Abstract
The pathogenic fungus, Histoplasma capsulatum, causes the respiratory and systemic disease ‘histoplasmosis’. This disease is primarily acquired via inhalation of aerosolized microconidia or hyphal fragments of H. capsulatum. Evolution of this respiratory disease depends on the ability of H. capsulatum yeasts to survive and replicate within alveolar macrophages. It is known that adhesion to host cells is the first step in colonization and biofilm formation. Some microorganisms become attached to biological and non-biological surfaces due to the formation of biofilms. Based on the importance of biofilms and their persistence on host tissues and cell surfaces, the present study was designed to investigate biofilm formation by H. capsulatum yeasts, as well as their ability to adhere to pneumocyte cells. H. capsulatum biofilm assays were performed in vitro using two different clinical strains of the fungus and biofilms were characterized using scanning electron microscopy. The biofilms were measured using a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium-hydroxide (XTT) reduction assay. The results showed that both the H. capsulatum strains tested were very efficient at adhering to host cells and forming biofilm. Therefore, this is a possible survival strategy adopted by this fungus.
Keywords:
Introduction
Histoplasma capsulatum var. capsulatum is a dimorphic fungus that causes the respiratory and systemic disease ‘histoplasmosis’ (Pervez et al. Citation2010). Histoplasmosis is endemic in areas of the USA (Ohio and Mississippi valleys) and in most of the Latin American countries, where a mild to severe form of the disease has frequently occurred (Taylor et al. Citation1996, Citation2005; Zancopé-Oliveira et al. Citation2005; Muñoz et al. Citation2010). Outbreaks of acute histoplasmosis have been reported, in the following Brazilian states after airborne fungal-propagules exposure: Sao Paulo, Rio de Janeiro, Espírito Santo, Mato Grosso, and Minas Gerais (Ferreira and Borges Citation2009). H. capsulatum is primarily acquired via inhalation of infective microconidia or hyphal fragments. The course of the associated respiratory disease depends on the ability of the H. capsulatum yeast to survive and replicate within the alveolar macrophages. After infection of the lungs, and depending on the immunological status of the host, the yeast can disseminate to other organs of the phagocytic mononuclear system, specifically the spleen and liver, causing the most lethal form of histoplasmosis (Ajello Citation1998; Deepe Citation2009; Dias et al. Citation2011). Clinical manifestations of this disease range from asymptomatic infection in an immune-competent host after low-inoculum exposure to a rapidly fatal and disseminated infection in the severely immunocompromised host. The role of cellular immunity in the defense against H. capsulatum has been well documented by several authors (Deepe Citation2009; Antonello et al. Citation2011; Kroetz and Deepe Citation2012). As a saprobe fungus, its strategies for successful pathogenesis include mold-to-yeast transition, entry into host phagocytes (primarily macrophages and dendritic cells), intracellular survival, proliferation during clinically unapparent infection, and sometimes promoting a reactivation mechanism. The ability to grow with different morphologies implies that the microorganism is able to adapt to different living conditions by expressing specific genes, whose products are critical to survival and colonization in a particular environment (López Citation2006).
H. capsulatum has two different morphotypes, a saprobe-geophilic and infective morphotype (mycelial-phase) in special environments of accumulated bird and bat guano or a parasitic and virulent morphotype (yeast-phase) preferentially found in an intracellular microenvironment of host mammals, including humans (Rooney et al. Citation2001).
To date, a few virulence factors have been identified for H. capsulatum, viz. mechanisms for iron acquisition, a secreted small protein that is able to bind Ca++ at a low concentration (Cbp1), an extracellular yeast phase specific protein (Yps3), and the cell wall polysaccharide, α-(1,3)-glucan. The last two factors are localized to the surface of H. capsulatum strains (Weaver et al. Citation1996; Rappleye et al. Citation2004; Bohse and Woods Citation2007) and are associated with its adhesion mechanism to host cells.
H. capsulatum yeasts may adhere to lung, spleen, liver, gut, and have been seen in trachea cryosections of Artibeus hirsutus bats and BALB/c mice (Suárez-Alvarez et al. Citation2010). A study performed by Suárez-Alvarez et al. (Citation2010) evaluated the adherence pattern of H. capsulatum yeasts with tissue sections from different organs of bats, in vitro. The results showed that lung tissue had the largest number of adhered yeasts and the trachea had the smallest number. In addition, many H. capsulatum yeasts that adhered to the lung parenchyma were predominantly distributed in the alveolar epithelium. This suggests that many adhesion sites prevail in the lung and are probably associated with the cell surface or extracellular matrix components (adhesins), which recognize the H. capsulatum yeasts.
Some yeasts, such as Candida albicans, non-Candida species, and Cryptococcus neoformans, and including filamentous molds such as Aspergillus fumigatus, have been shown to be implicated in biofilm-associated infections (Ramage et al. Citation2009). Biofilms are dynamic communities of microorganisms that are persistently attached to biological and nonbiological surfaces. Biofilm formation represents the most common method of growth for microorganisms in nature, a state that presumably allows microbial cells to survive in hostile environments and to disperse for colonizing new niches.
Biofilms are embedded in a matrix, forming a complex three-dimensional architecture (Chandra et al. Citation2001), characterized by a mesh of fibrillar polysaccharides (matrix) with microorganisms (Man et al. Citation1998; Cappelli et al. Citation2000). The physiological characteristics of these organisms include an altered growth rate and the fact that biofilm organisms transcribe genes not expressed in planktonic organisms (Donlan and Costerton Citation2002). In addition, there are reports indicating that microorganisms present in biofilms are able to release compounds such as endotoxins, polysaccharides, muramyl peptides, and DNA (Lonnemann Citation2000; Cappelli et al. Citation2007; Pierce et al. Citation2008).
During early biofilm formation, yeast cells adhere to an appropriate surface. The intermediate phase is characterized by continued adherence and production of an extracellular matrix, which consists of cell wall polysaccharides and proteins (Baillie and Douglas Citation2000).
Several methods for biofilm analysis and characterization have evolved, although scanning electron microscopy (SEM) is still heavily relied upon (Martinez and Casadevall 2007). Colorimetric methods can be used to evaluate cell viability. In this context, the use of tetrazolium salts (hydroxide tetrazolium -2,3-bis (2-methoxy-4-nitro-5-sulfenyl)-5- [(phenylamino) carbonyl]-2H-tetrazoliumhydroxide-) (XTT) represents one of the most widespread methods (Hawser et al. Citation1998; Nett et al. Citation2011; Zhou et al. 2012).
Biofilms have been described as a protective niche against microbial predators in nature and have been shown to enhance resistance against antifungal agents and specific mediators of host immune responses. Additionally, the biology of H. capsulatum is complex and determining the mechanisms involved in biofilm formation will help to establish potential targets and new antifungal drug regimens and preventing infection. Based on the ability of biofilms to survive in hostile environments and their role for spreading and colonizing host tissues, this study focused on investigating the ability of H. capsulatum yeasts to adhere to pneumocytes as well as their ability to form biofilm.
Materials and methods
Strains and growth conditions
Two H. capsulatum strains (EH-315 and 60I) were used for all experimental assays. The EH-315 strain was isolated from the intestine of infected bats captured in a cave in the state of Guerrero, Mexico. This strain is deposited in the H. capsulatum Culture Collection of the Fungal Immunology Laboratory of the Department of Microbiology and Parasitology, from the School of Medicine, National Autonomous University of Mexico (UNAM) ( www.histoplas-mex.unam.mx). This collection is registered in the database of the World Federation for Culture Collections under the number LIH-UNAM WDCM817. The 60I strain was isolated from the mouth of a patient infected in the city of Araraquara, São Paulo, Brazil, and it is deposited in the collection of strains at the Clinical Mycology Laboratory of the Faculty of Pharmaceutical Sciences, UNESP, Brazil.
The yeast-phase of each strain was maintained at 37°C in brain–heart infusion (BHI-broth) (Difco™) supplemented with 0.1% l-cysteine, and 1% glucose.
Adhesion assays
The continuous epithelial cell line, A549 cells, characterized as pneumocytes was used for adhesion testing to evaluate the pattern of adherence by H. capsulatum, as described by Mendes-Giannini et al. (Citation2004).
The epithelial cells were grown in glass bottles, in a culture medium specific for this cell line, and maintained at 36.5°C. After 3–4 days, the cell line monolayer formed was trypsinized. Briefly, the cell monolayer was washed with 1 ml of 0.2% trypsin solution and 0.02% Versene (ATV), and then 1 ml of ATV was added. In the following 1 to 2 min, the cells were mixed with variable amounts of their corresponding medium plus 10% fetal calf serum. At this stage, the trypsin was neutralized by the fetal bovine serum present in the culture medium. The total volume of cell suspension obtained was transferred to new bottles to obtain cell concentrations of 106 cells ml−1.
Cells were subcultured 2 days in advance. This procedure included trypsinization with 2 ml of ATV and the addition of 20 ml of culture medium. Five hundred μl of this mixture were placed on coverslips in each well of a 24-well plate (50,000 cells well−1).
After adjusting the yeast inoculum (1 × 106–5 × 106), the infection was initiated. Initially, the old medium was removed from the wells containing the cell line monolayer and 500 μl of fresh medium were added. Then, 500 μl of yeast suspension were added to each corresponding plate-well, followed by incubation at 37°C, for 5 h. The supernatant was then removed and the wells were washed three times with PBS.
Finally, 500 μl of 4% formaldehyde were added to each well and incubated overnight at 4°C. Then, the infected plates were washed three times with PBS and the potential fungal infection of pneumocytes was observed by using images processed by the IN Cell Analyzer 2000 System. All nuclei were DAPI stained and actin filaments were revealed with phalloidin-FITC. H. capsulatum was detected with an Alexa 594-conjugated antibody. The assay was performed in duplicate.
Biofilm assay
The assay was performed as described by Peeters et al. (Citation2008), with slight modifications. Initially, 500 μl of a 5 × 106 yeast cells ml−1 culture in saline were added to the wells of a 24-well plate (TPP®, Trasadingen, Switzerland) containing coverslips. The plates were incubated in an orbital shaker at 37°C for 7 h at 80 rpm for biofilm pre-adhesion. After pre-adhesion for 7 h the supernatant was removed from each well. Subsequently, 2000 μl of supplemented BHI-broth were added to each well and the plates were further incubated for 72 h. Then, after pre-adhesion for 7 h and biofilm formation for 72 h, the supernatant was again removed and the wells were rinsed using 200 μl of physiological saline. Six wells were filled with sterile medium as a control. All assays were repeated at least three times per strain. H. capsulatum biofilm formation in vitro on the coverslips was characterized at the structural level using scanning electron microscopy (SEM).
Measurement of biofilm metabolic activity
A quantitative measurement of H. capsulatum biofilm formation was obtained from the XTT reduction assay. For H. capsulatum yeasts, 50 μl of XTT salt solution (1 mg ml−1 in PBS) and 4 μl of menadione solution (1 mM in ethanol; Sigma) were added to each well of microtiter plates and incubated at 37°C for 3 h. Fungal mitochondrial dehydrogenase activity reduces XTT tetrazolium salt to XTT formazan, resulting in a colorimetric reaction that correlates with cell viability. This reaction was measured using a microtiter reader (iMark™ Microplate Reader; BIO-RAD) at 490 nm. In all the assays BHI-broth samples were included as negative controls (Martinez and Casadevall Citation2007).
SEM
Biofilms formed on coverslips were processed as described by Morris et al. (Citation1997) and modified by Ells and Truelstrup Hansen (2006). Briefly, the specimens were washed three times with PBS to remove planktonic cells and then fixed with 1% glutaraldehyde in a sodium cacodylate buffer (0,2 M, pH 7.2) for 18 h at 4°C. After three PBS washes of 10 min each, the biofilms adhered on the coverslips were fixed with 1% osmium tetroxide for 2 h. The specimens were washed with PBS and dehydrated with an increasing gradient of ethanol, from 50% to 100% ethanol, at room temperature. The samples were dried using the critical point method in a Samdri 780A desiccator (Rockville, MD, USA) using CO2. The samples were mounted on aluminum cylinders with silver paste and placed in a high vacuum evaporator for gold coating for 6 min in a metal ionizing Balzer Sputtering SCD-030 (Balzers Instruments, Balzers, Liechtenstein). Topographic features of biofilms were analyzed with a Zeiss-Leica/440 SEM at the Institute of Chemistry of Sao Carlos, University of Sao Paulo, using a 25 kV and a 10 mm working distance.
Statistical analysis
All data were subjected to statistical analysis using Origin 7.0 (Origin Lab Corporation, Northampton, MA). P values were calculated by t test. P values of <0.05 were considered significant.
Results
Adhesion of H. capsulatum to pneumocytes A549
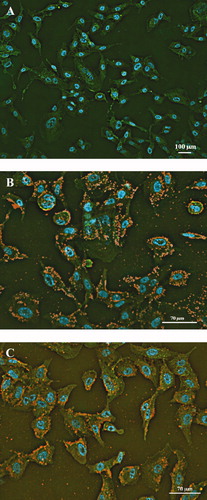
Adhesion of two H. capsulatum yeast cells (EH-315 and 60I) to pneumocytes was evaluated using IN Cell Analyzer 2000 System light microscopy (). Both strains were able to adhere to the A549 pneumocytes and both strains produced an adherent pattern of H. capsulatum showing a mass of yeast cells that resulted in compact structures tenaciously adhered to the pneumocytes (). A shows the non-infected pneumocyte controls.
Figure 1. Adherence patterns of H. capsulatum yeast to pneumocytes. Immunofluorescence staining of non-infected pneumocytes (A). Infection after incubation for 7 h with the EH-315 strain in pneumocytes (B) and the 60I strain in pneumocytes (C). Phalloidin-FITC (green); DAPI (blue); anti-Histoplasma polyclonal serum plus Alexa 594-conjugated antibody (red-to-yellow). The assays were conducted in an IN Cell Analyzer 2000 using light microscopy.
H. capsulatumbiofilms
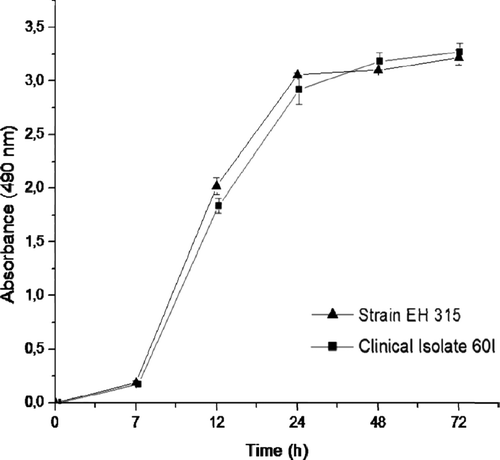
The kinetics of biofilm formation by EH-315 and 60I H.capsulatum strains on polystyrene microtiter plates were compared using the colorimetric XTT reduction assay to determine metabolic activity. Interestingly, both strains produced consistent biofilms from 24 h to 72 h. The results show that the initial formation of the biofilm was observed earlier than 7 h, which included the adhesion period. Over a period of 12–24 h, an increasingly larger biofilm was observed. The metabolic activity of the biofilm increased over time as the cellular mass increased (). The kinetics of biofilm formation was similar for both strains and did not show statistically significant differences (P < 0.005).
Figure 2. Kinetics of biofilm formation by H. capsulatum on polystyrene microtiter plates. The two H. capsulatum strains were processed by the colorimetric XTT reduction assay as described in Materials and methods. The measurement average data of three XTT assays was considered to generate the figure. The assay was performed twice, with similar results. P values of <0.05 were considered significant.
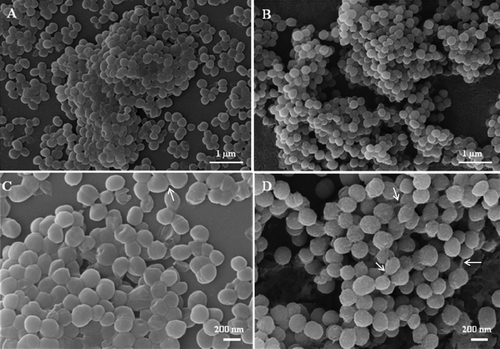
During the adhesion period (7 h), the H. capsulatum yeasts became attached to the plastic surface in a monolayer arrangement and eventually formed a surface attached community (). At the intermediate stage (12 h), the fungal population increased and consisted of yeast cells spread over the plastic surface, forming microcolonies. During the maturation stage (24 to 72 h), the microarchitecture of the H. capsulatum biofilms became more complex due to an increase in the amount of extracellular material surrounding the cells and resulted in compact structures that persistently adhered to the plastic surface (). SEM revealed the biofilm formation to be a highly organized network of fungal cells ().
Figure 3. Microphotograph taken using a camera attached to an inverted microscope of the kinetics of biofilm formation by H. capsulatum in polystyrene microtiter plates after aspiration of BHI medium and subsequent washings with PBS, as determined by the colorimetric XTT reduction assay. The average of three XTT assay measurements was taken. This experiment was performed twice, with similar results each time. Bars = 100 μm for panels A, B, C, and D.
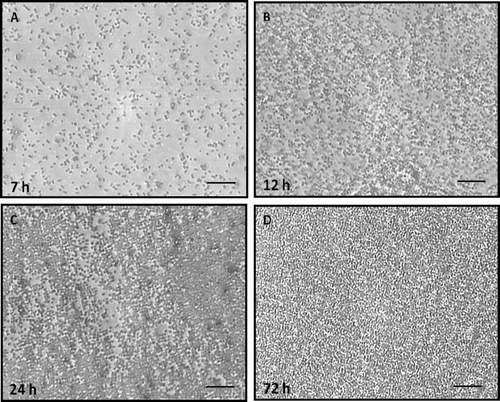
Figure 4. SEM images of mature biofilms of the EH-315 Mexican bat strain of H. capsulatum formed after incubation for 72 h at 37°C (A, C) and of the 60I Brazilian clinical strain of H. capsulatum also formed after incubation for 72 h at 37°C (B, D). Arrows denote yeasts cells bound by an exopolymeric matrix.
Discussion
Interactions of human pathogenic fungi with the host tissues are key factors in the pathogenesis of mycoses (Tronchin et al. Citation2008). This study demonstrated the ability of H. capsulatum cells to adhere to pneumocytes and their capability for biofilm formation. The two strains of H. capsulatum were able to adhere to epithelial lineage cells. This feature has not received much attention in the literature. The majority of studies have shown macrophages in phagocytosis of this fungus (Kurokawa et al. Citation1998). H. capsulatum infects either professional (neutrophils, macrophages, and dendritic cells) or non-professional phagocytes (endothelial and epithelial cells). Because of its intracellular preference, the fungus needs to avoid exposure to toxic reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Nittler et al. Citation2005) for successful parasitism, and opsonization has no effect on the ability of the organisms to proliferate within macrophages Yeasts were demonstrated both in alveolar epithelial cells and endothelial cells, suggesting that infected endothelial cells can facilitate the lymphohematogenous spread of the organism (Kurokawa et al. Citation1998).
The demonstration that H. capsulatum yeasts can adhere to different organ cryosections also provides evidence of an important mechanism for yeast colonization and dissemination that involves yeast cell adherence to host tissue architecture, probably including cellular surfaces and extracellular components (Suárez-Alvarez et al. Citation2010).
The present results indicate that both strains of H. capsulatum were able to form biofilm and adhere to epithelial A549 cells (pneumocytes). A large number of yeast cells were observed to be adhered to the cells in the cell line ().
Microbial adhesion can be divided into primary and secondary stages. The first stage is reversible and is determined by physico-chemical interactions such as hydrophobicity, electrostatic and Van der Waals forces, temperature and hydrodynamic forces, all of which determine the adhesion between the microbial cell and the area of interest. Secondary adherence occurs between molecular adhesins for a specific surface, in which the microorganism consolidates attachment by producing an exopolysaccharide complex and/or ligand-specific receptors with the material surface/ or cells. At the end of this stage, the attachment is considered irreversible (Costerton et al. Citation1987; Vesterlund et al. Citation2005). Several adhesins for different host ligands, sometimes with multifunctional properties, have been described. Some have been extensively characterized, and their expressions analyzed according to morphological changes or culture conditions. For some ligands, the amino acid or carbohydrate motifs participating in these interactions have been identified. Various host proteins or glycoproteins have been suggested as ligands, including components of biological fluids, extracellular matrix and basement membrane proteins. There is equal adherence to several cell types, mainly epithelial and endothelial cells, or to biomaterials (Tronchin et al. Citation2008). Adherence is a precondition for colonization and an essential step towards establishing an infection. Several studies have demonstrated the potential adhesion of isolates of epithelial cells of Candida spp. (Chaffin et al. Citation1998; Tronchin et al. Citation2008). In this current study, this feature of adhesion was demonstrated for H. capsulatum.
Verstrepen and Klis (Citation2006) emphasized the remarkable ability of biofilm formation due to the property of adhesion by some fungi, with the most studied to date being C. albicans. This characteristic has great medical importance, since the presence of a mature biofilm negatively affects the action of antifungal agents and the biofilm can become a reservoir of cells with resistance to certain drugs. The cell walls of fungi are extremely important in the pathogenesis of these microorganisms by providing adherence to host tissues and they also participate in the immunomodulation of the immune response of the host (Chaffin et al. Citation1998).
Biofilm formation represents the most common growth mode of microorganisms in nature, a state that presumably allows microbial cells to survive in hostile environments and to spread and colonize new niches.
Recently, there has been a lot of interest regarding the role of biofilms in infectious diseases. According to the Centers for Disease Control, 65% of human infections result from pathogenic biofilms (Costerton Citation2001). Whereas, a public announcement from The National Institutes of Health estimates that 80% of human infections are related to biofilms (Williams and Costerton Citation2012). Fungal biofilms are an increasing clinical problem associated with significant rates of mortality.
In this assay the quantification of H. capsulatum biofilm in vitro was performed as described previously by Martinez and Casadevall (Citation2007). The use of this assay is explained by Zhou et al.(2012) who report that the XTT reduction assay has been widely used in recent years. XTT is a new yellow tetrazolium salt which can be converted to a colored formazan in the presence of metabolic activity. Since the formazan product is water soluble, it is easily measured in cellular supernatants, which is important in biofilm research because it allows the study of intact biofilms, as well as examination of biofilm drug susceptibility without disruption of biofilm structure. So, the spectrophotometric method and the XTT reduction assay used in this study made it possible to evaluate fungal biofilm growth accurately.
The characteristics of some surface molecules of a parasite could be important in parasite-adherence patterns and in the selection of the route of internalization into the host cells, as well as in extracellular dissemination (Suárez-Alvarez et al. Citation2010). According to Long et al. (Citation2003) alveolar macrophages are able to recognize unopsonized H. capsulatum yeasts and microconidia during a infection via the β-chain (CD18) family of adhesion-promoting glycoproteins, LFA-1 (CD11a/CD18), CR3 (CD11b/CD18), and CR4 (CD11c/CD18). The binding of H. capsulatum to phagocytes may be mediated by the cell wall carbohydrates or glucans of the pathogen, which consist of glucose homo- and hetero-polymers, and whose glycosydic linkage types differ between the yeast and mycelial phases (Gorocica et al. Citation2009). A lectin-mediated interaction between H. capsulatum yeast and host cells has been considered, in which lectin activity is associated with a component present on the yeast cell surface. This lectin-like activity is specific to galactosylated surface molecules (mainly β-anomer) on murine macrophages (Taylor et al. Citation1998; Mendes-Giannini et al. Citation2000; Duarte-Escalante et al. Citation2003). H. capsulatum yeast also has the ability to bind and agglutinate human erythrocytes through this lectin-like component (Taylor et al. Citation2004). The biological significance of these findings seems to be related to aspects of dissemination and pathogenesis of the associated clinical disease (Pérez-Torres et al. Citation2009).
The current finding of biofilm formation with H. capsulatum yeasts could be related to the ability of the microorganism to avoid the immune system cells through grouping of yeast cells, contrasting with single of H. capsulatum yeast cells that would be very easy to eliminate by the immune system. However, further studies are needed to better understand the role of this mechanism on the severity and pathogenesis of histoplasmosis. For instance, it would be very interesting to know the association between a strains virulence and biofilm formation, considering that low-virulence strains of H. capsulatum require more time for the mycelium-to-yeast-phase transition at 37°C, in contrast to more virulent strains that are capable of resisting temperature changes and transform more quickly to the yeast phase (Medoff et al. Citation1986).
This is the first report on H. capsulatum yeast biofilm formation and describes a possible association between the ability of H. capsulatum to form biofilm and the pattern of yeast adhesion to epithelial A549 cells.
Acknowledgements
The authors acknowledge receipt of grant No. DGAPA (PAPIIT IN204210).
References
- Ajello , L . 1998 . Italian contributions to the history of general and medical mycology . Med Mycol , 36 ( Suppl. 1 ) : 1 – 11 .
- Antonello , VS , Zaltron , VF , Vial , M , Oliveira , FM and Severo , LC . 2011 . Oropharyngeal histoplasmosis: report of eleven cases and review of the literature . Rev Soc Bras Med Trop , 44 : 26 – 29 .
- Baillie , GS and Douglas , LJ . 2000 . Matrix polymers of candida biofilms and their possible role in biofilm resistance to antifungal agents . J Antimicrob Chemother , 46 : 397 – 403 .
- Bohse , ML and Woods , JP . 2007 . Expression and interstrain variability of the yps3 gene of Histoplasma capsulatum . Eukaryot Cell , 6 : 609 – 615 .
- Cappelli , G , Ballestri , M , Perrone , S , Ciuffreda , A , Inguaggiato , P and Albertazzi , A . 2000 . Biofilms invade nephrology: effects in hemodialysis . Blood Purif , 18 : 224 – 230 .
- Cappelli , G , Ricardi , M , Ravera , F , Ligabue , G , Ballestri , M , Bonucchi , D and Bondi , M . 2007 . Biofilm on artificial surfaces . Contrib Nephrol , 154 : 61 – 71 .
- Chaffin , WL , López-Ribot , JL , Casanova , M , Gozalbo , D and Martínez , JP . 1998 . Cell wall and secreted proteins of Candida albicans: identification, function, and expression . Microbiol Mol Biol Rev , 62 : 130 – 180 .
- Chandra , J , Kuhn , DM , Mukherjee , PK , Hoyer , LL , Mccormick , T and Ghannoum , MA . 2001 . Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance . J Bacteriol , 183 : 5385 – 5394 .
- Costerton , JW . 2001 . Cystic fibrosis pathogenesis and the role of biofilms in persistent infection . Trends Microbiol , 9 : 50 – 52 .
- Costerton , JW , Cheng , KJ , Geesey , GG , Ladd , TI , Nickel , JC , Dasgupta , M and Marrie , TJ . 1987 . Bacterial biofilms in nature and disease . Annu Rev Microbiol , 41 : 435 – 464 .
- Deepe , GSJ . 2009 . “ Histoplasma capsulatum ” . In Principles and practice of infectious diseases , Edited by: Mandell , GI , Bennet , JE and Dolin , RE . 3305 – 3318 . Philadelphia , PA : Churchill Livingstone .
- Dias , MA , Oliveira , RM , Giudice , MC , Netto , HM , Jordão , LR , Grigorio , IM , Rosa , AR , Amorim , J , Nosanchuk , JD Travassos , LR . 2011 . Isolation of Histoplasma capsulatum from bats in the urban area of São Paulo state, Brazil . Epidemiol Infect , 139 : 1642 – 1644 .
- Donlan , RM and Costerton , JW . 2002 . Biofilms: survival mechanisms of clinically relevant microorganisms . Clin Microbiol Rev , 15 : 167 – 193 .
- Duarte-Escalante , E , Zenteno , E and Taylor , ML . 2003 . Interaction of Histoplasma capsulatum yeasts with galactosylated surface molecules of murine macrophages . Arch Med Res , 34 : 176 – 183 .
- Ells , TC and Truelstrup , Hansen L . 2006 . Strain and growth temperature influence Listeria spp. attachment to intact and cut cabbage . Int J Food Microbiol , 111 : 34 – 42 .
- Ferreira , MS and Borges , AS . 2009 . Histoplasmosis . Rev Soc Bras Med Trop , 42 : 192 – 198 .
- Gorocica , P , Taylor , ML , Alvarado-Vásquez , N , Pérez-Torres , A , Lascurain , R and Zenteno , E . 2009 . The interaction between Histoplasma capsulatum cell wall carbohydrates and host components: relevance in the immunomodulatory role of histoplasmosis . Mem Inst Oswaldo Cruz , 104 : 492 – 496 .
- Hawser , SP , Norris , H , Jessup , CJ and Ghannoum , MA . 1998 . Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2h-t etrazolium hydroxide (xtt) colorimetric method with the standardized national committee for clinical laboratory standards method of testing clinical yeast isolates for susceptibility to antifungal agents . J Clin Microbiol , 36 : 1450 – 1452 .
- Kroetz DN, Deepe GS. 2012. The role of cytokines and chemokines in Histoplasma capsulatum infection. Cytokine 58:112–117
- Kurokawa , CS , Sugizaki , MF and Peraçoli , MT . 1998 . Virulence factors in fungi of systemic mycoses . Rev Inst Med Trop Sao Paulo , 40 : 125 – 135 .
- Long , KH , Gomez , FJ , Morris , RE and Newman , SL . 2003 . Identification of heat shock protein 60 as the ligand on Histoplasma capsulatum that mediates binding to cd18 receptors on human macrophages . J Immunol , 170 : 487 – 494 .
- Lonnemann , G . 2000 . The quality of dialysate: an integrated approach . Kidney Int Suppl , 76 : S112 – 119 .
- López , CE . 2006 . Dimorphism and pathogenesis of Histoplasma capsulatum . Rev Argent Microbiol , 38 : 235 – 242 .
- Man , NK , Degremont , A , Darbord , JC , Collet , M and Vaillant , P . 1998 . Evidence of bacterial biofilm in tubing from hydraulic pathway of hemodialysis system . Artif Organs , 22 : 596 – 600 .
- Martinez , LR and Casadevall , A . 2007 . Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light . Appl Environ Microbiol , 73 : 4592 – 4601 .
- Medoff , G , Maresca , B , Lambowitz , AM , Kobayashi , G , Painter , A , M. Sacco , M and Carratu , L . 1986 . Correlation between pathogenicity and temperature sensitivity in different strains of Histoplasma capsulatum . J Clin Invest , 78 : 1638 – 1647 .
- Mendes-Giannini , MJ , Hanna , SA , Da Silva , JL , Andreotti , PF , Vincenzi , LR , Benard , G , Lenzi , HL and Soares , CP . 2004 . Invasion of epithelial mammalian cells by Paracoccidioides brasiliensis leads to cytoskeletal rearrangement and apoptosis of the host cell . Microbes Infect , 6 : 882 – 891 .
- Mendes-Giannini , MJ , Taylor , ML , Bouchara , JB , Burger , E , Calich , VL , Escalante , ED , Hanna , SA , Lenzi , HL , Machado , MP Miyaji , M . 2000 . Pathogenesis ii: Fungal responses to host responses: interaction of host cells with fungi . Med Mycol , 38 ( Suppl. 1 ) : 113 – 123 .
- Morris , CE , Monier , J and Jacques , M . 1997 . Methods for observing microbial biofilms directly on leaf surfaces and recovering them for isolation of culturable microorganisms . Appl Environ Microbiol , 63 : 1570 – 1576 .
- Muñoz , B , Martínez , MA , Palma , G , Ramírez , A , Frías , MG , Reyes , MR , Taylor , ML , Higuera , AL , Corcho , A and Manjarrez , ME . 2010 . Molecular characterization of Histoplasma capsulatum isolated from an outbreak in treasure hunters . BMC Infect Dis , 10 : 264
- Nett , JE , Cain , MT , Crawford , K and Andes , DR . 2011 . Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by tetrazolium salt assay . J Clin Microbiol , 49 : 1426 – 1433 .
- Nittler , MP , Hocking-Murray , D , Foo , CK and Sil , A . 2005 . Identification of Histoplasma capsulatum transcripts induced in response to reactive nitrogen species . Mol Biol Cell , 16 : 4792 – 4813 .
- Peeters , E , Nelis , HJ and Coenye , T . 2008 . Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates . J Microbiol Meth , 72 : 157 – 165 .
- Pérez-Torres , A , Rosas-Rosas , A , Parás-García , A , Juan-Sallés , C and Taylor , ML . 2009 . Second case of histoplasmosis in a captive mara (Dolichotis patagonum): pathological findings . Mycopathologia , 168 : 95 – 100 .
- Pervez , MM , Cobb , B , Matin , N , Shahrin , L , Ford , ER and Pietroni , M . 2010 . Disseminated histoplasmosis in a patient with advanced HIV disease-lessons learnt from Bangladesh . J Health Popul Nutr , 28 : 305 – 307 .
- Pierce , CG , Uppuluri , P , Tristan , AR , Wormley , FL , Mowat , E , Ramage , G and Lopez-Ribot , JL . 2008 . A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing . Nat Protoc , 3 : 1494 – 1500 .
- Ramage , G , Mowat , E , Jones , B , Williams , C and Lopez-Ribot , J . 2009 . Our current understanding of fungal biofilms . Crit Rev Microbiol , 35 : 340 – 355 .
- Rappleye , CA , Engle , JT and Goldman , WE . 2004 . RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence . Mol Microbiol , 53 : 153 – 165 .
- Rooney , PJ , Sullivan , TD and Klein , BS . 2001 . Selective expression of the virulence factor bad1 upon morphogenesis to the pathogenic yeast form of blastomyces dermatitidis: evidence for transcriptional regulation by a conserved mechanism . Mol Microbiol , 39 : 875 – 889 .
- Suárez-Alvarez , RO , Pérez-Torres , A and Taylor , ML . 2010 . Adherence patterns of Histoplasma capsulatum yeasts to bat tissue sections . Mycopathologia , 170 : 79 – 87 .
- Taylor , ML , Granados , J and Toriello , C . 1996 . Biological and sociocultural approaches of histoplasmosis in the state of Guerrero, Mexico . Mycoses , 39 : 375 – 379 .
- Taylor , ML , Duarte-Escalante , E , Pérez , A , Zenteno , E and Toriello , C . 2004 . Histoplasma capsulatum yeast cells attach and agglutinate human erythrocytes . Med Mycol , 42 : 287 – 292 .
- Taylor , ML , Duarte-Escalante , E , Reyes-Montes , MR , Elizondo , N , Maldonado , G and Zenteno , E . 1998 . Interaction of murine macrophage-membrane proteins with components of the pathogenic fungus Histoplasma capsulatum . Clin Exp Immunol , 113 : 423 – 428 .
- Taylor , ML , Ruíz-Palacios , GM , Del , Rocío Reyes-Montes M , Rodríguez-Arellanes , G , Carreto-Binaghi , LE , Duarte-Escalante , E , Hernández-Ramírez , A , Pérez , A , Suárez-Alvarez , RO Roldán-Aragón , YA . 2005 . Identification of the source of an unusual outbreak of histoplasmosis, in a hotel in Acapulco, state of Guerrero, Mexico . FEMS Immunol Med Microbiol , 45 : 435 – 441 .
- Tronchin , G , Pihet , M , Lopes-Bezerra , LM and Bouchara , JP . 2008 . Adherence mechanisms in human pathogenic fungi . Med Mycol , 46 : 749 – 772 .
- Verstrepen , KJ and Klis , FM . 2006 . Flocculation, adhesion and biofilm formation in yeasts . Mol Microbiol , 60 : 5 – 15 .
- Vesterlund , S , Paltta , J , Karp , M and Ouwehand , AC . 2005 . Measurement of bacterial adhesion – in vitro evaluation of different methods . J Microbiol Meth , 60 : 225 – 233 .
- Weaver , CH , Sheehan , KC and Keath , EJ . 1996 . Localization of a yeast-phase-specific gene product to the cell wall in Histoplasma capsulatum . Infect Immun , 64 : 3048 – 3054 .
- Williams DL, Costerton JW. 2012. Using biofilms as initial inocula in animal models of biofilm-related infections. J Biomed Mater Res B Appl Biomater 100:1163–1169
- Zancopé-Oliveira , RM , Morais , P , Silva Tavares , E and Muniz , MM . 2005 . Genetic diversity of Histoplasma capsulatum strains in brazil . FEMS Immunol Med Microbiol , 45 : 443 – 449 .
- Zhou Y, Wang G, Li Y, Liu Y, Song Y, Zheng W, Zhang N, Hu X, Yan S, Jia J. 2012. In vitro interactions between aspirin and amphotericin b against planktonic cells and biofilm cells of Candida albicans and C. parapsilosis. Antimicrob Agents Chemother 56:3250–3260