Abstract
The presence of intrauterine contraceptive devices (IUDs) gives a solid surface for attachment and an ideal niche for biofilm to form and flourish. Pelvic actinomycosis is often associated with the use of IUDs. Treatment of IUD-associated pelvic actinomycosis requires the immediate removal of the IUD. Therefore, this article presents in vitro evidence to support the use of novel antibiotics in the treatment of actinomycete biofilms. Twenty one clinical actinomycetes isolates from endocervical swabs of IUD wearers were assessed for their biofilm forming ability. An in vitro biofilm model with three isolates, Streptomyces strain A4, Nocardia strain C15 and Nocardia strain C17 was subjected to treatment with nystatin. Inhibition of biofilm formation by nystatin was found to be concentration dependent, with MBIC50 values in the range 0.08–0.16 mg ml−1. Furthermore, at a concentration of 0.16 mg ml−1, nystatin inhibited the twitching motility of the isolates, providing evidence for a possible mechanism of biofilm inhibition.
Introduction
Temporary or permanent insertion of medical devices plays an important role in common diagnostics, therapeutic procedures and in the management of critically ill patients (von Eiff et al. Citation2005) and the number of these devices is steadily increasing. Accordingly, device infection is an issue of growing importance which compromises the performance of medical devices and leads to increased morbidity and mortality (Raad et al. Citation2007). These infections are usually caused by microorganisms present on the medical device after sterilization and/or from contact with the skin or mucosa of the patient at the moment of insertion (Dwyer Citation2008).
The surfaces of most polymers can be colonized by bacteria and fungi. Microbial biofilm develops when microorganisms irreversibly adhere to a surface and produce extracellular polymers that facilitate adhesion and provide a structural matrix (Pruthi et al. Citation2003). Consequently, biofilm associated infections caused by sessile microorganisms have gradually replaced the acute epidemic infections caused by planktonic microorganisms at many clinical sites (Costerton and Wilson Citation2004). Microbial cells residing in a biofilm show marked genotypic and phenotypic differences when compared with their planktonic counterparts, including increased antimicrobial resistance, slow growth (decreased oxygen and nutrient supply) and a decreased metabolic rate (Costerton Citation1999; Reid Citation1999; Donlan Citation2001). The proliferation of microorganisms can result in dissemination to other regions of the host body, provoking bloodstream infections that are particularly dangerous in patients with compromised immune systems (Mah and O'Toole Citation2001; Donlan and Costerton Citation2002; Tenke et al. Citation2004).
Actinomycetes also form biofilms. Actinomycetes are present as commensal organisms in healthy humans and are considered as opportunistic pathogens (Westoff Citation2007). The most common infectious sites for clinical actinomycosis include cervicofacial (60%), thoracic (15%) and abdominal/pelvic (25%). Female genital actinomycosis is associated with the use of intrauterine devices (IUD) and vaginal pessaries. Evidence for biofilm formation on IUDs has been demonstrated in the last 5 years (Pruthi et al. 2003; Carrillo et al. Citation2004; Pal et al. 2005; Chassot et al. Citation2008). Approximately 7% of women with an IUD have actinomycetes on their pap smear examination. The prolonged presence of an IUD is associated with the pelvic presence of actinomycetes. The rate of colonization with actinomycetes in cytological smears of IUD users ranges from 1.6% to 44% (Jonas et al. Citation2002). Pelvic acitnomycosis most commonly occurs in current or previous users of an IUD, with the risk of infection increasing with duration of use (O'Connor et al. 1989; Elsayed et al. 2006).
When host defence mechanisms and systemic antibacterial chemotherapy are unable to stop the infection, removal of the IUD is often required. However, in some cases removal/replacement of the IUD was associated with significant economic and quality of life costs (von Eiff et al. 2005). In order to reduce the number and/or the impact of device related infections, prophylactic strategies leading to reduced colonization and proliferation are required (Schierholz and Beuth Citation2001; Raad and Hanna Citation2002; von Eiff et al. Citation2005). The aim of this study was to evaluate the antibacterial activity of nystatin and its effect on biofilms generated by actinomycetes isolated from endocervical swabs of IUD users.
Materials and methods
Study subjects
A total of 118 married women (Group A) were selected from outpatients referred to the Gynaecological and Obstetrics unit of the Pankajam Seetharaman Hospital, Tiruchirapalli, Tamilnadu, India for removal of IUD. The reasons for removal were: pelvic inflammatory disease (PID), haemorrhage, pelvic pain, vaginal discharge, desire to conceive or an IUD in utero for 2 years or more. One hundred and forty two women attending the same hospital at the same time for advice on contraception were recruited as controls (Group B). The criteria for the controls were: non-IUD users, not currently pregnant, an intact uterus, no current referral of hysterectomy or cervical conisation, no reported use of vaginal medication, no reported treatment of gynaecological disease in the previous 6 months, no reported diagnosis of anogenital cancer or tobacco related disease. All the recruited women received detailed information regarding the objective of the study and gave written consent to participate. The study was approved by the Institutional Ethics committee of Bharathidasan University (DM/2011/101/20).
Collection of specimens
The cervix of each patient was exposed by sterile bivalve speculum and endocervical swabs were obtained with sterile cotton swabs. These sites were sampled as they represent areas in contact with the IUD. The swabs were then placed on sterile thioglycolate broth and transported to the laboratory on ice for further processing on the same day.
Microbiological methods
The transported swabs were streaked directly on actinomycete isolation agar containing nalidixic acid and nystatin (80 mg l−1) and incubated at 37°C for 3 weeks (Lechevalier 1989). The microbial growth from the case patients and control subjects was evaluated qualitatively every week for 4 weeks.
Biofilm formation
To study the initial stages of biofilm formation, the static biofilm model (O'Toole and Kolter Citation1998a, Citation1998b; Deziel et al. 2001; Schaber et al. 2004) was adopted with some modifications. Nunc Thermanox polyolefin polymer plastic cell-culture treated (TMX) coverslips, 13 mm diameter ×0.2 mm thick, (Nalge Nunc International, Rochester, NY) were placed into each well of sterile Falcon 24-well polystyrene plates. Seven day old cultures of actinomycetes were inoculated into wells containing actinomycete isolation broth to an initial OD600 of 0.02–0.03 (Lechevalier 1989). Wells containing broth alone were used as negative controls. The plates were covered and incubated at 27°C for 7 days.
Crystal violet assay
The assay was conducted as previously described (Schaber et al. 2004) with minor modifications. TMX coverslips were carefully removed from each well of the 24-well plates (static biofilm formation), rinsed gently with double distilled water, and placed into the corresponding wells of a new 24-well plate containing 1 ml of 4.0% crystal violet solution (wt/vol). After incubation at room temperature for 30 min, the crystal violet solution was discarded and the coverslips rinsed, and placed in the corresponding wells containing 1 ml of 95% ethanol. The plates were incubated at room temperature for 1 h and the extracted crystal violet, which indicates the amount of biofilm, was measured at an absorbance of 595 nm.
Characterization of the biofilm producers
The biochemical and physiological characteristics of the potential biofilm formers were determined using standard methods (Lechevalier 1989). For identification based on their morphology and culture characteristics, the isolates were streaked directly on various culture media (including nutrient agar, tryptone iron agar, oat meal agar and starch casein agar) and grown at 27°C for 14 days (Lechevalier 1989). Color comparisons and identification of the isolates were made with reference to Lechevalier (Citation1989).
Twitching motility
This was done for the actinomycete isolates as previously described (Deziel et al. Citation2001; Schaber et al. Citation2004) using tryptone based agar containing 0.5% agar. The twitching plates were stab-inoculated and incubated at 27°C for 7 days. The agar plates were visually examined for twitching motility.
Antibiotic susceptibility of the isolates
The antimicrobial susceptibility was tested according to the guidelines of the CLSI (2010) by the disk diffusion technique using commercial disks. The antimicrobials and concentrations tested in μg were: ampicillin (20), amoxicillin (25), chloramphenicol (25), nystatin (30), co-trimaxazole (25), ceftriaxone (20), cephatoaxime (25), gentamicin (30), nalidixic acid (30) clavulonic acid (30), erythromycin (30), tetracycline (10), trimethoprim (30), kanamycin (30) and streptomycin (30).
Biofilm inhibition assay
The effect of nystatin on actinomycete biofilm formation was examined using the microdilution method (Liu et al. Citation2011). In brief, two-fold serial dilutions of nystatin were prepared, with the final concentration of nystatin ranging from 0.04 mg ml−1 to 1.28 mg ml−1. Nystatin, PBS and the medium alone were used as the positive, non-treated and blank controls, respectively. A cell suspension of actinomycete isolates A4, A15 and A17 was prepared (7 day old culture) and 10% of the cell suspensions were inoculated into the wells of a 24-well polystyrene tissue culture plate except for the wells with medium alone (blank control). Following incubation at 27°C for 172 h, biofilm formation was quantified by the crystal violet assay. The percentage inhibition was calculated using the Equation [1 − (A590 of the test/A590 of non-treated control)] × 100. The minimum biofilm inhibition concentration (MBIC50) was defined as the lowest concentration that showed ≥50% inhibition of biofilm formation.
Examination of biofilm formation
Copper coupons measuring 2 × 2 cm were prepared at the physical workshop of Bharathidasan University. Copper coupons were polished with an alumina suspension and immersed in a solution of 5% commercial detergent and pre-warmed distilled water for 30 min with gentle mixing. To remove the detergent, coupons were rinsed five times with ultra pure water and air-dried. The air-dried materials were autoclaved. Coupons were finally placed in a 24 well plate. Cells from a 7 day old culture were harvested and resuspended in actinomycete isolation broth. The suspensions were dispensed into each of these wells containing the coupons. After exposure for 10 days coupons were viewed by scanning electron microscopy (SEM). Briefly, the copper coupons were submerged in 1 ml of fixative solution (3% formaldehyde and 1.5% glutaraldehyde) for 30 min, rinsed with dH2O and dehydrated using a series of ethanol washes of increasing concentrations. Then they were immersed in hexamethyldisilazane for 5–15 min, allowed to dry at room temperature, mounted on to SEM stubs and immediately sputter coated with gold. Biofilms were visualized using a Hitachi S-3400N scanning electron microscopy at ×11k magnification.
Results
Biofilm forming ability
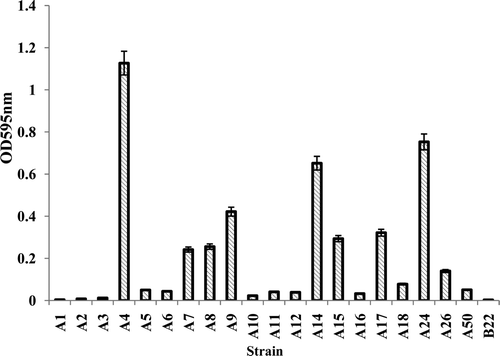
A total of 21 actinomycete isolates was obtained in culture from the endocervical swabs. Out of the 21 isolates, 20 were isolated from Group A and only one from Group B. Under the conditions of the study, the 21 actinomycete strains showed different levels of biofilm forming ability (). Three isolates showed high biofilm forming ability (from Group A), 6 were found to have low biofilm forming ability (from Group A) and 12 had no ability to form biofilm (11 from Group A and 1 from Group B). The one isolate from Group B, the isolate from non-IUD users, showed no biofilm forming ability. The results indicate that the isolates A4, A15 and A17 were more prone to biofilm formation, recording ODs of 0.652–1.127, an indication of higher biofilm forming ability. Hence these three isolates were used for further analysis.
Figure 1. Biofilm forming ability of the isolates. The static biofilm formation method was used to determine the biofilm forming ability of the isolates and biofilm formation was quantified by crystal violet assay. The amount of crystal violet was measured at an absorbance of 595 nm. The X-axis gives the isolate number and the Y axis the OD at 595 nm.
Culture characteristics
Based on their morphology and the patterns of the isolates on the various media, the high biofilm forming actinomycetes were characterised as Streptomyces strain A4, Nocardia strain A15 and Nocardia strain A17.
Twitching motility
It has been reported that motility can be involved with both biofilm formation (on biotic and abiotic surfaces) and pathogenesis. The assessment of twitching motility phenotype on 0.5% agar showed that out of the 21 actinomycete strains, 10 were motile. Out of the 10 motile strains, 9 showed the ability to form biofilms, including 3 strains with mature biofilm forming ability and 6 strains with immature biofilm forming ability. This indicates that surface based twitching motility plays an important role in biofilm formation.
Antibiotic sensitivity pattern of the isolates
The antibiotic resistance pattern of all the isolates revealed that they were resistant at least to one of the 15 antibiotics used (). The isolates showed 87.5% sensitivity to nystatin and 81.25% resistance against penicillin, clindamycin and erythromycin. The next highest incidence of resistance was observed against oxacillin and chloramphenicol (50%). Low resistance was observed against tetracycline, linezolid and gentamycin indicating that these antibiotics were promising for the treatment of actinomycete infections among IUD users.
Table 1. Antibiogram for 21 actinomycete isolates.
Actinomycete biofilm inhibition
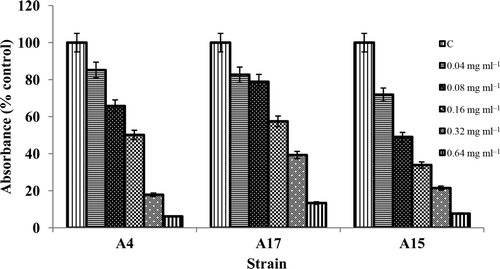
Based on the antibiotic sensitivity pattern of the isolates, nystatin was selected for the biofilm inhibition assay. Nystatin exhibited an inhibitory effect on actinomycete biofilm formation. Inhibition by nystatin was found to be concentration dependent with MBIC50 values in the range 0.08 to 0.16 mg ml−1 (). Of the three isolates, the Nocardia A15 strain showed strong biofilm inhibition, ranging from 7.7 to 71.9%, followed by Streptomyces A4 (6.2 to 85.2%) and Nocardia A17 (13.4 to 82.7%). The strain Nocardia A17 was selected for the microscopic evaluation of biofilm development and inhibition.
Figure 2. Effect of nystatin on actinomycete biofilm formation. Results are expressed as a percentage of non-treated control cell growth and are depicted by solid bars. Data represent the mean ± SD of two independent tests performed in duplicate. The X-axis gives the isolate number and the Y axis the percentage inhibition.
Microscopic evaluation of biofilm development and inhibition
shows an SEM micrograph of copper sheets and the biofilm formed on them. The surface of the copper sheets showed no significant features with SEM analysis (a). In contrast the Nocardia A17 incubated copper sheets revealed the formation of dense biofilm covering the sheets. The SEM analysis indicated exopolysaccharide or exopolymeric substance (EPS) production. The biofilm was characterised as porous, presumably enabling transport of water, nutrients and copper ions. The growth of Nocardia A17 revealed a well-structured biofilm consisting of several layers of bacteria and containing microcolonies which are characteristic features of mature biofilms (b). The results demonstrated the ability of the organism to survive copper toxicity due to the formation of a biofilm, which could affect IUD function.
Figure 3. Analysis of A17 biofilm formation using a static system: (a) uninoculated copper sheets visualised by SEM (×11k); (b) biofilm formed by A17 on copper sheets visualised by SEM (×11k); (c) inhibition of the formation of biofilm by 0.16 mg ml−1 nystatin visualised by SEM (×11k) on copper sheets. Scale bar = 100 μm.
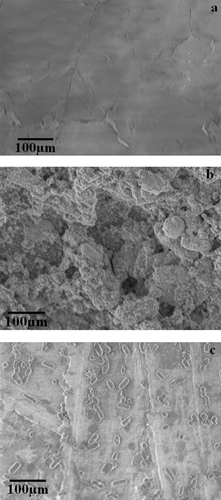
Nystatin was able to inhibit biofilm formation compared to the non-treated control. However, several individually attached bacterial cells were observed on the copper sheet when treated with nystatin at a concentration of 0.16 mg ml−1, suggesting most of the cells were killed by nystatin (c).
Possible mechanism of biofilm inhibition
As shown in , the addition of nystatin at a concentration of 0.16 mg ml−1 substantially decreased the twitching motility of Nocardia strain A17. These results suggest that part of the inhibitory effect of nystatin on biofilm formation is due to the decreased twitching motility.
Discussion
The biofilm mode of growth has been demonstrated as an important aspect of many bacterial diseases, including native valve endocarditis, osteomyelitis, dental caries, middle ear infections, medical device related infections, ocular implant infection and chronic lung infections in patients with cystic fibrosis (Jefferson Citation2004). The list of bacterial biofilm-related diseases has extended to include chronic wounds associated with diabetic and cardiovascular disease (James et al. Citation2008) and infections associated with the use of IUDs. The use of the latter is highly effective in preventing pregnancy and it is also very cost-effective. It is one of the most popular methods of contraception with more than 80 million people using IUDs for contraception worldwide. The effectiveness of IUDs rivals tubal ligation (Peterson et al. 1990). The use of IUDs is known to be associated with a risk of pelvic infection, heavier periods, muscular cramps (Lee et al. Citation1983) and above all complications associated with colonization of microbes on these implanted devices. The incidence of biofilm formation on IUDs has been thought to be routed by the upward migration of the microbes from vagina to the uterus. The vagina and the surrounding regions of the reproductive tract are known to support a large number of bacteria and fungi (Lewis Citation1988). The migration of these to upper part of the female urinogenital tract often leads to discomfort and infection. Although uterine secretions under normal conditions actively deal with such migrations, the presence of IUDs gives a solid surface for attachment and an ideal niche for the biofilm to form and flourish.
IUDs removed from women were shown to harbour Streptococcus epidermidis, other streptococci, Corynebacterium sp., Micrococcus sp., and anaerobic lactobacilli (Marrie and Costerton Citation1983; Wolf and Kreiger Citation1986). Furthermore, Actinomyces species in the endometrial cavity have been found to be associated with long periods of IUD use and are commonly associated with chronic endometritis (Valicenti et al. 1982). With this viewpoint, in the present study the authors sought to isolate actinomycete strains from the endocervical swabs of IUD users. In total 21 actinomycetes were obtained and assessed for their twitching motility and biofilm forming ability. Of the 21 strains, 10 showed twitching motility and 9 (3 high biofilm producers; 6 low biofilm producers) showed biofilm forming ability. This indicates that twitching motility may have a role in biofilm formation by actinomycetes. Nocardia strain A17 strain was incubated with copper sheets to determine the capacity of the strain to develop biofilms on copper surfaces as most of the IUDs used contain copper as their major component. The growth of the strain on copper surfaces showed that the organism resisted the local effect of copper ions released during the oxidation process. The antibiotic pattern of the isolates showed 87.5% sensitivity to nystatin. Thus, the ability of nystatin, a polyene antibiotic, to inhibit biofilm formation by actinomycetes was evaluated. The results showed that the growth levels observed in this study were similar to those of the untreated controls until the concentration of 0.16 mg ml−1 nystatin was reached. This indicated that there was no inhibition of biofilm growth at lower nystatin concentrations (0.04–0.16 mg ml−1). However, when the planktonic cultures used or biofilm production were evaluated, there was a significant inhibition of biofilm formation by nystatin even at concentration as low as 0.08 mg ml−1. Therefore nystatin can inhibit the formation of actinomycete biofilms at a concentration which is well below that required to kill or inhibit the growth of actinomycetes. A concentration dependent inhibition in the formation of biofilm was noted.
The effect of nystatin on the twitching motility of the strains showed that at the MBIC50 value of nystatin (0.16 mg ml−1) there was a considerable decrease in the motility, indicating that the mechanism of interference of biofilm development may have been due to the inhibition of twitching motility of the strains by nystatin. Thus it could be suggested that nystatin may inhibit type IV- pili and type IV pili mediated twitching motility. In this regard, nystatin may prevent the movement of the strains across the surface, thus inhibiting recruitment of adjacent cells to form microcolonies, thereby preventing biofilm formation.
Conclusions
Bacteria capable of forming biofilm are usually resistant to attack by antimicrobial agents and host phagocytes. To treat PIDs which develop in connection with IUD use requires the immediate removal of the IUD and treatment of the infection with an antibiotic that is active against the bacteria colonising the IUDs. With the advent of technologies involving antiseptic bonded biomaterials that are now in common use, future work on such biomaterials is likely to yield improved devices. The results of the present study suggest that the coating of IUDs with polyene antibiotics such as nystatin, which can have a broad spectrum of antimicrobial activity, may prevent biofilm formation by actinomycetes.
Authors' contributions
KN and SS designed the study and drafted the MS. SS and AL carried out the experiments. SK and AL collected the clinical samples and KN revised the manuscript critically.
Acknowledgments
The authors greatly acknowledge the Indian Council of Medical Research, New Delhi for financial support (3/2/2/63/2011/NCD-III; 5/13/88/06/NCD-III). The authors also acknowledge the Vice-Chancellor of Bharathidasan University for the providing the infrastructure facility to carry out the research work.
Additional information
Notes on contributors
Kalimuthusamy Natarajaseenivasan
The first two authors contributed equally to this workReferences
- Carrillo , M , Valdez , B , Veleva , L , Perez , T , Vargas , L and Schorr , M . 2004 . Microbiologically induced corrosion of copper intrauterine device by Enterobacter sp. in a synthetic intrauterine medium . Anti-Corros Method M , 51 : 331 – 338 .
- Chassot , F , Negri , M and Svidzinski , AE . 2008 . Can intrauterine contraceptive devices be a Candida albicans reservoir? . Contraception , 77 : 357 – 360 .
- 2010 . “ Clinical and Laboratory Standards Institute Quality Manual ” . Wayne (PA) : Clinical and Laboratory Standards Institute. 177 pp .
- Costerton , WJ . 1999 . Introduction to biofilm . Int J Antimicrob Ag , 11 : 217 – 221 .
- Costerton , WJ and Wilson , M . 2004 . Introducing biofilms . Biofilms , 1 : 1 – 4 .
- Deziel , DE , Comeau , Y and Villemur , R . 2001 . Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities . J Bacteriol , 183 : 1195 – 1204 .
- Donlan , RM . 2001 . Biofilm formation. A clinically relevant microbiological process . Health Epidemiol , 33 : 1387 – 1392 .
- Donlan , RM and Costerton , JW . 2002 . Biofilms: survival mechanisms of clinically relevant microorganisms . Clin Microbiol Rev , 15 : 167 – 193 .
- Dwyer , A . 2008 . Surface-treated catheters – a review . Semin Dialysis , 21 : 542 – 546 .
- Elsayed , S , George , A and Zhang , K . 2006 . Intrauterine contraceptive device associated pelvic actinomycosis caused by Actinomyces urogenitalis . Anaerobe , 12 : 67 – 70 .
- James , GA , Swogger , E , Wolcott , R , Pulcini , ED , Secor , P and Sestrich , J . 2008 . Biofilms in chronic wounds . Wound Repair Regen , 16 : 37 – 44 .
- Jefferson , KK . 2004 . What drives bacteria to produce a biofilm? . FEMS Microbiol Lett , 236 : 163 – 173 .
- Jonas , L , Baguhl , F , Wilken , HP , Jass , HJ and Nizze , H . 2002 . Copper accumulation in Actinomyces druses during endometritis after long-term use of an intrauterine contraceptive device . Ultrastruct Pathol , 26 : 323 – 329 .
- Lechevalier , HA . 1989 . “ The actinomycetes III. A practical guide to generic identification of actinomycetes ” . In Bergey's manual of systematic bacteriology , Edited by: Williams , ST . 2344 – 2347 . Baltimore (MA) : Wilkins Company. p .
- Lee , NC , Rubin , GL , Ory , HW and Burkman , RT . 1983 . Type of intrauterine device and the risk of pelvic inflammatory diseases . Obstet Gynecol , 62 : 1 – 6 .
- Lewis , R . 1988 . A review of bacteriological culture of removed intrauterine contraceptive devices . Brit J Fam Plan , 24 : 95 – 97 .
- Liu , Y , Wanga , L , Zhoub , X , Huc , S , Zhanga , S and Wub , H . 2011 . Effect of the antimicrobial decapeptide KSL on the growth of oral pathogens and Streptococcus mutans biofilm . Int J Antimicrob Ag , 37 : 33 – 38 .
- Mah , TF and O'Toole , GA . 2001 . Mechanisms of biofilm resistance to antimicrobial agents . Trends Microbiol , 9 : 34 – 39 .
- Marrie , TJ and Costerton , JW . 1983 . A scanning and transmission electron microscopic study of the surfaces of intrauterine contraceptive devices . Am J Obstet Gynecol , 146 : 384 – 394 .
- O'Toole , GA and Kolter , R . 1998a . Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development . Mol Microbiol , 30 : 295 – 304 .
- O'Toole , GA and Kolter , R . 1998b . Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis . Mol Microbiol , 28
- Pal , Z , Urban , E , Dosa , E , Pal , A and Nagy , E . 2005 . Biofilm formation on intrauterine devices in relation to duration of use . Med Microbiol , 54 : 1199 – 1203 .
- Peterson , HB , Xia , Z , Hughes , JM , Wilcox , LS , Tylor , LR and Trussell , J . 1996 . The risk of pregnancy after tubal sterilization: findings from the US Collaborative Preview of Sterilization . Am J Obstet Gynecol , 174 : 1161 – 1170 .
- Pruthi , V , Al-Janabi , A and Pereira . 2003 . Characterization of biofilm formed on intrauterine devices . Indian J Med Microbiol , 21 : 161 – 165 .
- O'Connor , KF , Bagg , MN , Croley , MR and Schabel , SI . 1989 . Pelvic actinomycosis associated with intrauterine devices . Radiology , 170 : 559 – 560 .
- Raad , I and Hanna , HA . 2002 . Intravascular catheter-related infections: new horizons and recent advances . Arch Intern Med , 162 : 871 – 878 .
- Raad , I , Hanna , H and Maki , D . 2007 . Intravascular catheter-related infections: advances in diagnosis, prevention, and management . Lancet Infect Dis , 7 : 645 – 657 .
- Reid , G . 1999 . Biofilms in infectious diseases and on medical devices . Int J Antimicrob Ag , 11 : 223 – 226 .
- Schaber , JA , Carty , NL , McDonald , NA , Graham , ED , Cheluvappa , R , Griswold , JA and Hamood , AN . 2004 . Analysis of quorum sensing-deficient clinical isolates of Pseudomonas aeruginosa . J Med Microbiol , 53 : 841 – 853 .
- Schierholz , JM and Beuth , J . 2001 . Implant infections: a haven for opportunistic bacteria . J Hosp Infect , 49 : 87 – 93 .
- Tenke , P , Riedl , CR , Jones , GL , Williams , GJ , Strickler , D and Nagy , E . 2004 . Bacterial biofilm formation on urologic devices and heparin coatings as preventive strategy . Int J Antimicrob Ag , 23S : S67 – 74 .
- 1982 . Detection and prevalence of IUD-associated actinomyces colonization and related morbidity . JAMA , 247 : 1149 – 1152 .
- von Eiff , C , Jansen , B , Kohnene , W and Becker , K . 2005 . Infections associated with medical devices. Pathogenesis, management and prophylaxis . Drugs , 65 : 179 – 214 .
- Westoff , C . 2007 . IUDs and colonization or infection with Actinomyces . Contraception , 75 : 48 – 50 .
- Wolf , AS and Kreiger , D . 1986 . Bacterial colonization of intrauterine devices (IUDs) . Arch Gynecol , 239 : 31 – 37 .