Abstract
Context
Effects of liposomal particles on immune function have not been adequately investigated. Earlier reports indicate that intravenous injection of rats with pegylated liposomes comprising chemically defined specific lipids produces myeloid derived suppressor-cell (MDSC)-like cells in the spleen.
Objectives
After liposome injection, we sought a cell surface marker expressed specifically on splenic macrophages. Then we assessed the immunosuppressive activity of macrophages positive for the marker. Furthermore, we investigated whether immunosuppression induction is an immunopharmacological action specific to this pegylated liposome, or not.
Materials and Methods
After using a microarray system to screen genes enhanced by this liposome, we evaluated cell surface expression of gene products using flow cytometry. Liposomes of several kinds, each comprising one type of phospholipid, were prepared and evaluated for their ability to induce T-cell suppression.
Results
Microarray analysis indicated enhanced B7-H3 expression. Flow cytometry revealed that the B7-H3 molecule was expressed on splenic macrophages after liposome injection. B7-H3+ macrophages were positive for iNOS. Removing B7-H3+ cells restored T-cell proliferation. Similarly to this liposome, various liposomes with different long chain fatty acids induced T-cell suppression when accumulated in the spleen.
Conclusions
Immunosuppressive cells induced by this pegylated liposome closely resemble MDSCs, especially B7-H3+ MDSCs. Immunosuppression induction is not a phenomenon specific to this liposome. Accumulation of long chain fatty acid in macrophages by internalization of liposomal nanoparticles might be related to macrophage acquisition of immunosuppressive activity in vivo.
Keywords:
Introduction
Myeloid-derived suppressor cells (MDSCs) are heterogeneous immature myeloid cells induced in humans and animals under various pathological conditions, especially under a tumor burden. They function as immunosuppressive cells, with immunosuppressive mechanisms ranging from direct cell-to-cell contact to secretion of humoral factor [Citation1]. Evaluation of suppressive activity against T-cell proliferation response is the only universally applied method to identify these cells [Citation2]. Morphologically, they are divisible into two groups: those resembling monocytes (M-MDSCs) and those resembling polymorphonuclear cells (PMN-MDSCs). The latter suppress antigen-specific T-cell proliferation. The former suppress both antigen-specific and antigen nonspecific T-cell proliferation via nitric oxide (NO) produced by inducible NO synthase (iNOS) [Citation3]. When an MDSC is defined by a cell surface marker, the expression patterns of several cell surface markers are used [Citation4–7]. For human and mouse, it was recently reported that B7 homolog 3 protein (B7-H3), a member of the B7-family, is expressed on MDSCs that are present in tumor tissues, but not on MDSCs that are present in normal tissue around the tumor or in the bloodstream [Citation8]. Therefore, expression of B7-H3 can be a marker for a subset of MDSCs present within tumor tissues.
Based on ex vivo experiments, MDSCs are induced from immature myeloid cells by cytokines: tumor-derived GM-CSFs, etc. for their expansion, and IL-1β, etc. for their acquisition of immunosuppressive function [Citation9–12]. Reportedly, when macrophages or monocytes internalize cellular-membrane-derived microvesicles (MVs) released from tumor cells or inflammatory cells, etc. in vivo, those cells become MDSC-like cells [Citation13–15]. Proteins or nucleic acids incorporated in MVs are thought to contribute to this phenomenon.
Reportedly, excessive lipid accumulation and utilization of oxidized long chain fatty acids as energy sources are important for immunosuppressive functions of MDSCs [Citation16,Citation17]. These earlier reports, coupled with the fact that lipid is the main component of cellular membrane, lead us to speculate that lipids present in MV might contribute to this phenomenon.
When assessing the biocompatibility of artificial red blood cells, which are pegylated liposomal particles encapsulating human hemoglobin molecules, injection of a considerable amount of this liposome was found to induce immunosuppressive cells transiently in a rat spleen [Citation18]. Careful examination subsequently revealed that liposome-internalized CD11b/c+ macrophages suppress T-cell proliferation in an antigen nonspecific manner, that cell-to-cell contact is necessary for suppression, and that NO is a direct effector for T-cell suppression. These data suggest that immunosuppressive cells induced by this liposome resemble M-MDSCs [Citation19]. Considering that liposomes are generally composed of phospholipids that consist of two long chain fatty acids (e.g. palmitic acid, oleic acid) and one phosphate (e.g. phosphatidylcholine), one might speculate that accumulation of long chain fatty acids into macrophages via internalization of liposome contributes to their development of immunosuppressive activity [Citation19].
This study demonstrated that the immunosuppressive cells express B7-H3 molecules and that the NFκB signaling pathway in these cells is activated. Furthermore, results indicate that T-cell suppression occurs not only by this liposome but also by other liposomes that include different fatty acids. These results suggest that this liposome has potential for in vivo inducement of MDSC-like cells that are present in tumor tissues. Moreover, this is apparently not a phenomenon that is specific to this liposome. Herein, we also assess the possible involvement of long-chain fatty acids in the induction of macrophages with immunosuppressive functionality.
Materials and methods
Liposome suspension preparation
The liposome used for this study, the same as that used with artificial red blood cells [Citation20], comprises 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC)/cholesterol (CHOL)/1,5-O-dihexadecyl-N-succinyl-L-glutamate (DHSG)/polyethylene glycol-conjugated 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine (PEG5000-DSPE) at a molar composition of 5/4/0.9/0.03. For this discussion, we designate this pegylated liposome as DPPC/CHOL/DHSG/PEG-DSPE-liposome or ‘Liposome’. It comprises one lipid bilayer with no enclosed lamellar structure [Citation20]. The mean particle size is approximately 250 nm.
The ζ-potential of the liposome suspended in phosphate-buffered saline (PBS, pH 7.4) was measured as −17.52 ± 0.21 mV (Zeta-potential and Particle Analyzer ELSZ-2000; Otsuka Electronics Co. Ltd., Osaka, Japan). The negatively charged succinic acid moiety of DHSG contributes to the negative ζ-potential value [Citation21]. Liposomes composed of DPPC only and of DPPC and cholesterol (at a molar ratio of PC versus cholesterol of 5:4) were prepared and designated respectively as DPPC-liposome and DPPC/CHOL-liposome. All lipids were purchased from Nippon Fine Chemical Co. Ltd. (Osaka, Japan), except PEG5000-DSPE (NOF Corp., Tokyo, Japan). Each liposome was prepared using extrusion (Lipex Extruder; Northern Lipids, Inc., Canada) by stepwise permeation through nitrocellulose membrane filters with successively smaller pores (3.0, 1.0, 0.6, 0.45, 0.33, and 0.22 μm pore size; Merck Millipore Ltd., Cork, Ireland) and suspended in normal saline, which contained 5.7 g of lipid/dl and less than 0.1 endotoxin units/ml.
For some experiments, liposomes comprising 1,2-dimyrisytoyl-sn-glycero-3-phosphatidylcholine (DMPC) only, 1-palmitoyl-2-oleyl-sn-glycero-3-phosphatidylcholine (POPC) only, and 1,2-oleyl-sn-glycero-3-phosphatidylcholine (DOPC) only were prepared and designated respectively as DMPC-liposome, POPC-liposome, and DOPC-liposome. These three phospholipids were purchased from NOF Corp. To increase the liposome stability, cholesterol was incorporated into each liposome at a molar ratio of PC versus cholesterol of 5:4. Such liposomes were designated respectively as DMPC/CHOL-liposome, etc. Each liposome was suspended in saline at approximately 5.6 g of lipid/dl. The mean particle size of these liposomes was approximately 250 nm. The respective phase transition temperatures (Tc) of DPPC, DMPC, POPC, and DOPC were 41, 23, −2, and −20 °C. The liposomes and their components are presented in . The ζ-potential of DPPC-liposome in PBS was nearly zero (−0.39 ± 0.4 mV). The DPPC/CHOL-liposome (5:4 molar ratio) had an almost neutral surface (ζ-potential: −2.58 ± 0.46 mV). Therefore, the ζ-potentials of DOPC-, DMPC- and POPC-liposomes and the respective cholesterol-mixed liposomes were presumed to be almost neutral also.
Table 1. Components of each liposome used for this study.
Liposome injection and preparation of spleen cell suspension
All experimental procedures using rats were approved by the Institutional Animal Care and Use Committee at Asahikawa Medical University (No: 19076). Wistar-King Aptekman Hokkaido male rats (8–12 weeks old) were purchased from Japan SLC, Inc. (Shizuoka, Japan). The total whole-blood volume was estimated as 56 ml/kg of body weight (b.w.). Under sevoflurane anesthesia, 11.2 ml/kg b.w. of a liposome suspension was injected through the tail vein, which corresponded to about 20% of total whole-blood volume (20% (v/v)) and which was equivalent to approximately 640 mg lipid/kg b.w. For example, 2.2 ml of liposome suspension was injected into a rat with 0.2 kg b.w. For comparison, an equal volume of saline was injected into control rats. At 18 h after injection of liposome suspension (or saline), cervical dislocation was executed in rats that had been anesthetized with sevoflurane. Subsequently, spleens were excised aseptically, immersed in plain RPMI 1640 medium, placed in a plastic dish with 20 ml of plain RPMI 1640 and were pressed gently and repeatedly with a 5-ml syringe plug in the plastic dish. Then, the whole-cell suspension was transferred to a 50-ml tube and let to stand for a few minutes for sedimentation of large cell clumps. The produced cell suspension was collected and washed twice with PBS. Subsequently, 3–5 ml of lysing buffer (IBL International GmbH; Hamburg, Germany) was added to the pellet. Then the cell pellet was resuspended by vortexing. Five minutes later, lysis was stopped by adding RPMI 1640 containing 10% fetal calf serum (FCS) and 50 µM mercaptoethanol, designated as complete medium (CM). After the cell suspension was filtered through a preseparation filter, cells were washed twice using 1%FCS/PBS. They were finally suspended in CM at 2 × 106/ml. These were designated as liposome-loaded splenocytes.
Evaluating proliferation of T cells stimulated with Con A
The liposome-loaded splenocytes suspended at 2 × 106/ml in CM were plated in 96 well microplate wells to a volume of 0.2 ml/well. Then, they were cultured in triplicate in the presence of Con A at the indicated concentrations for 72 h. After they were pulsed with BrdU for the final 18 h of incubation, the BrdU incorporated into DNA was detected using a BrdU cell proliferation assay kit (Millipore Corp.). In some experiments, 3H-thymidine (18.5 kBq) was pulsed. The 3H-thymidine incorporated into DNA was measured using a liquid scintillation counter (LS5000 TD; Beckman Coulter Inc., Fullerton, CA).
Analysis of cell surface marker expression on CD11b/c+ cells
Cell surface markers were analyzed using a flow cytometer (FACSCanto II; BD Biosciences, San Diego, CA). Splenocytes were mixed with PE-conjugated mouse anti-rat CD11b/c monoclonal antibody (OX42) (IgG2a; BD Pharmingen, Le Pont de Claix, France) and polyclonal rabbit anti-rat CD276 (B7-H3) antibody (H-300) (IgG; Santa Cruz Biotechnology Inc., San Diego, CA, USA) or polyclonal rabbit anti-CD274 (B7-H1) antibody (H-130) (IgG, SC-50298; Santa Cruz Biotechnology Inc.). After washing cells twice, they were stained with FITC-conjugated goat anti-rabbit IgG (H & L) (IgG; Abcam plc., Cambridge, UK) and were subjected to flow cytometry analysis. For each analysis, data of 10,000 events were collected and analyzed using software (FACSDiva.6.1; BD Biosciences).
Western blot analysis for detection of iNOS and Iκbα
Cell lysate derived from liposome-loaded splenocytes and control splenocytes were Western blotted on nitrocellulose membrane. Polyclonal rabbit anti-rat NOS2 (iNOS) antibody (1:500, IgG, sc-650; Santa Cruz Biotechnology Inc.) and polyclonal rabbit anti-Iκbα antibody (C-21) (1:500, IgG, sc-3719; Santa Cruz Biotechnology Inc.) were used as primary antibodies. Polyclonal rabbit anti-β-actin antibody (1:1000, IgG, CST-4976; Cell Signaling Technology Inc., Danvers, MA, USA) was also used as a primary antibody. The bound antibody was visualized using alkali phosphatase-conjugated goat anti-rabbit IgG second antibody (Promega Corp., Madison, WI, USA) and a kit (BCIP-NBT; Nacalai Tesque Inc., Kyoto, Japan). For some experiments, liposome-loaded splenocytes were stained with PE-conjugated mouse anti-rat CD11b/c antibody (OX-42; BD Pharmingen). Subsequently, CD11b/c+ were enriched using anti-PE Microbeads (Miltenyi Biotec). Then the CD11b/c+-cell enriched fraction and CD11b/c+-cell depleted fractions were Western blotted, respectively, to detect iNOS.
Separation of CD11b/c+ cells or B7-H3+ cells using magnetic beads
Liposome-loaded splenocytes were stained with PE conjugated mouse anti-rat CD11b/c antibody (OX-42; BD Pharmingen). Subsequently, CD11b/c+ cells were separated using microbeads (anti-PE Microbeads; Miltenyi Biotec). Then, the CD11b/c+-cell enriched fraction and CD11b/c+-cell depleted fractions were each Western blotted to detect iNOS.
For B7-H3+ cell-depletion, 3 µl of rabbit anti-rat CD276(B7-H3) antibody (H-300) (polyAb; Santa Cruz Biotechnology Inc.) was added to the cell pellet of 1 × 107 splenocytes. Subsequently, splenocytes were washed twice with PBS containing 1% FSC. Then, 30 µl of anti-rabbit IgG microbeads (Miltenyi Biotec) were added to the cell pellet, it was held in the dark for 15 min and was washed twice using PBS (1% FCS). Then, B7-H3+ cells and B7-H3--cell fractions were separated using a magnet (EasySepTM; STEMCELL Technologies). Part of the B7-H3- fraction was double-stained with PE-conjugated OX42 antibody and FITC-conjugated goat anti-rabbit IgG (H & L, polyAb; Abcam plc.) to confirm the B7-H3+-cell depletion using flow cytometry. Preliminary experiments revealed that microbead treatments did not interfere in the binding of this second antibody to the first antibody. As a control of anti-rat B7-H3 antibody, normal rabbit IgG (Santa Cruz Biotechnology Inc.) was used.
Analysis of gene expression
Spleens were excised from Liposome-loaded or control rats 24 h after Liposome or saline injection. Enrichment of MHC-class II-/CD11b/c+cells was performed using magnetic beads according to the manufacturer’s protocol. Cells were applied to anti-MHC class II(OX-6) microbeads (Miltenyi Biotec) for negative selection of MHC-class II+ cells. Subsequently, they were mixed with PE-conjugated anti-CD11b/c (OX-42), and were stained with anti-PE microbeads. They were applied to the MACS column for positive selection of CD11b/c+ cells. Total RNA extracted from MHC-class II negative and CD11b/c+-cell enriched fractions of both Liposome-loaded and saline-loaded rat were applied to a microarray system (Whole Rat Genome; Agilent Technologies Inc.) for gene expression analysis. Analyses were conducted twice. Genes that were expressed reproducibly twice or more compared to control were regarded as genes that were enhanced significantly. Gene expression data are available at the Gene Expression Omnibus (GEO) repository from the National Center for Biotechnology Information under accession number GSE126246 (https://www.ncbi.nlm.nih.gov/geo/).
Spleen weight comparison and histological staining
At 24 h after intravenous injection of liposome suspension, the rats were anesthetized using sevoflurane and were killed. Then, both the body weight and the resected spleen weight were measured. The spleen weight is expressed hereinafter as a ratio of spleen weight versus body weight. Then, the ratios were compared with those of control specimens. The resected spleens were fixed with formalin and were stained with hematoxylin (Sigma-Aldrich Corp.) and eosin (Merck Diagnostica, West Point, PA). Then, they were observed using light microscopy. Microscopic images captured using a digital camera (MP5Mc/OL; Olympus Corp.) were processed using software (Win Roof ver. 5.5; Mitani Corp., Tokyo, Japan).
Statistical analyses
To estimate the effects of B7-H3+-cell depletion on T-cell proliferation, repeated measures ANOVA was used. Dunnett tests were applied as post hoc tests. To assess various liposomes’ effects on T-cell proliferation and spleen weight, unpaired Student t-tests were used. Software was used for statistical analyses (ystat2004; Igaku Tosho Press, Co. Ltd., Tokyo, Japan). Results for which p < .05 were inferred as significant.
Results
Analysis of the gene expression profile
Our earlier report, as presented in , revealed that injection of liposome comprising DPPC/CHOL/DHSG/PEG-DSPE induces immunosuppressive cells that suppress Con A stimulated T-cell proliferation. It is noteworthy that injection of even 5% (vol/vol) of liposome suspension was sufficient to induce immunosuppressive effects [Citation19]. However, to achieve definitive results, we applied 20% (vol/vol) injection in the experimental setting. The immunosuppressive cells, which were liposome-internalized cells, were found to be positive for CD11b/c and negative or weakly positive for MHC-class II [Citation18,Citation19]. Based on these results, we enriched the CD11b/c+ MHC-class II--cell fraction using magnetic beads and conducted gene expression analyses to find genes that increase in expression after liposome loading. In fact, 32 genes were enhanced repeatedly more than five-fold compared to controls (Supplemental Table 1). Among them, four genes presumed to play some role in immune response are presented in . Considering that cell-to-cell contact is necessary for T-cell suppression, CD11b/c+-cells with T-cell suppressive functionality must express cell surface molecules that are involved in cell-to-cell contact between these cells and activated T cells. Therefore, we are specifically interested in B7-H3(CD276) because, among the four molecules, only B7-H3 is expressed on the cell surface and because it reportedly plays a role in modulating immune responses [Citation22–24]. At 24 h after injection of liposome into rats, splenocytes derived from the rats were analyzed for B7-H3+ cells using flow cytometry. Results demonstrate that some CD11b/c+ cells express B7-H3 after Liposome loading. In contrast, enhancement of B7-H1 expression on CD11b/c+ cells was not apparent (). This result led us to infer that infusion of this liposome preferentially enhances expression of B7-H3 on CD11b/c+ cells.
Figure 1. Induction of T-cell suppression and enhanced expression of B7-H3 on CD11b/c+ cells by liposome-loading. After liposome suspension was injected into rats, splenic T-cell proliferation assay was conducted as described in Materials and Methods. (a) 24 h later, splenocytes were stimulated with Con A at the noted concentrations. T-cell proliferation was suppressed significantly (p < .01, unpaired Student t-tests). (b) 24 h later, splenocytes derived from rats were stained with polyclonal rabbit anti-B7-H3 antibody or B7-H1 antibody along with PE-conjugated anti-CD11b/c as described in Materials and Methods. Then, flow cytometry analysis was conducted. Comparison with B7-H1 demonstrated that the expression of B7-H3 was more enhanced by liposome injection. Data are representative of three independently conducted experiments. (c) Bar chart showing flow cytometry data of three independently conducted experiments. The percentage of CD11b/c+/B7-H3+-cell (Q2 area) was significantly greater after Liposome loading.
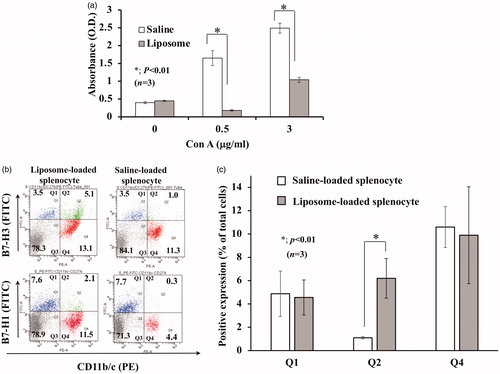
Table 2. Enhanced genes involved in immune response. The gene expression profile of CD11b/c+ cell enriched cells derived from liposome-injected rat spleen was compared with that of a saline-injected rat spleen. Genes which repeatedly showed more than five times enhancement and which were presumed to play some role in immune response are shown in the table.
B7-H3-expressing CD11b/c+ cells possess suppressive activity against T-cell proliferation
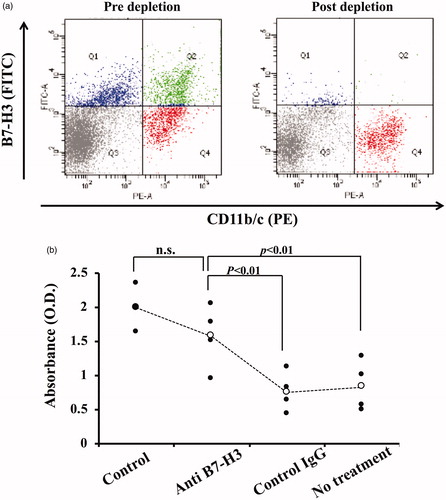
Our earlier study demonstrated that the CD11b/c+-cell fraction of Liposome-loaded splenocytes is responsible for T-cell suppression. Based on the appearance of B7-H3-expressing CD11b/c+ cells after Liposome-loading, it is likely that these cells are responsible for T-cell suppression. To validate this idea, B7-H3+ cells were removed from splenocytes using magnetic beads. Sufficient removal of B7-H3 expressing CD11b/c+ cells was confirmed (). T-cell proliferation was clearly restored to nearly normal levels (), which indicates that B7-H3-expressing CD11b/c+ cells play a key role in T-cell suppressive activity.
Figure 2. Restoration of T-cell proliferation by depletion of B7-H3-expressing cells. (a) B7-H3-expressing cells were depleted from liposome-loaded splenocytes, as described in Materials and Methods. Most B7-H3-expressing cells were depleted. Data presented in the figure are representative of at least three independently conducted experiments. (b) B7-H3-expressing cell depleted splenocytes were cultured in the presence of Con A (3 µg/ml) for 3 days. T-cell proliferation was assayed. Results of four independent experiments are shown. Significant differences were found among four groups (p < .01, non-repeated measures ANOVA). Depletion of B7-H3 expressing cells significantly restored T-cell proliferation (p < .01, post-hoc test). Control, splenocytes derived from saline-loaded rat; Anti-B7-H3, B7-H3-expressing cells were depleted from liposome-loaded splenocytes using rabbit polyclonal anti-rat B7-H3 IgG; Control IgG, B7-H3-expressing cells were depleted from liposome-loaded splenocytes using normal rabbit IgG; No treatment, liposome-loaded splenocytes with no treatment.
Inducible NOS (iNOS) is expressed in B7-H3-expressing CD11b/c+ cells
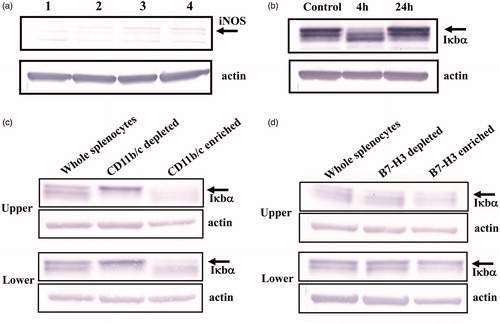
An earlier experiment demonstrated that T-cell suppression observed in Liposome-loaded splenocytes was mediated by NO, possibly produced by immunosuppressive cells [Citation19]. If B7-H3 expressing CD11b/c+ cells are involved in T-cell suppression, then these cells must be positive for iNOS. Using Western blotting, we investigated whether B7-H3-expressing cells express iNOS, or not. The signal for iNOS ceased after removal of B7-H3 positive cells from Liposome-loaded splenocytes (), indicating that B7-H3-expressing cells are positive for iNOS.
Figure 3. Preferential expression of iNOS and degradation of Iκbα in CD11b/c+-cell fraction. a) Saline-loaded splenocyte: lane 1 – B7-H3-expressing cells were depleted from Liposome-loaded splenocytes using anti-B7-H3 antibody; lane 2 – or control antibody (IgG); lane 3 – liposome-loaded splenocytes with no depletion treatment; lane 4 – iNOS was detected in liposome-loaded splenocytes. Depletion of B7-H3-expressing cell reduces the signal intensity of iNOS. Data are representative of three independently conducted experiments. (b) At 4 h after the Liposome suspension was injected into rats, spleens were excised. Splenocyte lysate was Western blotted to show the Ikbα signal. The signal intensity for Iκbα was reduced clearly at 4 h after liposome-loading; it had recovered completely by 24 h. (c, d) CD11b/c+ cells (c) or B7-H3+ cells (d) were enriched from splenocytes taken at 4 h after Liposome-loading. Then they were Western blotted to detect Iκbα signal. The signal intensities for Iκbα in both CD11b/c+ and B7-H3+-cell enriched fraction were markedly weaker in Liposome-loaded splenocytes than in saline-loaded splenocytes . Data are representative of at least two independently conducted experiments with similar results: Upper, liposome-loaded splenocytes; Lower, saline-loaded splenocytes.
NFκB signaling pathway in B7-H3 expressing CD11b/c+ cells is activated after liposome injection
Reportedly, iNOS is induced through NFκB activation [Citation25]. Additionally, translocation of NFκB from the cytosol to nucleus is well known to enhance MDSC-suppressive functions [Citation26–29]. Therefore, NFκB signaling pathway in CD11b/c+ cells of Liposome-loaded splenocytes is likely to be activated. As depicted in , the signal intensity of Ikbα in liposome-loaded whole splenocytes taken at 4 h after injection of Liposome was apparently weaker than that of control. This result suggests that NFκB signaling pathway is activated in liposome-loaded splenocytes. Furthermore, when the signal intensity of Iκbα in CD11b/c+-cell enriched fraction derived from liposome-loaded splenocytes was compared with that from saline-loaded splenocytes, the signal intensity of Ikbα in the former was weaker than that in the latter, indicating that NFκB pathway in CD11b/c+ cells was activated (). Furthermore, when the signal intensity of Iκbα in the B7-H3+-cell enriched fraction derived from Liposome-loaded splenocytes was compared with that from saline-loaded splenocytes, it was readily apparent that the former was weaker than the latter (). Considering that the B7-H3+-cell enriched fraction of saline-loaded splenocytes contains few CD11b/c+ cells and considering that of Liposome-loaded splenocytes contains both CD11b/c+ and CD11b/c− cells (), this finding suggests that the NFκB signaling pathway in B7-H3 expressing CD11b/c+ is highly activated.
Induction of T-cell suppression is not specific to liposomes containing DPPC
Our earlier report indicate that the T-cell suppressive effect is also observed with liposome composed of DPPC alone (DPPC-liposome) [Citation18], indicating that the DPPC component of Liposome is sufficient for induction of T-cell suppressive activity.
This result raised the question of whether this phenomenon is specific to the liposomes containing DPPC or not. To address this question, three other chemically defined liposomes were prepared: DMPC-liposome, POPC-liposome, and DOPC-liposome. Their T-cell suppressive effects were compared with those of DPPC-liposome. Except for DOPC-liposome, both DMPC-liposome and POPC-liposome were also shown to induce T-cell suppression, although the suppressive activity was weaker than that of DPPC-liposome (). Furthermore, both pathological examination and evaluation of spleen weight showed that these three liposomes, but not DOPC-liposome, accumulated in the spleen (, Citation5(a)).
Figure 4. Different liposome effects on T-cell suppressive effect induction. DOPC-, DMPC-, POPC-, DPPC-, DOPC/CHOL-, POPC/CHOL-, and DPPC/CHOL-liposome suspensions were prepared as described in Materials and Methods. They were injected intravenously into rats. The spleens were excised 18 h later. Then the 3H-thymidine uptake was observed in the presence of Con A (0.3 µg/ml). (a) Aside from the DOPC-liposome, three other liposomes induced T-cell suppression, with the strongest suppression found for DPPC-liposome. Data from several experiments were collected and expressed as mean ± SD. The numbers of experiments are presented in parentheses. (b) When cholesterol was incorporated, all tested liposomes induced significant T-cell suppression. c) When POPC/CHOL-liposome or DOPC/CHOL-liposome was injected, unique cells with enlarged cytoplasm appeared in the spleen similarly to DPPC/CHOL-liposome. Nevertheless, no apparent change was observed when DOPC-liposome or saline was injected. Each image was obtained from one independent experiment. Images were taken using a light microscope (BX50; Olympus Corp.) with a 20 × /0.50 objective lens to give original magnification at the time of photomicroscopy as × 200.
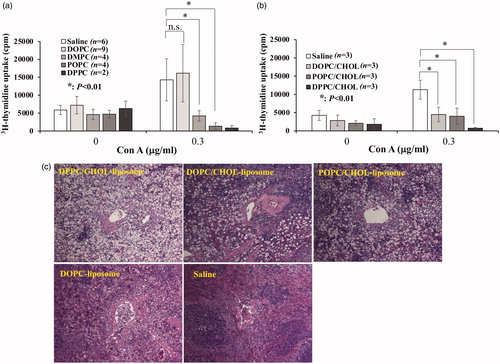
Although DOPC-liposome failed to induce T-cell suppression, DOPC/CHOL-liposome was able to induce T-cell suppression, as were all cholesterol-containing liposomes (). Furthermore, DOPC/CHOL-liposome was shown to be accumulated in the spleen (). These results indicate that, irrespective of the type of long chain fatty acids, chemically defined lipid vesicles used for this study can induce immunosuppression when accumulating in the spleen.
Discussion
Cells responsible for T-cell suppression are positive for B7-H3
Earlier reports have demonstrated that proliferation of Con A stimulated splenic T cells was suppressed when Liposome was injected into rats. Liposome-internalized cells with a phenotype of CD11b/c+, class II−, are responsible for that suppression. They meet some features of MDSCs, including the possible requirement of cell-to-cell contact between activated T cells and liposome-internalized cells [Citation18,Citation19]. We hypothesized that, when splenic macrophages internalize liposome, they express some unknown molecules on their surface, which is necessary for them to interact with activated T cells and to execute T-cell suppressive functions. Gene expression analysis screening yielded 32 genes that were upregulated more than fivefold (Supplemental Table). Herein, four genes that might be involved in immune reactions are presented (). Among them, we specifically examined the B7-H3 molecules because this molecule can be expressed on the cell surface and because it belongs to the B7-family, which function as immunoregulators [Citation22,Citation23]. Additionally, it is noteworthy that they are reportedly expressed in a subset of MDSCs in humans and in mice [Citation8]. The molecules encoded by other three genes presented in are associated with chemokines, cytokines or their receptors. They have no reported or recognizable potential to suppress T-cell proliferation.
As we expected, after the injection of Liposome, B7-H3 expressing CD11b/c+ cells appeared in Liposome-loaded splenocytes (). Furthermore, when B7-H3+ cells were removed from liposome-loaded splenocytes, Con A-induced T-cell proliferation was restored considerably (), indicating that cells with T-cell suppressing activity are present within B7-H3 expressing cells. It is noteworthy that we had earlier reported that depletion of CD11b/c+-cell fraction can restore T-cell proliferation completely [Citation19]. Therefore, one can reasonably infer that cells which are positive for B7-H3 and CD11b/c are responsible for T-cell suppression. The presence of iNOS in B7-H3 positive cells () supports this inference. Unfortunately, a blocking experiment using anti-B7-H3 antibody failed to restore T-cell proliferation. Nevertheless, this result does not rule out the possible involvement of B7-H3 molecule in T-cell suppression.
Internalization of liposome might activate NFκB signaling pathway
Activation of NFκB signaling pathway reportedly enhances MDSC immunosuppressive activity [Citation26–29]. Based on these reports, we speculated that the NFκB pathway in CD11b/c+ cells or B7-H3 expressing CD11b/c+ cells is activated. As expected, the reduced signal intensity of Iκbα in whole splenocytes and CD11b/c+-cell enriched fraction derived from Liposome-loaded splenocytes was observed compared to those of normal or saline-loaded splenocytes (). These findings indicate that the NFκB signaling pathway in Liposome-internalized macrophage is activated. Furthermore, it must be emphasized that, as described in the Results section, NFκB signaling pathways in B7-H3 expressing CD11b/c+ cells appeared to be activated (), although the data do not lead to conclusive findings. Taken together, the findings described above appear to be consistent with data indicating that B7-H3 expressing cells have T-cell suppressive activity (). Activation of NFκB signaling pathway is likely to promote iNOS production () and the release of NO from the cells in question.
Possible mechanisms underlying the activation of NFκB signaling pathway by Liposome can be considered. First, along with bacteria-derived LPS or lipopeptide, saturated fatty acids such as palmitic acid have been implicated as directly inducing Toll-like receptor 2 (TLR 2)-dependent or TLR 4-dependent signaling pathways [Citation30,Citation31]. Liposomes are internalized by macrophages via phagocytosis because of their particle size (250 nm). They must be digested within a phagolysosome. Considering that the acyl chain of DPPC is palmitic acid, excessive amounts of exogenous palmitic acid might accumulate in cytosol as a result of digestion. Consequently, a part of palmitic acid might be effluxed from the cell, thereby spurring activation of TLR 2 or TLR 4 expressed on the cell surface. Second, the accumulation of excessive amounts of palmitic acid through phagocytosis of Liposome might induce endoplasmic reticulum (ER) stress [Citation32,Citation33]. In fact, saturated fatty acids such as palmitic acid are known to induce ER stress [Citation34,Citation35], which might trigger unfolded protein response, thereby leading to degradation of Iκbα [Citation36,Citation37]. Consequently, the NFκB signaling pathway is activated. The complete mechanism involved in NFκB activation remains to be investigated. Finally, although liposome suspension used in experiments contained less than 0.1 endotoxin units/ml, it cannot be excluded that undetectable amounts of endotoxin contaminating the reagents used for experiments might stimulate TLRs, consequently leading to NFκB signaling activation [Citation38,Citation39].
Importance of stable vesicular formation for inducing immunosuppressive cells
An earlier study reported that, along with Liposome, DPPC-liposome was able to induce splenic T-cell suppression, suggesting that, among the four components of Liposome (DPPC/CHOL/DHSG/ PEG5000-DSPE), DPPC is necessary to induce suppressive activity [Citation18]. To extend this study, we addressed the effects of DMPC-liposome, POPC-liposome, and DOPC-liposome on T-cell proliferation. It was interesting that both DMPC-liposome and POPC-liposome induced significant T-cell suppressive activity, although the suppressive activity was weaker than that of DPPC-liposome (). In addition, judging from pathological changes and weight changes after these liposomes were injected, all these liposomes were confirmed to accumulate in the spleen (). Therefore, induction of T-cell suppression might not necessarily be a phenomenon specific to Liposome or DPPC-liposome. Apparently, accumulation of liposome particles in the spleen (splenic macrophage) is important for T-cell suppression, irrespective of the long chain fatty acid type.
Figure 5. Spleen weight at 18 h after injection of liposome suspension. POPC-, DOPC-, DPPC-, DMPC-, POPC/CHOL-, DOPC/CHOL, and DPPC/CHOL-liposome suspensions were injected into rats as described in Materials and Methods. Then, 18 h later, the body weight and spleen weight were measured. The spleen weight was expressed as a ratio (spleen weight vs. body weight). (a) Except for DOPC-liposome, the weight increase of spleens was observed by injection of POPC-, DPPC-, or DMPC-liposome. The weight increase of the spleens by DPPC-liposome appeared to be the most prominent. In contrast, no significant weight gain of spleen was found from DOPC-liposome injection. (b) Significant weight gain occurred after injection of all cholesterol-containing liposomes.
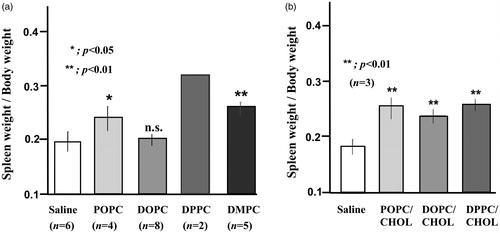
However, DOPC-liposome did not induce T-cell suppressive activity (). The following explanation can be put forward. Among the four liposomes, DOPC-liposome has the lowest Tc (-20 °C). Therefore, DOPC-liposome is the most unstable liposome. It might be unable to retain its vesicular form in the bloodstream. Therefore, internalization of DOPC-liposome by macrophage might occur only slightly. The observation that accumulation in the spleen was not observed supports this speculation (, Citation5(a)). In contrast, DOPC/CHOL-liposome can retain its vesicular form because cholesterol is known to enhance liposome stability. Therefore, similarly to other liposomes, it can be internalized by splenic macrophages (, Citation5(b)). This finding can explain why DOPC/CHOL-liposome, but not DOPC-liposome, induced T-cell suppression (). Cholesterol might be associated somehow with T-cell suppressive effects. Nevertheless, this subject is beyond the scope of this study.
Accumulation of long-chain fatty acids might be involved in inducing immunosuppressive function
Recent reports indicate that excessive accumulation of lipid makes the cells reprogram metabolic pathway from glycolysis to fatty acid oxidation and explain that utilization of oxidized lipid as an energy source is crucially important for the immunosuppressive function of MDSCs [Citation16,Citation17]. Reportedly, uncontrolled lipid accumulation is higher in MDSCs from patients and mice with tumor burdens than in their tumor-free counterparts, although the lipid source is unclear [Citation40]. Furthermore, similarly to our experimentally obtained results, NO is apparently a fundamentally important effector for the suppressive activity of this lipid-induced suppressive activity of MDSCs [Citation40]. Therefore, accumulation of long chain fatty acids into macrophages as a component of liposome must be associated somehow with the macrophage acquisition of immunosuppressive activity.
Regarding the fatty acid type, MDSCs cultured with unsaturated fatty acid (linoleic acid or oleic acid) but not saturated fatty acid (palmitic acid) for 4 days reportedly increased T-cell suppressive activity [Citation40]. According to that earlier report, unsaturated fatty acid but not saturated fatty acid appears to be more important for inducing T-cell suppressive activity. Induction of T-cell suppressive activity by POPC-liposome and DOPC/Chol-liposome () is in line with that earlier report [Citation40] because it is readily apparent that cells which internalize these liposomes must contain plenty of oleic acid, which is monounsaturated and susceptible to oxidation. The presence of large quantities of oleic acid might therefore cause the shift of the main metabolic pathway from glycolysis to fatty acid oxidation. However, results showing that DPPC-liposomes or DMPC-liposomes induce T-cell suppressive activity () contradict those presented herein because both palmitic acid and myristic acid are saturated fatty acids that are even more resistant to oxidation than unsaturated fatty acids are. The following explanation can be put forward. In that earlier study [Citation40], fatty acids were possibly present in the culture medium as albumin-binding free fatty acid. Therefore, they must be internalized into cells via long chain fatty acid receptors such as CD36. In contrast, in our experimental condition, fatty acids are incorporated into liposomes. Therefore, they are internalized via phagocytosis by macrophages as a liposome component. Based on this speculation, it might be true that even palmitic acid or myristic acid is oxidized by myeloperoxidase or NADPH oxidase present in the phagosome membrane due to the activity of reactive oxygen species. These oxidized fatty acids might be used as an energy source, leading to the induction of T-cell suppressive function [Citation17].
Cellular membrane-derived MVs released from tumor cells can be taken up into cells via endocytosis. In the case of macrophages, phagocytosis is apparently the main mechanism for their uptake [Citation41]. In addition, recent reports demonstrate that tumor-associated macrophages acquire immunosuppressive functions via uptake of MVs and reveal that proteins and nucleic acids involved in MVs are responsible for these effects [Citation13–15]. Considering that the liposome used for this study comprises a phospholipid bilayer out of which all cell membranes are constructed, the liposomes used for this study might be regarded as artificial MVs. Therefore, it is fascinating to speculate that our experimentally obtained results reflect the effects of the lipid component of MVs on immunological functions of macrophages. In other words, our results suggest that tumor-derived MV can be a source of lipids that accumulate in tumor-associated MDSCs, leading to their increased T-cell suppressive activity. In this sense, the precise mechanism of liposome-internalization and the subsequent cell signaling event or ER stress response that might be caused by lipid accumulation must be addressed.
In summary, we report that the chemically defined pegylated lipid vesicle (Liposome) can affect macrophage function by being internalized by macrophages. The internalization of this liposome can transform macrophages into a B7-H3 expressing MDSCs. Induction of immunosuppression is not a phenomenon that is specific to this liposome. It is most likely that, irrespective of the saturation status, direct internalization of long chain fatty acids as a lipid vesicle into macrophages might shift energy utilization from glycolysis to fatty acid oxidization, leading to conferral of immunosuppressive activity to the macrophages.
| Abbreviations | ||
| B7-H3 | = | B7 homolog 3 protein |
| DMPC | = | 1,2-dimyrisytoyl-sn-glycero-3-phosphatidylcholine |
| DOPC | = | 1,2-oleyl-sn-glycero-3-phosphatidylcholine |
| DPPC | = | 1, 2-dipalmitoyl-sn-glycero-3-phosphatidylcholine |
| ER | = | endoplasmic reticulum |
| GM-CSF | = | granulocytes-macrophage colony stimulating-factor |
| IL-1β | = | Interleukin-1β |
| iNOS | = | inducible nitric oxide synthase |
| MDSC | = | myeloid-derived suppressor cell |
| MV | = | cellular membrane-derived microvesicles |
| NADPH | = | nicotinamide adenine dinucleotide phosphate oxidase |
| PEG | = | polyethylene glycol |
| POPC | = | 1-palmitoyl-2-oleyl-sn-glycero-3-phosphatidylcholine |
Supplemental Material
Download PDF (240.7 KB)Acknowledgments
The authors thank Mr. Yoshitaka Tateishi for his technical assistance in cell culture and Western blotting.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mantovani A, Sica A, Allavena P, et al. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70(5):325–330.
- Bruger AM, Dorhoi A, Esendagli G, et al. How to measure the immunosuppressive activity of MDSC: assays, problems and potential solutions. Cancer Immunol Immunother. 2019;68(4):631–644.
- Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–3364.
- Youn JI, Nagaraj S, Collazo M, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802.
- Zhu B, Kennedy JK, Wang Y, et al. Plasticity of Ly-6C(hi) myeloid cells in T cell regulation. J Immunol. 2011;187(5):2418–2432.
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174.
- Brandau S, Moses K, Lang S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: Cousins, siblings or twins? Semin Cancer Biol. 2013;23(3):171–182.
- Zhang G, Huang H, Zhu Y, et al. A novel subset of B7-H3 + CD14 + HLA-DR-/low myeloid-derived suppressor cells are associated with progression of human NSCLC. Oncoimmunology. 2015;4(2):e977164.
- Condamin T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32(1):19–25.
- Bayne LJ, Beatty GL, Jhala N, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer . Cancer Cell. 2012;21(6):822–835.
- Marigo I, Bosio E, Solito S, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32(6):790–802.
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268.
- Schrey CD, Ckabcy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Gene Dev. 2012;26:1287–1299.
- Sadallah S, Eken C, Schifferli JA. Ectosomes as modulators of inflammation and immunity. Clin Exp Immunol. 2011;163(1):26–32.
- Xiang X, Poliakov A, Liu C, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–2633.
- Veglia F, Tyurin V, Kagan V, et al. Oxidized lipids contribute to the suppression function of myeloid derived suppressor cell in cancer. In: Proceeding of the 106th Annual Meeting of the American Association for Cancer Research. 2015. Philadelphia, PA: AACR.
- Yan D, Adeshakin AO, Xu M, et al. Lipid metabolic pathways confer the immunosuppressive function of myeloid-derived suppressor cells in tumor. Front Immunol. 2019;10:1399.
- Takahashi D, Azuma H, Sakai H, et al. Phagocytosis of liposome particles by rat splenic immature monocytes makes them transiently and highly immunosuppressive in ex vivo culture conditions. J Pharmacol Exp Ther. 2011;337(1):42–49.
- Azuma H, Yoshida Y, Takahashi H, et al. Liposomal microparticle injection can induce myeloid-derived suppressor cells (MDSC)-like cells in vivo. Immunopharmacol Immunotoxicol. 2017;39(3):140–147.
- Sato T, Sakai S, Sou K, et al. Static structures and dynamics of hemoglobin vesicle (HBV) developed as a transfusion alternative. J Phys Chem B. 2009;113(24):8418–8428.
- Sou K, Tsuchida E. Electrostatic interactions and complement activation on the surface of phospholipid vesicle containing acidic lipids: Effect of the structure of acidic groups. Biochim Biophys Acta. 2008;1778(4):1035–1041.
- Chapoval AI, Ni J, Lau JS, et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2(3):269–274.
- Prasad DV, Nguyen T, Li Z, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173(4):2500–2506.
- Lemke D, Pfenning PN, Sahm F, et al. Costimulatory protein 4IgB7H3 drives the malignant phenotype of glioblastoma by mediating immune escape and invasiveness. Clin Cancer Res. 2012;18(1):105–117.
- Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75(6):635–639.
- Ostrand-Rosenberg S, Fenselau C. Myeloid-derived suppressor cells: Immune-suppressive cells that impair antitumor immunity and are sculpted by their environment. J Immunol. 2018;200(2):422–431.
- Liu Y, Xiang X, Zhuang X, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176(5):2490–2499.
- Bunt SK, Clements VK, Hanson EM, et al. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85(6):996–1004.
- Hong EH, Chang SY, Lee BR, et al. Blockade of MYD88 signaling induces antitumor effects by skewing the immunosuppressive function of myeloid-derived suppressor cells. Int J Cancer. 2013;132(12):2839–2848.
- Huang S, Rutkowsky JM, Snodgrass RG, et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53(9):2002–2013.
- Suganami T, Koyama KT, Nishida J, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27(1):84–91.
- Halbleib K, Pesek K, Covino R, et al. Activation of the unfolded protein response by lipid bilayer stress. Mol Cell. 2017;67(4):673–684.
- Tam AB, Mercado EL, Hoffmann A, et al. ER stress activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One. 2012;7(10):e45078.
- Guo W, Wong S, Xie W, et al. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiology. 2007;293:576–586.
- Wei Y, Wang D, Topczewski F, et al. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol. 2006;291:275–281.
- Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462.
- Hu O, Couvillon AD, Kaufman RJ, et al. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26(8):3071–3084.
- Erridge C, Nilesh J, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29(11):1944–1949.
- Lancaster GI, Langley KG, Berglund NA, et al. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab. 2018;27(5):1096–1110.e5.
- Al-Khami AA, Zheng L, Del Valle L, et al. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology. 2017;6(10):e1344804.
- Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3(1):24641.
