Abstract
The adhesional properties of contaminating particles of scales of various lengths were investigated for a wide range of micro- and nanostructured insect wing cuticles. The contaminating particles consisted of artificial hydrophilic (silica) and spherical hydrophobic (C18) particles, and natural pollen grains. Insect wing cuticle architectures with an open micro-/nanostructure framework demonstrated topographies for minimising solid–solid and solid–liquid contact areas. Such structuring of the wing membranes allows for a variety of removal mechanisms to contend with particle contact, such as wind and self-cleaning droplet interactions. Cuticles exhibiting high contact angles showed considerably lower particle adhesional forces than more hydrophilic insect surfaces. Values as low as 3 nN were recorded in air for silica of ∼28 nm in diameter and <25 nN for silica particles 30 μm in diameter. A similar adhesional trend was also observed for contact with pollen particles.
Introduction
Many naturally occurring nano-structures have functional efficiencies which are superior to man-made technologies. One of the most striking nano-composite materials is the insect cuticle (Vincent and Wegst Citation2004). Recently micro- and nanostructures found on insect cuticles have been shown to exhibit a range of properties such as superhydrophobicity, directed wetting, ultra-low adhesion and self-cleaning (Parker and Lawrence Citation2001; Watson et al. Citation2008, Citation2010a, Citation2011a; Hu et al. Citation2011).
The field of self-cleaning surfaces generally falls into two categories: hydrophobic and hydrophilic. The mechanism of removing contaminants is by rolling droplets (superhydrophobic) and sheeting water (hydrophilic) (Blossey Citation2003). The self-cleaning action on hydrophobic surfaces is a consequence of their high water contact angles where almost spherical droplets form, which can easily roll away carrying foreign particles off the surface (Blossey Citation2003; Bhushan and Jung Citation2011; Liu and Jiang Citation2011). This form of self-cleaning via rolling droplets is thought to be one of the principle mechanism for removing contaminants from natural and artificial surfaces (Blossey Citation2003; Bhushan et al. Citation2009; Koch and Barthlott Citation2009; Bhushan and Jung Citation2011; Liu and Jiang Citation2011).
If the contacting droplets are small in size they may coalesce into larger droplets and then roll off the surface (Furstner and Barthlott Citation2005). The kinetic energy from droplet impacts has also been shown to be effective in removing contaminants from superhydrophobic surfaces (Furstner and Barthlott Citation2005; Bhushan et al. Citation2009). For the droplets to roll away easily, a low hysteresis is required. The lotus leaf and many other plant surfaces demonstrate this property and have provided a model for many self-cleaning materials (Barthlott and Neinhuis Citation1997; Sun et al. Citation2005; Liu and Jiang Citation2011). These leaves possess a micro/nano architecture which reduces the contact area of particles with the surface and thus physical adhesion forces via van der Waals are also reduced. As the water droplet rolls over the surface, particles/contaminants are absorbed into or adhered to the water droplet surface via the interaction of stronger capillary forces (Koch and Barthlott Citation2009).
The need for insects to maintain functionality upon exposure to contaminants suggests that there may be some structural components and mechanisms to aid the removal of particles. Additional weight due to contamination (water and/or contaminating particles) can potentially have a detrimental effect on the insects’ flight capabilities (Wagner et al. Citation1996). Wagner et al. (Citation1996) demonstrated a correlation between contaminability, wettability and the wing area of many insects. They exposed insect specimens to a fine silica dust and fogging conditions. The study showed that large winged insects such as butterflies exhibited contamination resistant surfaces with very unwettable wings, while smaller winged insects (eg flies) showed the opposite trend. In addition, insects with a very high wing surface area to body mass ratio (WSA/BM) showed lower contamination.
The atmospheric environment surrounding insects contains a multitude of biological and anthropogenic particulate matter that can potentially contaminate the wing cuticle, eg silica dust and plant material. Pollen grains are the most abundant component amongst the floating particles in the air (aeroplankton) surrounding most terrestrial organisms including human beings (Linskens and Cresti Citation2000). It has been known for some time that pollens and mould spores, or at least components of them, can trigger symptoms of allergic respiratory diseases such as asthma and hay fever (Yli-Panula and Rantio-Lehtimäki Citation1995; Linskens and Cresti Citation2000; D’Amato Citation2002; Cariñanos et al. Citation2004; Burney et al. Citation2008). A rise in respiratory allergic symptoms has also been attributed to pollen fragments which can adhere to other pollutant surfaces (for example diesel exhaust particles). Thus, the interaction of pollen grains with various surfaces is of interest in terms of distribution, transport and capture of these pollen allergens. Interestingly, recent studies have shown that pollen of genetically engineered and modified plants has the potential to kill certain insects (Losey et al. Citation1999).
Other potential airborne contaminants can originate from soils. Naturally occurring silicate particles composed principally of silicon dioxide (SiO2) such as quartz can comprise as much as 90–95% of the sand and silt fraction of soil (Smith and Lee Citation2003), and present the highest health risks (Ormstad et al. Citation1997; Smith and Lee Citation2003). Respirable quartz is commonly found in soil dust, although weathering and chemical reactions may make it less fibrogenic than the freshly fractured quartz found in occupational dust from quarrying and sandblasting (Ormstad et al. Citation1997). Exposure to, and inhalation of, a combination of various air-borne particulates have been found to contribute to various diseases including lung cancer (Knaapen et al. Citation2004). Silica dust has not only been linked to lung disease, but also as a contributor to lung cancer, silicosis, pulmonary tuberculosis, emphysema and immunologic reaction (Ding et al. Citation2002; Hnizdo and Vallyathan Citation2003). Thus, like pollen, enhanced mechanisms for shedding silica particles is of great interest. The capture and isolation of these particles (eg silica and pollen) is advantageous in some circumstances (eg air-conditioning filters and cleaning systems), but in most human habitats, adhesion is highly undesirable (furniture, flooring, fixtures and clothing).
As well as the health concerns associated with silica and pollen, these bodies also represent an ubiquitous source of hydrophobic and hydrophilic particles which come into contact with insects and can potentially foul the surface. Fouling in both the marine and terrestrial environments (especially where water can accumulate) is a significant economic and environmental problem (eg Yebra et al. Citation2004; Schultz et al. Citation2011). Until recently, biofouling control has focused on biocidal antifouling (AF) coatings, but there is now an urgency to develop effective non-biocidal AF alternatives. Increasingly, non-biocidal solutions to biofouling are being sought from nature (eg de Nys and Steinberg Citation2002; Scardino et al. Citation2009; Scardino and de Nys Citation2011). New surface-based technologies in particular are being inspired from biomimetics (eg the Lotus leaf) as physical surface effects, or surface bound chemical signals, have the advantage of eliminating effects on non-target organisms (Genzer and Efimenko Citation2006; Ralston and Swain Citation2011; Scardino and de Nys Citation2011). While these superhydrophobic surfaces may provide useful templates for biofouling prevention, other structuring such as hydrophilic micro-nano patterned surfaces where contact with water is preferred over biological interactions may also guide the development of new AF coatings (Marmur Citation2006).
In this study the micro-/nano-adhesion of contaminating particles (silica, C18 and a variety of pollen grains) on a number of insect species displaying a range of cuticle nanostructures and wetting properties (superhydrophobic and hydrophilic) have been measured. The focus was on insect species with large wings and/or a high WSA/BM value and/or a close association with water in their life history. Insect species were also chosen based on the differing topographical landscape of their wing cuticle and also their hydrophobicity.
Materials and methods
Atomic force microscope investigations
Fresh insect wings were surgically separated using a scalpel. The forewings were cut into smaller sections (3 × 5 mm2) and attached by adhesive tape, or by an epoxy resin, to AFM mounted stubs.
The investigations were carried out with a ThermoMicroscope TMX-2000 Explorer/Discoverer AFM. The instrument is based on detection of tip-to-surface forces through the monitoring of the optical deflection of a laser beam incident on a force-sensing/imposing lever. Scanners were calibrated on atomic scale surfaces (Watson et al. Citation2002). The analyses were carried out under air-ambient conditions (temperature of 23–25°C and 65–75% RH) and thus one of the major contributing forces of attraction was from capillary interactions from the surfaces (Blach-Watson et al. Citation2004).
‘Beam-shaped’ probes (NT-MDT Ultrasharp) were used throughout the study. Typical parameters, as reported by the manufacturer, included: a normal force constant, kN , of 0.03–4.5 Nm−1, an integrated probe and conical tip shape with a cone angle <20°, a radius of curvature of the tip <10 nm and a tip height of 17.5 μm. The actual tip parameters such as the normal force constant was determined by using resonance methods, and the torsional force constant was calculated from the expression for a long and thin lever (Cleveland et al. Citation1993).
Force distance adhesion data
Force vs distance (f-d) analysis was used to obtain adhesion data. The tip/particle was held stationary at an x-y (sample plane) location and ramped along the z-axis, first in the direction of approach and contact with the surface, and then in the reverse direction. F-d curves were acquired at rates of translation in the z-direction in the range of 5–10 μm s−1. Each f-d curve consisted of 600 data points. The attachment procedure for particle adhesion has been utilised in the literature (eg Watson et al. Citation2004). In short, the procedure consisted of contacting a tipless lever at the terminal end into a small glue reservoir of micron dimensions (Selleys Araldite two part epoxy) and then using the in-house translational x, y, z stage to make contact with the microsphere.
Twenty five measurements per particle (micro particle or nano tip)-substratum size combination were acquired. A total of five particles were attached to cantilevers for each particle type (eg five silica beads of ∼4.5 μm diameter were used for adhesion measurements each yielding 25 measurements). Only pollen grains that exhibited the same orientation upon fixing to a lever were used for adhesion measurements. The f-d curves were analysed using TopoMetrix analysis software package (TopoMetrix SPMLab, v 4.0). The values were then entered into SigmaPlot 10.0 whereby standard error (SE) values were obtained and presented as error bars.
Adhesion was measured under the conditions of the two surfaces coming into contact with no applied loading force, that is, adhesion represented the force of attraction that the particle-cuticle would experience where deformation of structures was minimised and where the main contributing force involved was simply that of the adhesion of the particle to the surface. For f-d curves on the moth wing, adhesion was also measured under a low loading force of 23 nN.
Two relevant parameters, namely the radius of curvature and the nano-scale roughness for the AFM tip/microsphere, were determined quantitatively by SEM and reverse imaging on sharp spikey projections (NT-MDT). The latter technique affords rapid and convenient topographical characterization of a microsphere attached to an AFM probe. Individual AFM tips were characterised by reverse imaging on a spikey biological array (cicada wing species, Cicadetta oldfieldi).
Frictional forces were measured using friction loops (Watson et al. Citation2006). Analysis was carried out by monitoring the torsional deformation of the lever during forward and reverse line scans 20 μm in length. The linear scan speed was 5 Hz. Friction measurements were carried out at the adhesional force loading (ie at a loading force that represented the adhesional force of contact between the wing membrane and the spherical particle).
Scanning electron microscopy
In the case of scanning electron microscope (SEM) imaging, a square of dried wing tissue (∼3 × 5 mm2) was excised and mounted on an aluminium pin-type stub with carbon-impregnated double-sided adhesive, then sputter coated with 7–10 nm of platinum (Pt), before being imaged using a JEOL 6300 field emission SEM at 8 kV.
Insect and particle features such as periodicity, height, width and spacing values for the nanostructuring were determined from SEM imaging of the top view and cross-sectional profiles of the wing cuticle. The Pt coating layer (7–10 nm in thickness) was taken into account when determining the width and density of the surface structures.
Contact angle measurements
A horizontal microscope (AIS-OPTICAL, model: AIS-V8G, magnification: 40X) with digital capturing (Panasonic Colour CCTV Camera, model: WV-CP410/G) of the images was used to obtain precise measurements of static contact angles. Ten measurements per droplet were taken on images captured at ambient conditions of 21°C and RH of 65–75%. Left and right angles between the sample surface and the tangent line to the droplet were considered as one measurement. For droplets on the insect wing surfaces, apparent static contact angles were measured. Five droplets of 10 μl Milli-Q water were applied to each of the wing membranes, where possible, near the dorsal cell region between the wing veins. This region was used in order to accommodate the droplet footprint without the effects/influence of the vein structure. Smaller sized droplets were difficult to place on the superhydrophobic insect cuticle surfaces due to the adhesion between the water droplet and the syringe needle being stronger than the force of gravity and adhesion of the cuticle surface. To remove the effects of the fine hairs levitating the water droplet above the lacewing cuticle membrane, smaller sized droplets were placed by spraying a fine water mist onto the membrane.
Results and discussion
Topographical characterisation of insect wing cuticles
The surfaces of the insect species studied in this investigation showed a wide range of wing membrane structuring (see ). This diversity in surface structuring as well as insect behaviour provided the impetus for the selection of species in this study. The micro-nano structuring is characterised in , listing the relevant geometrical parameters such as shape, spacing, depth and width of the structures.
Figure 1. Topographical SEM images of: (a) and (b) Cicadetta oldfieldi (top and cross-section, respectively) and, (c) and (d) black coloured region of Gudanga sp. nr adamsi (Black cicada) wing membrane (top and cross-section, respectively). (e) Structuring found on the dragonfly (Rhyothemis phyllis chloe) wing membrane. (f) Micron, and (g) sub-micron structures found on a moth wing (Prasinocyma albicosta). (h) and (i) Lacewing (Glenoleon pulchellus) and flower wasp (Scolia soror) nanostructures, respectively, and (j) broad bump structuring of the bladder cicada (Cystosoma schemltzi).
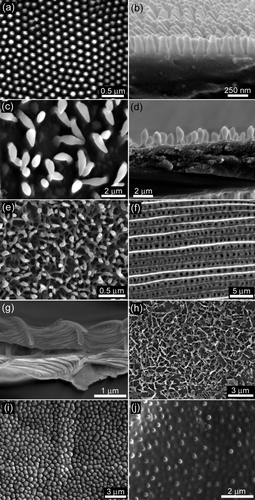
Table 1. Geometrical parameters of cuticle from insect species investigated in this study.
Superhydrophobic cuticles
The transparent cicada wing membranes of Cicadetta oldfieldi are covered with a periodic topography. The structures are shown in the topographical SEM images in showing top views, and the respective membrane cross-sections. The arrays consist of hexagonally packed spherically capped conical protuberances with a spacing and height of ∼200 nm and radius of curvature of ∼25–45 nm at the apex. Similar features have been found on the wings of a number of other cicada species including Aleeta curvicosta, Tamasa tristigma, Macrotristria angularis, Psaltoda claripennis and Thopha saccata (eg Watson et al. Citation2008). The features are present on all areas of the dorsal and ventral wing membrane. A previous study has demonstrated the functional effectiveness of similar structures as an antireflective surface which presumably helps to camouflage the insect from predators (Watson et al. Citation2008). The surfaces also demonstrated low adhesion with hydrophilic particles (Watson et al. Citation2008). The multi-functional nature of the structures appears to be a common feature of the cuticle found on many insect species.
The other superhydrophobic cicada species examined (Black cicada – Gudanga sp. nr adamsi) had coloured as well as non-transparent wing regions as opposed to the species discussed above which all have transparent fore and hind wings. The transparent regions on the black cicada wing membrane (hind wing) showed a similar well-ordered structure size, shape and periodicity as found on other cicada with completely transparent wings. Interestingly, the non-transparent coloured regions of the forewing (black in colour) showed substantially different structuring. It was composed of a less ordered surface with individual diamond-shaped structures almost one order of magnitude larger in height and width (width at widest point) (). This provides strong evidence for specific dimensional structure size/shape for specific functionality on selected regions of the wings. Regions of the wing where the antireflection property is required have the necessary structure dimensions (less than the wavelength of light [Watson et al. Citation2008]) while other coloured regions are not restricted by this wavelength condition. Suggested functioning of these structures on coloured regions is discussed below.
The dragonfly (Rhyothemis phyllis chloe) exhibited a surface topography with rod-like structures forming a layer of structured matting as shown in . These structures are similar to those found on damselflies (Gorb et al. Citation2000). Gorb et al. (Citation2000) suggested a number of possible functions for the wax-like covering including intra/inter-specific communication based on ultraviolet light reflection of the layer. The covering was also suggested to protect the insect when in contact with water.
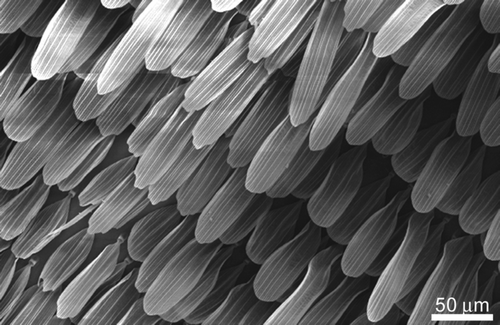
The moth (Prasinocyma albicosta) topography showed scales with a typical overlaying tile type arrangement as found on the wings of many butterfly/moth species. These scales exhibited micron () and submicron structuring in the form of longitudinal and lateral ridges as seen in the cross-sectional SEM image of a single scale in . A number of functional properties have been attributed to scales on butterflies including camouflage display, signalling and possibly thermo-regulation control (Gorb Citation2001; Vukusic and Sambles Citation2003; Cong et al. Citation2004). Moth and butterfly scales are typically superhydrophobic and can detach as an aid for protection against highly adhesive surfaces such as spiders’ webs.
The surface of the lacewing (Glenoleon pulchellus) showed interconnected ridges forming a dense netting on the cuticle surface (). The vein regions were characterised by an array of macrotrichia (fine hairs). Macrotrichia have been found to add a secondary layer of protection against surfaces with which the insect may come into contact (Watson et al. Citation2010b). The lacewing surface of the species Chrysoperla carnea has been previously studied where the wing was exposed to a fine dust of silica particles under fogging conditions and was shown to exhibit a high degree of non-contaminability (Wagner et al. Citation1996).
Hydrophilic cuticles
The flower wasp (Scolia soror) () and the bladder cicada (Cystosoma schmeltzi) () both exhibited surfaces with similar features represented by relatively large sized curved projections (bumps), flat (low in height) and spaced many hundred of nanometres apart (centre-centre distance). A similar type of insect topography (broad bumps) to those of these two species have been reported on a previous study of micro/nano structuring on the termite (Schedorhinotermes sp.) wing membrane (Watson and Watson Citation2004). The study suggested that the wing membrane topography (array of bumps) may improve flight efficiency by acting as a series of stabilizing elements designed to handle loading forces.
Wettability
Separating the contributing effects of topographical structuring and chemistry is not always a trivial exercise. Wettability however incorporates both the chemical and topographical components of the surface and is a useful parameter to relate to other measureable properties such as adhesion and friction. The result of these two contributing components (structure plus chemistry) will thus influence the interfacial properties and functional efficiency of insect wing membranes.
Many insects (especially superhydrophobic species) already have chemistries which result in contact angles that have reached the near upper limit of what can be achieved on flat surfaces (Holdgate Citation1955). Thus topographical architectures resulting in increased roughness is one way to improve on an already low energy surface. The non-wetting surfaces offer survival value to insects as they afford resistance to wetting by rain and other water surfaces they may encounter.
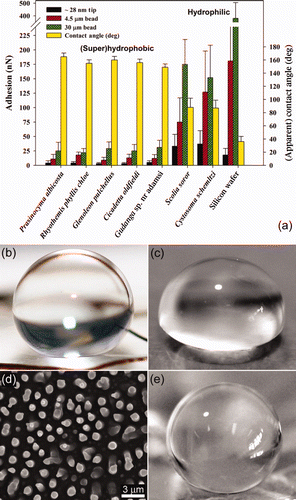
The wettability (apparent contact angles [CAs]) of the insect wing cuticles in this study are shown in . Many of the membranes represent superhydrophobic surfaces with static CAs close to or >150°. A typical example is shown in (cicada with structure height and periodicity ∼200 nm) where it is evident from the image that the water droplet gains negligible energy through absorption to compensate for any enlargement of its surface area (see also Supplementary Figure S1). [Supplementary figures are available via a multimedia link on the online article webpage.] For comparison, shows the interaction of water with a flat (non-structured) polydimethylsiloxane (PDMS) sample. This polymer demonstrates a hydrophobic surface with a measured CA of ca 101–105° (in good agreement with values reported in the literature [Sun et al. Citation2005]) and highlights the hydrophobic character of the wing membranes.
Figure 2. (a) Graph displaying the species type as a function of the apparent contact angle (right axis) and adhesion (left axis). The adhesion measurements were obtained using three different particles (4.5 and 30 μm silica beads, and an AFM silica tip (ca 28 nm)). Error bars represent SEs. Photographs showing a 10 μl droplet deposited on (b) a cicada wing membrane, and (c) hydrophobic PDMS surface. (d) and (e) show the PDMS replica of the cicada Tosena sybilla and the interaction of a water droplet with a polymer (PDMS) replica of the cicada Gaeana cheni, respectively.
There are a number of theories to express the superhydrophobic condition all of which have certain assumptions and limitations (Wenzel Citation1936; Cassie and Baxter Citation1944; Herminghaus Citation2000; Gao and McCarthy Citation2007; Shirtcliffe et al. Citation2010). Cassie and Baxter (Citation1944) express the superhydrophobic state in terms of a number of interfaces; a liquid–air interface with the ambient environment surrounding the droplet and a surface under the droplet involving solid-air, solid-liquid and liquid-air interfaces. Equation (1) expresses the CA (θ C ) formed with a rough surface.
The Cassie–Baxter model can also be applied to determine the CA of an array of hemispherical-top protrusions (a close approximation to the structures shown in for the cicada Cicadetta oldfieldi and also the transparent regions of the black cicada) using Equation (2):
Many of the other insect species in this study demonstrated specialised topographies for minimising the solid–liquid CA and maximising the liquid-air contact resulting in high CAs. The non-transparent cicada wing cuticles of Gudanga sp. for example, present structures that satisfy a number of the above contact conditions. The diamond-like shape demonstrates ‘model’ features for lowering the solid–liquid contact area and allowing a larger pocket/volume of air to occupy the volume beneath the water droplet. In addition, the shape minimises the amount of material required and hence reduces the wing and thus insect weight. The largest spacing between individual structures as seen in is <5 μm and this may suggest that this might be near a critical distance for structures of these heights (several μm) interacting with water. Indeed, a study on fabricated surfaces shows that larger spacings may be more susceptible to wetting (Bhushan and Jung Citation2008). It may be advantageous for the cicada membrane to resist water under a variety of conditions so as not to invade or make contact with the underlying surface between asperities increasing the solid–liquid contact or promoting a transition to the fully wetted state.
It has been suggested that cicada microstructures on the wings could act as natural templates to transfer wetting properties onto materials such as polymers (Watson and Watson Citation2004). A polymer (PDMS) was previously tailored using this templating procedure on the wing membrane of the cicada A. curvicosta (Watson et al. Citation2008). Attempts to duplicate the black cicada membrane with a polymer replica (PDMS) proved difficult due to the unusual structure (diamond) shape. The replica features only formed to a height of several hundred nanometres (see Supplementary Figure S2a and S2b). [Supplementary figures are available via a multimedia link on the online article webpage.] Interestingly, these sized features (height and spacing) did not result in the polymer exhibiting superhydrophobic behaviour. However, structures of similar dimensions (height and spacing) to Gudanga sp. can be found on wings of other coloured cicada species (Tosena sybilla and Gaeana cheni in Supplementary Figure S3a, S3b and S3c, S3d, respectively). [Supplementary figures are available via a multimedia link on the online article webpage.] shows a PDMS replica of the cicada Tosena sybilla (structure height ∼2 μm, spacing ∼2 μm). Droplets on these features showed superhydrophobic CAs. Even PDMS replicated structure heights approximately half those of Gudanga sp. (produced from Gaeana cheni ∼750 nm), which produced superhydrophobic interactions . The images in illustrate the increased hydrophobicity when roughness is introduced to the polymer surface at these dimensions. The diamond structuring and colouring found on the black cicada may also be multi-functional providing similar attributes to some butterflies, eg camouflage display, signalling and thermo-regulation control.
The dragonfly and lacewing demonstrated superhydrophobic contact angles. Even though the asperity density is high, the small width of the asperities () will increase the air pocket volume by the 3-dimensional ‘undergrowth’ of surface matting on the membrane. The moth also demonstrated a superhydrophobic contact with water. The topography has an anisotropic hierarchy of roughness with the scales and ridges of different length scales allowing minimisation of contact with water bodies.
The bladder cicada and flower wasp exhibited surfaces which were more easily wetted than the other insect species examined with CAs <90°. The hydrophilic nature of these surfaces is in stark contrast to the superhydrophobic topographies examined. The habit of the bladder cicada is sedentary in nature with limited flying (Moulds Citation1990). The large green wings are said to serve as a camouflaging blanket for the insect in dense foliage. This cicada has intense green wings similar in shape to leaves. The camouflage is so effective that it can take a few minutes to locate a ‘singing’ specimen <0.5 metres away (Moulds Citation1990). Interestingly, their camouflaging green colour is lost to a significant degree when dehydrated (Moulds Citation1990). (This feature is shown in Supplementary Figure S4a and S4b). [Supplementary figures are available via a multimedia link on the online article webpage.] Thus the present observation of a hydrophilic wing cuticle is in agreement with these factors and possibly functions by retaining condensed moisture and thus camouflage.
The hydrophilic black flower wasp has a similar cuticle structuring to the bladder cicada. The hydrophilicity of the flower wasp wing is highlighted by the interaction with moving droplets as would be experienced under conditions of precipitation (see Supplementary movies S1 and S2). [Supplementary movies are available via a multimedia link on the online article webpage.] In contrast, interaction of similar sized droplets with the superhydrophobic species results in water droplets bouncing off the surface. The wasp is generally solitary and does not make communal nests. In mid to late summer, they often form small swarms flying low over areas such as turf, shrubs and compost heaps and are commonly seen taking nectar from flowers. A common feature of this insect is that it is an extremely strong flyer. This wasp species has been observed in light rain conditions where the mobility of the insect seemed unhindered. Thus the insect appears to have sufficient strength and/or wing flapping frequency to remove water droplets quickly upon contact and thus maintain flight even though the wing membrane is hydrophilic.
A recent study has examined the wetting properties of wings of the termite Schedorhinotermes sp. which have a topography very similar to the flower wasp (Watson et al. Citation2011b). These termites typically fly after rain periods (typically at night) and thus do not come into contact with mobile droplets during flight. Unlike the flower wasp, the termite is a very weak flier and cannot displace water droplets easily from the wing. The termite displays a hydrophilic structuring on the wings with a small scale roughness which is not dimensionally sufficient to introduce an increase in hydrophobicity. The lack of hydrophobicity allows the termite to be hydrophilically captured at locations (for example on wetted leaves and rocks) where water may be present in large quantities; sufficient for the initial colonization period (Watson et al. Citation2011b).
Adhesion and friction
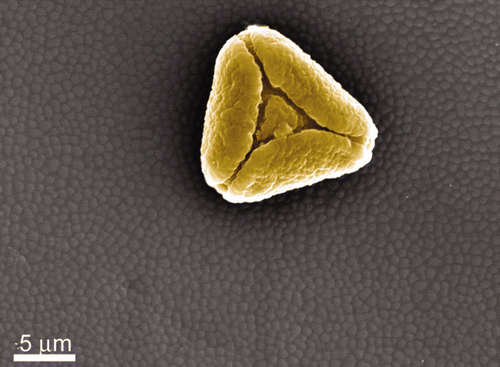
The adhesion and friction properties were measured on the selected insect species based on the hypothesis that contamination and wettability of wings are related to insect habit and/or WSA/BM values. The AFM is ideally suited for particle adhesion measurements in air or aqueous environments (Watson et al. Citation2004, Citation2008). Four different sized particles (three with hydrophilic chemistries) were used to measure adhesion on the insect wing membranes. The dimensional and chemical differences (see ) were chosen to mimic contact conditions of particles that could potentially contaminant the structured insect cuticle surfaces. In addition, natural particles (three different pollens) were also introduced on the surfaces. shows SEM images of both the orientation of the particles used for adhesion measurements and their surface topographies (pollens and ca 30 μm silica beads). The pollens were chosen based on the three distinct topographies with various levels of roughness. As shown in the spherical pollen of Pimelea linifolia ssp, presented one to several asperities with the insect cuticles upon contact. The pyramidal shaped pollen of Grevillea () demonstrated a homogeneous surface topography/roughness on all sides, comprising small holes and bumps. The pollen of Acacia fimbriata (Golden wattle) () demonstrated a patterned surface with two distinct levels of roughness. The outer layer of the pollen grains comprises carboxylic acids cross-linked with saturated and unsaturated aliphatic chains with varying amounts of aromatics resulting in a hydrophobic surface (Thio et al. Citation2009). The dimensional parameters of the pollens are shown in . Supplementary Figure S5 shows higher resolution SEM images of the three pollen surfaces investigated. [Supplementary figures are available via a multimedia link on the online article webpage.] The surface features of the artificial contaminants were analysed (eg surface roughness) using delta-like shaped projections (a calibration grid). An example is shown in Supplementary Figure S6 (silica bead). [Supplementary figures are available via a multimedia link on the online article webpage.]
Figure 3. SEM images showing the orientation of the contaminating particulates attached to AFM levers. (a) Pimelea linifolia ssp., (b) Grevillea ‘Red Sunset’, (c) Acacia fimbriata, and (d) 30 μm silica bead.
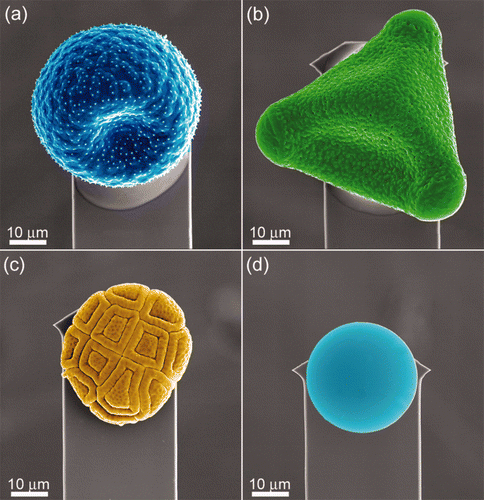
Table 2. Dimensional parameters of all the particles used for adhesion measurements.
The adhesion between the various particles and a flat hydrophilic silicon sample with a native oxide layer was used for comparison as it highlights the adhesional differences between the various surfaces. For the case where the silica sphere comes into contact with the silicon dioxide surface, meniscus forces at the point of contact between the tip/particle and the surface accounted for the high adhesive forces. The meniscus force between a sphere and a flat surface can be expressed as a function of ‘true’ CA and other parameters (McFarlane and Tabor Citation1950; Bhushan Citation2002).
The adhesion between the silica tip/microsphere and the insect cuticles represents a high surface energy contaminant particle coming into contact with low energy hydrophobic and higher energy hydrophilic micro/nano-structures/arrays. This is highlighted in , which shows the differences in adhesion and wetting between the two groups of insect structuring. Particle adhesion on the (super)hydrophobic insect cuticles was much lower in comparison with that for the flat hydrophilic Si surface and hydrophilic insect species. The higher adhesion values measured between the contacting surfaces of the hydrophilic insect membranes and the hydrophilic contaminants reflects the formation of menisci from liquid present on the surfaces. In addition, the relatively flattened and broadened structures of the hydrophilic insect group do not minimise the contact area to the degree of the hydrophobic species.
The larger the particle contacts (eg compare silica AFM tip (ca 28 nm) to 4.5 and 30 μm sphere in ), the higher the adhesion, which reflects the increase in the radius of curvature and the increased contact points. Thus, the real contact area increases along with the meniscus contributions.
Adhesion was also measured for the 30 μm silica sphere on a poly(tetrafluoroethylene (PTFE) surface (CA 108°) with a measured value of ∼50 nN. Thus the adhesion values for the superhydrophobic insect cuticle membranes () represent lower surface energy materials than the flat PTFE for interaction of particles at this length scale. Based on the Young–Dupré equation, the surface energy of the cicada membrane is ∼12 mJ m−2. For comparison, the work of adhesion for water on a silica (glass) surface is ∼120 mJ m−2 (Sklodowska et al. Citation1999). It is evident that the topography of the hydrophobic wing membranes results in minimal actual contact between the touching surfaces. The highly intricate hydrophobic patterning decreases the contact area, number of menisci, van de Waals attraction and thus the total adhesive force.
AFM manipulation of the cicada wing membrane (by contact imaging with an AFM probe with a tip with a large radius of curvature (>150 nm)) resulted in partial crushing of the array structures. The crushed array presented a flatter surface and thus a greater contact area for adhering particles in comparison with the intact array, thus leading to greater adhesion. For example the 30 μm sphere showed an adhesion almost twice that of the intact membrane (∼45 nN; a similar adhesional value to that of the PTFE surface). An AFM silica tip with a radius of curvature of ∼14 nm was also used to measure adhesion on the crushed membrane. This showed the same adhesion (±0.3 nN) as the intact cicada membrane. This observation reveals that for particles with a radius of the curvatures smaller than that of the membrane surface protrusions, the contacting areas between particles, whether with intact or flattened membranes, are similar. Thus, the size of the particle (silica microsphere/tip) determines the adhesion and the chemistry of the crushed regions does not significantly alter from intact membranes (significant chemical changes would most likely manifest in greater differences in adhesion).
Adhesion of the C18 particles with the insect cuticles was generally less than the comparably sized silica particles (compare data from and ). This represents the difference in adhesion of a hydrophobic and hydrophilic particle coming into contact with the insect wing membranes. In addition, the differences in roughness may also contribute to this effect.
The contact of contaminant particles with the wing membrane surface of Cicadetta oldfieldi showed ultra-low adhesion forces as small as 3 nN for AFM tip sized spherical bodies (ca 28 nm diameter) and <30 nN for 30 μm sized silica particles.
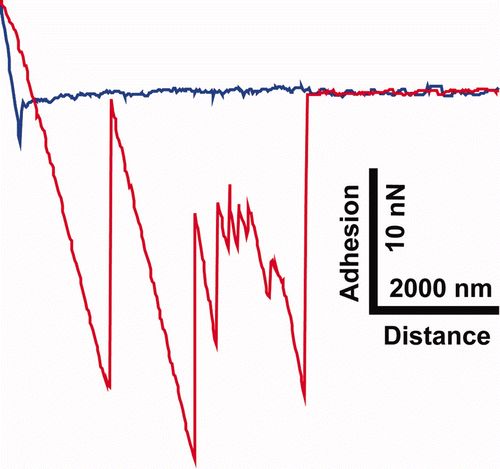
The adhesion values measured on the dragonfly cuticle for the 30 μm sized silica particle were the lowest of all the insects. The open structure framework of the membrane minimises the contact points and thus the adhesion. Adhesion curves on the moth surface with larger particle sizes often showed multiple releases during the retract section of the force curve (eg Supplementary Figure S7). [Supplementary figures are available via a multimedia link on the online article webpage.] This type of adhesion was in stark contrast to the other insect surfaces where adhesion was generally characterised by a single release mechanism (with the exception of Pimelea linifolia ssp. where release from multiple contacts from the pollen sometimes occurred). Release of the particles from the rough scale surface may be the result of the detachment of the varying pollen protuberance contact points with the scale roughness making multiple adhesional points between the two surfaces.
However, butterfly and moth flattered macrotrichia (scales) are known to fully detach from the wing. This attribute can serve as a defence mechanism upon contact with other surfaces (eg spider web). The multiple release steps as indicated by the adhesion curves may also suggest a number of possible mechanisms of detachment between the particle and scale: (1) the overlapping scale morphology (see Supplementary Figure S8) [Supplementary figures are available via a multimedia link on the online article webpage.] provides a ratchet-type release in sequential steps; (2) the pedicel attached to the scale releases in steps from the socket; (3) the scale is compressed upon contact and follows the particle upon retraction. The sequential release mechanism of the scales may also indicate a unique way upon which these type of insects contend with small contact adhesion without full detachment of the scale occurring.
The pollen grains were of a similar scale to the 30 μm silica beads. However, due to the rougher morphology and more hydrophobic nature of the long chain polymers that compose the pollen sporopollenin (outer layer), adhesion between the pollen grains and the insect cuticles was lower (see ). This Figure shows that the spherical shaped profile with small asperities on Pimelea linifolia ssp. exhibited the lowest adhesion. Thus, the reduced contacting area resulted in lower adhesion. The pollen of Acacia fimbriata demonstrated similar adhesion values to the pyramidal shaped Grevillea pollen.
Figure 4. Graph displaying the relationship between the apparent contact angle and adhesion with the C18 particles on the hydrophilic and super/hydrophobic insect wing surfaces. Error bars represent SEs.
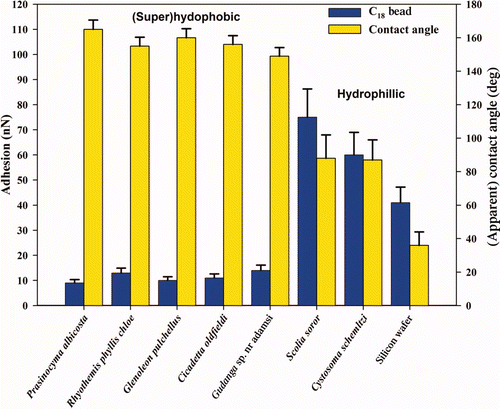
Figure 5. Graph displaying the apparent contact angle and adhesion with the three different pollen particles – ⋄ Pimelea linifolia ssp., ▵ Grevillea ‘Red Sunset’ and ˆ Acacia fimbriata ‘Golden wattle’. Error bars represent SEs.
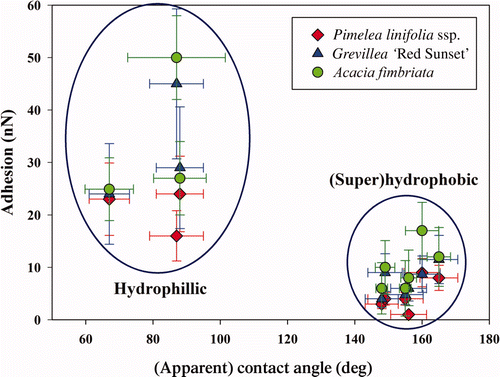
The highest adhesion between hydrophilic insect membranes and the pollens was 50 nN. These high values were also reflected in the frequency of contaminating particles as seen on freshly caught specimens using optical and SEM imaging (for example see pollen grain on a flower wasp wing membrane in Supplementary Figure S9). [Supplementary figures are available via a multimedia link on the online article webpage.] Pollen grains were also interacted with an Australian stingless native bee (Trigona carbonaria) pollen basket located on the hind leg. Adhesion values of pollen on this region were up to 70 nN. This value is of a similar order to adhesion of pollens to stigma cells (in the order on 10−7 N).
Frictional forces were also measured on three insect species (Cicadetta oldfieldi, Flower wasp and Bladder cicada) with a silica tip at loading forces represented by the adhesional force. The frictional force obtained on the insect cuticle of the cicada was 5.5 ± 2.5 nN. This value demonstrates that only very low frictional forces are required to remove contaminants of this size from the cicada membrane. In contrast to the frictional force on the cicada, the flower wasp and bladder cicada yielded values of 47 ± 15 and 65 ± 15 nN, respectively. This demonstrates the higher forces required to remove particles which are more strongly attached to the membrane.
Conclusions
The contact forces of contaminating bodies of different length scales (and chemistry) were evaluated by measuring the strength of interaction between particles with selected insect cuticle micro/nanostructuring. An open framed intricate structuring characteristic of hydrophobic insect species showed minimal adhesion with water and particles of all sizes and chemistry. Indeed many of the insect species demonstrated a superhydrophobic interaction with water. As the growth of most microorganisms is provided by permanent or temporary water availability, which can lead to the formation of biofilms, this superhydrophobic property may well protect the insects from pathogens such as fungi and bacteria by limiting water availability. Some of these insects may also encounter periods without rainfall and fogging conditions for self-cleaning of wing surfaces. Low adhesion and friction may aid in these circumstances to minimise contamination from foreign bodies and facilitate removal. In contrast to the hydrophobic species examined, hydrophilic cuticles showed lower contact angles and higher adhesion with particles.
A different size range of particles was used to ascertain the contact conditions of bodies that would normally come into contact with the cuticle surfaces. The unique topographical micro- and nanostructures found on the insect surfaces demonstrate design characteristics and features for surfaces with low/high wettability, adhesion and friction.
The diversity of topographies demonstrates a range of architectures suitable for optimising surface properties and replication for man-made structures/applications. Indeed, a range of new materials, for example self cleaning, AF, anti-reflective surfaces, could be utilised in environments where the surfaces are intermittently submerged. Thus, functionality must be maintained in both terrestrial and aquatic environments. These new materials may constitute a marriage of structural and chemical components/properties gleaned from both aquatic and terrestrial organisms. The insect structures provide a set of well characterised ‘technologies’ which incorporate a range of properties primarily focused on reducing adhesion with solid and water bodies. Many of the insects displaying unique structural architecture also represent good candidates for investigations in aqueous environments.
gbif_a_637187_sup_22798932.pdf
Download PDF (1 MB)gbif_a_637187_sup_22740132.pdf
Download PDF (1 MB)gbif_a_637187_sup_22740126.mpg
Download MPEG Video (2.9 MB)gbif_a_637187_sup_22740125.mpg
Download MPEG Video (2.5 MB)References
- Barthlott , W and Neinhuis , C . 1997 . Purity of the sacred lotus, or escape from contamination in biological surfaces . Planta , 202 : 1 – 8 .
- Bhushan , B . 2002 . Introduction to tribology , 732 – 751 . New York : Wiley. pp .
- Bhushan , B and Jung , Y C . 2008 . Wetting, adhesion and friction of superhydrophobic and hydrophilic leaves and fabricated micro/nanopatterned surfaces . J Phys: Condens Matter , 20 : 225010
- Bhushan , B and Jung , Y C . 2011 . Natural and biomimetic artificial surfaces for superhydrophobicity, self cleaning, low adhesion, and drag reduction . Prog Mater Sci , 56 : 1 – 108 .
- Bhushan , B , Jung , Y C and Koch , K . 2009 . Micro-, nano- and hierarchical structures for superhydrophobicity, self-cleaning and low adhesion . Philos T Roy Soc A , 367 : 1631 – 1672 .
- Blach-Watson , J A , Watson , G S , Brown , C L and Myhra , S . 2004 . UV patterning of polyimide: differentiation and characterization of surface chemistry and structure . App Surf Sci , 235 : 164 – 169 .
- Blossey , R . 2003 . Self-cleaning surfaces-virtual realities . Nature Mater , 2 : 301 – 306 .
- Burney , P GJ , Newson , R B , Burrows , M S and Wheeler , D M . 2008 . The effects of allergens in outdoor air on both atopic and nonatopic subjects with airway disease . Allergy , 63 : 542 – 546 .
- Cariñanos , P , Alcázar , P , Galán , C , Navarro , R and Domínguez , E . 2004 . Aerobiology as a tool to help in episodes of occupational allergy in work places . J Invest Allerg Clin , 14 : 300 – 308 .
- Cassie , A BD and Baxter , S . 1944 . Wettability of porous surfaces . Trans Faraday Soc , 49 : 546 – 551 .
- Cleveland , J P , Manne , S , Bocek , D and Hansma , P K . 1993 . A non-destructive method for determining the spring constant of cantilevers for scanning force microscopy . Rev Sci Instrum , 64 : 403 – 405 .
- Cong , Q , Chen , G-H , Fang , Y and Ren , L-Q . 2004 . Study on the super-hydrophobic characteristic of butterfly wing surface . J Bionics Eng , 1 : 249 – 255 .
- D’Amato , G . 2002 . Environmental urban factors (air pollution and allergens) and the rising trends in allergic respiratory diseases . Allergy , 57 : 30 – 33 .
- de Nys , R and Steinberg , P D . 2002 . Linking marine biology and biotechnology . Curr Opin Biotechnol , 13 : 244 – 248 .
- Ding , M , Chen , F , Shi , X , Yucesoy , B , Mossman , B and Vallyathan , V . 2002 . Diseases caused by silica: mechanisms of injury and disease development . Int Immunopharmacol , 2 : 173 – 182 .
- Furstner , R and Barthlott , W . 2005 . Wetting and self-cleaning properties of artificial superhydrophobic surfaces . Langmuir , 21 : 956 – 961 .
- Gao , L and McCarthy , T J . 2007 . How Wenzel and Cassie were wrong . Langmuir , 23 : 3762 – 3765 .
- Genzer , J and Efimenko , K . 2006 . Recent developments in superhydrophobic surfaces and their relevance to marine fouling: a review . Biofouling , 22 : 339 – 360 .
- Gorb , S . 2001 . Attachment devices of insect cuticle , 21 – 36 . New York : Kluwer Academic Publishers .
- Gorb , S N , Kesel , A and Berger , J . 2000 . Microsculpture of the wing surface in Odonata: evidence for cuticular wax covering . Arthropod Struct Devel , 29 : 129 – 135 .
- Herminghaus , S . 2000 . Roughness-induced non-wetting . Europhys Lett , 52 : 165 – 170 .
- Hnizdo , E and Vallyathan , V . 2003 . Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence . Occup Environ Med , 60 : 237 – 243 .
- Holdgate , M W . 1955 . The wetting of insect cuticles by water . J Exp Biol , 32 : 591 – 617 .
- Hu , H M , Watson , G S , Cribb , B W and Watson , J A . 2011 . Non wetting wings and legs of the cranefly aided by fine structures of the cuticle . J Exp Biol , 214 : 915 – 920 .
- Knaapen , A M , Borm , P JA , Albrecht , C and Schins , P F . 2004 . Inhaled particles and lung cancer. Part A: Mechanisms . Int J Cancer , 109 : 799 – 809 .
- Koch , K and Barthlott , W . 2009 . Superhydrophobic and superhydrophilic plant surfaces: an inspiration for biomimetic materials . Philos T Roy Soc A , 367 : 1487 – 1509 .
- Linskens , H E and Cresti , M . 2000 . Pollen-allergy as an ecological phenomenon: a review . Plant Biosyst , 134 : 341 – 352 .
- Liu , K and Jiang , L . 2011 . Bio-inspired design of multiscale structures for function integration . Nano Today , 6 : 155 – 175 .
- Losey , J E , Rayor , L S and Carter , M E . 1999 . Transgenic pollen harms monarch larvae . Nature , 399 : 214
- Marmur , A . 2006 . Super-hydrophobicity fundamentals: implications to biofouling prevention . Biofouling , 22 : 107 – 115 .
- Martines , E , Seunarine , K , Morgan , H , Gadegaard , N , Wilkinson , C DW and Riehle , O . 2005 . Superhydrophobicity and superhydrophilicity of regular nanopatterns . Nano Lett , 5 : 2097 – 2103 .
- McFarlane , J S and Tabor , D . 1950 . Adhesion of solids and the effect of surface films . P Roy Soc Lond A Mat , 202 : 224 – 243 .
- Moulds , M S . 1990 . Australian cicadas , 193 – 195 . Australia : New South Wales University Press .
- Ormstad , H , Gaarder , P I and Johansen , B V . 1997 . Quantification and characterisation of suspended particulate matter in indoor air . Sci Total Environ , 193 : 185 – 196 .
- Parker , A R and Lawrence , C R . 2001 . Water capture by a desert beetle . Nature , 414 : 33 – 34 .
- Ralston , E and Swain , G . 2011 . Can biomimicry and bioinspiration provide solutions for fouling control? . Mar Technol Soc J , 45 : 216 – 227 .
- Scardino , A J and de Nys , R . 2011 . Mini review: biomimetic models and bioinspired surfaces for fouling control . Biofouling , 27 : 73 – 86 .
- Scardino , A J , Zhang , H , Cookson , D J , Lamb , R N and deNys , R . 2009 . The role of nanoroughness in antifouling . Biofouling , 25 : 749 – 756 .
- Schultz , M P , Bendick , J A , Holm , E R and Hertel , W M . 2011 . Economic impact of biofouling on a naval surface ship . Biofouling , 27 : 87 – 98 .
- Shirtcliffe , N J , McHale , G and Newton , M I . 2010 . An introduction to superhydrophobicity . Adv Colloid Interface Sci , 161 : 124 – 138 .
- Sklodowska , A , Wozniak , M and Matlakowska , R . 1999 . The method of contact angle measurements and estimation of work of adhesion in bioleaching of metals . Biol Proced Online , 1 : 114 – 121 .
- Smith , J L and Lee , K . 2003 . Soil as a source of dust and implications for human health . Adv Agronomy , 80 : 1 – 32 .
- Sun , T , Feng , L , Gao , X and Jiang , L . 2005 . Bioinspired surfaces with special wettability . Acc Chem Res , 38 : 644 – 652 .
- Thio , B JR , Lee , J-H and Meredith , J C . 2009 . Characterization of ragweed pollen adhesion to polyamides and polystyrene using atomic force microscopy . Environ Sci Technol , 43 : 4308 – 4313 .
- Vincent , J FV and Wegst , U GK . 2004 . Design and mechanical properties of insect cuticle . Arthropod Struct Devel , 33 : 187 – 199 .
- Vukusic , P and Sambles , J R . 2003 . Photonic structures in biology . Nature , 424 : 852 – 855 .
- Wagner , P , Neinhuis , C and Barthlott , W . 1996 . Wettability and contaminability of insect wings as a function of their surface sculptures . Acta Zool , 77 : 213 – 225 .
- Watson , G S and Watson , J A . 2004 . Natural nano-structures on insects - possible functions of ordered arrays characterized by atomic force microscopy . App Surf Sci , 235 : 139 – 144 .
- Watson , G S , Cribb , B W and Watson , J A . 2010a . How micro/nanoarchitecture facilitates anti-wetting: an elegant hierarchical design on the termite wing . ACS Nano , 4 : 129 – 136 .
- Watson , G S , Cribb , B W and Watson , J A . 2010b . The role of micro/nano channel structuring in repelling water on cuticle arrays of the lacewing . J Struc Biol , 171 : 44 – 51 .
- Watson , G S , Cribb , B W and Watson , J A . 2011b . Contrasting micro/nano architecture on termite wings: two divergent strategies for optimising success of colonisation flights . PLoS ONE , 6 : e24368 doi:10.1371/journal.pone.0024368
- Watson , G S , Dinte , B P , Blach , J A and Myhra , S . 2002 . Demonstration of atomic scale stick-slip events stimulated by the force versus distance mode using atomic force microscopy . J Phys D Appl Phys , 35 : 2066 – 2074 .
- Watson , G S , Myhra , S , Cribb , B W and Watson , J A . 2008 . Putative function(s) and functional efficiency of ordered cuticular nano-arrays on insect wings . Biophys J , 94 : 3352 – 3360 .
- Watson , G S , Blach , J A , Cahill , C , Nicolau , D V , Pham , D K , Wright , J and Myhra , S . 2004 . Interactions of poly(amino acids) in aqueous solution with charged model surfaces – analysis by colloidal probe . Biosens Bioelectron , 19 : 1355 – 1362 .
- Watson , J A , Brown , C L , Myhra , S and Watson , G S . 2006 . Two-dimensional stick-slip on a soft elastic polymer: pattern generation using atomic force microscopy . Nanotechnology , 17 : 2581 – 2589 .
- Watson , J A , Cribb , B W , Hu , H M and Watson , G S . 2011a . A dual layer hair array of the brown lacewing: repelling water at different length scales . Biophys J , 100 : 1149 – 1155 .
- Wenzel , R N . 1936 . Resistance of solid surfaces to wetting by water . Ind Eng Chem , 28 : 988 – 994 .
- Yebra , D M , Kiil , S and Dam-Johansen , K . 2004 . Antifouling technology – past, present and future steps towards efficient and environmentally friendly antifouling coatings . Prog Org Coat , 50 : 75 – 104 .
- Yli-Panula , E and Rantio-Lehtimäki , A . 1995 . Birch-pollen antigenic activity of settled dust in rural and urban homes . Allergy , 50 : 303 – 307 .
Figure S1. An optical microscope image of a water droplet resting on the wing membrane of a cicada revealing an apparent contact angle (CA) of ca 150° with the surface.
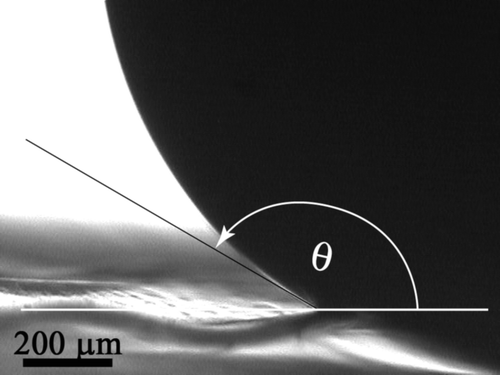
Figure S2. Top (a) and side (b) view, SEM images of a PDMS replica of the black cicada (Gudanga sp.).
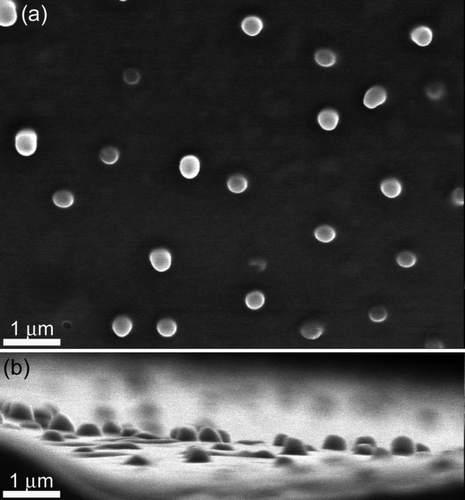
Figure S3. Top and side SEM images of the coloured regions found on the cicada wings of Tosena sybilla ((a) and (b), respectively) and Gaeana cheni ((c) and (d), respectively).
Figure S5. SEM images of the finer surface features of the three pollen surfaces used. (a) Pimelea linifolia ssp., (b) Gravillea ‘Red Sunset’ and (c) Acacia fimbriata.
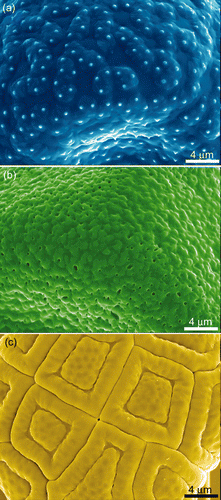
Figure S6. AFM images of a silica bead, revealing the finer surface structures. (a) A topographical image, (b) a 3-d representation, and (c) an artificially shaded and leveled 3-d image showing the fine surface features. The imaging was performed with the bead attached to a lever and scanned over a calibration grid, ie reverse imaging.
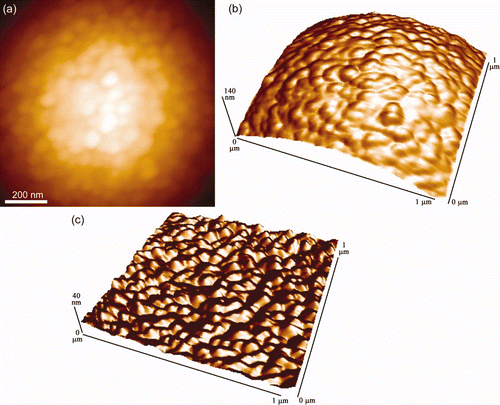
Figure S7. A single representative f-d curve showing multiple ‘jumps’ on the retract curve (red) obtained on the moth wing (Prasinocyma albicosta). The approach curve (blue) shows a distinct snap-on feature (and a general downward trend) where the scales are presumably statically attracted to the surface of the Si bead.
Movie S1. A water droplet interacting with the surface of a flower wasp wing, revealing its hydrophilic nature. The wing is fixed at one end.
Movie S2. A water droplet interacting with the surface of a flower wasp wing which is fixed flat onto a surface.