Abstract
The presence of adult barnacles of Fistulobalanus pallidus (Darwin) and Fistulobalanus albicostatus (Pilsbry) attached to field-readable plastic leg rings on the Lesser Black-backed Gull Larus fuscus in Northern Europe is reported. L. fuscus is a long-distance palaearctic migrant, breeding in temperate areas spreading widely over inland and marine habitats outside the breeding season. The species is known to perform long-distance migration to Africa and the Middle East. Combining present knowledge on the birds' migratory pattern and the home range of the barnacle species, it is concluded that the cypris larvae of F. pallidus must have settled in African waters, whereas the area where F. albicostatus settled on the bird leg rings is less certain. The barnacles were of adult size and must thus have been attached for a period of no less than 2 months. More than 30 individual barnacles could occur together on a single field-readable plastic leg ring. The barnacles could therefore, if ported alive to a new area, reproduce successfully and thus either introduce the species or genetically affect other native populations. This may pose a new and wholly unexpected transportation pathway for barnacles as invasive species.
Introduction
Stalked and sessile barnacles (Cirripedia; Thoracica) are important marine animals. They are abundant and key components in many marine ecosystems, notably the upper rocky intertidal, and their biology with pelagic larvae and sessile, suspension feeding adults means that they are among the most important marine fouling organism. As a result of human activity, barnacles are often introduced to new areas which they could not reach by natural dispersal of larvae from their home range. This can occur either by transporting larvae in ballast water tanks or by adults attached to ships' hulls (Foster and Willan Citation1979; Piola and Johnston Citation2008; Yamaguchi et al. Citation2009). Dispersal by ballast water can introduce barnacles through barriers, such as freshwater, where the adults cannot survive. Amphibalanus improvisus was introduced to the Caspian Sea by ballast water transport through the freshwater filled Volga-Don Canal (Riedel et al. Citation2006) and Amphibalanus amphitrite was introduced to the Salton Sea in S. California by similar means (Newman and Abbott Citation1980). [For alternative generic names of A. improvisus and A. amphitrite, see Clare and Høeg (Citation2008) and Carlton and Newman (Citation2009).] Several species of barnacle, including A. amphitrite, Megabalanus tintinnabulum and Megabalanus coccopoma have been dispersed by shipping throughout the subtropic and tropic waters of the world due to increasing marine traffic (Yamaguchi et al. Citation2009). However, the most notable example of dispersal to an entirely new area is the introduction of the Australian Austrominius modestus (formerly Elminius modestus) to European temperate waters in the middle of the twentieth century (see Bishop (Citation1947); O'Riordan et al. (Citation2009)). This process was followed closely and the species is now a naturalized component of the local European intertidal systems. The dispersal was possible only because of the fast sailing of modern ships through hot tropical waters, which this species cannot endure for extended periods. Transport of adult barnacles over land barriers not linked by water has not previously been believed possible.
Lesser black-backed gulls, Larus fuscus, are Palaearctic migrants breeding in temperate areas and spreading widely over inland and marine habitats in the non-breeding period (). Ring-recoveries have shown that the L. fuscus fuscus populations breeding in the Baltic Sea and Northern Scandinavia perform a long-distance migration to Africa and the Middle East (Bakken et al. Citation2003; Bønløkke et al. Citation2006; Pütz et al. Citation2007, 2008; Fransson et al. Citation2008). Furthermore, satellite tracking has shown that L. fuscus is able to migrate long distances in a short period of time. One bird traveled non-stop from the Mediterranean Sea to Lake Victoria in 92 h (∼38 km h−1) (Bønløkke et al. Citation2006; Rahbek (unpublished data)). L. fuscus is a generalist feeder and feeds in many different terrestrial and marine habitats eg estuary soft shores, marshes, and mangroves (Cramp and Simmons Citation1983).
Figure 1. (A) F. pallidus from Gambia, West Africa (Stn 155, Atlantide-Exp. West Africa, Zoological Museum, Denmark) showing the variations in the colour patterns, from tinged purple striations to white. (B) Shell of F. albicostatus collected from Taiwan, showing the pronounced white ridges on surface. (C) A clump of F. albicostatus on a tree trunk of a mangrove in Taiwan; note the shell surface has pronounced white ridges. (D) F. albicostatus (enlarged in E) to the surface of which the another individual was attached, on a field-readable plastic leg rings on L. fuscus (photo: Hannu Koskinen). (E) L. fuscus, ringed ‘CU19’ (10 June 2007), seen at Tara on this date only. Ringed as a pullus, 3 July 2005 at Hauho. (F) L. fuscus individual ‘CU19’, 1 year older as a third-summer (4cy) (6 July 2008). Note the barnacles have mostly disappeared, and only some basal parts remain visible. (G, H) Third summer (4cy) L. fuscus, ringed ‘CNKH’, with several F. pallidus individuals (20 July 2006). ‘CNKH’ was seen 16.07 to 3 September 2006 at Tarastenjärvi (but not during 2003–2005 and not after 2006). Ringed as a pullus at Valkeakoski on 27 June 2003. (I) Second summer L. fuscus, ringed ‘CZ21’ (25 May 2007) with F. albicostatus attached on leg ring. Ringed as pullus at Luopioinen 04-07-2005. (J) L. fuscus, ‘CZ21’ at 12 June 2007. (K, L) L. fuscus ‘C32A’ individual at 7 June 2009, with F. pallidus attached. (M) Third summer L. fuscus (4cy), ringed ‘CUCE’, with one F. pallidus individual (23 May 2009). (N) Adult L. fuscus with F. albicostatus attached at leg ring at Lemmingsvaer, Tromsø, N. Norway (30 July 2009). Photographed while flying over the Froholm breeding colony. (O) L. fuscus individuals ‘White CX20’ with a clump of F. pallidus on leg ring at Tarastenjärv dump, SW Finland 10 April 2010. This White CX20 leg ring visited Tarastenjärv dump annually, also in the summer of 2009, but without barnacles attached. Insert shows the opposite view of the leg ring.
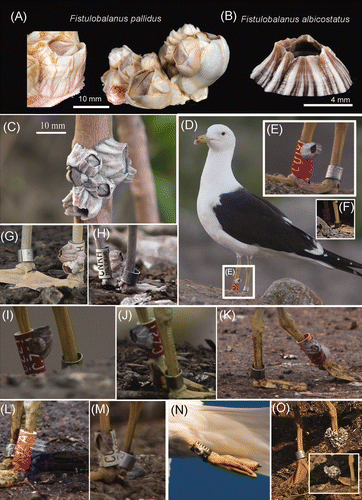
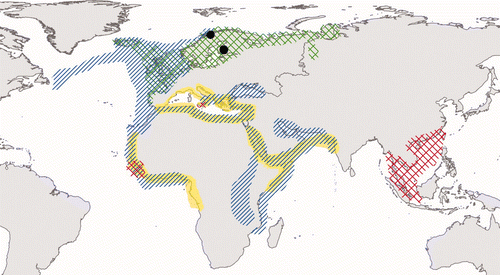
Figure 2. Breeding (green chequered area) and non-breeding (blue striped area) range of the L. fuscus (following Cramp and Simmons (Citation1983)). The recorded distribution of F. pallidus (yellow area) and F. albicostatus (red chequered area; see Stubbings (Citation1967); Newman and Ross (Citation1976); Zevina and Litvinova (Citation1970); Henry and Mclaughlin (Citation1975)) are also presented. Black dots indicate the two locations where L. fuscus were found carrying barnacles on the field-readable plastic rings ( ).
This paper reports on a new epibiotic substratum for intertidal barnacles, field-readable plastic leg rings on the migratory L. fuscus, and the potential for long-distance dispersal of barnacle species by air.
Materials and methods
As part of the Finnish and Norwegian ringing schemes L. fuscus were ringed with field-readable plastic leg rings and subsequently recovered by visual observations. This method is applied to increase the number of recoveries otherwise limited by re-trapping of the individuals to achieve the ring information (traditional metal rings are used; Bakken et al. (Citation2003)). In this study, information is presented on L. fuscus individuals observed and photographed in southern Finland (61.33N, 23.59E) and northern Norway (69.03N, 16.20E) with barnacles attached to the field-readable leg rings. From the digital photographs, the leg rings were further magnified in the computer to facilitate barnacle species identification, based on morphology of external parietal plates. Comparative materials of balanid barnacles from African waters were examined from the collections of the Zoological Museum, Copenhagen, Denmark ( ) and Asian barnacles were collected from Taiwan ( and ; also see Chan et al. (Citation2009)). From the photographs the rostral-carinal basal diameter of the barnacles attached to the bird leg ring was measured using the computer software, Sigma Scan Pro (methodology follows Chan et al. (Citation2007a,Citationb)). Calibrations were based on the known length of the leg ring (25 mm). The number of barnacles per leg ring was also recorded.
Results
Barnacles were observed attached to field-readable plastic rings on L. fuscus in the Baltic Sea and in northern Norway ( , ). A total of nine barnacle specimens was identified on the leg rings of seven birds, excepting a single bird individual (White CX20) that had a large clump of barnacles (> 30 individuals) attached on one of the leg ( ). L. fuscus usually spend their first year away from the breeding area (Cramp and Simmons Citation1983) and the individuals included in this study were not seen in Finland and Norway before their second- and third-summers, indicating that they acquired these barnacles during their stay at wintering grounds. The number of barnacles attached on the birds' leg rings ranged from 1 to 3 per leg ring, excepting the individual with one leg ring carrying > 30 barnacles ( ; ), and the basal (rostro-carinal) diameter ranged from 11.0 to 20.1 mm.
Table 1. Seven L. fuscus individuals observed with barnacles attached to their field-readable plastic rings, including information on the age, date of observation/photography and notes on the barnacles on the plastic rings.
Based on the appearance of the barnacle parietal wall plates, they were identified as two species of the genus Fistulobalanus (see and ; ). Fistulobalanus pallidus (on bird individuals CNKH, CUCE, White CX20 and C32A; , H, L, M, and O; ) had smooth conical shells and the color pattern varied from white, tinged purple or with faint purple stripes (Harding Citation1962; Henry and Mclaughlin Citation1975; ). Fistulobalanus albicostatus (on bird individuals CZ21, CU19, 1010; , , , , , and ; ) is characterized by having strong white ribbings on the surface of the parietal plates and occasionally with purple strips or are pale grey in color.
Discussion
Darwin (1859) suggested that migratory water birds can act as a long-distance transport for aquatic invertebrates and plants. Recent studies indicate birds can play a major role in the expansion of exotic species (Green and Figuerola Citation2005; Gittenberger et al. Citation2006). Invertebrates are often transported by birds by staying in their digestive tracts. External long-distance transport of aquatic invertebrates by birds has been reported for bivalves clipped onto birds' legs and for bryozoans attached to their plumage (Green and Figuerola Citation2005). This study is the first report of long-distance dispersal of barnacles by birds.
The barnacles identified on the rings are not European species. F. pallidus is common in mangroves and estuary waters (Sandison Citation1966a,Citationb; Sandison and Mill Citation1966; Stubbings Citation1967). It occurs in west (Stubbings Citation1967) and east African waters, the north Indian Ocean (including the Red Sea, Zevina and Litvinova (Citation1970)), the Mediterranean Sea (see review in Henry and Mclaughlin (Citation1975)), South West Australia, the Gulf of Mexico and Argentina (Newman and Ross Citation1976). F. albicostatus is common in mangroves ( ), estuary waters and also as a fouling species on ships' bottoms (Chan et al. Citation2006) and occurs in Japan, S. China and Malaysian waters (Newman and Ross Citation1976; Chan (unpublished data); ). F. albicostatus has also been recorded in Catania, the Mediterranean sea and at Senegal, West Africa by Kolosvary (Citation1943), but Stubbings (Citation1967) did not find this species in west Africa and suggested that such records need further verification.
Settlement of barnacles appears to have occurred when L. fuscus individuals were in the sub-tropical and tropical non-breeding area, during feeding or resting, with their legs immersed in the water for considerable periods of time. Prior to settlement, barnacle cyprids actively explore the substratum walking on the antennules, during which period they can react to a variety of physical, chemical, and biological properties and use these to select where and when to attach permanently (Crisp Citation1979; Clare Citation1995; Walker Citation1995; Lagersson and Høeg Citation2002; Aldred and Clare Citation2008; Bielecki et al. Citation2009; ). This exploratory phase of the cyprids can last for minutes, but they can remain and survive on a field-readable plastic leg ring even if the bird alights and flies for a short distance as long as the larva remains reasonably wet. A cemented cyprid cannot detach and will also survive provided desiccation does not become a problem (see Foster (Citation1971)). Settled Semibalanus cyprids have 50% mortality in their population when maintained in 0% humidity and 15°C air temperature for 7 h (Foster Citation1971).
The largest of the barnacles observed on the field-readable plastic leg rings had almost certainly grown to sexual maturity. The biggest rostral-carinal basal diameter of ring settled barnacles was 20 mm. The age of barnacles attached could be <1 year. The leg ring White CX20 ( ) carried no barnacles in the photograph from the summer of 2009, but it had a whole clump of barnacles in the photograph from April 2010 ( ). In Nago harbour, W. Africa, settlement of F. pallidus occurs in both summer and winter. The post-settlement growth rate during winter months ranges from 2 to 4 mm per month (Sandison Citation1966a,Citationb), and F. pallidus can therefore grow to maturity in < 1 year. Barnacles in lower salinity water have a higher growth rate (Sandison Citation1966a,Citationb). Ring-recovery data indicate that Northern European L. fuscus fuscus stay in tropical and sub-tropical habitats for at least 1–2 months (Bønløkke et al. Citation2006; Fransson et al. Citation2008) during which period the birds will have regularly frequented waters suitable for the barnacle and long enough for sufficient suspension feeding to take place. Balanomorphan barnacles are cross fertilizing hermaphrodites that normally can reproduce only if settled at least two together within reach of their exceptionally long penises. Self-fertilization is known for few species (Barnes and Crisp Citation1956; Landau Citation1976; Furman and Yule Citation1990), but could also happen for Fistulobalanus species. In the present study, two or more individuals, or even a large clump of barnacles (> 30 individuals) were observed settled on the rings and such small groups of breeding barnacles are similarly common on their natural substratum in the mangroves ( ). This suggests that barnacles attached to a field-readable plastic leg ring on birds cannot only reach adult size but also reproduce and release larvae into the water. The observation of multiple specimens attached close together on the field readable leg rings furthermore shows that the gregarious settlement common to barnacles occurs also on this substratum.
The barnacles growing on the field-readable plastic rings must obviously have been out of the water frequently. Species of Fistulobalanus can easily survive out of water for at least 6 h at low tide and, by comparison with other intertidal barnacles, very likely much longer (Foster Citation1971; Chan et al. Citation2006). For example, ∼ 50% of a Semibalanus balanoides population suffered mortality when exposed to 0% relative humidity, 10°C air temperature for 192 h (Foster Citation1971). Staying for short periods in freshwater would not kill the barnacles, since they can close the opercular valves to keep the mantle water isolated from the outside environment (Chan et al. Citation2001). This enables balanomorphans to survive even in the harsh environment of the splash zone, where they are not regularly submerged at each high tide and where they are occasionally exposed to either very long periods of desiccation or immersion in fresh water by rain showers. Intertidal barnacles are therefore eminently suited to survive on field-readable plastic leg rings of marine birds, where they are often and at random exposed to both desiccation (during flight and stays on land) and immersion in fresh water. In the present case it is unknown if the barnacles survived transportation all the way to the Baltic. If so, they most likely did not survive long after arrival because the birds on which they occurred were breeding in pure freshwater localities. Several leg rings with empty barnacle shells were observed, suggesting that some barnacles cannot survive well upon arrival in the Baltic ( , , , and ). The cold winter regime would also prevent establishment of a permanent breeding population even though Fistulobalanus larvae are known to survive the salinity conditions prevalent in the Baltic Sea (Chan and Leung Citation2007). Whether or not live barnacles reached the Baltic, the observation shows that specimens settled on field-readable plastic leg rings can survive and grow for several months. During this period they could be transported considerable distances through air and survive and breed if they arrived in suitable habitats. This offers a new and unexpected means for the dispersal of barnacles that can even pass land barriers. This might conceivably result either in the introduction of unwanted marine immigrants or genetic exchange between populations otherwise separated by climatic or geographic barriers. Such ‘air’ transport of barnacles differs from the common invasive pathway via ballast water and ship fouling in being, at present, beyond human control measures.
Acknowledgements
The authors are grateful to Morten Helberg for data from Norway, and Markku Kangasniemi for additional photos from Finland and Jouko Astor, Risto Juvaste for ringing the birds. JTH gratefully acknowledges grants from the Danish Natural Science Research Council (FNU), the Carlsberg Foundation and from the European Union FP6 SYNTHESYS programme. BKKC acknowledge grants from the Career Development Award in the Academia Sinica, Taiwan (AS-98-CDA-L15). APT acknowledges the Danish National Research Foundation for support to the Center for Macroecology, Evolution and Climate.
References
- Aldred , N and Clare , A S . 2008 . The adhesive strategies of cyprids and development of barnacle resistant marine coatings . Biofouling , 24 : 351 – 363 .
- Bakken , V , Runde , O and Tjørve , E . 2003 . Norsk ringmærkningsatlas , Vol. 1 , Stavanger : Stavanger Museum .
- Barnes , M and Crisp , D J . 1956 . Evidence of self-fertilization in certain species of barnacles . J Mar Biol Assoc UK , 35 : 631 – 639 .
- Bielecki , J , Chan , B KK , Høeg , J T and Sari , A . 2009 . Antennular sensory organs in cyprids of balanomorphan cirripedes: standardizing terminology using Megabalanus rosa . Biofouling , 25 : 203 – 214 .
- Bishop , M WH . 1947 . Establishment of an immigrant barnacle in British coastal waters . Nature , 159 : 501 – 502 .
- Bønløkke , J , Madsen , J , Thorup , K , Bjerrum , M and Rahbek , C . 2006 . Dansk Trækfugleatlas , Humlebæk : Rhodos .
- Carlton , J and Newman , N . 2009 . Reply to Clare and Høeg 2008. Balanus amphitrite or Amphibalanus amphitrite? A note on barnacle nomenclature . Biofouling , 25 : 77 – 80 .
- Chan , B KK and Leung , P TY . 2007 . Antennular morphology of the cypris larvae of the mangrove barnacle Fistulobalanus albicostatus (Cirripedia: Thoracica: Balanomorpha) . J Mar Biol Assoc UK , 87 : 913 – 915 .
- Chan , B KK , Morritt , D and Williams , G A . 2001 . Effect of salinity and recruitment on the distribution of Tetraclita squamosa and Tetraclita japonica (Cirripedia: Balanomorpha) in Hong Kong . Mar Biol , 138 : 999 – 1009 .
- Chan , B KK , Tsang , L M and Chu , K H . 2007a . Morphological and genetic differentiation of the acorn barnacle Tetraclita squamosa (Crustacea, Cirripedia) in East Asia and description of a new species of Tetraclita . Zool Scr , 36 : 79 – 91 .
- Chan , B KK , Tsang , L M and Chu , K H . 2007b . Cryptic diversity of Tetraclita squamosa complex (Crustacea, Cirripedia) in Asia: description of a new species from Singapore . Zool Stud , 46 : 46 – 56 .
- Chan , B KK , Prabowo , R E and Lee , K-S . 2009 . Crustacean fauna of Taiwan: barnacles, Vol 1 – Cirripedia: Thoracica excluding the Pyrgomatidae and Acastinae , 298 Taiwan : National Taiwan Ocean University Press .
- Chan , B KK , Morritt , D , De Pirro , M , Leung , K MY and Williams , G A . 2006 . Summer mortality: effects on the distribution and abundance of the acorn barnacle Tetraclita japonica on tropical shores . Mar Ecol Prog Ser , 328 : 195 – 204 .
- Clare , A S . 1995 . “ Chemical signals in barnacles: old problems, new approaches ” . In New frontiers in barnacle evolution. Crustacean issues 10 , Edited by: Schram , F R and Hoeg , J T . 49 – 67 . Rotterdam : AA Balkema .
- Clare , A S and Høeg , J T . 2008 . Balanus amphitrite or Amphibalanus amphitrite? A note on barnacle nomenclature . Biofouling , 24 : 55 – 57 .
- Cramp , S and Simmons , K EL . 1983 . Birds of Europe, the Middle East and North Africa: the birds of the western Palearctic , Vol. 3 , New York : Oxford University Press .
- Crisp , D J . 1979 . Dispersal and re-aggregation in sessile marine invertebrates particularly barnacles . Syst Ass Spec , 11 : 319 – 328 .
- Darwin , C . 1859 . On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life , London : John Murray .
- Foster , B A . 1971 . Desiccation as a factor in the intertidal zonation of barnacles . Mar Biol , 8 : 12 – 29 .
- Foster , B A and Willan , R C . 1979 . Foreign barnacles transported to New Zealand on an oil platform . NZ J Mar Freshwater Res , 13 : 143 – 149 .
- Furman , E R and Yule , A B . 1990 . Self-fertilization in Balanus improvisus . J Exp Mar Biol Ecol , 144 : 235 – 239 .
- Fransson , T , Österblom , H and Hall-Karlsson , S . 2008 . Swedish bird ringing atlas , Vol. 2 , Stockholm : Swedish Museum of Natural History .
- Gittenberger , E , Groenenberg , D SJ , Kokshoorn , B and Preece , R C . 2006 . Molecular trails from hitch-hiking snails . Nature , 439 : 409
- Green , A J and Figuerola , J . 2005 . Recent advances in long-distance dispersal of aquatic invertebrates via birds . Divers Distrib , 11 : 149 – 156 .
- Harding , J P . 1962 . Darwin's type specimens of varieties of Balanus amphitrite . Bull Br Mus Nat Hist Zool , 9 : 273 – 296 .
- Henry , D P and Mclaughlin , P A . 1975 . The barnacles of the Balanus amphitrite complex (Cirripedia, Thoracia) . Zool Verh , 141 : 1 – 254 .
- Kolosvary , G . 1943 . Cirripedia Thoracica in der Sammlung des ungarischen National-Museums . Ann Hist Nat Mus Nat Hung , 36 : 67 – 120 .
- Landau , M . 1976 . A comment on self-fertilization in the barnacle Balanus eburneus Gould (Cirripedia Thoracica) . Crustaceana , 30 : 105 – 106 .
- Lagersson , N and Høeg , J T . 2002 . Settlement behavior and antennulary biomechanics and in cypris larvae of Balanus amphitrite (Crustacea: Thecostraca: Cirripedia) . Mar Biol , 141 : 513 – 526 .
- Newman , W A and Ross , A . 1976 . Revision of the balanomorph barnacles; including a catalog of species . Mem San Diego Soc Nat Hist , 9 : 1 – 108 .
- Newman , W A and Abbott , D P . 1980 . “ Cirripedia: the barnacles ” . In Intertidal invertebrates of California , Edited by: Morris , R J , Abbott , D P and Haderlie , E C . 504 – 535 . Stanford, CA : Stanford University Press .
- O'Riordan , R M , Culloty , S , Davenport , J and McAllen , R . 2009 . Increases in the abundance of the invasive barnacle Austrominius modestus on the Isle of Cumbrae, Scotland . J Mar Biol Assoc UK 2 – Biodiversity Records , 6638 : 1 – 4 .
- Piola , R F and Johnston , E L . 2008 . The potential for translocation of marine species via small-scale disruption of antifouling surfaces . Biofouling , 24 : 157 – 161 .
- Pütz , K , Rahbek , C , Saurola , P , Pedersen , K T , Juvaste , R and Helbig , A J . 2007 . Satellite tracking of the migratory pathways of first-year Lesser Black-backed Gulls Larus fuscus departing from the breeding grounds of different subspecies . Vogelwelt , 128 : 141 – 148 .
- Pütz , K , Helbig , A J , Pedersen , K T , Rahbek , C , Saurola , P and Juvaste , R . 2008 . From fledging to breeding: long-term satellite tracking of the migratory behaviour of a Lesser Black-backed Gull Larus fuscus intermedius . Ringing Migr , 24 : 7 – 10 .
- Riedel , F , Audzijonyte , A and Mugue , N . 2006 . Aliens associating with Caspian Sea endemic bivalves . Biol Invasions , 8 : 1067 – 1071 .
- Sandison , E E . 1966a . The naupliar stages of Balanus pallidus stutsburi Darwin and Chthamalus aestuarii Stubbings (Cirripedia Thoracica) . Crustaceana , 13 : 161 – 174 .
- Sandison , E E . 1966b . The effect of salinity fluctuation on the life cycle of Balanus pallidus stutsburi Darwin in Lagos Harbour, Nigeria . J Anim Ecol , 35 : 363 – 378 .
- Sandison , E E and Hill , M B . 1966 . The distribution of Balanus pallidus stutsburi Darwin, Gryphaea gasae (Adanson) Dautzenberg), Mercierella enigmatica Fauvel and Hydrodes uncinata (Philippi) in relation to salinity in Lagos Harbour and adjacent creeks . J Anim Ecol , 35 : 235 – 250 .
- Stubbings , H G . 1967 . The Cirriped fauna of tropical west Africa . Bull Br Mus Nat Hist Zool , 15 : 20 – 317 .
- Walker , G . 1995 . “ Larval settlement: historical and future perspectives ” . In New frontiers in barnacle evolution. Crustacean Issues 10 , Edited by: Schram , F R and Hoeg , J T . 69 – 85 . Rotterdam : AA Balkema .
- Yamaguchi , T , Prabowo , R W , Ohshiro , Y , Shimono , T , Jones , D , Kawai , H , Otano , M , Inagawa , S , Akaya , T and Tamura , I . 2009 . The introduction to Japan of the Titan barnacle,Megabalanus coccopoma (Darwin, 1854) (Cirripedia: Balanomorpha) and the role of shipping in its translocation . Biofouling , 25 : 325 – 333 .
- Zevina , G B and Litvinova , N M . 1970 . “ Supplement to the fauna of the barnacles (Cirripedia, Thoracica) of the Red Sea ” . In Biology of the sea Edited by: Kovalefsky , A O . Acad Sci UKR SSR: Inst Biol Southern Seas. p. 172–181