Abstract
Salvadora persica sticks are used for chewing and oral-hygiene measures worldwide. The growth inhibition and anti-biofilm effects of various extracts on cariogenic Streptococcus mutans isolates were evaluated. Biofilm inhibition, gas chromatography–mass spectrometry (GC–MS) analyses for phytochemicals and their possible mode of interaction with biofilm response regulators were revealed using LigandFit docking protocols. All S. persica extracts showed considerable inhibitory activity and the cariogenic S. mutans showed varied susceptibility when compared with controls. The percentage reduction in biofilm inhibition obtained for methanol, ethanol, chloroform, acetone, and aqueous extracts were 87.92%, 85.75%, 72.44%, 61.66% and 58.68%, respectively. GC–MS analyses revealed >28 compounds, of which benzyl (6Z,9Z,12Z)-6,9,12-octadecatrienoate, 3-benzyloxy-1-nitro-butan-2-ol and 1,3-cyclohexane dicarbohydrazide interacted efficiently with the bacterial communication quorum-sensing (QS) regulators Streptococcus OmpP and Staphylococcus Lux proteins. The bioactive, dual-function, anti-biofilm agents in S. persica not only inhibit growth, but also control the colonization and accumulation of caries-causing S. mutans.
Introduction
Caries is one of the oldest dental diseases in humans (Han et al. Citation2010). This multifactorial disease has been found to be a major concern for almost all human populations. Dental plaque is an example of a biofilm that has a primary role in the pathogenesis of disease, and shows that the properties of bacteria associated with it have markedly different characteristics than those of identical cells growing in liquid broth (Babpour et al. Citation2009). The characteristics of biofilm and planktonic cells differ markedly due to the structure of the biofilm and the fact that the physiological attributes of biofilm organisms confer an inherent resistance to antimicrobial agents (Donlan and Costerton Citation2002). The mouth contains a wide variety of bacteria, but only a few specific species are believed to be the causative agents of caries. Dental caries are, however, preventable. Among the research strategies attempted for caries management, inhibition of cell growth has attracted a great deal of attention. Effective antimicrobial agents against the causative oral pathogens could play an important part in the prevention of dental caries. However, attempts to use such agents have been of minimal practical use so far. Antibiotics such as penicillin and erythromycin have been reported to effectively prevent dental caries in animals and humans (Kubo et al. Citation1992). However, they are not used in this context because of adverse effects such as hypersensitivity reactions, supra-infections and teeth staining (Tredwin et al. Citation2005). The main cariogenic bacteria in humans, viridans streptococci such as Streptococcus mitis, Streptococcus sanguis and Streptococcus mutans, are found to be moderately resistant to antibiotics. These drawbacks justify further research and development of natural antibacterial agents that are safe for the host and specific for oral pathogens (Singh et al. Citation2007). Many members of the genus Streptococcus that cause infections in humans use quorum-sensing (QS) systems to regulate several physiological properties, including incorporating foreign DNA, tolerating acids, forming biofilms, and becoming virulent (Cvitkovitch et al. Citation2003).
It has been well documented that several medicinal plants used in traditional medicine confer extensive antibacterial activity against various microorganisms, including bacteria that cause dental caries (Kelmanson et al. Citation2000). Prevention of dental caries can be achieved through maintaining oral hygiene by regular removal of food deposits and dental plaque, which is traditionally done by non-specific removal by mechanical means (Cowan Citation1999). Even though toothbrushes and toothpastes have found wide usage, natural methods of teeth-cleaning using chewing sticks selected and prepared from the twigs, stems, or roots from various plant species have been practiced for thousands of years in Asia, Africa, the Middle East, and the Americas (Wu et al. Citation2001). Chewing sticks come from different species of plants and, within one stick, the chemically active component may be heterogeneous (Cowan Citation1999).
Utilization of the desert plant miswak (Salvadora persica) is widespread in Saudi Arabia and in many other countries owing to religious and cultural reasons (Al-Otaibi Citation2004). Sticks plucked from the evergreen miswak tree, manipulated into “brushes”, and used appropriately have been effective “natural toothbrushes” for >1000 years. It has been demonstrated in vitro that the aqueous extracts of miswak inhibit the growth of several oral microorganisms (Al-Otaibi et al. Citation2004). They are therefore helpful in fighting oral bacteria and maintaining oral health. The efficiency of miswak use for oral hygiene was found to be comparable (or slightly better) than toothbrush use with respect to the periodontal status of miswak users in a Sudanese population (Darout et al. Citation2000). However, the observation of increased plaque formation and gingival bleeding in individuals using chewing sticks in comparison with toothbrush-users warrants further research on evaluation of the effectiveness of chewing sticks (Batwa et al. Citation2006). As knowledge of the mechanisms of oral disease increases, future treatment is likely to be more targeted (eg against small groups of organisms, single species or at key virulence factors that they produce). Though the organisms causing dental caries and other oral problems are mostly biofilm-forming, the activity of S. persica on specific biofilm-forming organisms is not known. Furthermore, there is potential for deriving active compounds having specific effects on the biofilm-forming, dental caries-causing organisms from this widely used plant. S. persica has also been found to have a myriad of phytochemical constituents. LuxR is the best-studied member of a family of bacterial transcription factors taking part in QS (Stevens et al. Citation1994). A form of “bacterial communication” among “biofilm communities” can be used for understanding the molecular interactions of plant phytochemicals with biofilm-specific receptors/activators. In this context, this report describes the activity of extracts of S. persica on dental caries-causing, biofilm-forming S. mutans, their bioactive compounds, and mode of action.
Materials and methods
Ethical approval of the study protocol
The study protocol was approved by the Ethics Committee of King Saud University (Riyadh, Saudi Arabia). All subjects provided written informed consent to be included in the study.
Cariogenic Streptococcus mutans and their biofilm-forming ability
Samples of dental plaque were collected from the anterior and molar teeth of both jaws from outpatients undergoing treatment at University Hospital, College of Dentistry of King Saud University (Riyadh, Saudi Arabia) following standard procedures. Plaque samples were stored in a 1.5-ml Eppendorf tubes containing 500 μl of 1 × phosphate-buffered saline (PBS) at –20°C until analyses. Samples were diluted 100-fold in PBS, plated onto Mitis-Salivarius agar (HiMedia, Mumbai, India) supplemented with freshly prepared 0.2 unit bacitracin plus sucrose (20% final concentration), and the organisms were isolated (Yoo et al. Citation2007). These isolates were identified (using Api-20 STREP) according to National Committee for Clinical Laboratory Standards (NCCLS) and maintained in Brain–Heart Infusion (BHI) Agar with 5% (v/v) defibrinated blood (Al-Anbori et al. Citation2008). The slime-forming ability of the isolates was evaluated using the tube adherence method devised by Christensen et al. (Citation1982) and graded as described (Murugan et al. Citation2010a). The extracellular polysaccharide substance and slime-producing ability of the isolates was determined with the Congo Red Agar (CRA) method (Freeman et al. Citation1989).
Biofilm-formation assay
Biofilm formation by all strains was assayed as previously reported (Motegi et al. Citation2006). S. mutans strains were assayed for biofilm formation by mixing 40 μl of cell suspension (8 × 106 colony-forming units (CFU)) and 160 μl of dextrose-free tryptic soy broth (TSB) supplemented with 0.25% sucrose in the wells of sterile 96-well U-bottom polystyrene tissue culture plates (Tarsons, Mumbai, India) that had previously been coated with whole saliva. After incubation at 37°C for 16 h under anaerobic conditions, the liquid medium was removed and wells rinsed again with sterilized distilled water. Plates were then air-dried and stained with 0.25% safranin for 15 min. This was followed by rinsing with distilled water (to remove excess dye) and air drying. Each biofilm was examined, without dissolving with solvent, using a microplate reader (model 680, Bio-Rad, Hercules, CA, USA) at 492 nm as the biofilm was uniformly formed onto the bottom of the wells of the 96-well plates.
Extraction of bioactive compounds from plant materials
Sticks of S. persica were collected fresh from the plant, shade dried, and cut into pieces using a sharp knife. These were ground to a powder with a commercially available food blender. Other plant materials (including leaves) were collected in polythene bags, labeled using a running number system, and authenticated by the Herbarium (Department of Botany, College of Science, King Saud University, Riyadh, Saudi Arabia). Ground stem samples (20 g) were extracted in 200 ml of aqueous, ethanol, methanol and hexane solvents using a soxhlet extractor (Quality Biological, Gaithersburg, MD, USA) for 24 h, filter-sterilized and stored at 4°C until use. The solvent from the extract was removed under reduced pressure at 40°C. The solid obtained was used in further studies after dissolving in dimethyl sulfoxide (DMSO) with a maximum concentration of DMSO in the test solution of ≤1%. Extracts were used to screen for antimicrobial activity against biofilm-forming S. mutans by a well-diffusion method carried out in Horse Blood Agar (HBA; Oxoid, Hampshire, UK). Briefly, 20 μl of young S. mutans standardized culture (1 × 108 CFU ml−1) were placed on a HBA plate and uniformly spread using a glass rod. Paper disks (diameter, 6 mm) soaked in 10 μl of each of the extracts at 2.5 g ml−1 were placed concentrically on the HBA plate. Inoculated HBA plates were incubated for 48 h under anaerobic conditions at 37°C. Their efficacy was also compared with solvent alone and amoxicillin antibiotics as controls. After incubation, the zone of inhibition was measured. The minimum inhibitory concentration (MIC) of the extract was then determined (Wong et al. Citation2010).
Determination of anti-biofilm activity
The anti-biofilm activity of methanol extracts of S. persica on biofilm formation by 18–20 h-old cultures of S. mutans was determined by microtiter plate analyses (Pitts et al. Citation2003). To 180 ml of freshly prepared TSB, 20 ml of 18 h-old broth culture of each isolate were added and mixed thoroughly. From this inoculated broth, 200 μl from each culture were transferred into every well and incubated at 37°C for 48 h. At 8 h intervals, utilized broth was replaced with freshly prepared broth. The wells were emptied, and 50 μl of the respective extracts as well as 150 μl of fresh medium added. Equal amounts of DMSO and amoxicillin at 50 g ml−1 were added as negative and positive controls, respectively. Treated wells were incubated for 24 h and stained with 0.1% crystal violet for 5 min. All wells were then rinsed with sterile distilled water four times to remove spent media, plant extracts, and free-floating cells. The absorbance of the wells was determined at 490 nm using a microplate reader (model 680, Bio-Rad). Values of optical density (OD) were used to calculate the standard deviation (SD) and coefficient of variation to determine the stability of the organism against the antimicrobial activity of S. persica, and the percentage inhibition calculated for each extract (Murugan et al. Citation2010b).
Statistical analyses
Growth and biofilm-inhibition data were analyzed with statistical analysis computer software (SPSS 15.0 for Windows; SPS Chicago, IL, USA). All experiments were conducted in triplicate. Data are mean ± SD. One-way ANOVA was used to compare the effects of different extracts.
Determination of QS inhibition
The ability of methanol extracts of S. persica to inhibit biofilm formation was determined using a modified soft-agar overlay protocol as described by McLean et al. (Citation2004). Briefly, BHI agar (HiMedia) plates were prepared and divided into two parts. The test isolates of S. mutans were streaked on one-half, and the plant extract added into the well made in the other half. After incubation for 24 h at 300°C, 5 ml of soft agar containing the indicator organism Chromobacterium violaceum (ATCC 12472; American Type Culture Collection, Manassas, VA, USA) were overlaid, incubated, and observed for growth and pigmentation.
Gas chromatography–mass spectrometry (GC–MS) analyses of plant extracts
Among the four extracts, the methanol extract was selected based on its high-level activity. This extract was further subjected to phytochemical analyses. The bioactive components of this extract were identified by GC–MS. Compounds were separated by GC. The structures of the components were identified using a mass spectrophotometer. GC–MS analyses were carried out in a Clarus 500 Mass Spectrometer (PerkinElmer, Waltham, MA, USA) coupled to a Clarus 500 Mass Spectrometer mass detector under electron impact ionization (70 eV). The interface temperature was 280°C and the scan range 40–450 atomic mass units (AMU). The chromatographic column used for analyses was a capillary column: Elite 5ms (5% phenyl 95% dimethylpolysiloxane); size, 250 μm; and ID, 0.25 μm × 30 m. The stepped temperature program was held at 70°C for 2 min, increased from 70°C to 280°C at 10°C min−1, and held for 5 min. The total run time was 30 min. Helium was used as the carrier gas and injected at 1 ml min−1. The injection volume was 1 μl. The solvent delay was 2 min and injected in a split ratio of 1:10. Peaks () were identified by computer searching in a commercial mass spectral reference library (Wiley NIST).
Figure 1. GC–MS chromatogram of antibiofilm methanol extract of S. persica showing the compounds and their percentage abundance (parenthesis) 1. benzaldehyde (13.2); 2. benzyl chloride (1.6); 3. ethanone, 2-hydroxy-1-phenyl-(0.09); 4. benzyl isocyanate (7.2); 5. benzyl nitrile (2.0); 6. benzenecarboxylic acid (24.5); 7. l-[-]-4-hydroxy-1-methylproline(0.1); 8. benzaldehyde, 4-methoxy-(0.1); 9. benzaldehyde, 3-methoxy-(0.5); 10. 2-coumaranone (0.4); 11. benzeneacetic acid (0.07); 12. 3-benzyloxy-1-nitro-butan-2-Ol (0.01); 13. benzaldehyde, 4-(phenylmethoxy)- (0.3); 14. benzene isothiocyanate (18.1); 15. 2-(3’-phenylpropyl)-5-ethylpyridine (0.8); 16. (Z,Z)-à-farnesene (0.3); 17. N- benzylacetamide (10.9); 18. benzyl (6Z,9Z,12Z)-6,9,12-octadecatrienoate (0.02); 19. 1,3-cyclohexanedicarbohydrazide (0.01); 20. benzylidenebenzylamine (12.1); 21. 3à-hydroxy-3-methyl-6-phenyl-4-piperidone (0.8); 22. decanoic acid, methyl ester (0.1); 23. docosanoic acid, ethyl ester (0.08); 24. acetophenone benzyloxime (0.43); 25. benzamide, N-(4-methylphenyl)- (0.45); 26. pyrrole-2-carboxylic acid, 4-hydrazonomethyl-3,5-dimethyl-, ethyl ester(0.3).
![Figure 1. GC–MS chromatogram of antibiofilm methanol extract of S. persica showing the compounds and their percentage abundance (parenthesis) 1. benzaldehyde (13.2); 2. benzyl chloride (1.6); 3. ethanone, 2-hydroxy-1-phenyl-(0.09); 4. benzyl isocyanate (7.2); 5. benzyl nitrile (2.0); 6. benzenecarboxylic acid (24.5); 7. l-[-]-4-hydroxy-1-methylproline(0.1); 8. benzaldehyde, 4-methoxy-(0.1); 9. benzaldehyde, 3-methoxy-(0.5); 10. 2-coumaranone (0.4); 11. benzeneacetic acid (0.07); 12. 3-benzyloxy-1-nitro-butan-2-Ol (0.01); 13. benzaldehyde, 4-(phenylmethoxy)- (0.3); 14. benzene isothiocyanate (18.1); 15. 2-(3’-phenylpropyl)-5-ethylpyridine (0.8); 16. (Z,Z)-à-farnesene (0.3); 17. N- benzylacetamide (10.9); 18. benzyl (6Z,9Z,12Z)-6,9,12-octadecatrienoate (0.02); 19. 1,3-cyclohexanedicarbohydrazide (0.01); 20. benzylidenebenzylamine (12.1); 21. 3à-hydroxy-3-methyl-6-phenyl-4-piperidone (0.8); 22. decanoic acid, methyl ester (0.1); 23. docosanoic acid, ethyl ester (0.08); 24. acetophenone benzyloxime (0.43); 25. benzamide, N-(4-methylphenyl)- (0.45); 26. pyrrole-2-carboxylic acid, 4-hydrazonomethyl-3,5-dimethyl-, ethyl ester(0.3).](/cms/asset/3883b89e-da7e-466c-9ccb-424278c6c6cd/gbif_a_647308_o_f0001g.jpg)
Molecular docking studies
The molecular interaction between the bioactive compounds in S. persica and QS signaling proteins were determined using the LigandFit protocol available in Accelrys Discovery studio 2.5 (Accelrys, San Diego, CA, USA) for accurately docking ligands into the active sites of proteins. The structure of the S. persica phytochemical ligands, the antibiotic chlorhexidine, and the QS DNA-binding response regulators (PDB ID: 1NXO) from the Streptococcus sp. OmpR subfamily response regulator () and PDB ID: 3B2N from the Staphylococcus aureus LuxR family () were retrieved from public databases. Before beginning docking, the binding site of the receptor was specified. The method involved a cavity-detection algorithm for finding invaginations in the protein as candidate active-site regions. A shape comparison filter was combined with a Monte Carlo conformational search for generating ligand poses consistent with the shape of the active site. Candidate poses were minimized in the context of the active site using a grid-based method for evaluating protein–ligand interaction energies as explained by Venkatachalam et al. (Citation2003).
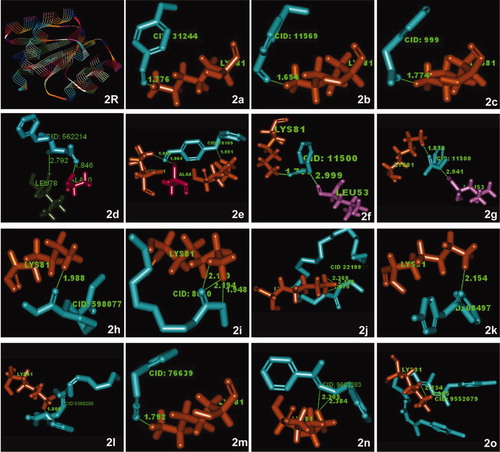
Figure 2. Crystal structure of quorum sensing response regulator (PDB ID: INXO) (R) and its super imposed structures of (a) benzaldehyde, 4-methoxy-; (b) benzaldehyde, 3-methoxy-; (c) benzeneacetic acid; (d) benzyloxy-1-nitro-butan-2-ol; (e) benzaldehyde, 4-(phenylmethoxy)-; (f,g) N-benzylacetamide; (h) 1,3-cyclohexane dicarbo hydrazide; (i) decanoic acid, methyl ester; (j) docosanoic acid, ethyl ester; (k) benzamide, N-(4-methylphenyl)-; (l) benzyl (6Z,9Z,12Z)-6,9,12-octadecatrienoate; (m) benzyl isocyanate; (n) acetophenone benzyloxime; (o) chlorohexidine. The illustration demonstrates close alignment of molecular components of S.persica phytochemicals and the QS protein. The antibiotic chlorhexidine (2o) also interacts in the same manner as most of the phytochemicals.
Results
The activity of S. persica phytochemicals on caries-causing isolates of S. mutans and their possible mode of action were determined through biofilm-specific methods. As the causative agent of dental caries and dental plaque, S. mutans was isolated and identified. Twenty isolates were obtained from samples from 25 patients. Isolates that produced blue-colored colonies, were Gram-positive rods in chains, and were catalase-negative were presumed to be Streptococcus spp. The identity of the selected isolates was confirmed by measuring enzymatic activity or using sugar fermentation tests. These data were then compared with the analytical profile index (API) of the API 20 STREP system. All 20 of these isolates were identified as S. mutans.
The tube adherence test, CRA plate method, and confirmation by microtiter plate assay revealed the biofilm-forming abilities of all the isolates of S. mutans. Fourteen out of the twenty isolates were moderately positive (+2), 5 were strongly positive (+3), and 1 isolate was biofilm-negative. Furthermore, the microtiter plate adherence assay indicated that biofilm formation by S. mutans SMS 09 recorded a higher OD value (1.14), and this was therefore selected for anti-biofilm studies. The results of the antimicrobial activity of S. persica extracts on biofilm-forming cariogenic S. mutans bacteria by the agar-well diffusion method revealed that all 5 extracts of S. persica showed anti-streptococcal activity (). The susceptibility of S. mutans to different extracts varied considerably, indicating the presence of more than one bioactive principle. Alcohol was found to be a better solvent for the extraction of antibacterially-active substances from S. persica against cariogenic bacteria as compared with water and hexane. The MIC of methanol extracts of S. persica that inhibited cariogenic S. mutans was 2.6 mg ml−1. When the anti-biofilm activity of methanol, ethanol, acetone, aqueous and hexane extracts were compared for their selective action on the selected biofilm-forming cariogenic isolate S. mutans SMS 09, biofilm formation was inhibited to 87.92% and 85.75% by the methanol and ethanol extracts, respectively. The lack of pigment production by the indicator organism (C. violaceum; ATCC 12472) around the growth of S. mutans and the extract-containing wells on BHI agar plates revealed quorum-sensing inhibition (QSI).
Table 1. Growth inhibition and biofilm inhibiting activity of S. persica extracts on caries causing S. mutans.
GC–MS analyses of the methanol extracts of S. persica resulted in the identification of 32 components (). The relative percentage of identified and isolated phytochemicals, their two-dimensional chemical structure, and their contributory important functional groups revealed more bioactive compounds and validated the use of S. persica as a natural toothbrush. Among the phytochemicals, benzaldehyde, benzyl isocyanate, benzenecarboxylic acid, benzene isothiocyanate, N-benzylacetamide, and benzylidene benzylamine were the major compounds. Most were phenolic, and these compounds proved to have varied biological activity. The present study confirmed the potentially rich antibacterial agents in S. persica and their activity against cariogenic organisms.
Table 2. Ligandfit docking scores of the bioactive compounds of S. persica and the antibiotic chlorhexidine with the active site of the DNA-binding response regulator (PDB ID: 1NXO).
Molecular interaction between the bioactive compounds of S. persica and QS regulatory proteins revealed a possible mechanism of biofilm inhibition through interference of the QS pathway. The three-dimensional structural information on QS transcriptional regulators, DNA-binding response regulators (PDB ID: 1NXO and PDB ID: 3B2N), and inhibitors from S. persica phytochemicals for the target proteins were obtained from public databases, and their molecular interaction was determined by computer-aided drug design to reveal their level of interaction. The LigandFit protocol analyses suggested that 15 compounds among 32 phytochemicals as well as the antibiotic chlorhexidine showed higher scores when docked with the QS DNA-binding response regulator (PDB ID: 1NXO) (). The receptor-site amino acids LEU 53, ALA 80, LYS 81 and LYS 101 interacted more than others with the ligands (). 3-Benzyloxy-1-nitro-butan-2-ol was perfectly docked with the DNA-binding response regulator (PDB ID: 3B2N) and scored 26.985 with low internal energy (–2.548). It docked with the receptor-site amino acid ARG 108 with a distance of 3.206 Å. Conversely, 1,3-cyclohexane dicarbohydrazide yielded a dock score of 24.032 when docked with the same target protein (Table 3). The receptor-site amino acid ARG108 interacted with a distance of 2.824 Å (). These results indicated that the phytochemicals found in S. persica could interact not only with the QS response regulators of Streptococcus spp. but also with other organisms such as S. aureus. Also, the molecular docking studies suggested that the compounds benzyl (6Z,9Z,12Z)-6,9,12-octadecatrienoate and 3-benzyloxy-1-nitro-butan-2-ol might interact more efficiently with QS system regulators than with other compounds.
Figure 3. The DNA-binding response regulator (PDB ID: 3B2N) (R), LuxR family from S. aureus that was used for docking experiments with the bioactive compounds of S. persica 3-benzyloxy-1-nitro-butan-2-ol (a) and 1,3-cyclohexanedicarbohydrazide (b). The illustration demonstrates close alignment of the molecular components.
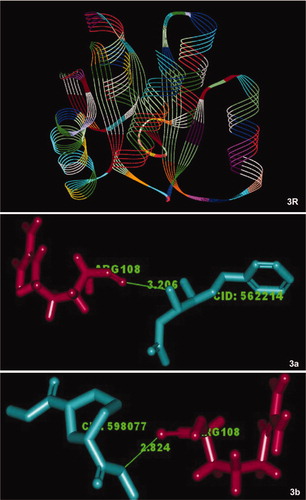
Table 3. Docking scores (highest) of the bioactive compounds of S. persica in the active site of 3B2N, the DNA-binding response regulator, Luxr family.
Discussion
The use of plants has been closely associated with dental hygiene and therapeutic practices for thousands of years (More et al. Citation2008). Many cultures do not use plastic-bristle brushes. Instead, herbal chewing sticks taken from plants, shrubs, or trees with high antimicrobial activity (Hobbs Citation2008) are used for relieving dental problems. Several clinical studies confirmed that chewing sticks, if used appropriately, can be as efficient as toothbrushes in removing dental plaque due to the combined effect of mechanical cleaning, enhanced salivation and leaching-out of antimicrobial substances (Wu et al. Citation2001). The relationship between the pathogenic state and the mode of growth of biofilms has been most clearly established with oral streptococci, which are known to initiate dental caries if they are living in the biofilm environment of dental plaque (Cvitkovitch et al. Citation2003). The lifecycle of biofilms of the S. mutans isolate has revealed its oral adaptation to recurrently exposed stresses such as extreme shortage or excess of nutrients, low pH, high osmolarity, oxidation, and consumption of antimicrobial agents or antibiotics (Donlan and Costerton Citation2002).
Investigations on antibacterial plants and their active constituents have focused entirely on planktonic bacteria with little emphasis on the more resistant and difficult to control biofilm forms (Sandasi et al. Citation2010). The present study highlighted not only the bioactive phytochemicals of S. persica but also revealed their importance in cleansing teeth through mechanical and antibacterial actions. Several studies reported the antibacterial activity and associated role of Asian (Almas Citation2001), African (Ndukwe et al. Citation2004; van Vuuren and Viljoen Citation2006), Ethiopian (Kassu et al. Citation1999), Indian (Hebbar et al. Citation2004), and Nigerian (Fadulu Citation1975) natural toothsticks in maintaining oral hygiene. In the present study, the susceptibility of S. mutans to different extracts varied considerably, indicating the presence of more than one bioactive principle. Alcohol was found to be a better solvent for the extraction of antibacterial substances from S.persica active against cariogenic bacteria compared with water and hexane, which corroborates the results obtained by other authors (Ahmad et al. Citation1998; Hwang et al. Citation2004; Murugan et al. Citation2010a) using other plants. Interestingly, the methanol extract was more potent than other extracts. The increased dissolution of active constituents in methanol indicated the presence of more phenolic compounds and extraction due to their release from cell wall-bound polyphenols by the low-polarity methanol (Lapornik et al. Citation2005). Isolation and identification of constituents that exhibit anti-biofilm properties may be important alternatives in the control of biofilms (Sandasi et al. Citation2010). GC–MS of S. persica methanol extracts revealed a large number of phytochemicals comprising many groups (although most were phenolic). Although numerous bioactive substances have been isolated from plants and their modes of action investigated, relatively few studies have been done in dental research (Song et al. Citation2007). In general, it is recognized that plant polyphenols bind strongly to proteins through reversible interactions. The ultimate fate of these reactive species has been relatively neglected (Robards Citation2003). Most of the phytochemicals in S. persica have polyphenolic structures and are therefore good donors of hydrogen. Hence, hydrogen bonding may enable them to bind strongly to proteins and nucleic acids. Earlier studies failed to predict the exact mechanism for the inhibitory effects of S. persica on biofilm formation. The use of quantitative structure–activity relationships (QSARs) and molecular modeling of bioactive species assessing structure–activity relationships can result in identification of compounds with a high level of bioactivity, low degree of toxicity, and optimal pharmacokinetic properties (Suntharalingam and Cvitkovitch Citation2005).
It has been observed that inactivation of any component of competence-stimulating peptide-quorum sensing (CSP-QS), ie the main S. mutans QS pathway, results in competence and biofilm-defective phenotypes, and that CSP appears to influence the initial stages of biofilm formation (Li et al. Citation2001, Citation2002). These pathways can be exploited for a selective and targeted approach to control dental caries-causing biofilms. Inactivation of any of the genes encoding the components of the QS signaling system results in formation of an abnormal biofilm (Cvitkovitch et al. Citation2003). Hence, biofilm formation can be prevented by searching for molecules that interfere with cell-to-cell communication. The ability to prevent or confuse QS might stop expression of biofilm and virulence genes. To date, no such techniques are available for clinical use (Cooper and Okhiria Citation2006). Bacterial adaptation to changing environmental conditions can be thought of as occurring on several different levels, including the multicellular level (cell aggregation and biofilm formation). These regulatory processes are undertaken by various response regulators, and the patterns of distribution of response regulators are generally conserved within key microbial lineages. For instance, Lux R shows a phylogenetic distribution among most bacterial phyla except for Chlamydiae and Thermotogae (Galperin Citation2010). Hence, compounds that can interact with these response regulators can be ideal candidates for multi-species biofilms. The present molecular docking studies revealed the close interaction between the QS response regulators of the cariogenic S. mutans and the biofilm-forming S. aureus.
The bioactive, dual-function, anti-biofilm agents in S. persica not only inhibit growth, but also control the colonization and accumulation of caries-causing S. mutans. Their use may offer a novel strategy to combat and reduce the development of dental caries. The widely adopted caries-reduction approach employing chemoprophylatic agents is associated with several undesirable side effects. Hence, the results of the present study may increase comprehension of the causes, incidence, prevention and control of dental caries. S. mutans does not occur in monoculture in vivo. Hence, the precise bioactive compounds to be used in the control of oral biofilms have to be investigated by employing in vivo models as well as biofilm models.
The results of the present study indicated that S. persica contains bioactive anti-biofilm agents with dual functionalities of growth inhibition and QS regulator interaction. S. persica may offer a novel strategy to reduce the development of dental caries by inhibiting the initial adhesion and subsequent biofilm formation by cariogenic bacteria. Several terrestrial plants are known to produce “QS mimics” capable of controlling bacterial QS. A recent study identified QS inhibitors in various medicinal plants commonly found in southern Florida (Vattem et al. Citation2007). Thus, S. persica could be a rich source of compounds that may affect (positively or negatively) at least two major QS systems in biofilm-forming cariogenic bacteria. If so, then the engineering or breeding of plants to produce specific QS mimics to affect specific bacterial pathogens or symbionts could be an important future application (Bauer and Robinson Citation2002). Further in vitro studies are necessary to clarify the anti-biofilm activity of the active constituents. Nevertheless, the present study demonstrated the anti-cariogenic potential and presence of possible QS inhibitors of S. persica.
Acknowledgements
The authors acknowledge the Rector, KSU and the Deanship of Scientific Research, College of Science Research Center, King Saud University for their support of this project.
References
- Ahmad , I , Mehmood , Z and Mohammad , F . 1998 . Screening of some Indian medicinal plants for their antimicrobial properties . J Ethnopharmacol , 62 : 183 – 193 .
- Al-Anbori , D KA , Al-Nimer , M SM and Al-Weheb , A M . 2008 . Antibacterial activity of ethanolic extract of Myrtus communis. L leaves against salivary mutans streptococci . Saudi Dental J , 20 : 82 – 87 .
- Almas , K . 2001 . The antimicrobial effects of seven different types of Asian chewing sticks . Odontostomatol Trop , 24 : 17 – 20 .
- Al-Otaibi , M . 2004 . The miswak (chewing stick) and oral health. Studies on oral hygiene practices of urban Saudi Arabians . Swed Dental J Suppl , 167 : 2 – 75 .
- Al-Otaibi , M , Al-Harthy , M , Gustafsson , A , Johansson , A , Claesson , R and Angmar-Månsson , B . 2004 . Subgingival plaque microbiota in Saudi Arabians after use of miswak chewing stick and toothbrush . J Clin Periodont , 31 : 1048 – 1053 .
- Babpour , E , Abdolhamid , Angaji S and Mahdi , Angaji S . 2009 . Antimicrobial effects of four medicinal plants on dental plaque . JMPR , 3 : 132 – 137 .
- Batwa , M , Bergström , J , Batwa , S and Al-Otaibi , M F . 2006 . The effectiveness of chewing stick miswak on plaque removal . Saudi Dental J , 18 : 125 – 133 .
- Bauer , W D and Robinson , J B . 2002 . Disruption of bacterial quorum sensing by other organisms . Curr Opin Biotechnol , 13 : 234 – 237 .
- Christensen , G D , Simpson , W A , Bisno , A L and Beachey , E H . 1982 . Adherence of slime producing strains of Staphylococcus epidermidis to smooth surfaces . Infect Immun , 37 : 318 – 326 .
- Cooper , R and Okhiria , O . 2006 . Biofilms, wound infection and the issue of control . Wounds UK , 2 : 48 – 57 .
- Cowan , M M . 1999 . Plant products as antimicrobial agents . Clin Microbiol Rev , 12 : 564 – 582 .
- Cvitkovitch , D G , Li , Y H and Ellen , R P . 2003 . Quorum sensing and biofilm formation in streptococcal infections . J Clin Invest , 112 : 1626 – 1632 .
- Darout , I A , Albandar , J M and Skaug , N . 2000 . Periodontal status of adult Sudanese habitual users of miswak chewing sticks or toothbrushes . Acta Odontol Scand , 58 : 25 – 30 .
- Donlan , R M and Costerton , J W . 2002 . Biofilms: survival mechanisms of clinically relevant microorganisms . Clin Microbiol Rev , 15 : 167 – 193 .
- Fadulu , S O . 1975 . The antibacterial properties of the buffer extracts of chewing sticks used in Nigeria . Planta Med , 27 : 122 – 126 .
- Freeman , D J , Falkiner , F R and Keane , C T . 1989 . New method for detecting slime production by coagulase negative staphylococci . J Clin Pathol , 42 : 872 – 874 .
- Galperin , M Y . 2010 . Diversity of structure and function of response regulator output domains . Curr Opin Microbiol , 13 : 150 – 159 .
- Han , S S , Baek , K W , Shin , M H , Kim , J , Oh , C S , Lee , S J and Shin , D H . 2010 . Dental caries prevalence of medieval Korean people . Arch Oral Biol , 55 : 535 – 540 .
- Hebbar , S S , Harsha , V H , Shripathi , V and Hegde , G R . 2004 . Ethnomedicine of Dharwad district in Karnataka, India – plants used in oral health care . J Ethnopharmacol , 94 : 261 – 266 .
- Hobbs , C . 2008 . Importance of herbs for dental health Available from: http://worldental.org/oralhygiene/importance-of-herbs-for-dental-health/121/
- Hwang , J H , Shim , J S and Chung , J Y . 2004 . Anticariogenic activity of some tropical medicinal plants against Streptococcus mutans . Fitoterapia , 75 : 596 – 598 .
- Kassu , A , Dagne , E , Abate , D , De Castro , A and van Wyk , BE . 1999 . Ethnomedical aspects of the commonly used toothbrush sticks in Ethiopia . East Afr Med J , 76 : 651 – 653 .
- Kelmanson , J E , Jager , A K and van Staden , J . 2000 . Zulu medicinal plants with antibacterial activity . J Ethnopharmacol , 69 : 241 – 246 .
- Kubo , I , Muroi , H and Himejima , M . 1992 . Antimicrobial activity against Streptococcus mutans of mate tea flavor components . J Agric Food Chem , 4 : 245 – 248 .
- Lapornik , A , Prosek , M and Wondra , G A . 2005 . Comparison of extracts prepared from plant byproducts using different solvents and extraction time . J Food Eng , 71 : 214 – 222 .
- Li , Y H , Lau , P CY , Lee , J H , Ellen , R P and Cvitkovitch , D G . 2001 . Natural genetic transformation of Streptococcus mutans growing in biofilms . J Bacteriol , 183 ( 3 ) : 897 – 908 . DOI: 10.1128/JB.183.3.897-908.2001
- Li , Y H , Tang , N , Aspiras , M B , Lau , P CY , Lee , J H , Ellen , R P and Cvitkovitch , D G . 2002 . A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation . J Bacteriol , 184 : 2699 – 2708 .
- McLean , R JC , Pierson , L S and Fuqua , C . 2004 . A simple screening protocol for the identification of quorum signal antagonists . J Microbiol Methods , 58 : 351 – 360 .
- More , G , Tshikalange , T E , Lall , N , Botha , F and Meyer , J JM . 2008 . Antimicrobial activity of medicinal plants against oral microorganisms . J Ethnopharmacol , 119 : 473 – 477 .
- Motegi , M , Takagi , Y , Yonezawa , H , Hanada , N , Terajima , J , Watanabe , H and Senpuku , H . 2006 . Assessment of genes associated with Streptococcus mutans biofilm morphology . Appl Environ Microbiol , 72 : 6277 – 6287 .
- Murugan , K , Selvanayaki , K and Al-Sohaibani , S . 2010a . Antibiofilm activity of Andrographis paniculata against cystic fibrosis clinical isolate Pseudomonas aeruginosa . World J Microbiol Biotechnol , 27 : 1661 – 1668 .
- Murugan , K , Usha , M , Malathi , P , Al-Sohaibani , S and Chandrasekaran , M . 2010b . Biofilm forming multi drug resistant Staphylococcus spp. among patients with conjunctivitis . Pol J Microbiol , 59 : 233–239
- Ndukwe , K C , Lamikanra , A and Okeke , I N . 2004 . Antibacterial activity in plants used as chewing sticks in Africa . Drugs Future , 29 : 1221 – 1233 .
- Pitts , B , Hamilton , M A , Zelver , N and Stewart , P S . 2003 . A microtiter plate method for biofilm disinfection and removal . J Microbiol Methods , 54 : 269 – 276 .
- Robards , K . 2003 . Strategies for the determination of bioactive phenols in plants, fruit and vegetables . J Chromatogr A , 1000 : 657 – 691 .
- Sandasi , M , Leonard , C M and Viljoen , A M . 2010 . The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes . Lett Appl Microbiol , 50 : 30 – 35 .
- Singh , J , Kumar , A , Budhiraja , S and Hooda , A . 2007 . Ethnomedicine: use in dental caries . Braz J Oral Sci , 6 : 1308 – 1312 .
- Song , J H , Yang , T C , Chang , K W , Han , S K , Yi , H K and Jeon , J G . 2007 . In vitro effects of a fraction separated from Polygonum cuspidatum root on the viability, in suspension and biofilms, and biofilm formation of mutans streptococci . J Ethnopharmacol , 112 : 419 – 425 .
- Stevens , A M , Dolan , K M and Greenberg , E P . 1994 . Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region . P Natl Acad Sci USA , 91 : 12619 – 12623 .
- Suntharalingam , P and Cvitkovitch , D G . 2005 . Quorum sensing in streptococcal biofilm formation . Trends Microbiol , 13 : 3 – 6 .
- Tredwin , C J , Scully , C and Bagan-Sebastian , J V . 2005 . Drug-induced disorders of teeth . J Dent Res 2005 , 84 : 596 – 602 .
- van Vuuren , SF and Viljoen , A M . 2006 . The in vitro antimicrobial activity of toothbrush sticks used in Ethiopia . S Afr J Bot , 72 : 646 – 648 .
- Vattem , D A , Mihalik , K , Crixell , S H and McLean , R JC . 2007 . Dietary phytochemicals as quorum sensing inhibitors . Fitoterapia , 78 : 302 – 310 .
- Venkatachalam , C M , Jiang , X , Oldfield , T and Waldman , M . 2003 . LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites . J Mol Graph Model , 21 : 289 – 307 .
- Wong , R WK , Hägg , U , Samaranayake , L , Yuen , M KZ , Seneviratne , C J and Kao , R . 2010 . Antimicrobial activity of Chinese medicine herbs against common bacteria in oral biofilm. A pilot study . Int J Oral Maxillofac Surg , 39 : 599 – 605 .
- Wu , C D , Darout , I A and Skaug , N . 2001 . Chewing sticks: timeless natural toothbrushes for oral cleaning . J Periodont Res , 36 : 275 – 284 .
- Yoo , S Y , Park , S J , Jeong , D K , Kim , K W , Lim , S H , Lee , S H , Choe , S J , Chang , Y H , Park , I and Kook , J K . 2007 . Isolation and characterization of the mutans streptococci from the dental plaques in Koreans . J Microbiol , 45 : 246 – 255 .