Abstract
Modern antifouling coatings use heavy metals and toxic organic molecules to prevent biofouling, the undesirable growth of marine organisms on man-made substrata. In an ongoing survey of deep-sea microorganisms aimed at finding low toxic antifouling metabolites, an actinomycete bacterium was isolated from the Pacific sediment at the depth of about 5000 m. The bacterium was closely related to Streptomyces fungicidicus (99% similarity) according to 16S ribosomal RNA sequence information. The spent culture medium of this bacterium inhibited barnacle larval attachment. Bioassay-guided fractionation was employed to isolate antifouling compounds. The ethyl acetate extract was fractionated by using an open silica gel column. Active fractions were further purified on a HPLC C18 column. Five diketopiperazines, cyclo-(L-Leu-L-Pro), cyclo-(L-Phe-L-Pro), cyclo-(L-Val-L-Pro), cyclo-(L-Trp-L-Pro), and cyclo-(L-Leu-L-Val) were isolated for the first time from a deep sea bacterium, and the structures of the compounds were elucidated by nuclear magnetic resonance spectroscopy and mass spectrometry. The pure diketopiperazines were tested for antilarval activity using the barnacle Balanus amphitrite. Effective concentrations that inhibited 50% larval attachment (EC50) after 24 h ranged from 0.10 – 0.27 mM. The data suggest that diketopiperazines and other compounds from deep-sea bacteria may be used as novel antifoulants.
Introduction
Natural and artificial substrata in the marine environment are quickly colonised by microfoulers (bacteria, diatoms and protozoans) and macrofoulers (invertebrate larvae, and macroalgal spores) (Wahl, Citation1989; Little & Wagner, Citation1997). Both micro- and macrofouling in the world's oceans cause huge material and economic losses in maintenance of mariculture, shipping industries, naval vessels, and seawater pipelines (Yebra et al. Citation2004). In order to minimise the impacts of foulers, many underwater structures are protected by toxic antifouling coatings (Evans, Citation1999; Yebra et al. Citation2004). The International Maritime Organisation (IMO) and Marine Environmental Protection Committee (MEPC) decided to ban the usage of TBT or other substances containing tin as biocides in antifouling paints, effective in January 2008. Therefore, there is a clear need for the development of “environmentally-friendly” antifoulants.
In recent years, extensive search for novel antifouling compounds against biofouling have been carried out (Clare et al. Citation1992; Holmstroem & Kjelleberg, Citation1999; Burgess et al. Citation2003; Dobretsov & Qian, Citation2004). At present, most marketed marine products are derived from shallow waters. Increasing economic and scientific interest has now shifted to the biotechnological potentials of deep-sea microorganisms that have high biodiversity and have possibly developed unique adaptations to live in dark, cold, and high-pressurised environments. Novel metabolic pathway of the deep-sea microbes offers a wealth of opportunities for bioactive marine natural products research. Deep-sea bacteria seem nearly boundless in their potential as a source of bioactive compounds with novel structures, since the discovery that genetically identical organisms living in different environments produce a host of differing chemical defence compounds (Venter et al. Citation2004). Numerous efforts have been made to isolate new enzymes and bioactive compounds from deep-sea microorganisms. However, the potential application of metabolites produced by deep-sea bacteria in antifouling has hardly been explored (Dobretsov et al. Citation2006).
In searching for new antifouling compounds from marine microbes, five diketopiperazines (DKPs) were isolated from a deep-sea bacterium, Streptomyces fungicidicus, using a bioassay-guided fractionation. This represents the first report that documents the production of these DKPs by the deep-sea bacterial strain S. fungicidicus and their antifouling properties.
Materials and methods
Isolation and identification of the bacterium
Sediment samples were collected from the Western Pacific (N 10°50′35′′; W 154°05′28′′) at a depth of 5000 m. The sediments were kept at 0°C, transported to the laboratory, and stored at 4°C before processing. One pure strain of the bacterium was obtained by successive dilutions of the samples. After primary biological screening, the ethyl acetate extraction of the spent bacterial culture medium had strong antifouling properties. A molecular approach was adopted to identify this bacterium. The 16S rRNA gene was amplified by the forward primers 8F (5′-AGAGTTTGATCCTGGCTCAG-3′), 26F (5′-AGAGTTTGATCCTGGCTCA-3′), 355F (5′-ACTCCTACGGGAGGCAGC-3′) and the reverse primers 1055R (5′-CACGAGCTGACGACAGCCAT-3′), 1492R (5′-GGYTACCTTGTTACGACTT-3′) and 1525R (5′-AAGGAGGTGWTCCARCC-3′). The primers were purchased from Life Technologies Inc. The PCR amplification procedure included 5 min at 94°C followed by 30 cycles of 50 sec at 94°C, 60s at 58°C, and 60s at 72°C. In the last cycle, the 72°C step was extended for 10 min, and the samples were finally cooled down to 4°C. The PCR products were purified and sequenced with both the forward and the reverse primers by a Megabace® capillary genetic analyzer (USA) using a dye terminator method according to the manufacturer's protocol. DNA sequences were assembled using the Sequencer® software package (Gene Codes, USA). The homologies of the resulting sequences were searched for using the BLAST program that is available from the National Center for Biotechnology Information (NCBI, www.ncbi.nlm.nih.gov/BLAST/).
Preparation of spent culture medium
The bacterium Streptomyces fungicidicus was inoculated in 3 l Erlenmeyer flasks containing culture medium (malt extract 20 g, yeast extract 8 g and glucose 8 g, 1.5 l filtered seawater). The pH of the culture medium was adjusted to 7.8 before sterilisation. The cultures were incubated at room temperature for 10 d on an orbital shaker at 110 rpm.
Isolation of diketopiperazines (DKPs)
About 60 l of the bacterial culture was centrifuged to separate bacterial cells and spent culture medium. Both cell pellets and spent culture medium were extracted three times with ethyl acetate. Later, the extracts were combined together and concentrated under reduced pressure. This resulted in 8 g of a dark yellow oily extract which was dissolved in 150 ml of methanol. This solution was extracted with 150 ml of hexane and both phases collected separately and evaporated until dryness under reduced pressure.
The methanol phase was pre-separated by column chromatography on silica gel (3×30 cm) with a CH2Cl2/MeOH gradient into nine fractions. Fractions with typical 1H NMR signals for DKPs were further purified by a reverse phase HPLC (Lichrospher RP-18 Endcapped, 100A 5 μ 250×4.6 mm) using a water/methanol gradient (flow rate: 1 ml min−1; UV detection at 220 nm and 254 nm). HPLC purification was performed on a Waters 600 pump with a Waters 717 autosampler. The eluted fractions were monitored by Waters 996 PDA Detector and collected by Waters Fraction Collector II. Fractions showing antifouling activity in the bioassays were dried and used for structure elucidation.
Structure determination of DKPs
The structure of the DKPs was determined using nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). A JEOL DRX 400 NMR Spectrometer using CDCl3 was deployed to measure 1H and 13C and 2D NMR. All spectra were recorded at 23°C, and one-dimensional 1HNMR, 13CNMR, as well as two-dimensional 1H-1H correlation spectroscopy and 1H-13C heteronuclear multiple bond correlation experiments were performed according to JEOL standard pulse sequences. Chemical shifts are reported relative to the solvent peaks (CDCl3: 1H δ 7.24 and 13C δ 77.23).
The mass spectra of the DKPs were determined on a Waters Micromass ZQ 2000, coupled with an electrospray source. The probe voltage was maintained at 2.5 kV, the cone voltage maintained at 18 V and the extractor voltage at 2 V. The source temperature was kept at 100°C, the desolvation temperature was 300°C, and the drying gas flow rate was 200 l h−1.
The absolute configuration of the DKPs was determined by analysis of DKP hydrolyates on TLC plates (Gunther, Citation1988) and comparison of NMR data with those reported from the literature (Young et al. Citation1976; Schmitz et al. Citation1983; Adamczeski et al. Citation1995; Jayatilake et al. Citation1996; Fdhila et al. Citation2003; Mitova et al. Citation2004).
Anti-larval attachment assays
Adult brood stocks of the barnacle Balanus amphitrite Darwin, were collected from piling and floating rafts in Port Shelter Bay, Hong Kong (22°19′N, 114°16′E). Barnacle nauplii were reared according to Harder et al. (Citation2001). Briefly, cyprids were aged in darkness for 4 days at 8°C prior to attachment bioassays. Prior to the bioassay, samples were evaporated and re-dissolved in dimethyl sulfoxide (DMSO). Still-water laboratory bioassays were performed with 4 replicates (n = 4) in the sterile polystyrene Petri dishes (# 1006, Falcon, USA) containing 20 larvae and the sample under investigation dissolved in 4 ml of filtered (0.22 μm) seawater (FSW). Larval attachment assays were run at 28°C under continuous illumination for 24 h. After the experiment, the number of attached and swimming larvae was counted with microscope.
In all bioassays, FSW and FSW with solvent (DMSO) served as controls. The percentage values of larval attachment in response to experimental treatments were arcsine-transformed. To improve the arcsine-transformation, those replicates with no attachment were given the value of 1/(4 n) (n = number of larvae in a single replicate) (Zar, Citation1996). The homogeneity and normality of data sets were analysed with Levene's and Shapiro-Wilk's W test, respectively, at a confidence level of 95%. The median effective concentration (EC50) of the five compounds was calculated by fitting the data to a sigmoidal inhibition model:
The median lethal concentration (LC50) of the compounds after 24 h for B. amphitrite were calculated with Probit® software.
Results
Identification of the strain of deep-sea bacterium
The comparison of the 16S rRNA sequence of the isolate with those sequences submitted to GenBank demonstrated that the strain was 99% similar to the 16S rRNA sequence of S. fungicidicus strain YH04 (Accession no. AY636155). (See Appendix.)
Purification and characterisation of DKPs from S. fungicidicus
The ethyl acetate fraction of the extract was first isolated by silica gel column chromatography. The active fractions were further purified by reversed phase HPLC to obtain five pure compounds that were all colourless amorphous solids. The compounds were identified as cyclo-(L-Leu-L-Pro), cyclo-(L-Phe-L-Pro), cyclo-(L-Val-L-Pro), cyclo-(L-Trp-L-Pro), and cyclo-(L-Leu-L-Val) ( ). The absolute stereochemistry of the DKPs indicated that all amino acids were of the L-configuration. Below is chemical structure information of the isolated DKPs ().
Cyclo-(L-Leu-L-Pro)
In total, 13.5 mg of this compound were obtained. ESI-MS m/z [M + H]+ 210.8. 1HNMR (400 MHz, CDCl3) δ 0.95 (3H, d J = 6.6 Hz, CH3), 0.99 (3H, d J = 6.6 Hz, CH3), 1.53 (1H, m, H-10a), 1.75 (1H, m, H-10b), 1.86 – 1.94 (1H, m, H-11), 2.00 – 2.18 (2H, m, H-4), 2.32 – 2.38 (2H, m, H-5), 3.48 – 3.64 (2H, m, H-3), 4.02 (1H, dd J = 9.3Hz/3.9Hz, H-9), 4.12 (1H, t J = 8.3, H-6), 6.10 (1H, brs, NH). 13CNMR (100 MHz, CDCl3) δ 170.25 (s, C-1), 45.77 (t, C-3), 23.60 (t, C-4), 28.40 (t, C-5), 59.22 (d, C-6), 166.23 (s, C-7), 53.64 (d, C-9), 38.88 (t, C-10), 24.99 (d, C-11), 23.06 (q, C-12), 21.53 (q, C-12′).
Cyclo-(L-Phe-L-Pro)
In total, 19.0 mg of this compound were obtained. ESI-MS m/z 244.9 ([M + H]+). 1HNMR (400 MHz, CDCl3) δ1.88 – 1.93 (1H, m, H-4a); 1.97 – 2.05 (1H, m, H-4b); 2.31 – 2.37 (2H, m, H-5); 2.78 (2H, dd J = 14.4/10.5, H-10); 3.55 – 3.65 (2H, m, H-3); 4.08 (1H, t J = 7.6Hz, H-6); 4.27 (1H, dd J = 10.6/2.7, H-9); 5.63 (1H, brs, N-H); 7.22∼7.38 (5H, m, Ar-H). 13C NMR (100 MHz, CDCl3) δ 169.04 (s, C-1), 45.38 (t, C-3), 22.52 (t, C-4), 28.31 (t, C-5), 59.03 (d, C-6), 164.74 (s, C-7), 56.07 (d, C-9), 36.71 (t, C-10), 135.65 (s, C-1′), 129.04 (d, C-2′), 128.84 (d, C-3′), 127.32 (d, C-4′).
Cyclo-(L-Val-L-Pro)
In total, 20 mg of this compound were obtained. ESI-MS m/z [M + H]+ 196.9. 1HNMR (400 MHz, CDCl3) δ0.92 (3H, d J = 7.6Hz, CH3), 1.07 (3H, d J = 7.3, CH3), 1.87 – 1.95 (1H, m, H-4a), 2.00 – 2.11 (1H, m, H-4b), 2.35 – 2.41 (2H, m, H-5), 2.59 – 2.67 (1H, m, H-10), 3.51 – 3.68 (2H, m, H-3), 3.94 (1H, s, H-9), 4.08 (1H, t J = 7.6Hz, H-6), 5.99 (1H, brs, N-H). 13C NMR (100 MHz, CDCl3) δ169.71 (s, C-1), 45.07 (t, C-3), 22.33 (t, C-4), 28.48 (t, C-5), 60.27 (d, C-6), 164.60 (s, C-7), 58.72 (d, C-9), 28.32 (d, C-10), 19.22 (q, C-11), 16.03 (q, C-11′).
Cyclo-(L-Trp-L-Pro)
In total, 15 mg of this compound were obtained. ESI-MS m/z 283.9 [M + H]+. 1HNMR (400 MHz, CDCl3) δ 1.89 – 2.05 (2H, m, H-4), 2.30 – 2.37 (2H, m, H-5), 2.97 (1H, dd J = 10.7Hz/15.0Hz, Ha-10), 3.60 (2H, m, H-3), 3.76 (1H, dd J = 3.9Hz/15.0Hz, Hb-10), 4.08 (1H, t J = 8.0Hz, H-6), 4.38 (1H, dd J = 2.7Hz/10.6Hz, H-9); 5.74 (1H, brs, N-H), 7.12 – 7.60 (5H, m, Ar-H), 8.24 (1H, brs, N-H (Indole)). 13C NMR (100 MHz, CDCl3) δ 168.99 (s, C-1), 45.34 (t, C-3), 22.58 (t, C-4), 28.25 (t, C-5), 59.12 (d, C-6), 165.22 (s, C-7), 54.44 (d, C-9), 26.79 (t, C-10), 123.05 (d, C-2′), 109.76 (s, C-3′), 118.27 (d, C-4′), 119.77 (d, C-5′), 122.56 (d, C-6′), 111.32 (d, C-7′), 126.44 (s, C-8′), 136.38 (s, C-9′).
Cyclo-(L-Leu-L-Val)
In total, 50 mg of this compound were obtained. ESI-MS m/z 212.9 [M + H]+. 1HNMR (400 MHz, CDCl3) δ0.95 (3H, d J = 6.8 Hz, H-3), 1.00 (3H, d J = 6.6 Hz, H-11), 1.05 (3H, d J = 7.1 Hz, H-3′), 1.27 (3H, d J = 6.3 Hz, H-11′), 1.62 (1H, m, H-5a), 1.79 (1H, m, H-4), 1.87 – 1.94 (1H, m, H-5b), 2.42 (1H, m, H-10), 3.34 (1H, m, H-9), 4.02 (1H, m, H-6), 6.04 (1H, brs, N-H), 6.20 (1H, brs, N-H). 13C NMR (100 MHz, CDCl3) δ168.24 (s, C-1), 43.54 (t, C-5), 60.08 (d, C-6), 166.70 (s, C-7), 52.97 (d, C-9), 31.34 (d, C-10), 18.80 (q, C-11), 16.34 (q, C-11′), 24.23 (d, C-4), 20.93 (q, C-3), 23.26 (q, C-3′).
Anti-larval attachment activity
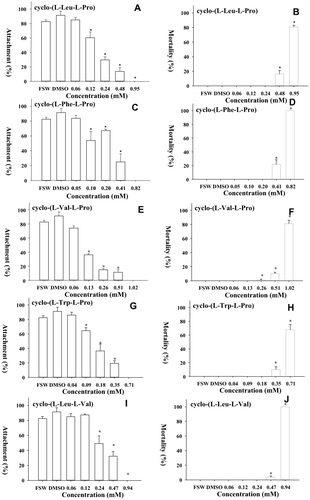
The activity of the five DKPs against barnacle larval attachment and mortality is presented in and . The EC50 of the five compounds ranged from 0.1 – 0.28 mM, while the LC50 ranged from 0.47 – 0.75 mM and the therapeutic ratio of LC50/EC50 ranged from 1.7 – 7.5. Among the five compounds, cyclo-(L-Val-L-Pro) had the maximal therapeutic ratio (7.5), and the lowest EC50 and the highest LC50.
Table I. EC50 and LC50 values for diketopiperazines on the larvae of the barnacle B. amphitrite
Discussion
In this study, five DKPs from S. fungicidicus were isolated and identified and their antifouling properties determined. DKP identification was supported by the presence of characteristic 13C NMR chemical shifts for the amide carbonyl groups (δC165 – 170) and the 1H NMR signals of the amino acids. Proline was easily deduced from the presence of three broad methylene multiplets (δH1.8 – 3.7). The NMR spectra clearly indicated that valine, leucine, tryptophan and phenylalanine were the other amino acid residues in cyclo-(L-Val-L-Pro), cyclo-(L-Leu-L-Pro), cyclo-(L-Trp-L-Pro) and cyclo-(L-Phe-L-Pro) correspondingly.
DKPs comprise an important family of the secondary metabolites that are mainly produced by microorganisms (Kelecom, Citation2002). The marine bacterium S. fungicidicus is a new source of bioactive DKPs. Preliminary trials showed that the biosynthesis of DKPs by S. fungicidicus relied on the optimisation of growth parameters such as temperature, pH, micronutrients, and carbon sources (data not shown).
DKPs have long been disregarded, however, more recently they have received an increasing amount of attention in drug discovery (Krchnak et al. Citation1996). Many investigations reveal DKPs to be antitumour, antibacterial and antifungal agents (Prasad, Citation1995; Milne et al. Citation1998; Ström et al. Citation2002; McCleland et al. Citation2004). For instance, cyclo-(Trp-Pro) has been therapeutically used in the modulation of membrane potassium ion channels (Jamie et al. Citation2002). However, the present study is the first to show the antifouling activity of these compounds. Among all five DKPs that inhibited larval attachment of B. amphitrite, cyclo-(L-Val-L-Pro) had the highest antifouling activity. The therapeutic ratio (Rittschof, Citation1999) of this compound was >7, which suggests that it has low toxicity towards to larvae and a low effective concentration.
So far, only a few anti-larval attachment compounds have been isolated and identified from bacteria (Dobretsov et al. Citation2006). For instance, the marine bacterium Alteromonas sp. isolated from the marine sponge Halichondria okadai produces the antifouling compound ubiquinone, which inhibits larval attachment of the barnacle B. amphitrite (Kon-ya et al. Citation1995). An epibiotic bacterium, Acinetobacter sp., isolated from the surface of the ascidian Stomozoa murrayi produces 6-bromindole-3-carbaldehyde that inhibits attachment of the barnacle B. amphitrite (Olguin-Uribe et al. Citation1997). These strains are epibiotic bacteria associated with soft-bodied marine invertebrates and are from shallow water environments. The present investigation is the first report demonstrating that deep-sea bacteria could be a potential source of novel antifouling compounds and thus deserve more intensive study in the future.
Acknowledgements
This work was supported by grants (CAG04/05.SC01) from the Research Grants Council of the Hong Kong government and a Croucher Foundation Fund grant (CAS-CF03/04.SC01) and grant (COMRRDA 03/04 SC01) from the China Ocean Mineral Resources Research and Development Association to Pei-Yuan Qian.
References
- Adamczeski , M , Reed , A R and Crews , P . 1995 . New and known diketopiperazines from the Caribbean sponge Calyx Cf. podatypa . J Nat Prod , 58 : 201 – 208 .
- Burgess , J G , Boyd , K G , Armstrong , E , Jiang , Z , Yan , L , Berggren , M , May , U , Pisacane , T , Granmo , A and Adams , D R . 2003 . The development of a marine natural product-based antifouling paint . Biofouling , 19 : 197 – 205 .
- Clare , A S , Rittschof , D , Gerhart , D J and Maki , J S . 1992 . Molecular approaches to non-toxic antifouling . Invert Reprod Dev , 22 : 67 – 76 .
- Dobretsov , S and Qian , P Y . 2004 . The role of epibotic bacteria from the surface of the soft coral Dendronephthya sp. in the inhibition of larval attachment . J Exp Mar Biol Ecol , 299 : 35 – 50 .
- Dobretsov , S , Dahms , H U and Qian , P Y . 2006 . Inhibition of biofouling by marine microorganisms and their metabolites . Biofouling , 22 : 43 – 54 .
- Evans , S M . 1999 . TBT or not TBT?: that is the question . Biofouling , 14 : 117 – 129 .
- Fdhila , F , Vázquez , V , Sánchez , J L and Riguera , R . 2003 . DD-diketopiperazines: antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus . J Nat Prod , 66 : 1299 – 1301 .
- Gunther , K . 1988 . Thin-layer chromatographic enantiomeric resolution via ligand exchange . J Chromatogr , 448 : 11 – 30 .
- Harder , T , Vengatesen , T and Qian , P Y . 2001 . Effect of cyprid age on the attachment of Balanus amphitrite Darwin in response to natural biofilms . Biofouling , 17 : 211 – 219 .
- Holmstroem , C and Kjelleberg , S . 1999 . Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents . FEMS Microbiol Ecol , 30 : 285 – 293 .
- Jamie , H , Kilian , G , Dyason , K and Milne , P J . 2002 . The effect of the isomers of cyclo-(Trp-Pro) on heart and ion-channel activity . J Pharm Pharmacol , 54 : 1659 – 1665 .
- Jayatilake , G S , Thornton , M P , Leonard , A C , Grimwade , J E and Baker , B J . 1996 . Metabolites from an Antarctic sponge-associated bacterium, Pseudomonas aeruginosa . J Nat Prod , 59 : 293 – 296 .
- Kelecom , A . 2002 . Secondary metabolites from marine microorganisms . Ann Brazil Acad Sci , 74 : 151 – 170 .
- Kon-ya , K , Shimidzu , N , Otaki , N , Yokoyama , A , Adachi , K and Miki , W . 1995 . Inhibitory effect of bacterial ubiquinones on the settling of barnacle, Balanus amphitrite . Experientia , 51 : 153 – 155 .
- Krchnak , V , Weichse , A S , Cabe , D , Flegelova , Z and Leb , M . 1996 . Structurally homogeneous and heterogeneous synthetic combinatorial libraries . Mol Divers , 1 : 149 – 164 .
- Little , B J and Wagner , P A . 1997 . Spatial relationships between bacteria and mineral surfaces . Rev Mineral , 35 : 123 – 160 .
- McCleland , K , Milne , P J , Lucieto , F R , Frost , C , Brauns , S C , Van De Venter , M , Du Plessiss , J and Dyason , K . 2004 . An investigation into the biological activity of the selected histidine-containing diketopiperazines cyclo(His-Phe) and cyclo(His-Tyr) . J Pharm Pharmacol , 56 : 1143 – 1153 .
- Milne , P J , Hunt , A L , Rostoll , K , van der Walt , J J and Graz , C JM . 1998 . The biological activity of selected cyclic dipeptides . J Pharm Pharmacol , 50 : 1331 – 1335 .
- Mitova , M , Tommonaro , G , Hentschel , U , Müller , W EG and De Rosa , S . 2004 . Exocellular cyclic dipeptides from a Ruegeria strain associated with cell cultures of Suberites domuncula . Mar Biotechnol , 6 : 95 – 103 .
- Olguin-Uribe , G , Abou-Mansour , E , Boulander , A , Débard , H , Francisco , C and Combaut , G . 1997 . 6-bromoindole-3-carbaldehyde, from an Acinetobacter sp. bacterium associated with the ascidian Stomozoa murrayi . J Chem Ecol , 23 : 2507 – 2521 .
- Prasad , C . 1995 . Bioactive cyclic dipeptides . Peptides , 1 : 151 – 164 .
- Rittschof , D . 1999 . “ Fouling and natural products as antifoulants ” . In Recent advances in marine biotechnology , Edited by: Fingerman , M , Nagabhushanam , R and Thompson , M F . Vol. 3 , 245 – 257 . Enfield, USA : Science Publishers Inc. . Biofilms, bioadhesion, corrosion, and biofouling
- Schmitz , F J , Vanderah , D J , Hollenbeak , K H , Enwall , C E , Gopichand , Y , Sengupta , P K , Hossain , M B and van der Helm , D . 1983 . Metabolites from the marine sponge Tedania ignis. A new atisanediol and several known diketopiperazines . J Org Chem , 48 : 3941 – 3945 .
- Ström , K , Sjögren , J , Broberg , A and Schnürer , J . 2002 . Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo- (L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid . Appl Environ Microbiol , 68 : 4322 – 4327 .
- Venter , J C , Remington , K , Heidelberg , J F , Halpern , A L , Rusch , D , Eisen , J A , Wu , D , Paulsen , I , Nelson , K E , Nelson , W , Fouts , D E , Levy , S , Knap , A H , Lomas , M W , Nealson , K , White , O , Peterson , J , Hoffman , J , Parsons , R , Baden-Tillson , H , Pfannkoch , C , Roger , Y H and Smith , H O . 2004 . Environmental genome shotgun sequencing of the Sargasso Sea . Science , 304 : 66 – 74 .
- Wahl , M . 1989 . Marine epibiosis. I. Fouling and antifouling: some basic aspects . Mar Ecol Prog Ser , 58 : 175 – 189 .
- Yebra , D M , Kill , S and Dam-Johansen , K . 2004 . Antifouling technology: past, present and future steps towards efficient and environmentally friendly antifouling coatings . Prog Org Coat , 50 : 75 – 104 .
- Young , P E , Madison , V and Blout , E R . 1976 . Cyclic peptides. 15. Lanthanide-assisted 13C and 1H NMR analysis of preferred side-chain rotamers in proline-containing cyclic dipeptides . J Am Chem Soc , 98 : 5365 – 5371 .
- Zar , J H . 1996 . Biostatistical analysis. , 3rd ed. , Upper Saddle River, NJ, , USA : Prentice Hall .
Appendix
The 16s rDNA sequence of the deep-sea strain of the bacterium
GTCGAACGATGAACCGCTTTCGGGCGGGGATTAGTGGCGAACGGGTGAGTAACACGTGGGCAATCTGCCCTGCACTCTGGGACAAGCCCTGGAAACGGGGTCTAATACCGGATATGACCGTCTGCCGCATGGTGGATGGTGTAAAGCTCCGGCGGTGCAGGATGAGCCCGCGGCCTATCAGCTTGTTGGTGAGGTAGTGGCTCACCAAGGCGACGACGGGTAGCCGGCCTGAGAGGGCGACCGGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTGGGGAATATTGCACAATGGGCGAAAGCCTGATGCAGCGACGCCGCGTGAGGGATGACGGCCTTCGGGTTGTAAACCTCTTTCAGCAGGGAAGAAGCGAAAGTGACGGTACCTGCAGAAGAAGCGCCGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGGCGCAAGCGTTGTCCGGAATTATTGGGCGTAAAGAGCTCGTAGGCGGCTTGTCACGTCGGTTGTGAAAGCCCGGGGCTTAACCCCGGGTCTGCAGTCGATACGGGCAGGCTAGAGTTCGGTAGGGGAGATCGGAATTCCTGGTGTAGCGGTGAAATGCGCAGATATCAGGAGGAACACCGGTGGCGAAGGCGGATCTCTGGGCCGATACTGACGCTGAGGAGCGAAAGCGTGGGGAGCGAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGGTGGGCACTAGGTGTGGGCAACATTCCACGTTGTCCGTGCCGCAGCTAACGCATTAAGTGCCCCGCCTGGGGAGTACGGCCGCAAGGCTAAAACTCAAAGGAATTGACGGGGGCCCGCACAAGCGGCGGAGCATGTGGCTTAATTCGACGCAACGCGAAGAACCTTACCAAGGCTTGACATACACCGGAAACGTCTGGAGACAGGCGCCCCCTTGTGGTCGGTGTACAGGTGGTGCATGGCTGTCGTCAGCTCGTGTCGTGAGATGGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTTGTCCCGTGTTGCCAGCAGGCCCTTGTGGTGCTGGGGACTCACGGGAGACCGCCGGGGTCAACTCGGAGGAAGGTGGGGACGACGTCAAGTCATCATGCCCCTTATGTCTTGGGCTGCACACGTGCTACAATGGCCGGTACAATGAGCTGCGATACCGTGAGGTGGAGCGAATCTCAAAAAGCCGGTCTCAGTTCGGATTGGGGTCTGCAACTCGACCCCATGAAGTCGGAGTCGCTAGTAATCGCAGATCAGCATTGCTGCGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACGTCACGAAAGTCGGTAACACCCGAAGCCGGTGGCCCAACCCCTTGTGGGAGGGAGCTTGTCGAAGGTGGGACTGGCGATTGGGACGAAGTCGT
1423/1425 99% similar to Streptomyces fungicidicus strain YH04 (Accession no. AY636155)