski Citation2007). All these big ports along the southeast coast of the North Sea, including Hamburg as the major port of Germany, have a low salinity and highly turbulent water because of river outflow ( and ). The threat of secondary spread of invasive species within the North Sea via the international/major hub ports is another issue (). We recommend giving routes a prominent place in ballast water risk assessments.
Conclusions
Within the North Sea, ships' ballast water transfers marine organisms with invasive potential. Based on actual models for the North Sea, we have discussed the major parameters that need to be combined and adapted in order to evaluate the exemption for BWM requirements of particular routes in the North Sea.
Exemptions in the North Sea area have been claimed by shipping companies. This paper has investigated the proposition that the North Sea is an ecologically homogeneous zone, where organisms are transported by natural pathways. The hydrological conditions of the North Sea result in an east–west barrier for transport by water currents. Salinity distribution results in a salinity barrier for coast-to-coast transfer of coastal and estuarine organisms, while within the coastal regions salinities remain in a similar range. Various conditions do result in distinct habitats and species distribution patterns within the area, however.
We would note that the North Sea is far from homogeneous in terms of hydrological and biological conditions. The risk of non-indigenous species from other parts of the North Sea being redistributed is considerable, and this is already happening. We conclude that exemptions from BWM are therefore not recommended for the North Sea area. Anticipating the BWMC, ship owners would do well to study the feasibility of installing BWM systems onboard.
Acknowledgments
We thank the partners in the project for the provision of relevant data and literature. We would especially like to thank the captain of motor tank vessel Dutch Spirit and the chief officer of motor tank vessel Dutch Navigator for supplying the salinity data for various North Sea ports. Our thanks also go to Dick Visser for the graphics. The authors thank the reviewers for their valuable comments on the manuscript.
Funding
This work was performed within the Interreg North Sea Ballast Water Opportunity Project. The project aims to reach regional cohesion and innovation, and to develop future strategies in ballast water policies and BWM. The project was co-funded by the Interreg IVB North Sea Region Programme of the European Regional Development Fund.
References
- AAPA. 2010. World Port Rankings. http://www.aapa-ports.org/ (accessed December 7, 2012).
- Anil, A. C., K. Venkat, S. S. Sawant, M. Dileep Kumar, V. K. Dhargalkar, N. Ramaiah, S. N. Harkantra, and Z. A. Ansari. 2002. Marine bioinvasion: Concern for ecology and shipping. Current Science 83:214–218.
- Balaji, R., and O. B. Yaakob. 2011. Emerging ballast water treatment technologies: A review. Journal of Sustainability Science and Management 11:126–138.
- Barry, S. C., K. R. Hayes, C. L. Hewitt, H. L. Behrens, E. Dragsund, and S. M. Bakke. 2008. Ballast water risk assessment: Principles, processes, and methods. ICES Journal of Marine Science 65:121–131.
- Behrens, H. L., E. Leppäkoski, and S. Olenin. 2005. Ballast water risk assessment guidelines for the North Sea and Baltic Sea. Nordic Innovation Centre Report no TR 587, Nordic Innovation Centre, Oslo, Norway.
- BIMCO. 2014. Ballast water management. https://www.bimco.org/About/Viewpoint/02_Ballast_Water_Management.aspx (accessed February 10, 2015).
- Boesch, D. F. 1977. A new look at the zonation of benthos along the estuarine gradient. In Ecology of marine benthos, ed. B. C. Coull, 245–266. Columbia, SC: Belle, W. Baruch Library in Marine Science, University of South Carolina Press.
- British Admiralty. 2009. Admiralty sailing directions: North Sea (West) Pilot NP 54. 8th ed. Taunton: the UK Hydrographic Office.
- British Admiralty. 2010. Admiralty sailing directions: North Sea (East) Pilot NP 55. 7th ed. Taunton: the UK Hydrographic Office.
- British Admiralty. 2011. Admiralty sailing directions: Dover Strait Pilot NP 55. 7th ed. Taunton: the UK Hydrographic Office.
- British Admiralty. 2009. Admiralty sailing directions: Baltic Pilot vol. 1 NP 55. 15th ed. Taunton: the UK Hydrographic Office.
- British Admiralty. 2011. Admiralty sailing directions: Norway Pilot vol. 2A NP 57A. 7th ed. Taunton: the UK Hydrographic Office.
- Callaway, R., J. Alsvåg, I. de Boois, J. Cotter, A. Ford, H. Hinz, S. Jennings, I. Kröncke, J. Lancaster, G. Piet, and P. Prince. 2002. Diversity and community structure of epibenthic invertebrates and fish in the North Sea. ICES Journal of Marine Science 59:1199–1214.
- Campbell, M. L. 2009. An overview of risk assessment in a marine biosecurity context. In Biological invasions in marine ecosystems: Ecological, management, and geographic perspectives, Ecological Studies 204, ed. G. Rilov and J. A. Crooks, 353–370. Berlin/Heidelberg: Springer-Verlag.
- Carlton, J. T. 1996. Biological invasions and cryptogenic species. Ecology 77:1653–1655.
- Carlton, J. T. 1999. The scale and ecological consequences of biological invasions in the world's oceans. In Invasive species and biodiversity management, ed. O. T. Sandlund, P. J. Schei and Å. Viken, 195–212. Dordrecht: Kluwer Academic Publishers.
- Carlton, J. T., and J. B. Geller. 1993. Ecological roulette: The global transport on non-indigenous marine organisms. Science 261:78–82.
- Clark, I. C. 2002. The management of merchant ship stability, trim and strength. London: The Nautical Institute.
- Dahlstrom, A., C. L. Hewitt, and M. L. Campbell. 2011. A review of international, regional and national biosecurity risk assessment frameworks. Marine Policy 35:208–217.
- DAISIE. 2015. Delivering Alien Invasive Species Inventories for Europe. 100 of the worst, show aquatic marine. http://www.europe-aliens.org/speciesTheWorst.do (accessed August 20, 2015).
- David, M. 2015. Vessels and ballast water. In Global maritime transport and ballast water management, Invading Nature, Springer Series in Invasion Ecology 8, ed. M. David and S. Gollasch. 13–34. Dordrecht: Springer Science+Business Media.
- David, M., S. Gollasch, and M. Pavliha. 2013. Global ballst water management and the “same location” concept: A clear term or a clear issue? Ecological Applications 32:331–338.
- Dunstan, P. K., and N. J. Bax. 2008. Management of an invasive marine species: Defining and testing the effectiveness of ballast-water management options using management strategy evaluation. ICES Journal of Marine Science 65:841–850.
- EEA. 2012. Natura 2000 network viewer. http://natura2000.eea.europa.eu/# (accessed March 5, 2015).
- Eisma, D. 1981. Supply and deposition of suspended matter in the North Sea. Special publication of the International Association of Sedimentologists 5:415–428.
- ESA. 2009. ESA map reveals European shipping routes like never before. http://www.esa.int/Our_Activities/Observing_the_Earth/Envisat/ESA_map_reveals_European_shipping_routes_like_never_before (accessed April 2, 2015).
- European Commission. 2014. Eurostat, Maritime transport statistics—Short sea shipping of goods. http://ec.europa.eu/eurostat/statistics-explained/index.php/Maritime_transport_statistics_-_short_sea_shipping_of_goods (accessed February 16, 2015).
- Flagella, M. M., M. Verlaque, A. Soria, and M. C. Buia. 2007. Macroalgal survival in ballast water tanks. Marine Pollution Bulletin 54:1395–1401.
- Forrest, B. M., J. P. A. Gardner, and M. D. Taylor. 2009. Internal borders for managing invasive marine species. Journal of Applied Ecology 46:46–54.
- Gallardo, B., and D. C. Aldridge. 2014. Is Great Britain heading for a Ponto-Caspian invasional meltdown? Journal of Applied Ecology 52:41–49.
- GiMaRIS. 2014. Port of Rotterdam survey and monitoring non-native species conform HELCOM/OSPAR protocol. Report GiMaRIS 2014_31. Gittenberger Marine Research Inventory Strategy (GiMaRIS), Leiden, the Netherlands.
- Gollasch, S. 2002. The importance of ship hull fouling as a vector of species introductions into the North Sea. Biofouling 18:105–121.
- Gollasch, S., and M. David. 2012. A unique aspect of ballast water management requirements—The same location concept. Marine Pollution Bulletin 64:1774–1775.
- Gollasch, S., M. David, and E. Leppäkoski. 2011. Pilot risk assessments of alien species transfer on intra-Baltic ship voyages. HELCOM Project No. 11.36. Helsinki Commission—Baltic Marine Environment Protection Commission HELCOM, Hamburg, Germany.
- Gollasch, S., D. Haydar, D. Minchin, W. J. Wolff, and K. Reise. 2009. Introduced aquatic species of the North Sea coasts and adjacent brackish waters. In Biological invasions in marine ecosystems: Ecological, management, and geographic perspectives, Ecological Studies 204, ed. G. Rilov and J. A. Crooks, 507–528. Berlin/Heidelberg: Springer-Verlag.
- Gollasch, S., and E. Leppäkoski. 2007. Risk assessment and management scenarios for ballast water mediated species introductions into the Baltic Sea. Aquatic Invasions 2:313–340.
- Gollasch, S., H. Rosenthal, H. Botnen, J. Hamer, I. Laing, E. Leppäkoski, E. Macdonald, D. Minchin, M. Nauke, S. Olenin, S. Utting, M. Voigt, and I. Wallentinus. 2000. Fluctuations of zooplankton taxa in ballast water during short-term and long-term ocean-going voyages. International Review of Hydrobiology 85:597–608.
- Gregg, M., G. Rigby, and G. M. Hallegraeff. 2009. Review of two decades of progress in the development of management options for reducing or eradicating phytoplankton, zooplankton and bacteria in ship's ballast water. Aquatic Invasions 4:521–565.
- Haydar, D., and W. J. Wolff. 2011. Predicting invasion patterns in coastal ecosystems: Relationship between vector strength and vector tempo. Marine Ecology Progress Series 431:1–10.
- HELCOM/OSPAR. 2013. Joint harmonized procedure for the contracting parties of OSPAR and HELCOM on the granting of exemptions under International Convention for the control and management of ships' ballast water and sediments, Regulation A-4. http://www.helcom.fi/Documents/HELCOM%20at%20work/Groups/MARITIME/TG%20BALLAST/HELCOM-OSPAR%20Joint%20Harmonized%20Procedure%20for%20BWMC%20A-4%20exemptions.pdf (accessed March 19, 2015).
- HELCOM/OSPAR Harmonized Procedure. 2014. Risk assessment tool under the HELCOM/OSPAR Harmonised Procedure on exemptions under the Ballast Water Management Convention: Joint decision support tool on alien species introductions via Ballast Water. http://jointbwmexemptions.org/ballast_water_RA/apex/f?p=100:LOGIN:15385849801424 (accessed April 2, 2015).
- Helsinki and OSPAR Commissions. 2014. Outcome of the fifth meeting of the joint HELCOM/OSPAR task group on Ballast Water Management Convention Exemptions (HELCOM/OSPAR tg Ballast). https://portal.helcom.fi/meetings/HELCOM-OSPAR%20TG%20BALLAST%205-2014-173/MeetingDocuments/Outcome%20of%20HELCOM-OSPAR%20TG%20BALLAST%205-2014.pdf (accessed March 20, 2015).
- ICES. 2008. Report of the ICES advisory committee 2008. Book 6. North Sea. Copenhagen: ICES advice.
- ICONA. 1992. Noordzee-atlas voor Nederlands beleid en beheer (in Dutch). Amsterdam: Stadsuitgeverij.
- IMO. 2004. International convention for the control and management of ship's ballast water and sediments. London: International Maritime Organization (IMO).
- IMO. 2007. Annex 2 Resolution MEPC.162(56). http://www.imo.org/blast/blastDataHelper.asp?data_id=19689&filename=162%2856%29.pdf (accessed April 2, 2015).
- IMO. 2013. Explore the world of PSSAs. http://pssa.imo.org/#/globe (accessed March 5, 2015).
- IMO. 2014. List of Ballast Water Management Systems that make use of active substances which received basic approval from IMO. http://www.imo.org/OurWork/Environment/BallastWaterManagement/Documents/20of%20BA%20FA%20TA%20updated%20in%20Oct%202014.pdf (accessed February 20, 2015).
- IMO. 2015a. Status of multilateral conventions and instruments in respect of which the International Maritime Organization or its secretary-general performs depositary or other functions. http://www.imo.org/About/Conventions/StatusOfConventions/Pages/Default.aspx (accessed March 19, 2015)
- IMO. 2015b. Technical notice BWM.1–BWM.2/Circ.56 Communication received from the government of the Netherlands (accessed August 20, 2015).
- Jackson, M. C., and J. Grey. 2013. Accelerating rates of freshwater invasions in the catchment of the River Thames. Biological Invasions 15:945–951.
- Johnston, E. L., R. F. Piola, and F. C. Clark. 2009. The role of propagule pressure in invasion success. In Biological invasions in marine ecosystems. Ecological Studies 204, ed. G. Rilov and J. A. Crooks, 133–151. Berlin/Heidelberg: Springer-Verlag.
- Kaiser, M. J., M. J. Attril, S. Jennings, D. N. Thomas, D. K. A. Barnes, A. S. Brierly, N. V. C. Polunin, D. G. Raffaelli, P. J. Le, and B. Williams. 2005. Marine ecology. Processes, systems and impacts. Oxford: Oxford University Press.
- Kaluza, P., A. Kölzsch, M. T. Gastner, and B. Blasius. 2010. The complex network of global cargo ship movements. Journal of the Royal Society Interface 7:1093–1103.
- Keller, R. P., J. M. Drake, M. B. Drew, and D. M. Lodge. 2011. Linking environmental conditions and ship movements to estimate invasive species transport across the global shipping network. Diversity and Distribution 17:93–102.
- Ketchum, B. H. 1983. Estuarine characteristics. In Estuaries and enclosed seas. Ecosystems of the world 26, ed. B. H. Ketchum, 1–14. Amsterdam: Elsevier.
- Kideys, A. E. 1994. Recent dramatic changes in the Black Sea ecosystem: The reason for the sharp decline of the Turkish anchovy fisheries. Journal of Marine Systems 5:171–181.
- Kunnitzer, A., D. Basford, J. A. Craeymeersch, J. M. Dewarumex, J. Dorjes, G. C. A. Duineveld, A. Eleftheriou, C. Heip, P. Herman, P. Kingston, U Niermann, E. Rachor, H. Rumohr, and P. A. J. de Wilde. 1992. The benthic infauna of the North-Sea—Species distribution and assemblages. ICES Journal of Marine Science 49:127–143.
- Lockwood, J. L., P. Cassey, and T. Blackburn. 2005. The role of propagule pressure in explaining species invasions. Trends in Ecology and Evolution 20:223–228.
- Lovell, S. J., S. F. Stone, and L. Fernandez. 2006. The economic impacts of aquatic invasive species: A review of the literature. Agricultural and Resource Economics Review 35:195–208.
- Lüning, K. 1990. Seaweeds. Their environment, biogeography and ecophysiology. New York: John Wiley and sons.
- Nehring, S. 2006. Four arguments why so many alien species settle into estuaries, with special references to the German river Elbe. Helgoland Marine Research 60:127–134.
- NIOZ. 2015. GETM-ERSEM. Royal Netherlands Institute for Sear Research. https://www.nioz.nl/ersem-getm (accessed August 25, 2015).
- NRC. 2011. Assessing the relationship between propagule pressure and invasion risk in ballast water. Washington, DC: The National Academies Press. Committee on Assessing Numeric Limits for Living Organisms in Ballast Water.
- OSPAR. 2013. 2012 Status report on the OSPAR network of Marine Protected Areas. Report no. 618, Oslo Paris Conventions, North East Atlantic Ocean (OSPAR), London, UK.
- Otto, L. 1983. Currents and water balance in the North Sea. In North Sea dynamics, ed. J. Sündermann and W. Lenz, 26–43. Berlin: Springer.
- Otto, L., J. T. F. Zimmermann, G.K, Furnes, M. Mork, R. Saetre, and G. Becker. 1990. Review of the physical oceanography of the North Sea. Netherlands Journal of Sea Research 26:161–238.
- Paramor, O. A. L., K. A. Allen, M. Aanesen, C. Armstrong, T. Hegland, W. Le Quesne, G. J. Piet, J. Raakær, S. Rogers, R. van Hal, L. J. W. van Hoof, H. J. M. van Overzee, and C. L. J. Frid. 2009. MEFEBO North Sea Atlas. University of Liverpool, Liverpool, UK. Making the European Fisheries Ecosystem Plan Operational (MEFEBO).
- Radziejewska, T., P. Gruszka, and J. Rokicka-Praxmajer. 2006. A home away from home: A meiobenthic assemblage in a ship's ballast water tank sediment. Oceanologia 48:259–265.
- Rees, H. L., J. D. Eggleton, E. Rachor, and E. Vanden Berghe. 2007. Structure and dynamics of the North Sea benthos. Report no 288. International Council for the Exploration of the Seas (ICES), Copenhagen, Denmark.
- Reise, K., S. Gollasch, and W. J. Wolff. 1999. Introduced marine species of the North Sea coasts. Helgoländer Meeresuntersuchungen 52:219–234.
- Roy, H. E., J Peyton, D. C. Aldridge, T. Bantock, T. M. Blackburn, R. Britton, P. Clark, E. Cook, K. Dehnen-Schmutz, T. Dines et al. 2014. Horizon scanning for invasive alien species with the potential to threaten biodiversity in Great Britain. Global Change Biology 20:3859–3871.
- Ruiz, G. M., P. W. Fofonoff, G. Ashton, M. S. Minton, and A. W. Miller. 2013. Geographic variation in marine invasions among large estuaries: Effects of ships and time. Ecological Applications 23:311–320.
- Sea-distances.org. 2015. Sea distances/Port distances online tool for calculation distances between sea ports. http://sea-distances.org/ (accessed February 9, 2015).
- Shipping Guides Ltd. 2010. Guide to Port Entry 2011/2012 edition. Reigate: Shipping Guides Limited.
- Spalding, M. D., H. E. Fox, G. R. Allen, N. Davidson, Z. A. Ferdana, M. Finlayson, B. S. Halpern, M. A. Jorge, A. Lombana, S. A. Lourie, K. D. Martin, E. McManus, J. Molnar, C. A. Recchia, and J. Robertson. 2007. Marine Ecoregions of the world: A bioregionalization of coastal and shelf seas. Bioscience 57:573–583.
- Stachowicz, J. J., J. F. Bruno, and J. Emmett Duffy. 2007. Understanding the effects of marine biodiversity on communities and ecosystems. Annual Review of Ecology, Evolution and Systematics 38:739–766.
- Stelzer, K. 2010. DUE innovator II ballast water product user guide. Geesthacht: Brockmann Consult & ESA.
- Ten Hallers-Tjabbes, C. C., J. W. Wegener, A. G. M. Van Hattum, J. F. Kemp, E. Ten Hallers, T. J. Reitsema, and J. P. Boon. 2003. Imposex and organotin concentrations in Buccinum undatum and Neptunea antiqua from the North Sea: Relationship to shipping density and hydrographical conditions. Marine Environmental Research 55:203–233.
- Tsolaki E., and E. Diamadopoulos. 2010. Technologies for ballast water treatment: A review. Journal of Chemical Technology and Biotechnology 85:19–32.
- Turrell, W. R. 1992. New hypotheses concerning the circulation of the northern North Sea and its relation to North Sea fish stock recruitment. ICES Journal of Marine Sciences 49:107–123.
- United Kingdom Marine Monitoring and Assessment Strategy (UKMMASS). 2010. Charting Progress 2 Feeder report: Ocean Processess. http://chartingprogress.defra.gov.uk/feeder/Section_3.2_Temperature_and_Salinity.pdf (accessed February 24, 2015).
- Van Aaken, H. M. 1990. Natuurkunde. In de Noordzee (in Dutch), ed. P. de Wolf, 42–54. Zutphen: Terra.
- Van Beusekom, J. E. E., S. Diel-Christiansen. 2009. Global change and the biochemistry of the North Sea: The possible role of phytoplankton and phytoplankton grazing. International Journal of Earth Sciences 98:269–280.
- Van Dokkum, K. 2003. Ship knowledge. Enkhuizen: Dokmar.
- Van Weering, T., K. Kramer. 1984. De Noordzee. Het bedreigde zeemilieu (in Dutch). Utrecht: Werkgroep Ekologie.
- Verling, E., G. M. Ruiz, L. D. Smith, B. Galil, A. W. Miller, and K. R. Murphy. 2005. Supply-side invasion ecology: Characterizing propagule pressure in coastal ecosystems. Proceedings of the Royal Society B 272:1249–1256.
- Vila, M., C. Basnou, P. Pyšek, M. Josefsson, P. Genovesi, S. Gollasch, W. Nentwig, S. Olenin, A. Roques, D. Roy, P. E. Hulmes, and DAISIE partners. 2010. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Frontiers in Ecology and the Environment 8:135–144.
- Wolff, W. J. 1983. Estuarine benthos. In: Estuaries and enclosed seas. Ecosystems of the world 26, ed. B. H. Ketchum, 1–14. Amsterdam: Elsevier.
Appendix A. Overview of the result of the questionnaire on ballast water use and management.
