Abstract
3, 4-Dihydroxyphenylanine (Dopa)-containing proteins are key to wet adhesion in mussels and possibly other sessile organisms also. However, Dopa-mediated adhesive bonding is a hard act to follow in that, at least in mussels, bonding depends on Dopa in both reduced and oxidized forms, for adhesion and cohesion, respectively. Given the vulnerability of Dopa to spontaneous oxidation, the most significant challenge to using it in practical adhesion is controlling Dopa redox in a temporally- and spatially defined manner. Mussels appear to achieve such control in their byssal attachment plaques, and factors involved in redox control can be measured with precision using redox probes such as the diphenylpicryl hydrazyl (DPPH) free radical. Understanding the specifics of natural redox control may provide fundamentally important insights for adhesive polymer engineering and antifouling strategies.
Abbreviations
| Dopa | = |
(3, 4-dihydroxyphenylalanine) |
| DPPH | = |
(diphenyl picryl hydrazyl) |
| E° | = |
(standard reduction potential relative to SHE at pH 0) |
| E°′ | = |
(standard reduction potential relative to SHE at pH 7) |
| E o′ | = |
(standard reduction potential relative to SHE at pH 7 and 1 M reagent concentations) |
| E ad | = |
(adhesion energy); Fad (force of adhesion) |
| mfp | = |
(mussel foot protein) |
| precol | = |
(prepolymerized collagen) |
| SFA | = |
(surface forces apparatus) |
| SHE | = |
(standard hydrogen electrode) |
| tmp | = |
(thread matrix protein) |
Introduction
There is growing evidence that 3, 4-dihydroxyphenylalanine (Dopa) and related catechols endow various natural and synthetic polymers with wet adhesion. The holdfast proteins of marine mussels, and other sessile aquatic invertebrates including dreissenids (Rzepecki and Waite Citation1993; Gilbert and Sone Citation2010), sabellariid polychaetes (Zhao et al. Citation2005), turbellarians (Huggins and Waite Citation1993), hydroids (Knight Citation1970) and tunicates (Dorsett et al. Citation1987) contain significant amounts of Dopa. In mussel holdfasts, the Dopa levels are highest at the interface between the adhesive plaque and the substratum (Rubin et al. Citation2010). The most adhesive proteins isolated from mussel (Mytilus sp.) holdfasts (byssus), namely mfp-3 and mfp-5, are Dopa-rich with 20 and 30 mol% Dopa, respectively (Lee et al. Citation2011). There is no clear consensus as to how Dopa imparts adhesiveness. Adhesivity has been attributed to Dopa-mediated bidentate H-bonding (Anderson et al. Citation2010; Yu et al. Citation2011a; Bahri et al. Citation2011), metal/metal oxide coordination (Lee et al. Citation2006; Anderson et al. Citation2010), or oxidative cross-linking (Wilker Citation2010), but a comprehensive, broadly applicable answer has yet to be articulated.
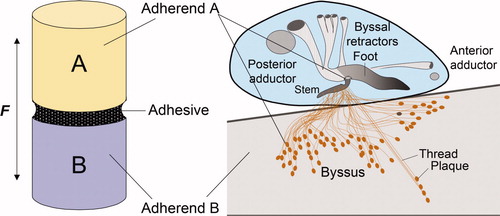
Practical adhesive bonding in sessile organisms is defined here as a biopolymer-mediated process for coupling two different adherends, A and B (Figure 1a). In the case of mussel adhesion, A is a part of the organism and B is the surface of a foreign solid, and coupling the two is the adhesive holdfast (b). But where adherend A begins and ends is strictly a matter of perspective and length scale. For example, in a macroscale view, adherend A might represent the living soft tissue of the mussel in which case the adhesive would consist of the entire holdfast or collection of byssal threads between the insertion point into soft tissues and the surface of B. In contrast, in the present micro- to nano scale perspective, adherend A represents a plaque, while the adhesive represents those plaque proteins that predominate in the plaque footprint on any given foreign surface (b). In either context, the prerequisites for good adhesive bonding are threefold: (1) the adhesive biopolymers must spread over both surfaces, (2) the biopolymers must interact strongly with both surfaces, and (3) the biopolymers must be cured or cross-linked for adequate cohesion (Houwink and Salomon Citation1965; Schonhorn Citation1981). Although these prerequisites have been recognized for some time, they easily become blurred in the case of mussel adhesion, because the surface interactions (adhesion) and cohesion (cross-linking) both show critical dependence on Dopa (Lee et al. Citation2006; Wilker Citation2010). Yu and Deming (Citation1998) were the first to appreciate the precarious balance between cohesive and adhesive roles of Dopa in synthetic adhesive polypeptides on metal surfaces: too much Dopa oxidation (and associated cross-linking) contributes to interfacial failure, whereas too little cross-linking leads to cohesive failure. It is proposed that Dopa can serve both the adhesive and cohesive capacities of adhesive bonding if the Dopa redox is positionally and temporally controlled, and that mussels exercise this level of control in their byssal adhesion.
Figure 1. Comparison of an adhesive butt joint with mussel adhesion. (A) A layer of adhesive polymer mediates the force (F) transfer between adherend A and adherend B. (B) There are two length scales of byssal adhesion in a mussel (inset). At the macroscale, adherend A is the soft living tissue of the mussel, the adhesive polymer is the byssus and adherend B is a foreign substratum. In contrast, at a length scale of micrometers, adherend A is represented by the proteins in the plaque, the adhesive polymers are a consortium of mostly interfacial plaque proteins, and adherend B remains a foreign substratum. Both perspectives are important to understanding mussel fouling.
Arena of adhesion – mussel plaque
The initial arena for byssal adhesion is the interfacial contact area negotiated by the mussel foot on a substratum surface during plaque formation (a). The best studied adhesion has been in Mytilus edulis L (Me) and Mytilus californianus Conrad (Mc). The foot deposits a complex mixture of proteins onto the surface, and after a few minutes, detracts (a). A freshly formed byssal thread and plaque is disengaged from the foot and, then, independently of the mussel, undergoes further maturation in the ambient seawater. Indeed, in the larger perspective, the entire byssus is an arena in that a weak link anywhere in the threads will lead to mussel detachment. As reviewed by Lee et al. (Citation2011), there are nine known protein families in each byssal plaque, viz. mfp-1, mfp-2, mfp-3, mfp-4, mfp-5, mfp-6, the collagenous precol-D and precol-NG, and thread matrix protein (b). The protein families are summarized to highlight their range in Dopa content, molecular weight, and adhesive properties (). Of these, mfp-3 is by far the most heterogeneous with two electrophoretically distinct subfamilies viz. fast and slow, each with as many as 30 different sequence variants. There has been some attempt to pinpoint distributions of the known proteins in the plaque but, given the insolubility of the structure and protein heterogeneity, significant uncertainty remains. Based on matrix-assisted laser desorption analysis of footprints, mfp-3 (fast variants = f; slow = s) and -5 appear to be present at the substratum surface with mfp-6 over, or comingled with, them (Zhao et al. Citation2006), but this should not be taken to suggest that the proteins do not persist into the plaque bulk (b). On top of mfp-3 and -5, and representing much of the plaque bulk, are mfp-3 (slow) and mfp-2. Finally, approaching the load bearing fibers that contain precols are mfp-4 and the thread matrix proteins (tmps).
Figure 2. Schematic view of a mussel byssal plaque. (a) each plaque is made by the mussel (inset) in a few minutes in the distal depression of the foot; (b) molecular model of the plaque. Approximate distribution of known plaque proteins is shown in relation to the substratum (adherend B). Dopa-containing proteins nearest the interface with the substratum and with known adhesion to mica are mfp-3 (fast and slow), mfp-5, and mfp-6. Adapted from Hwang et al. (2010). ©The American Society of Biochemistry & Molecular Biology.
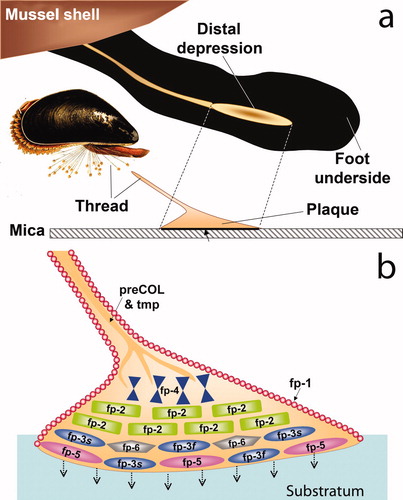
Table 1. Tally of Dopa-containing proteins present in the byssal plaques of Mytilus species (Me = M. edulis; Mc = M. californianus) and their respective adhesion to mica as measured by the surface forces apparatus. For best comparison, testing conditions in all cases were at pH 5.5, ionic strength 0.10 M, 1 atm and 20°C after a 60 min contact, and were selected to enable comparisons of all mfps. Note: maximal asymmetric adhesion to mica always occurs at pH 3.
Conditions of plaque formation
Plaque formation especially in its initial stages has attracted much recent scrutiny. Advances in mass spectrometry and Raman microscopy, for instance, have enabled the molecular dissection of some protein and metal distributions in byssus with picomole and micrometer precision (Zhao et al. Citation2006; Harrington et al. Citation2010; Hwang et al. Citation2010). Ideally, the logical next step would be to follow the deposition of each protein in real time, that is, to design probes that will report on proteins and solution conditions as they appear between the foot and a substratum surface during plaque formation. This aim has to overcome significant limitations that have to do with the organisms as well as technical issues. For example, with respect to probes, can appropriate test surfaces be modified with pH- and redox-sensitive probes such that they are stable in seawater and can be efficiently interrogated by microscopy and lasers during plaque formation? With regard to the mussel, how can the probability that the foot will deposit new plaques onto a patch of surface that has been modified with tethered pH or redox reporter probes be improved? Mussels appear to be capricious about plaque placements on surfaces generally.
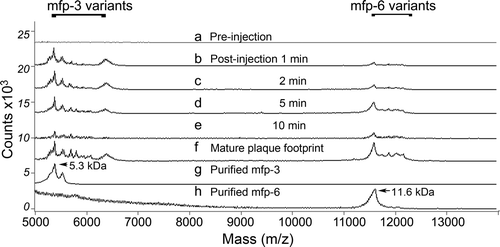
In the absence of mussel cooperation, the chemically induced secretion of adhesive proteins has been investigated in real time (Yu et al. Citation2011b). Induced plaques contain the same proteins as natural plaques, though whether processing is identical with unperturbed plaque formation remains unknown and improbable. Plaques are induced ‘on demand’ by injecting a small volume of 0.55 M KCl solution into the pedal ganglion at the base of the foot (Tamarin et al. Citation1976; Yu et al. Citation2011b). Secretion typically takes place within a minute or less in the distal depression (Yu et al. Citation2011b). The first secreted proteins detected by MALDI TOF mass spectrometry are mfp-3 followed by mfp-6 30 s later and are identical with the byssal secretions of the unperturbed animal (b–e, compared with 3f). Mfp-5 was separately detected within the first minute but typically requires much higher laser power to desorb and ionize it from surfaces (Zhao and Waite Citation2006). Because mussel secretions generally vary from individual to individual, each investigation of secreted adhesive proteins must compare the native footprint proteins with those induced by KCl in the same individual ().
Figure 3. Mass analysis of proteins in the distal depression during plaque formation induced by stimulating the pedal nerve. (a) pre-injection swab of proteins in the distal depression; (b) 1 min post-injection swab of distal depression; (c) 2 min post-injection swab; (d) 5 min post-injection swab; (e) 10 min post-injection swab; (f) proteins of a mature plaque footprint; (g) purified mfp-3 protein variants (5.3 kDa peak mass) used in SFA experiments; (h) purified mfp-6 variants (11.6 kDa) used in SFA experiments. Note absence of mfp-5 (9.5 kDa) which requires much higher laser power to desorb during analysis by MALDI. Adapted from Yu et al. (2011b). © Nature Publishing Group.
The surface forces apparatus measures adhesion and cohesion
Any research investigating the relationship between structure and function must possess a sensitive and precise methodology for measuring some property associated with function. As plaque proteins are associated with byssal adhesion, they need to be assessed singly and in combination for their adhesive properties. Surface interactions between various mussel foot proteins (mfps) and mica are routinely assayed in a surface forces apparatus (SFA), which has a 0.1 nm distance and nanoNewton force resolution (Israelachvili et al. Citation2010). The chief parameters measured are the hardwall and the adhesion force Fad. The hardwall represents the minimum separation distance possible between mica surfaces coated with films; with monolayer protein films, the hardwall approximates the hydrodynamic diameter of the protein. The adhesion force represents the resistance to separation, but given the dependence of force on, for example, the rate of separation, Fad is usually converted to the more robust adhesion energy E ad typically using the Derjaguin approximation E ad = Fad/2πR (Israelachvili Citation2010).
Two modes of SFA analysis are particularly revealing with respect to adhesion measurements. In symmetric mode (a), a monolayer of protein is absorbed to one mica surface. When this is brought into contact with another freshly cleaved mica surface, the molecules can rearrange to bridge from one side to the other. If this happens, a force (sometimes considerable) is required to separate the two surfaces and it can be concluded that the protein adsorbs strongly to both surfaces. This analysis has discerned intriguing differences among mfps: Mfp-1 adsorbs strongly to mica surfaces but does not bridge between two mica surfaces, whereas mfp-3 (fast) both coats mica and bridges between mica surfaces (Lin et al. Citation2007). A protein capable of bridging adsorption is behaving like an adhesive, whereas one that adsorbs to a surface and, in doing so loses its stickiness, is a coating. The reasons for the inability of mfp-1 to bridge are not known, but it can be made to bridge by shearing (Lin et al. Citation2007) and addition of FeIII (Zeng et al. Citation2010).
Figure 4. Adhesion and cohesion of mfps as determined by the surface forces apparatus (SFA). (a) Deposition of protein films by adsorption to freshly cleaved mica. A monolayer adsorbed onto one mica surface is denoted as asymmetric mode and is suitable for measuring adhesion during approach-separation runs in the SFA because adhesion will only occur if the protein engages in energetic interactions with both surfaces. In symmetric mode, a protein monolayer is adsorbed to both mica surfaces such that in approach-separation runs, cohesive interactions of mfps can be explored. (b) Three approach (in) –separation (out) runs of an asymmetric mefp-3 film on mica at pH 3, 5.5 and 7.5 that reveal the high pH-dependence of mfp-3f adhesion. (c) Rescue of adhesion by addition of pmole amounts of mfp-6 at pH 5.5. (d) Enhanced rescue of adhesion at pH 3 by mfp-6 with nearly full recovery after preincubation for 60 min. (e) Dependence of adhesion rescue by mfp-6 on its cysteine thiolates. CM-mfp-6 denotes protein with cysteines that have been S-alkylated by iodoacetic acid. Adapted from Yu et al. (2011b). © Nature Publishing Group.
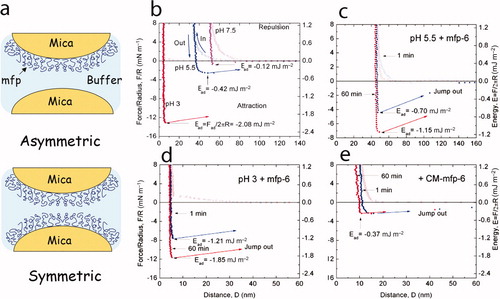
The symmetric mode (a) is well adapted to measure cohesive interactions between similar or different proteins. Protein is applied usually as a monolayer to both mica surfaces and the two surfaces are again brought into contact. Two things can happen upon contact: (1) if the proteins bind to mica better than to one another, they may rearrange to make room for all the proteins to bind both underlying surfaces. Mfp-3 apparently does this until more than a monolayer is present (Yu et al. Citation2011a). (2) If the binding to mica is strong and fixed, then the measured force of separation may approximate protein–protein cohesion, that is, how well the proteins bind to one another. These two scenarios are distinguishable by the hardwall thickness introduced earlier, in that the hardwall represents the limit of protein compressibility and approaches the hydrodynamic diameter of the protein that is independently measurable by dynamic light scattering (Yu et al. Citation2011a). Two nondisplaceable monolayers will be twice as thick as one layer.
Adhesion
Of all the proteins tested by SFA, monolayers of mfp-3 and -5 require the highest bridging force to separate two mica surfaces brought into contact in asymmetric mode at pH 5.5 (). Maximal forces of adhesion in mfps, however, are only detected in the SFA under acidic solution conditions, eg pH 3–4, and can achieve impressive levels, eg in mfp-5, adhesion (asymmetric) approaches Ead = −14 mJm−2 at pH 3, which exceeds even the adhesion between well-oriented films of avidin and biotin (Danner et al. Citation2012). At pH 5.5, adhesion reduced by at least 75% was evident and decreased rapidly with time; at pH 7.5, adhesion was effectively abolished (b). This result was at first mystifying because mussels were thought to do their business in seawater at pH 8.2. However, oxidation of Dopa residues in the proteins is highly pH sensitive, and the quinones formed have little affinity for the largely H-bond accepting polysiloxanes on the mica surface (Anderson et al. Citation2010; Yu et al. Citation2011a). Inferior quinone adhesion to mica was confirmed by the periodate-mediated oxidation of Dopa at low pH where pH-dependent auto-oxidation cannot occur (Yu et al. Citation2011a).
Cohesion
Analysis by the SFA has distinguished three potential types of cohesive mfp interactions in the plaque: (1) metal-mediated, (2) intrinsic binding, and (3) covalent. Metal- mediated interactions between mfps eg, mfp-2-mfp-2 interactions, are Fe3+- and Ca2+-mediated at specific binding sites (Hwang et al. Citation2010). In addition, mfp-1-mfp-1 interactions are strongly Fe3+-mediated (). Iron binding is Dopa-dependent but not every mfp Dopa binds iron with equal affinity (Hwang et al. Citation2010). Intrinsic binding between mfps, eg mfp-5 and mfp-2 and mfp-5 and mfp-3 is noncovalent, reversible and independent of metal ions (Hwang et al. Citation2010; Danner et al. Citation2012). Given the lack of structure in mfps in solution, the notion of intrinsic binding between mfps is one of the most peculiar results to emerge from the authors’ SFA-based studies. Affinity binding between proteins typically involves sites defined by secondary and tertiary structure neither of which has been detected in the majority of mfps. However, as observed in the bone binding protein osteocalcin (Hoang et al. Citation2003), structure may only be adopted following protein adsorption to a surface, and that would change everything. Nothing is known about surface dependent structure in mfps. Only one detail is known about intrinsic binding in mfp-5–mfp-5 interaction: it is Dopa-dependent and abolished or much diminished by periodate treatment (Hwang et al. Citation2010; Danner et al. Citation2012). Adhesion forces consistent with the formation of covalent cross-links can be observed following Dopa oxidation but it requires setting for 6–12 h (Danner et al. Citation2012). Although cysteinyl Dopa and diDopa cross-links have been isolated from the plaque (McDowell et al. Citation1999; Zhao et al. Citation2006), it has not been possible to identify cross-links formed during SFA testing. In byssus, the formation of Dopaquinone, hence those covalent cross-links derived from it, is thought to be catalyzed by a byssal catechol oxidase (Waite Citation1985). Catechoxidase distribution in various regions, eg the thread cuticle, core, plaque, and interface, has yet to be determined but may represent yet another factor in the complex redox of the byssus. In summary, both metal-mediated and intrinsic binding interactions of mfps require Dopa, whereas the covalent cross-links depend on Dopa oxidation to quinone.
Conditions of plaque formation
The critical pH dependence of mussel mfp adhesion in the SFA prompted a fundamental reassessment of the working assumptions, chief of which was that because mussels live in seawater at a pH of ∼8.2 and ionic strength of 0.7 M, that these conditions necessarily pertain also to their adhesion. Recent measurement of solution conditions under the foot has revealed a significant deviation from seawater and underscores the importance of improving investigative strategies for directly probing intricate bioprocesses. The approach with mussels has relied on the use of microelectrodes to monitor solution conditions of secreted proteins in the distal depression of the foot following KCl injection into the pedal ganglion. On average, the pH in the distal depression dropped to ∼pH 5 and the ionic strength to ∼0.1 M (Yu et al. Citation2011b). These are conservative estimates of change since, the high viscosity and stickiness of the secretions led to substantial fouling and blockage of the electrode surfaces. Nonetheless, these are hardly seawater conditions and suggest that at least the initial stages of adhesive plaque formation may rely on carefully choreographed solution conditions for optimal protein adsorption to the surface.
Mussel foot regulation of pH during plaque formation is an important new insight into the requirements for exploiting the multifunctional capabilities of Dopa in adhesion. The control of pH however, may not be adequate by itself, eg at pH 5.5, significant Dopa losses to oxidation in the SFA reduced adhesion. A second level of damage control appears to be by redox cycling. The nonadhesive mfp-6 is co-secreted stoichiometrically with mfp-3 and mfp-5. In SFA studies, reduced adhesion in mfp-3 at pH 5.5 (compared with pH 3) is completely and rapidly recovered by adding pmole amounts of mfp-6 (d). The initial hardwall (5 nm at pH 3) is also largely restored by addition of mfp-6. This may seem a subtle point but reveals two important trends about mfp-3 adhesion to mica: (1) the pH-dependent hardwall increase is not due to protein cross-linking/polymerization; (2) hardwall increases may reflect pH-dependent conformational changes related to dehydroDopa, for example (Yu et al. Citation2011b). Recovery is blocked by iodoacetate alkylation of thiolates in mfp-6, ie carboxymethylation (CM) of cysteine (e). Apparently thiolates in mfp-6 can rescue adhesion in the SFA by providing reducing equivalents for the 2-electron reduction of Dopaquinone back to Dopa. Measuring the reducing power of mfp-6 is described in fuller detail below. Use of thiolates as antioxidants is widespread in living cells but ordinarily constrained to pH levels near the thiol pKa (∼8.0), that is to say, the thiolate not the thiol is the operative antioxidant (Jensen et al. Citation2000). Mfp-6 cysteine recruitment for redox at pH 5.5 or lower is unusual and portends that one or more of the cysteines in mfp-6 have pKa s in the range of 3–4.
Lee et al. (Citation2006) first demonstrated how oxidation of a single tethered Dopa to Dopaquinone compromised adhesion to titania surfaces. In a similar vein, in investigating the adhesion to mica by Dopa-containing proteins with the SFA, the present authors found that adhesion could be decreased in a stepwise manner by a stoichiometric periodate-mediated oxidation of Dopa. The strength of mfp-3 and -5 adhesion to mica, for example, was inversely correlated with the degree of Dopa oxidation to Dopaquinone (Yu et al. Citation2011b; Danner et al. Citation2012). Complete oxidation to the quinone abolished adhesion; reduction back to Dopa restored it. Mussels cosecrete Dopa-containing adhesives with thiolate antioxidants to preserve the Dopa-dependent sticking power. A recent survey of ‘mussel inspired’ adhesive applications (Lee et al. Citation2011), however, revealed that most or all rely on the intentional oxidation of catechol-functionalized polymers for adhesion to bioorganic surfaces. The reason usually given for this is that quinones have a better chance of reacting covalently with proteins in the target surface. Although quinones do form in the adhesive plaques and lead to cross-linking between different plaque proteins, the authors have found no evidence so far that mussels present quinones to any foreign surface. Indeed, in the SFA, oxidation of mfp-3 or -5 abolishes adhesion and cohesion between symmetrically deposited protein films except after very long contact times (∼12 h) (Danner et al. Citation2012). Failure of such cross-links to form rapidly between oxidized protein films as well as evidence of the strongly reducing environments created by mussels during plaque formation argues against the widely held view that mussels exploit Dopaquinones for covalent adhesion to biopolymeric surfaces. Future research will need to explore this more critically.
Measuring redox of mfp-6
The DPPH coupled assay
A standard assay for redox activity in biological molecules is an essential metric for measuring rates and stoichiometries as well as for comparison with other known redox agents. The antioxidant activity of any given substance X is typically defined as the efficiency by which it reduces another oxidized substance Y. Because antioxidants involve the movement of electrons, activity would be most directly assayed by measuring the flow of electrons between X and Y, but practically, indirect measurement with appropriate molecules at absorbing or fluorescing wavelengths is easier and more sensitive. To this end, free radical redox indicators with high molar absorptivities at visible wavelengths are becoming increasingly popular for chromogenic detection of antioxidants (for a detailed review of methods for testing antioxidant activity see Antolovich et al. Citation2002).
A well-established protocol for the determination of antioxidant activity in biological samples is the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay. In contrast to other ‘one-dimensional’ antioxidant tests that involve prior oxidative generation of a metastable radical, the DPPH free radical has an unpaired valence electron at one atom of the nitrogen bridge that is stable over a broad range of pH (Takebayashi et al. Citation2006). The reduction potential E° of the DPPH•/DPPH-H redox couple vs the standard hydrogen electrode (SHE) in pure methanol or 60:40 (v/v) methanol/water solvents are +0.47V and +0.45V, respectively (Chen et al. Citation2011). In its free radical form in methanol, DPPH has a maximum absorbance centered at about 515 nm (ϵ = 12,509 M−1 cm−1 as per Brand-Williams et al. Citation1995) but upon reduction by an antioxidant or another radical species, the absorption decreases with a shift to lower λmax. Redox exchange between DPPH and a broad range of mono- and polyphenolic compounds has already been demonstrated (Villaño et al. Citation2007). However, the details involving DPPH and thiol/thiolate groups of biomolecules are largely unexplored (Wu et al. Citation2011).
Rather than the methanol–water cocktails of the standard protocols, a mild non-ionic detergent (0.3% v/v Triton X-100) is recommended to keep both the hydrophobic hydrazyl radical and the strongly cationic mussel protein soluble. A typical reaction is started by adding 5 μM of purified mfp-6 (equivalent to ∼45 μM in thiol groups) or other control antioxidants (eg ascorbic acid) to 100 μM of freshly prepared DPPH in 0.1 M citrate phosphate buffer. The decrease in absorbance at 515 nm is followed over 60 min to monitor fast and slow reduction kinetics (a). By normalizing the absorbance reduction at 515 nm against the respective DPPH control (as %), the relative DPPH reduction at any given buffer pH and antioxidant concentration can be determined over time. b shows the 60 min time course of the relative amount of remaining DPPH radical following addition of different antioxidants in a molar ratio of 1:20 (antioxidant/DPPH) at a pH of 4. In contrast to cysteine and ascorbic acid control antioxidants, which decrease the initial DPPH radical concentration by about 10% after a 60 min incubation time, the addition of molar equivalents of mfp6 protein result in a final DPPH reduction of about 60%.
Figure 5. Free radical reporter of antioxidant activity 1,1-diphenyl-2-picrylhydrazyl (DPPH•). (a) UV-Vis spectrum (300–800 nm) of DPPH• in its oxidized free radical (purple spectrum) and reduced (yellow spectrum) forms. The absorbance maximum (molar absorptivity) of DPPH at ∼515 nm decreases to its yellow-orange hydrazine counterpart (DPPH-H) upon the addition of an antioxidant. Inset: the reaction mixture (40% v/v 0.1M sodium acetate and 60% v/v methanol, pH 5.5) with 50 μM DPPH radical alone and in the presence of 10 μM L-ascorbic acid or 10 μM mfp6 after incubation for 30 min. (b) Time course of DPPH• reduction with the antioxidants L-cysteine, L-ascorbic acid and mfp6 protein at pH 4.0. 1 ml of a 0.1 M citrate phosphate reaction buffer contained 0.3% (v/v) Triton X-100 and 100 μM DPPH• alone or supplemented with 5 μM of L-cysteine, L-ascorbic acid or mfp6. The reaction was started by the addition of the antioxidant and the decrease in absorbance was monitored at 515 nm. The absorbance of DPPH• without antioxidant addition was stable over 60 min. For clarity, the control absorbance of 100 μM DPPH is displayed as 100% DPPH radical.
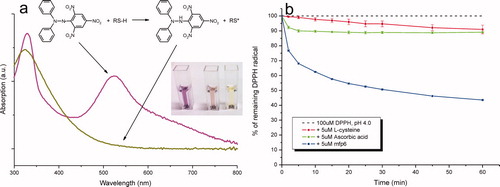
With typical pKa s of 8.2–8.3 for the thiol group in free cysteine and pKa s of 4.2 and 11.6 for the two enolic OH groups of ascorbic acid, the reactive groups of these antioxidant compounds are significantly protonated at a pH of 4. As already suggested by Brand-Williams et al. (Citation1995), the rapid kinetics of ascorbic acid and perhaps those of mfp6 may correspond to the number of ionized hydroxyl and thiol groups, respectively. In addition, in polar solvents the rate determining step of DPPH radical reduction by, for example, phenol-containing antioxidants is a fast electron transfer process (ET mechanism) from the dissociated antioxidant anion (eg phenoxide) to DPPH free radical (Foti et al. Citation2004). However, if the ratio of thiolates (RS⊖) or enolates (RO⊖) to undissociated thiols (RSH) or enols (ROH) is calculated according to the equation A⊖/AH = 10(pH-pKa), ratios of 0.05 × 10−3 for a typical free cysteine as well as 2.5 × 10−8 and 0.63 for the two enolic alcohols of ascorbic acid at pH 4 are obtained. This again suggests that at least some of the cysteines in mfp6 must have lower pKa s in the range of 4 or less as mentioned above. Interestingly, both thiol-containing antioxidants (L-cysteine and mfp6) show a continuous DPPH reduction with a negative slope even after incubation for 60 min. Besides the primary reaction of the DPPH radical (=DPPH•) with the thiol-group of the antioxidant (Reaction 1), this subsequent steady reaction might be attributed to the products of secondary reactions such as complexation (Reaction 2), dimerization (Reaction 3), or other radical adduct formations (Villaño et al. Citation2007):
Ascorbic acid shows a fast initial decrease (5–10 min) but no net change in absorption over the whole time course. Similar reaction kinetics were obtained by Sharma and Bhat (Citation2009) and suggest a fast (completed) antioxidant activity and the absence of any side reactions of oxidized ascorbic acid with the residual DPPH radicals.
Besides the comparative reaction of L-cysteine, ascorbic acid and mfp6 at a single buffer condition, a more thorough analysis of mfp6 and its recombinant derivatives will enable more quantitative determination of the antioxidant activity of mfp6 under different pH conditions as well as a detailed analysis of the underlying molecular mechanisms (data not shown). Future investigations will also include the total and specific antioxidant capacity in the mussel plaque over time, ie the persistence of antioxidant activity of crude extracts as well as purified mfp6 in differently aged plaques (data not shown). In this regard, the clear discrimination of putative co-antioxidant effects of the poly-phenolic mfp-3 and mfp-5 as well as possible recycling properties of mfp-6 during plaque formation need to be evaluated.
A model of redox and adhesion
Redox potentials, E° (pH 0) and its physiological (pH 7) surrogate E°′, are useful indicators of directional electron flow in oxidation–reduction reactions. The more positive the magnitude of E° is, the stronger the electron pulling power of the reaction. The following half-reactions represent characteristic redox chemistry that might occur in mussel byssus when Dopa residues in an adhesive protein encounter dissolved oxygen at equilibrium and standard physiological conditions (1 atm, 1M, 25°C and pH 7) (Segel Citation1976; Proudfoot and Ritchie Citation1983):
One Dopa (QH2) is converted to one Dopaquinone (Q) by the extraction of 2 protons and 2 electrons (Reaction 4), which are transferred to the reduction of O2, which requires an equal quantity to form each molecule of water (Reaction 5) (Segel Citation1976). The positive overall ΔE ′o (+0.60 V relative to SHE at pH 7) for the reactions predicts a favorable formation of Dopaquinone and water (ΔG =−nFΔE = −116 kJ mol−1) from Dopa and oxygen.
Because it is now known that the pH of plaque formation is likely to be lower than pH 7, the half reaction redox potentials can be adjusted to suit any pH, eg E′pH5 = E′pH7 −0.059 ΔpH or + 0.938V for O2 reduction and −0.338 V for Dopa (QH2) oxidation and no net change in ΔE at + 0.60 V (Segel Citation1976). Although, in this light, Dopaquinone formation appears to be favorable over a wide range of pH in the presence of oxygen, reaction rates must also be considered. The rate constant of O2 reduction on hydroquinone/quinone modified surfaces is significantly decreased at lower solution pHs, and this is usually attributed to the higher activation energy of the reaction intermediate (Johnsson Wass et al. Citation2006). Notwithstanding this limitation, it is easy to understand why in SFA measurements done in the presence of O2, Dopa-dependent mfp-3 and mfp-5 adhesion to mica is inversely correlated with [Q]/[QH2] – the ratio of Dopaquinone (Q) to Dopa (QH2) in the protein (Yu et al. Citation2011a, 2011b).
As O2, transition metals, free radicals and other electron acceptors can oxidize Dopa, hence deprive it of adhesion, their effects must be effectively counteracted. What safeguards are in place against unwanted Dopa oxidation during plaque formation by the mussel foot? First, when the mussel foot makes contact with a surface, it extrudes all seawater and replaces it with an acidic fluid at pH ∼5, ionic strength 0.1 M (Yu et al. Citation2011a, 2011b) and low oxygen tension (PO2); mussel hemolymph PO2 can be as low as low as 10–15 torr (Booth and Mangum Citation1978). Second, the foot makes the acidic fluid strongly reducing with 2 electron donors, namely Dopa, contributed by Dopa-rich proteins, and the thiols from cysteine rich mfp-6 variants. Whenever quinones form in this environment, coupling the two standard half reactions for quinone (Q) reduction and thiol (R-SH) oxidation predicts that the cysteines in mfp-6 will have more than sufficient reducing power (ΔE o′= +0.47 V) to restore any Dopaquinone formed to Dopa (Reaction 9); in the present case, however, this prediction is suspect because the standard conditions for E o′ (1 M reactant concentrations, pH 7.0, 1 atm of pressure, and a temperature of 25°C) are far removed from those observed in the mussel.
Conditions under the foot during plaque formation are not yet known with any precision, but even without precision they deviate significantly from the assumed standard conditions. For example, equilibrium is not achieved, the temperature of seawater ranges from about 4 to 15°C, the pH under the foot is acidic (probably between pH 3 and 5), and all of the protein or oxygen concentrations are considerably less than 1 M. Clearly redox potentials appropriate to conditions of plaque formation need to be experimentally determined and modelled only after more specific conditions are known.
The major events of plaque formation by the distal depression of the foot are summarized in a: (i) the pH of the space defined by the cupped foot over the target surface is acidified. Whether [H+] is fixed or has a range of possible pH adjustments is not known. (ii) Dopa-containing proteins such as mfp-3 (with 10 Dopa residues) and thiol-containing mfp-6 (with 9 residues of cysteine) are co-secreted into the depression; the range of Dopa is from 30 mol% (mfp-5) to as little as 5% (in mfp-2 and mfp-6), whereas all variants of mfp-6 contain 11 mol% cysteine (with at least two of these coupled as a disulfide). (iii) Proteins adsorb readily to surfaces (in this case, to mica) by way of Dopa. Incredibly, even on mica, Dopa is absolutely critical to wet adsorption; without it, there is no measurable protein adhesion force in the SFA; (iv) with time, some losses to Dopaquinone occur perhaps linked to trace O2 or metals such as Fe3+ (Giles et al. 2003; Wilker Citation2010), but these are repaired, at least in the short-term, (v) by thiolates in thiol-containing proteins such as mfp-6 or by other Dopa proteins. The rescue is an interesting one in that it is directed at the unadsorbed Dopa residues; once surface-adsorbed by a bidentate H-bond, Dopa (iii) is 106 times more tightly bound than the single H-bond, hence appears to be safe from oxidation (Yu et al. Citation2011a). The conformational changes in the protein backbone necessary to bring other Dopa groups to the surface are time-dependent, leaving unbound Dopa exposed to solvent vulnerable to oxidation. Mfp-6 prolongs the lifetime of these Dopa with the benefit of more adsorption to the surface.
Figure 6. A model of redox control during the initial stages of adhesive plaque formation by the foot. (a) The cup-shaped ventral surface of a mussel foot placed over a patch of substratum defines the microenvironment over which a new regime of pH, ionic strength, and redox conditions distinct from the surrounding seawater will be imposed. KCl-induced protein secretion by the foot mimics plaque formation and exhibits a microenvironment that is acidic pH ∼5, low ionic strength <0.1 M and strongly reducing. Reducing activity comes mostly from thiolate groups in mfp-6, pairs of which are converted to disulfides for every quinone rescued as Dopa. (b) Integrated view of parameters influencing redox during plaque formation. Red parameters denote standard redox potential, equilibrium and ionization constants of Dopa; blue parameters denote standard redox, equilibrium and ionization constants of thiolates. Acidity [H+] is purple. The biggest and most interesting uncertainty is to what extent a mussel can tailor redox to specific surfaces.
![Figure 6. A model of redox control during the initial stages of adhesive plaque formation by the foot. (a) The cup-shaped ventral surface of a mussel foot placed over a patch of substratum defines the microenvironment over which a new regime of pH, ionic strength, and redox conditions distinct from the surrounding seawater will be imposed. KCl-induced protein secretion by the foot mimics plaque formation and exhibits a microenvironment that is acidic pH ∼5, low ionic strength <0.1 M and strongly reducing. Reducing activity comes mostly from thiolate groups in mfp-6, pairs of which are converted to disulfides for every quinone rescued as Dopa. (b) Integrated view of parameters influencing redox during plaque formation. Red parameters denote standard redox potential, equilibrium and ionization constants of Dopa; blue parameters denote standard redox, equilibrium and ionization constants of thiolates. Acidity [H+] is purple. The biggest and most interesting uncertainty is to what extent a mussel can tailor redox to specific surfaces.](/cms/asset/ca95cc01-baee-420a-b831-3e14df9c7289/gbif_a_719023_o_f0006g.jpg)
Is there a realistic experimental approach to redox in mussel adhesion? The equation in b defines a measurable redox potential Eh relative to the SHE that is derived from the generalized Nernst equation E h = E° + (RT/nF) ln [oxidized]/[reduced] for each half-reaction (where E° is the standard reduction potential for the reaction in question, R is the gas constant, F is Faraday's constant, n is the number of electrons exchanged, and [oxidized]/[reduced] is the ratio of chemical activities or concentrations at any measured point). Applied to the two half-reactions of redox in the adhesive footprint, special emphasis is given to the known fact that, in a hypothetical protein HS-P-SH with at least two thiols, thiolates (P-S⊖) and not thiols (P-SH) are the actual reduced and reducing species, that HS-P-SHtotal = [SH-P-SH] + [HS-P-S⊖] + [⊖S-P-S⊖], and that the Kas of the two ionization steps are not necessarily the same. Complete derivation is worked out in Dawes (Citation1972) and similar equations are applied to the ionization of the two phenolic hydroxyls of Dopa. The equation in b builds upon the standard E°'s of thiols and quinones while making specific allowances for differences in H+ ion concentrations (ie pH) and temperature (T), the steady state distribution of Dopa/Dopaquinone (Q/QH2) and disulfide/thiol (RSSR/RSH), and the dissociation constants (Ka1 , Ka2 ) of both the Dopa and cysteine residues. Note that at high [H+] ie low pH, the terms Ka1[H+] and Ka1Ka2 can be ignored, whereas at low [H+] or high pH, only the Ka1Ka2 term remains. Many of these parameters are already known or can be experimentally determined for a more relevant picture of redox in plaque formation. In the case of the Dopa as a free amino acid, the pKas for the two phenolic OH groups are 9.84 and 13.4 (Pettit Citation1984), well above pH 7. Dopa as a side-chain in a protein, however, can have dissociation constants significantly removed from these pKas (unpublished results), hence caution is advisable. In the case of cysteine, the dissociation constant Ka of cysteine thiols is known to vary considerably in proteins eg from the typical pKa 8–9 of solvent accessible cysteine side-chains to the pKa of 3.5 for Cys-30 in the redox protein, DsbA, of E. coli (Nelson and Creighton Citation1994). Titration of thiols in freshly purified mfp-6 with Ellman's reagent detected a significant proportion of cysteines with pKas in the vicinity of pH 3–4 (Yu et al. Citation2011b), but how each cysteine residue ionizes will require a detailed analysis by proton NMR. These are important differences: if the foot imposed a pH of, say, 4 during plaque formation, typical cysteines (eg pKa 8) would not be present as thiolates at four pH units below the pKa , hence they would lack significant reducing power. By lowering the thiol pKas, thiolate reducing power is maintained. This is value-added in addition to the decreased tendency for Dopa to become oxidized at acidic pH (Eact higher for transition state). By making slight changes in pH and in the concentrations of mfp-6, mfp-3 and/or mfp-5 during plaque formation, mussels could, in principle, prepare a microenvironment anywhere from strongly reducing to strongly oxidizing simply by increasing or decreasing the proportion of mfp-6 cosecreted with mfp-3 and mfp-5.
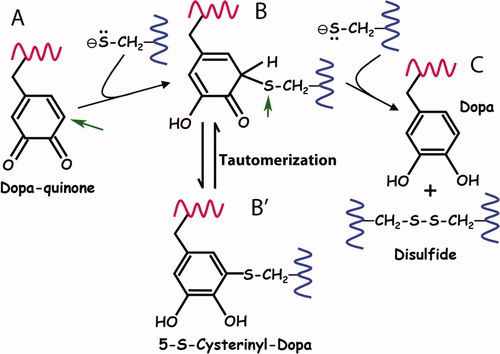
That thiols both reduce and react with quinones is not new to biochemistry. The involvement of thiols, for example, in depigmenting melanins (Lerner et al. Citation1950), in catechol toxicology (Wardman Citation1990), and in the coupling of protein disulfide formation with electron transport (Bader et al. Citation2000) is well known. Indeed, a most striking yet unappreciated aspect about thiol quinone chemistry is that covalent thio-quinone adducts are intermediates in the reduction mechanism (Inaba Citation2010). As pointed out in , in the first step of Reaction 9, a thiolate anion in a classic Michael reaction, attacks the methylene carbon (A, arrow) ortho to the keto group thereby creating a tetrahedral transition state. The second thiolate in Reaction 9 then is needed to couple with the thioether sulfur, split off the disulfide (B, arrow) and regenerate Dopa. Note that when thiolate availability is limiting, the first step may become trapped thereby accumulating quinone-thiol adducts. Also important is the fact that the thioether-catechol (structure B′ in ) is a tautomer of the initial adduct. The 5-S-cysteinyl-Dopa cross-links detected in hydrolysates of M. californianus byssal plaques (Zhao and Waite Citation2006) probably are derived by tautomerization from quinone thiol adducts trapped in the first step of reduction. Given this, Yu et al. (Citation2011b) were unable to resist the remark that even after expiration of its antioxidant function, mfp-6 is recruited into a second important function – cross-linking plaque proteins with Dopaquinone. Rigorously testing this interesting proposition remains for future investigations.
Figure 7. Proposed reaction mechanism for the reduction of quinone by thiolates. (A)The first thiolate attacks the o-quinone of Dopa at the 5-C position (arrow) in a Michael reaction. (B) The covalent thio-quinone adduct has a tetrahedral 5-C which is prone to further attack at the 5-S (arrow) by the second thiolate. (C) In the attack by the second thiolate, the 5-S leaves behind the electron pair it shared with 5-C and splits off as a disulfide thereby regenerating Dopa. (B’) The thio-quinone intermediate (structure B) is related by tautomerization with 5-S-cysteinylDopa (structure B′). Tautomers share the same oxidation level but exhibit relocation of electrons, ie double bonds. Adapted from Inaba (2010).
Challenges
The mussel foot evidently has all the attributes necessary for creating a nonequilibrium microenvironment optimized for adhesion to a surface such as mica, but does it use the same set of attributes to stick to different kinds of surfaces? Is the solution chemistry of secreted mfps deposited onto surfaces fixed or adjustable? If adjustable, what is the working range of pH and redox conditions, and how is the range of conditions exploited to adhere to different surfaces? How do the adjustments improve adhesion to different surfaces? Is interfering with adhesive redox a potentially effective antifouling strategy? The answers to these questions are not known, and future investigations need to invent microsensing devices to interrogate the space between the foot and the substratum surface during plaque formation in such a way that pH and redox control on different surfaces can be directly and dynamically measured. The issue of redox and adhesion may be much broader and not limited to mussel Dopa chemistry. Whether other sessile/fouling organisms exercise redox control during adhesion to surfaces merits closer scrutiny. Cross-linking of barnacle cement, for example, relies heavily on pH-triggered thiol redox chemistry even though Dopa is not involved (Nakano et al. 2007).
Acknowlegements
The authors thank the US National Institutes of Health (R01 DE-018468), the Human Frontiers of Science Program, and Materials Science & Engineering Center Program of the National Science Foundation (DMR 1121053) for their support.
References
- Anderson , TH , Yu , J , Estrada , A , Hammer , MU , Waite , JH and Israelachvili , JN . 2010 . The contribution of DOPA to substrate-peptide adhesion and internal cohesion of mussel-inspired synthetic peptide films . Adv Funct Mater , 20 : 4196 – 4205 .
- Antolovich , M , Prenzler , PD , Patsalides , E , McDonald , S and Robards , K . 2002 . Methods for testing antioxidant activity . Analyst , 127 : 183 – 198 .
- Bader , MW , Xie , T , Yu , C-A and Bardwell , JCA . 2000 . Disulfide bonds are generated by quinone reduction . J Biol Chem , 275 : 26082 – 26088 .
- Bahri , S , Jonsson , CM , Jonsson , CL , Azzolini , D , Sverjensky , DA and Hazen , RM . 2011 . Adsorption and surface complexation study of L-DOPA on rutile (α-TiO2) in NaCl solutions . Environ Sci Technol , 45 : 3959 – 3966 .
- Booth , CH and Mangum , CP . 1978 . Oxygen uptake and transport in the lamellibranch mollusk Modiolus demissus . Physiol Zool , 51 : 17 – 34 .
- Brand-Williams , W , Cuvelier , M and Berset , C . 1995 . Use of a free radical method to evaluate antioxidant activity . LWT-Food , 8 : 25 – 30 .
- Chen , W , Li , W , Fu , P and Yeh , A . 2011 . Reactivity of DPPH in the oxidation of catechol and catechin . Int J Chem Kinet 43: , : 147 – 153 .
- Danner , E , Kan , YJ , Hammer , M , Israelachvili , JN and Waite , JH . 2012 . Adhesion of mussel foot protein mefp-5 to mica: an underwater superglue . Biochemistry , doi:10.1021/bi3002538
- Dawes , EA . 1972 . Quantitative problems in biochemistry , 5th ed , Baltimore , MD : Williams & Wilkins Company. 470 pp .
- Dorsett , LC , Hawkins , CJ , Grice , JA , Lavin , MF , Merefield , PM , Parry , DL and Ross , IL . 1987 . Ferreascidin: a highly aromatic protein containing 3, 4-dihydroxyphenylalanine from the blood cells of a stolidobranch ascidian . Biochemistry , 26 : 8078 – 8082 .
- Foti , MC , Daquino , C and Geraci , C . 2004 . Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH radical in alcoholic solutions . J Org Chem , 69 : 2309 – 2314 .
- Gilbert , TW and Sone , ED . 2010 . The byssus of the zebra mussel (Dreissena polymorpha): spatial variations in protein composition . Biofouling , 26 : 829 – 836 .
- Giles , NM , Watts , AB , Giles , GI , Fry , FH , Littlechild , JA and Jacob , C . 2003 . Metal and redox modulation of cysteine protein function . Chem Biol , 10 : 677 – 693 .
- Harrington , MC , Masic , A , Holten-Andersen , N , Waite , JH and Fratzl , P . 2010 . Iron-clad fibers: a metal-based biological strategy for hard flexible coatings . Science , 328 : 216 – 220 .
- Hoang , QQ , Sicheri , F , Howard , AJ and Yang , DSC . 2003 . Bone recognition mechanism of porcine osteocalcin from crystal structure . Nature , 425 : 977 – 980 .
- Houwink , R and Salomon , G . 1965 . Adhesion and adhesives , 1, 2nd ed , Amsterdam , , The Netherlands : Elsevier. 548 pp .
- Huggins , LG and Waite , JH . 1993 . Eggshell formation in Bdelloura candida, an ectoparasitic turbellarian of the horseshoe crab Limulus polyphemus . J Exp Zool , 265 : 549 – 557 .
- Hwang , DS , Zeng , H , Masic , A , Harrington , MJ , Israelachvili , J and Waite , JH . 2010 . Fe3+-dependent cohesion of a prominent protein of mussel adhesive plaques . J Biol Chem , 285 : 25850 – 25858 .
- Inaba , K . 2010 . Structural basis of protein disulfide bond generation in the cell . Genes Cells , 15 : 935 – 943 .
- Israelachvili , JN . 2010 . Intermolecular and surface forces , 3rd ed , London , , UK : Elsevier. 674 pp .
- Israelachvil , JN , Min , Y , Akbulut , YM , Alig , A , Carver , G , Greene , W , Kristiansen , K , Meyer , E , Pesika , N , Rosenberg , K and Zeng , H . 2010 . Recent advances in the surface forces apparatus (SFA) technique . Rep Prog Phys , 73 : 036601
- Jensen , KS , Hansen , RE and Winther , JR . 2000 . Kinetic and thermodynamic aspects of cellular thiol-disulfide redox regulation . Antioxid Redox Signal , 11 : 1047 – 1058 .
- Johnsson Wass , KRT , Ahlberg , E , Panas , I and Schiffrin , DJ . 2006 . Quantum chemical modelling of the rate determining step for oxygen reduction on quinones . Phys Chem Chem Phys , 8 : 4189 – 4199 .
- Knight , DP . 1970 . Sclerotization of the perisarc of the calyptoblastic hydroid Laomedusa flexuosa . Tissue Cell , 2 : 467 – 477 .
- Lee , BP , Messersmith , PB , Israelachvili , JN and Waite , JH . 2011 . Mussel inspired wet adhesives and coatings . Ann Rev Mat Res , 41 : 99 – 132 .
- Lee , HS , Scherer , NF and Messersmith , PB . 2006 . Single-molecule mechanics of mussel adhesion . P Natl Acad Sci USA , 103 : 12999 – 13003 .
- Lerner , AB , Fitzpatrick , TB , Calkins , E and Summerson , WH . 1950 . Mammalian tyrosinase. The relationship of copper to enzyme activity . J Biol Chem , 187 : 793 – 802 .
- Lin , Q , Gourdon , D , Sun , CJ , Holten-Andersen , N , Anderson , TH , Waite , JH and Israelachvili , JN . 2007 . Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3 . P Natl Acad Sci USA , 104 : 3782 – 3786 .
- McDowell , LM , Burzio , LA , Waite , JH and Schaefer , J . 1999 . REDOR Detection of cross-links formed in mussel byssus under high flow stress . J Biol Chem , 274 : 20293 – 20295 .
- Nakano , M , Shen , J-R and Kamino , K . 2007 . Self-assembling peptide inspired by a barnacle underwater adhesive protein . Biomacromolecules , 8 : 1830 – 1835 .
- Nelson , JW and Creighton , TE . 1994 . Reactivity and ionization of the active site cysteine residues of DsbA, a protein required for disulfide bond formation in vivo . Biochemistry , 33 : 5974 – 5983 .
- Papov , VV , Diamond , TV , Biemann , K and Waite , JH . 1995 . Hydroxyarginine-containing polyphenolic proteins in the adhesive plaques of the marine mussel Mytilus edulis . J Biol Chem , 270 : 20183 – 20192 .
- Pettit , LD . 1984 . Critical survey of formation constants of complexes of histidine, phenylalanine, tyrosine, L-Dopa, and tryptophan . Pure Appl Chem , 56 : 247 – 292 .
- Proudfoot , GM and Ritchie , IM . 1983 . A cyclic voltammetric study of some 4-substituted benzene-1, 2-diols . Austral J Chem , 36 : 885 – 994 .
- Rubin , DJ , Miserez , A and Waite , JH . 2010 . Diverse strategies of protein sclerotization in marine invertebrates: structure–property relationships in natural biomaterials . Adv Insect Physiol , 38 : 75 – 133 .
- Rzepecki , LM and Waite , JH . 1993 . The byssus of the zebra mussel Dreissena polymorpha: morphology and in situ processing during maturation . Mol Mar Biol Biotech , 2 : 255 – 266 .
- Schonhorn , H . 1981 . “ Adhesion and adhesive interactions at interfaces. In ” . In Adhesion in cellulosic and wood-based composites , Edited by: Oliver , JF . 91 – 111 . New York , NY : Plenum Publishing Corp .
- Segel , I . 1976 . Biochemical calculations , 2nd ed , New York , NY : Wiley & Sons. 441 pp .
- Sharma , O and Bhat , T . 2009 . DPPH antioxidant assay revisited . Food Chem , 113 : 1202 – 1205 .
- Takebayashi , J , Tai , A , Gohda , E and Yamamoto , I . 2006 . Characterization of the radical-scavenging reaction of 2-O-substituted ascorbic acid derivatives, AA-2G, AA-2P, and AA-2S: a kinetic and stoichiometric study . Biol Pharm Bull , 29 : 766 – 771 .
- Tamarin , A , Lewis , P and Askey , J . 1976 . The structure and formation of the byssus attachment plaque in Mytilus . J Morphol , 149 : 199 – 221 .
- Villaño , D , Fernández-Pachón , MS , Moyá , ML , Troncoso , AM and García-Parrilla , MC . 2007 . Radical scavenging ability of polyphenolic compounds towards DPPH free radical . Talanta , 71 : 230 – 235 .
- Waite , JH . 1985 . Catecholoxidase activity in the byssus of the common mussel . J Mar Biol Ass UK , 65 : 359 – 371 .
- Waite , JH and Qin , XX . 2001 . Polyphosphoprotein from the adhesive pads of Mytilus edulis . Biochemistry , 40 : 2887 – 2893 .
- Waite , JH , Housley , TJ and Tanzer , ML . 1983 . Peptide repeats in a mussel adhesive protein: variations on a theme . Biochemistry , 24 : 5010 – 5014 .
- Wardman , P . 1990 . Bioreactive activation of quinones: redox properties and thiol reactivity . Free Radical Res Com , 8 : 219 – 229 .
- Wilker , JJ . 2010 . The iron fortified adhesive system of marine mussels . Angew Chem , 49 : 8076 – 8078 .
- Wu , MJ , Clarke , FM , Rogers , PJ , Young , P , Sales , N , O'Doherty , PJ and Higgins , V J . 2011 . Identification of a protein with antioxidant activity that is important for the protection against beer ageing . Int J Mol Sci , 12 : 6089 – 6103 .
- Yu , J , Wei , W , Danner , E , Israelachvili , JN and Waite , JH . 2011a . Effects of interfacial redox in mussel adhesive protein films on mica . Adv Mater , 23 : 2362 – 2366 .
- Yu , J , Wei , W , Danner , E , Ashley , RK , Israelachvili , JN and Waite , JH . 2011b . Mussel protein adhesion depends on interprotein thiol-mediated redox modulation . Nature Chem Biol , 7 : 588 – 590 .
- Yu , M and Deming , TJ . 1998 . Synthetic polypeptide mimics of marine adhesives . Macromolecules , 31 : 4739 – 4745 .
- Zeng , H , Hwang , DS , Israelachvili , JN and Waite , JH . 2010 . Strong reversible Fe3+-mediated bridging between dopa-containing protein films in water . P Natl Acad Sci USA , 107 : 12850 – 12853 .
- Zhao , H and Waite , JH . 2006 . Linking adhesive and structural proteins in the attachment plaque of Mytilus californianus . J Biol Chem , 281 : 26150 – 26158 .
- Zhao , H , Sun , CH , Stewart , RJ and Waite , JH . 2005 . Cement proteins of the tube-building polychaete Phragmatopoma californica . J Biol Chem , 280 : 42938 – 42944 .
- Zhao , H , Robertson , NB , Jewhurst , SA and Waite , JH . 2006 . Probing the adhesive footprints of Mytilus californianus byssus . J Biol Chem , 281 : 11090 – 11096 .