Abstract
Previous studies have shown that bacterial biofilms formed from natural seawater (NSW) enhance the settlement of spores of the green alga Ulva linza, while single-species biofilms may enhance or reduce settlement, or have no effect at all. However, the effect of biofilms on the adhesion strength of algae, and how that may be influenced by coating/surface properties, is not known. In this study, the effect of biofilms formed from natural seawater and the marine bacterium Cobetia marina, on the settlement and the adhesion strength of spores and sporelings of the macroalga U. linza and the diatom Navicula incerta, was evaluated on Intersleek® 700, Intersleek® 900, poly(dimethylsiloxane) and glass. The settlement and adhesion strength of these algae were strongly influenced by biofilms and their nature. Biofilms formed from NSW enhanced the settlement (attachment) of both algae on all the surfaces while the effect of biofilms formed from C. marina varied with the coating type. The adhesion strength of spores and sporelings of U. linza and diatoms was reduced on all the surfaces biofilmed with C. marina, while adhesion strength on biofilms formed from NSW was dependent on the alga (and on its stage of development in the case of U. linza), and coating type. The results illustrate the complexity of the relationships between fouling algae and bacterial biofilms and suggest the need for caution to avoid over-generalisation.
Introduction
The colonisation of immersed surfaces by many macrofouling organisms relies on motile dispersal stages of their life histories, viz. larvae of invertebrates, spores of algae, to actively seek out and attach to substrata. The ‘recruitment’ process is known to be influenced by a variety of surface-associated cues such as topography (Scardino and de Nys 2011), charge (Petrone et al. Citation2011) and wettability (Schilp et al. Citation2007; Finlay et al. Citation2010). In addition, in recognition of the fact that surfaces immersed in natural seawater (NSW) will normally be colonised by a microbial biofilm, there has been increasing emphasis on studies of the influence of such biofilms on surface colonisation.
Several studies have shown both positive and negative influences of marine bacterial biofilms (natural or single-species) on the settlement of larvae of a variety of invertebrates (Hadfield 2011; Mos et al. Citation2011; Wang et al. Citation2012) and spores of the marine macroalga Ulva linza (Dillon et al. Citation1989; Holmström et al. Citation1996; Joint et al. Citation2000; Patel et al. Citation2003). The mechanistic basis for these effects may include the indirect influence of the biofilm in modulating those physico-chemical properties of the immersed surface that provide recognition cues for settlement. However, there is considerable evidence for more specific effects based on the detection by larvae and spores of diffusible chemical signals produced by the biofilm (Joint et al. Citation2002; Wheeler et al. Citation2006; Dobretsov et al. Citation2009; Hadfield 2011). There is also evidence that some larvae detect the extracellular polymeric substances (EPS) secreted by bacteria and diatoms through lectin-like receptors located at the surface of the larvae (Kirchman et al. Citation1982; Khandeparker et al. 2003; Lau et al. Citation2003; Lam et al. Citation2005; Caldwell and Pagett Citation2010).
Once a motile larva or algal spore has detected a suitable surface, it settles, resulting in the secretion of adhesive polymers and the subsequent metamorphosis of the adhered organism/cell to form the sessile, adult stage. In addition to effects on recruitment, it may be envisaged that surface-associated microbial biofilms may also influence the adhesion strength of these sessile stages, yet there is only one systematic study of such effects reported in the literature. Zardus et al. (Citation2008) assessed the influence of microbial biofilms formed from NSW on glass slides, on the adhesion strength of newly settled larvae of four species of marine invertebrates (a polychaete worm, an ascidian, a barnacle and a bryozoan). Except for the bryozoan, settled larvae of all tested species showed significantly increased adhesion strength on the biofilmed surface. However, there has not been a comparable study of the effect of biofilms on the adhesion strength of marine algae, and how this interaction is influenced by the type of coating/surface. In the present paper, the results of such a study are reported.
The main objective was to assess the effect of primary bacterial biofilms on the adhesion strength of two common marine fouling algae: the green macroalga Ulva linza and the pennate diatom (microalga) Navicula incerta. These two species of alga differ in their adhesion biology. Spores of U. linza actively ‘choose’ where to settle on a surface and the transition from the swimming to permanently adhered, non-motile spores, is achieved by the secretion of a glycoprotein adhesive (Callow and Callow 2006a). In contrast, planktonic cells of raphid diatoms like N. incerta are immotile in the water column and reach a surface by gravity or water currents. On attachment they release adhesive polymers, some of which also serve to facilitate locomotion on the surface (Molino and Wetherbee Citation2008). Whilst the processes involved in initial attachment of these two algae are clearly different, for simplicity, in this paper both situations are referred to as ‘settlement’. Two types of bacterial biofilm were formed: biofilms formed from NSW produced a mixed bacterial assemblage whereas single-species biofilms were formed from cultures of the Gram-negative marine bacterium Cobetia marina, a hydrophilic species producing large quantities of EPS composed mainly of uronic acids (Shea et al. Citation1991). C. marina was chosen as it is extensively used as a model species in marine biofouling research.
A further novel feature of this study is that interactions with a range of characterised surfaces with different fouling-release (FR) properties were examined. Considerations of the effects of surface properties on adhesion strength of attached organisms are directly relevant to the evaluation of experimental coatings for their FR performance. An early stage in such evaluations is typically conducted in laboratory assays with organisms such as U. linza and N. incerta (Ekblad et al. Citation2008; Sundaram et al. Citation2011), such assays normally being conducted on pristine, non-biofilmed surfaces, which are therefore atypical of the natural environment. The test surfaces for the present study included two commercial, biocide-free, elastomeric FR coatings viz. Intersleek® 700 (IS700) and Intersleek® 900 (IS900), an unpigmented poly(dimethylsiloxane) (PDMS) elastomer and glass slides. Biofilms were formed on these test surfaces from either NSW or cultures of C. marina. Control (non-biofilmed) and biofilmed test surfaces were challenged with either zoospores of U. linza or cells of N. incerta to evaluate settlement (spores and diatoms) and adhesion strength (spores and sporelings of U. linza and diatoms). Confocal microscopic observations were also performed to explore the spatial relationships between the settled spores and the biofilms.
Materials and methods
Preparation of test surfaces
Coatings were formed on standard glass microscope slides (Thermo Scientific, 76 × 26 mm) which were also used as a reference. Slides were degreased with xylene before coating. For preparation of grey Intersleek® 700 (IS700), a hydrophobic silicone coating and Intersleek® 900 (IS900), an amphiphilic coating based on fluoropolymer technology and siloxane, slides were roller-coated with a commercial IS731 tie coat ( http://www.international-marine.com/PDS/664+M+eng + A4.pdf), according to manufacturer’s instructions and then dried overnight at room temperature. The three-pack IS700 and IS900 top coats (see http://www.international-marine.com/PDS/147+M+eng + A4.pdf and http://www.international-marine.com/PDS/4592+M+ eng + A4.pdf for specifications) were applied by roller.
Unpigmented PDMS standard test surfaces were prepared by first roller-coating slides with an unpigmented acrylic polymer tie coat and then dried overnight at room temperature. Slides were then roller-coated with a top coat, characterised by a blend of silicone polymer, crosslinking agent (tetraethyl orthosilicate (TEOS)), catalyst (IS700 part C) and solvent (xylene).
All the FR silicone-based coatings were allowed to cure for 2 days at room temperature before characterisation. The dry film thickness of the coatings ranged between 212 ± 34 μm and 311 ± 61 μm.
Before use in the bioassays the slides were placed in racks in a 50-l tank of deionised water recirculated through a carbon filter, for at least 4 weeks in order to remove residual curing agents. Prior to starting the assays, each test surface was gently wiped with a sterile sponge and sterile deionised water (DW) in order to remove any conditioning film and/or freshwater bacterial biofilm formed during the leaching-period.
Glass microscope slides without coating were used as reference test surfaces and were degreased with acetone, washed in sterile DW and then soaked overnight in sterile DW.
Characterisation of test surfaces
Since the FR performance of elastomeric coatings is considered to be linked to surface energy and elastic modulus (Brady and Singer Citation2000), these two parameters were determined for each test coating.
Contact angle
Static contact angles for DW and diiodomethane (DII) (three drops for each of three replicate slides per test surface) were measured using a Dataphysics OCA35 instrument and SCA22 software for each test surface. The drop size, provided by a micro-syringe, was 4 μl for DW and 1 μl for DII. Measurements were made after a relaxation time of 60 s for each liquid. From each contact angle measurements, polar and dispersive surface energy and total surface energy were calculated according to the Owens, Wendt, Rabel and Kaelble equation (Owens and Wendt 1962).
Elastic modulus
FR test surfaces were prepared with a thickness of ∼1 mm for these measurements as free films (not supported by glass slides). Dynamic Mechanical Analysis (DMA) was carried out on a Perkin Elmer Pyris Diamond Dynamic Mechanical Analyser and MUSE 4.2u software (one measure for each of the three replicate slides per test surface). Tension mode with sinusoidal oscillation at 1 Hz and heating at 3°C min−1 was used. The following additional parameters were also used: sample strain 10 μm, minimum tension force 5 mN, tension/compression gain 1.25, initial amplitude force 2000 mN and temperature range −140°C to 100°C.
Formation of biofilms from natural seawater (NSW)
NSW was collected from Hartlepool Marina, County Durham, UK (54°69′N; 1°20′E) in June 2011 (for assays with U. linza) and January 2012 (for assays with N. incerta) and was stored at 4°C until used for assays. For each bioassay, fifteen (U. linza) or nine (N. incerta) replicate slides per test surface were held in sterilised glass slide holders (slide staining trough, 112 mm × 112 mm × 50 mm, Fisher Scientific) with 150 ml of NSW and were placed on a rotary shaker for 12 days at 50 rpm at room temperature; the NSW was replaced every 2 days. At the end of the incubation period, slides were washed with 10 ml of filter-sterilized (0.22 μm) artificial seawater (ASW; Tropic Marin®). Non-biofilmed control slides were incubated as described above using NSW filtered through a 0.22 μm membrane filter.
To evaluate the bacterial density on biofilmed test surfaces and to verify the absence of contamination on control test surfaces, three replicate slides per test surface were fixed with 10 ml of 2.5% (v/v) glutaraldehyde in filtered ASW, for 20 min at room temperature, washed in DW and air-dried. Adhered bacterial cells were stained with 5 μm SYTO13® (Invitrogen, Molecular Probes) (λ excitation and emission: 488 and 509 nm, respectively), and covered with a glass coverslip (22 × 64 mm, VWR International). After 10 min in darkness, the density of bacterial cells was determined using an AxioVision 4 image analysis system attached to a Zeiss epifluorescence microscope (40 × objective; λ excitation and emission: 450/490 and 515/565 nm, respectively) and a video camera. A total of thirty fields of view (each 0.038 mm2) was counted for each of the three replicate slides, images being taken every 5 mm for three parallel rows, avoiding the slide edges.
Formation of single-species biofilms of Cobetia marina
Cobetia marina (C. marina; Cobet et al. Citation1970; Arahal et al. Citation2002) (ATCC 25374T, DSMZ, Germany) was revived from the original lyophilate and stored as frozen stock aliquots in glycerol and liquid ZoBell medium. Experimental stock cultures were maintained on ZoBell agar plates and stored at 4°C for up to 4 weeks. Bacterial pre-cultures were prepared from several colony forming units from the agar stock cultures and were suspended in 25 ml of ZoBell medium for 24 h at 18°C with shaking at 150 rpm. Sub-cultures containing 50 ml of ZoBell medium were prepared from the pre-cultures to obtain an OD600nm of 0.1 (4.0 × 107 cells ml−1) and were grown for 6 h at 18°C with shaking at 150 rpm.
From the centrifuged sub-cultures (1 min at 8000 rpm), pellets were washed twice in filtered ASW, and diluted if needed, to obtain a bacterial suspension with an OD600nm = 0.1. For each bioassay, fifteen and nine replicate slides per test surface for assays with U. linza and N. incerta, respectively, were placed into individual compartments of polystyrene 4-compartment plates (‘QuadriPerm’ dishes, Greiner Bio-One Ltd) and 10 ml of the bacterial suspension were added. The dishes were placed on a rotary shaker at room temperature for 1 h at 50 rpm to allow attachment of bacteria to the test surfaces. The bacterial suspension was removed and 10 ml of filtered ASW were added for 1 min at 50 rpm to remove any loosely bound cells. Test surfaces were transferred to sterilised glass slide holders with 150 ml of ZoBell medium, which were placed on a rotary shaker at room temperature for 18 h at 50 rpm. Non-biofilmed control slides were incubated with 150 ml of sterile ZoBell medium and then processed as described above. After 18 h all slides (biofilmed and control) were washed with 10 ml of filtered ASW for 1 min at 50 rpm.
To evaluate the bacterial densities on the biofilmed and control test surfaces, three replicate slides per test surface were fixed, stained and counted as described above.
All surfaces (biofilmed and control) were immersed in filtered ASW for 30 min prior to the start of the algal assays.
Adhesion strength of bacteria forming the biofilms
The adhesion strength of biofilms (NSW and C. marina) was evaluated in independent experiments. After formation of the biofilms (six replicates per test surface), three replicates were fixed in 2.5% (v/v) glutaraldehyde and another three replicate slides were exposed for 5 min to a wall shear stress of 52 Pa in a calibrated water channel (Schultz et al. Citation2000, Citation2003). After exposure to flow, slides were fixed and the bacterial density on all the slides was determined as previously described. The number of bacteria remaining after flow was compared to the unexposed samples as a measure of the adhesion strength of bacteria for each test surface and biofilm.
Settlement and adhesion strength of spores of U. linza
Standard protocols were used to measure the settlement of spores and their adhesion strength after settlement. In brief, motile spores (zoospores) were released from fertile plants of U. linza collected from Llantwit Major beach, Wales, UK (51°40′N; 3°48′W) as described by Callow et al. (1997) and Cooper et al. (Citation2011). The spore concentration was adjusted to OD600nm = 0.15 (1.0 × 106 spores ml−1) using filtered ASW. Twelve replicate slides for each biofilmed and control test surface were placed in individual compartments of QuadriPerm dishes (Greiner One) and 10 ml of spore suspension were added to each compartment. After incubation for 45 min in darkness at room temperature, all slides were washed in filtered ASW to remove unsettled (motile) spores. Three replicate slides of each biofilmed and control test surface were fixed with 10 ml of 2.5% (v/v) glutaraldehyde in filtered ASW and then washed and allowed to air-dry overnight (Callow et al. 1997; Cooper et al. Citation2011). Settled spores were counted by chlorophyll autofluorescence using epifluorescence microscopy (20 × objective; λ excitation and emission: 546 and 590 nm, respectively) as described above.
To assess the adhesion strength of the settled spores, three replicate slides of each biofilmed and control test surface were exposed for 5 min to a wall shear stress of 52 Pa in a calibrated water channel (Schultz et al. Citation2000, Citation2003). Settled spores were fixed and counted as previously described. The number of spores remaining after flow was compared to the unexposed samples and the percentage removal of spores was calculated for each biofilmed and control test surface. The six remaining slides for each test surface were used to cultivate sporelings (young plants). Ten millilitres of enriched seawater medium (Starr and Zeikus Citation1987) were added to each compartment of the QuadriPerm dishes, which were incubated at 18°C with a 16 h:8 h light:dark cycle with a light intensity of 40 μmol m−2 s−2, for 7 days to allow the growth of sporelings. The medium was refreshed after 24 h and then every 2 days.
Adhesion strength of sporelings of U. linza
The biomass of sporelings after 7 days growth on each biofilmed and control test surface was estimated by quantifying the fluorescence of chlorophyll in a plate reader (λ excitation and emission: 430 and 670 nm, respectively, TECAN Genios Plus). Fluorescence was measured as relative fluorescence units (RFU) and was the mean of a 168-point fluorescence reading taken within an area 6 × 1.9 cm along the middle of the slide. One slide for each test surface, previously hydrated in sterilised ASW, was used to blank the plate reader. RFU values from the TECAN are directly proportional to the concentration of DMSO-extractable chlorophyll a over the range of 0–2 μg chl a cm−2. The six replicate slides per test surface were exposed for 5 min to a shear stress within the range of 10–52 Pa (depending on the adhesion strength of the sporelings to each particular coating), in a calibrated water channel. Biomass remaining after flow was quantified as described above and was compared to the unexposed samples to evaluate the adhesion strength of sporelings as percentage removal of biomass on each test surface.
Settlement and adhesion strength of N. incerta
Standard protocols (Finlay et al. Citation2010) were used to measure the settlement and adhesion strength of cells of the diatom N. incerta which was isolated from rocks at Llantwit Major beach. In brief, cells in log phase were washed three times in filtered ASW, resuspended, then filtered through 20 and 50 μm nylon meshes. The culture was diluted to a chl a content of 0.25 μg ml−1. Six replicate slides of each biofilmed and control test surface were placed in compartments of QuadriPerm dishes and 10 ml of diatom suspension were added. After 2 h on the laboratory bench at room temperature, the slides were washed vigorously in filtered ASW to remove unsettled (unattached) diatoms. Three replicate slides of each biofilmed and control test surface were fixed and quantified as described previously for spores of U. linza while the other three replicates slides were exposed for 5 min to a wall shear stress of 40 Pa in a calibrated water channel and processed as above. The number of cells remaining after flow was compared to the unexposed samples to evaluate the adhesion strength of cells on each test surface.
Effect of biofilm densities on the adhesion strength of spores of U. linza and cells of N. incerta
To investigate whether there was an effect of biofilm on the adhesion strength of spores of U. linza and cells of N. incerta, different cell densities of C. marina were prepared to start the formation of biofilms using IS700 test surfaces (starting OD600 = 0.02, 0.1 and 0.2 leading respectively to a low, medium and high biofilm density). The adhesion strength of the algal cells was evaluated using a shear stress of 52 Pa for 5 min as previously described.
Confocal laser scanning microscopy and image analysis
Biofilms were stained with SYTO13® dye at 5 μm and incubated for 10 min in the dark. Slides were washed gently in filtered ASW and transferred into the compartments of QuadriPerm dishes. Spores of U. linza were settled on the biofilmed slides as described above (spore concentration was adjusted at OD600nm = 0.15).
A Bio-Rad Radiance 2000 confocal laser scanning microscope coupled to an upright microscope equipped with a Nikon x60 (NA 1.4) oil objective was used to analyse the biofilm structure together with settled spores. SYTO13® dye fluorescence and spore autofluorescence were excited at 488 nm and fluorescence emission collected at 515–530 nm and 570–590 nm, respectively. In some cases, sequences of images were taken along the optical Z axis (15 μm of depth) using the XY series facility to obtain an .avi file. Image acquisition was performed with Lasersharp 2000 software (v.3.2.). Minimal processing of samples was performed with the Image J software to compile composite images (projection in the xy plane) and to obtain sagittal/Z sections (projection in the xz or yz plane) from the .avi files.
Statistical analyses
The settlement densities of spores of U. linza and cells of N. incerta are presented as the number of settled spores/cells mm−2; the biomass of sporelings of U. linza is presented as relative fluorescence units (RFU). The adhesion strength of spores/cells and sporelings is presented as percentage removal with 95% confidence limits calculated from arcsine-transformed data. Using the software Minitab 15, one way ANOVA test was used to determine differences between biofilmed and control slides for each test surfaces. Data were checked for normality and most data conformed to normality assumptions. A small proportion of the data had a minor level of negative skewness but as explained previously (Patel et al. Citation2003), ANOVA tests were still performed on these data because Model I ANOVA, as used here, is extremely robust to non-normality as far as comparisons between means and their confidence intervals are concerned (Kendall et al. Citation1983). Therefore, the small amounts of skewness of the size detected here are very unlikely to result in spurious significance. Resort to non-parametric tests in such situations would have led to considerable and unnecessary loss of statistical power. One way ANOVA with a pairwise Tukey comparison test was used to determine differences between the four biofilmed or control test surfaces. Values were considered significantly different from each other when p-value (p) < 0.05.
Results
Characterisation of test surfaces
As expected, both IS700 and PDMS coatings were hydrophobic, characterised by high static water contact angles (CA), low total surface energies and low contributions of polar surface energies (). IS900, an amphiphilic coating, gave the lowest static water CA values of the three elastomeric coatings and although the total surface energy was intermediate between IS700 and PDMS, the contribution of the polar surface energy was much higher (45.7%) than for either of these coatings. As anticipated, glass was the most wettable surface with the lowest CA value, a high total surface energy and a high proportion of the polar surface energy component ().
Table 1. Wettability properties of the test surfaces used, as determined by static contact angle measurements.
The elastic modulus values for the FR coatings were determined as 1.2 MPa (IS700), 1.0 MPa (IS900) and 0.8 MPa (PDMS).
Bacterial biofilm densities
Biofilms formed from NSW
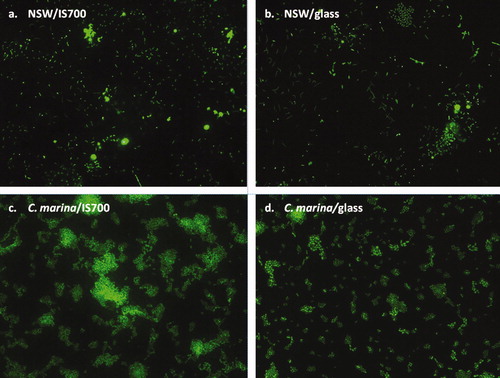
After biofilm growth for 12 days, the mean bacterial densities on IS700 and PDMS were not significantly different (a). Significantly higher and lower densities were obtained on IS900 and glass, respectively. However, the magnitude of the difference comparing IS900 with IS700 and PDMS was at most only 18 and 10.6%, respectively. The biofilms appeared to be mainly in the form of monolayers with a few small aggregates as illustrated in a and b for IS700 and glass. Only bacterial cells were routinely seen, apart from a sporadic diatom on some samples.
Figure 1. Mean density of bacterial cells obtained from biofilms formed from (a) NSW (n = 360 for each surface) and (b) C. marina (n = 630 for each surface) on a range of test surfaces. Error bars represent ± 2 × SE. For the two figures, values that are significantly different to each other at p < 0.05, are indicated by different letters above the bars.
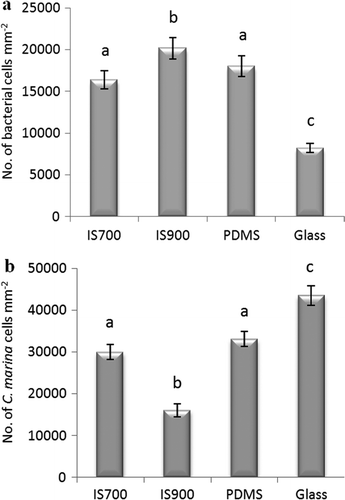
Figure 2. Epifluorescence micrographs showing a general view of biofilms formed from NSW (a and b) and from C. marina (c and d) on IS700 and glass stained with SYTO13® dye. Image size: 225 μm×170 μm.
The adhesion strength of the biofilms was assessed at the maximum shear stress obtainable, which was also the same as that used to assess adhesion strength of the test algae, ie 52 Pa for 5 min. Under these conditions the percentage removal (±2xSE) was relatively low being 4.7% (±2.6) for IS700, 12.6% (±4.0) for IS900, 14.2% (±4.2) for PDMS and 14.9% (±4.8) for glass.
Biofilms formed from C. marina
After biofilm growth for 18 h, as for NSW, the mean bacterial densities on IS700 and PDMS were not statistically different. Unlike NSW, IS900 supported the least dense biofilm and glass the highest (b). Representative views of these biofilms showing a monolayer of cells with some three dimensional aggregates are shown in c and d for IS700 and glass.
In comparison with biofilms formed from NSW, the adhesion strength of biofilms of C. marina was weak on all the surfaces with 76% (±3.7) removal for IS700, 80.1% (±4.1) removal for IS900, 76.2% (±4.3%) removal for PDMS and 60.4% (±8.5%) removal for glass.
Bioassays with U. linza
Bioassays were performed twice for each biofilm type and the pattern of results presented in this study was observed in both experiments.
Settlement and adhesion strength of spores on biofilms formed from NSW
Differences in spore settlement on control (non-biofilmed) test surfaces were observed being significantly greater on IS900 and PDMS compared with glass and IS700 (ANOVA, Tukey test p < 0.05) (a). The presence of biofilms significantly enhanced settlement on all the test surfaces by 1.7 (IS900) to 5.2 (IS700) fold compared with control surfaces (ANOVA, p < 0.05). The number of settled spores on biofilmed glass was significantly lower compared to the three biofilmed silicone surfaces (ANOVA, Tukey test p < 0.05).
Figure 3. Effect of bacterial biofilms formed from NSW on the settlement and the adhesion strength of spores of U. linza on a range of test surfaces. (a) Mean density of settled spores on control (non-biofilmed) and biofilmed surfaces obtained from three replicate slides (n = 90). Error bars represent ± 2×SE. (b) Mean percentage removal of spores from control and biofilmed surfaces, calculated from the counts of three replicates exposed to 52 Pa shear stress compared with the three unexposed replicates. Error bars represent ± 2×SE, calculated from arcsine-transformed data. For the two figures, asterisks show values that are significantly different to control surfaces (ANOVA, p < 0.05).
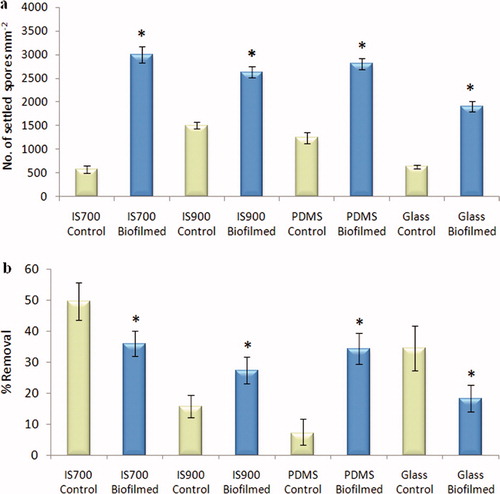
The adhesion strength of settled spores on the control surfaces, as assessed by percentage removal under a defined shear stress, varied between the test surfaces, with highest removal from IS700 and lowest removal from PDMS. Both significant positive and negative effects of the presence of a biofilm were observed (ANOVA, p < 0.05). Compared with the control test surfaces, the adhesion strength of settled spores was enhanced by the biofilm on IS700 and glass, whereas it was reduced on IS900 and PDMS (b). This was not correlated with the relatively small differences in bacterial density observed on the three elastomeric test surfaces (a). The adhesion strength of settled spores on biofilmed glass slides was significantly lower compared with the three biofilmed silicone surfaces (ANOVA, Tukey test p < 0.05) (b).
Adhesion strength of sporelings on biofilms formed from NSW
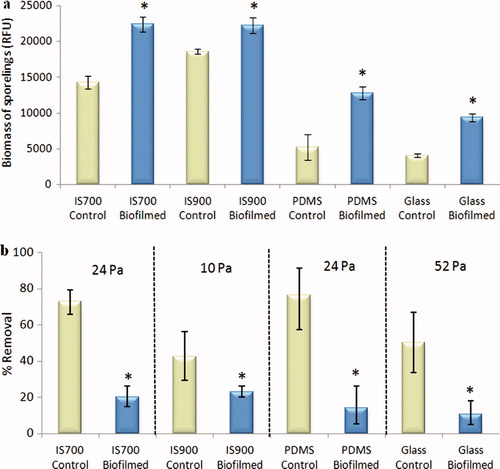
Sporelings are young plants formed by growth of germinated settled spores. After 7 days growth, the mean biomass of sporelings was higher on all biofilmed surfaces than on the control surfaces, with the greatest biomass on IS700 and IS900 (ANOVA, p < 0.05) (a). Because the adhesion strength of sporelings on this range of test coatings is known to vary considerably, different shear stresses were used to evaluate the effect of the biofilm. In all cases there was significantly less removal of sporelings from biofilmed surfaces (<23.5%) compared with the non-biofilmed controls (>42.5%), showing that the adhesion strength of sporelings was enhanced by the presence of biofilms formed from NSW whatever the test surface used (ANOVA, p < 0.05) (b).
Figure 4. Effect of bacterial biofilms formed from NSW on the settlement and the adhesion strength of sporelings of U. linza on a range of test surfaces. (a) Mean biomass of sporelings on control (non-biofilmed) and biofilmed surfaces before exposure to shear stress obtained from the measure of the chlorophyll fluorescence (RFU) on six replicate slides. Error bars represent ± 2×SE. (b) Mean percentage removal of sporelings from control and biofilmed surfaces, after exposure to a shear stress of 10 Pa for IS900, 24 Pa for PDMS and IS700 and 52 Pa for glass, obtained from RFU determinations on six replicate slides before and after exposure to flow. Error bars represent ± 2×SE and were calculated from arcsine-transformed data. For the two figures, asterisks show the values that are significantly different to control surfaces (ANOVA, p < 0.05).
Settlement and adhesion strength of spores on biofilms formed from C. marina
Differences in spore settlement on the control test surfaces were observed. The mean settled spore density between the three silicone elastomers was significantly different, with the highest density obtained for PDMS and the lowest for IS900. The mean density of settled spores on glass was not significantly different to that on PDMS (ANOVA, Tukey test p < 0.05) (a). It will be noted that the density of settled spores on the non-biofilmed control slides from this experiment was considerably higher than previously shown for non-biofilmed control slides in the experiments with NSW. Furthermore the ranking of settlement between surfaces is different (a). The reason for this probably lies in the different pre-treatment of control surfaces (sterile culture medium vs filtered NSW) which probably causes the formation of quantitatively and qualitatively different conditioning layers on these surfaces. It is known that settlement of spores of U. linza is sensitive to molecular conditioning of surfaces (Thomé et al. Citation2012).
Figure 5. Effect of bacterial biofilms of C. marina on the settlement and the adhesion strength of spores of U. linza on a range of test surfaces. (a) Mean density of settled spores on control (non-biofilmed) and biofilmed surfaces obtained from the count of three replicate slides (n = 90). Error bars represent ± 2×SE. (b) Mean percentage removal of spores on control and biofilmed surfaces, calculated from the counts of three replicate slides exposed to 52 Pa shear stress compared with the three unexposed replicate slides. Error bars represent ± 2×SE calculated from arcsine-transformed data. For the two figures, asterisks show the values that are significantly different to control surfaces (ANOVA, p < 0.05).
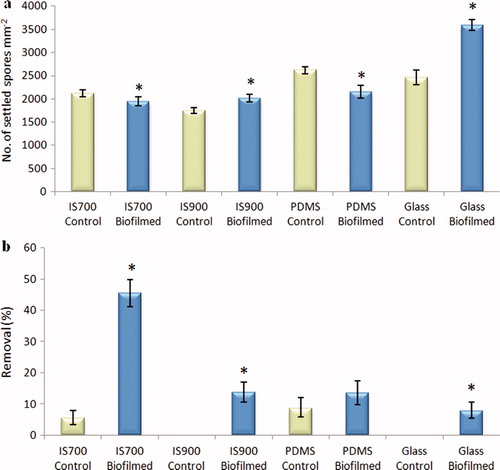
The presence of biofilms of C. marina significantly influenced the settlement of spores, but in contrast with biofilms formed from NSW, both negative (IS700 and PDMS) and positive (IS900 and glass) effects were obtained (ANOVA, p < 0.05) and although there was an enhanced settlement on IS900 the effect was relatively small. The number of settled spores on biofilmed glass was significantly higher compared with the three biofilmed silicone surfaces (a).
In the experiments with non-biofilmed control slides (pre-treated with sterile culture medium), the adhesion strength of settled spores was high, with a maximum removal of 8.6% at 52 Pa (b). By comparison, the maximum removal from non-biofilmed control slides in the experiments with NSW was ca 50%. Again, the reason for this probably lies in the differences in pre-conditioning of the surfaces, although it is also known that there is seasonal variation between different batches of spores.
In contrast with the results obtained for biofilms formed from NSW, the adhesion strength of settled spores was significantly and consistently reduced by the presence of biofilms on all the test surfaces (ANOVA, p < 0.05). The lowest adhesion strength was obtained on IS700 (45.5% removal) while the highest was obtained on glass (7.8% removal) (b).
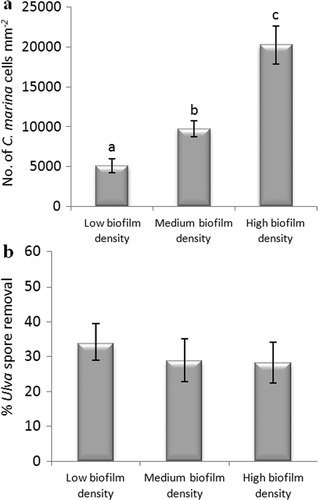
The effect of the density of the biofilm formed from C. marina on the adhesion strength of spores of U. linza was evaluated on IS700. Significant differences were obtained between the three biofilm densities (low, medium and high density) (ANOVA, Tukey test p < 0.05) (a). However, these different densities of cells of C. marina had no significant effect on the adhesion strength of spores (removal ranged between 28.1 and 33.6%) (ANOVA, Tukey test p > 0.05) (b).
Figure 6. Effect of biofilm densities of C. marina on the adhesion strength of spores of U. linza on IS700. (a) Mean density of bacterial cells obtained from the count of three replicate slides (n = 90). Error bars represent ± 2×SE. Values that are significantly different to each other at p < 0.05, are indicated by different letters above the bars. (b) Mean percentage removal of spores on biofilmed IS700 surfaces, calculated from the counts of three replicate slides exposed to 52 Pa shear stress compared with the three unexposed replicate slides. Error bars represent ± 2×SE, calculated from arcsine-transformed data.
Adhesion strength of sporelings on biofilms formed from C. marina
The mean biomass of sporelings on IS700 and PDMS was significantly lower on biofilmed surfaces than on control surfaces while in contrast, the mean biomass of sporelings on IS900 and glass was significantly higher on biofilmed surfaces than on control surfaces (ANOVA, p < 0.05) (a).
Figure 7. Effect of biofilms of C. marina on the settlement and the adhesion strength of sporelings of U. linza on a range of test surfaces. (a) Mean biomass of sporelings on control (non-biofilmed) and biofilmed surfaces before exposure to shear obtained from the measure of chlorophyll fluorescence (RFU) on six replicate slides. Error bars represent ± 2×SE. (b) Mean percentage removal of sporelings on control and biofilmed surfaces, after exposure to a shear stress of 10 Pa for IS900, 24 Pa for PDMS and IS700 and 52 Pa for glass obtained from RFU determinations on six replicate slides before and after exposure to flow. Error bars represent ± 2×SE calculated from arcsine-transformed data. For the two figures, asterisks show the values that are significantly different to control surfaces (ANOVA, p < 0.05).
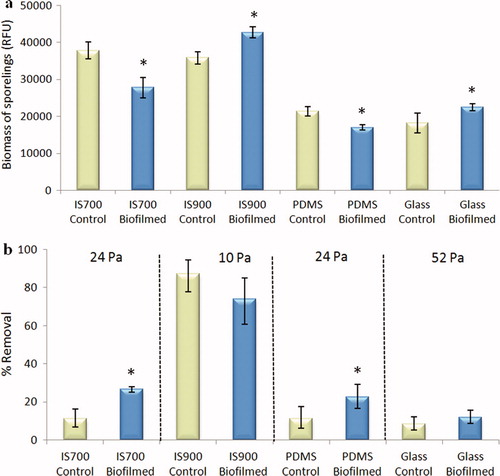
In the presence of biofilms, the adhesion strength of sporelings on IS700 and PDMS was significantly reduced (ANOVA, p < 0.05) while for IS900 and glass there were no significant effects of the biofilm (ANOVA, p > 0.05) (b).
Confocal laser scanning microscopy (CLSM)
For both types of biofilm, CLSM showed that spores of U. linza typically interacted with biofilms of both types by settling either adjacent to bacterial aggregates as previously observed by Joint et al. (Citation2000), or directly over the bacteria as observed by Patel et al. (Citation2003). However, reconstructed Z sections showed that some spores were also able to settle under the bacteria and to be in direct contact with the surface ( and ). Spores settling underneath the bacteria were more often observed with biofilms formed from C. marina which were adhered less strongly than those formed from NSW as described above.
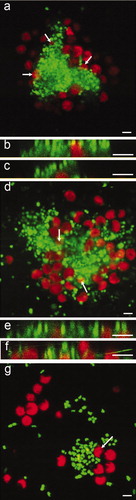
Figure 8. Confocal microscope micrographs showing settled spores of U. linza (revealed in red due to chlorophyll auto-fluorescence) on test surfaces with the presence of bacterial biofilm formed from NSW (revealed in green due to SYTO13® dye). (a and b) Composite images showing settled spores adjacent to bacterial aggregates on IS700, (c) Composite image showing settled spores underneath bacteria on glass and, (d and e) Sagittal/Z sections of the composite image (c), showing settled spores underneath bacteria. Scale bar = 5 μm.
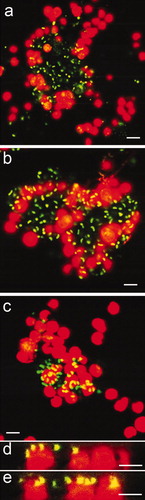
Figure 9. Confocal microscope micrographs showing settled spores of U. linza (revealed in red due to chlorophyll auto-fluorescence) on test surfaces with the presence of bacterial biofilm formed from C. marina (revealed in green due to SYTO13® dye). (a) Composite image showing settled spores adjacent to bacterial aggregates and partially settled under the bacteria (arrows) on IS700. (b and c) Sagittal/Z sections of the composite image (a), settled spores underneath bacteria. (d) Composite image showing settled spores adjacent to bacterial aggregates and under the bacteria (arrows) on IS900, (e and f) Sagittal/Z sections of the composite image (d), showing settled spores underneath bacteria and, (g) composite image showing settled spores adjacent to bacteria on glass. A spore undergoing settlement (arrow) was able to move bacteria from the settlement site by the motion of its flagella. Scale bar = 5 μm.
Bioassays with the diatom Navicula incerta
Bioassays with N. incerta were performed twice for each biofilm and the pattern of results presented below was observed in both experiments.
Settlement and adhesion strength of cells of N. incerta on biofilms formed from NSW
Relatively small differences in cell settlement on control silicone test surfaces were obtained and the lowest settlement was on glass (a). As observed for spores of U. linza, the presence of biofilms formed from NSW substantially enhanced settlement on all the surfaces by 1.7 (IS700) to 2.8 (glass) fold (ANOVA, p < 0.05) (a).
The adhesion strength of cells of N. incerta did not follow the same trend as observed with spores of U. linza. Indeed, the removal of settled cells under shear was significantly and strongly increased on all biofilmed silicone surfaces compared with control surfaces (ANOVA, p < 0.05) (b). Removal of cells from the control surfaces was very low, being almost zero in the case of IS900 and PDMS, whereas removal from biofilmed surfaces ranged from 37–93%. Cells attached rather weakly to control glass slides, but even here there was a small, but statistically significant increase in removal from the biofilmed glass slides (ANOVA, p < 0.05).
Settlement and adhesion strength of cells of N. incerta on biofilms formed from C. marina
Although biofilms formed from NSW strongly enhanced the settlement of diatom cells irrespective of coating type, the effects of biofilms formed from C. marina were smaller in magnitude and more variable between coatings (a). There was no statistically significant effect of biofilms formed on IS700 (ANOVA, p > 0.05). However, biofilms formed on IS900 and glass significantly increased settlement by 31–32%, but those formed on PDMS reduced settlement by 33.3%. The mean density of cells of N. incerta on control surfaces was significantly lower on IS900 than on IS700 (ANOVA, p < 0.05).
Figure 10. Effect of bacterial biofilms formed from NSW on settlement and adhesion strength of cells of N. incerta on a range of test surfaces. (a) Mean density of settled cells on control (non-biofilmed) and biofilmed surfaces obtained from the count of three replicate slides (n = 90). Error bars represent ± 2×SE. (b) Mean percentage removal of cells on control and biofilmed surfaces calculated from the counts of three replicates exposed to 40 Pa shear stress compared with three unexposed replicate slides. Error bars represent ± 2×SE were calculated from arcsine-transformed data. For the two figures, asterisks show the values that are significantly different to control surfaces (ANOVA, p < 0.05).
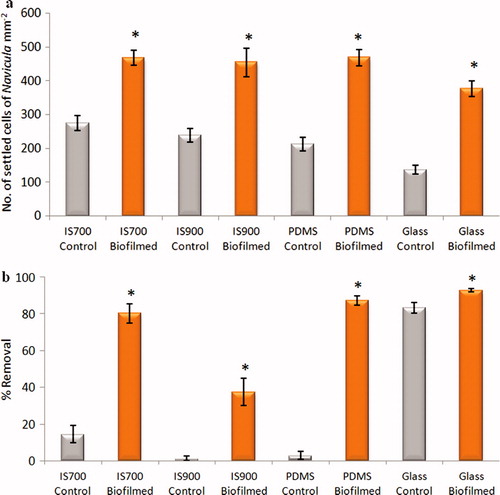
Figure 11. Effect of bacterial biofilms formed form C. marina on settlement and adhesion strength of cells of N. incerta on a range of test surfaces. (a) Mean density of settled cells on control (non-biofilmed) and biofilmed surfaces obtained from the count of three replicate slides (n = 90). Error bars represent ± 2×SE. (b) Mean percentage removal of cells from control and biofilmed surfaces calculated from the counts of three replicates exposed to 40 Pa shear stress compared with three unexposed replicate slides. Error bars represent ± 2×SE were calculated from arcsine-transformed data. For the two figures, asterisks show the values that are significantly different to control surfaces (ANOVA, p < 0.05).
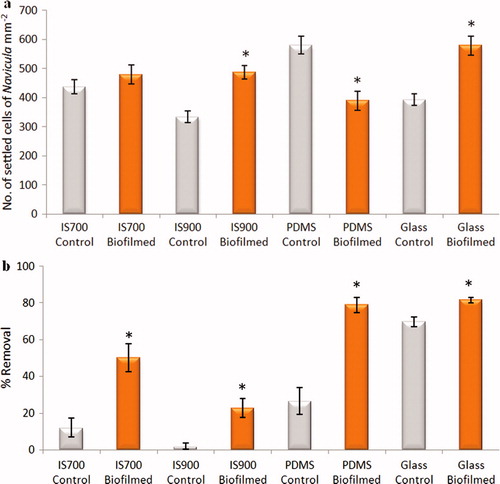
As observed with biofilms formed from NSW, the adhesion strength of N. incerta cells was significantly reduced on all biofilmed surfaces compared with the control surfaces although the effect was small on glass slides (ANOVA, p < 0.05) (b).
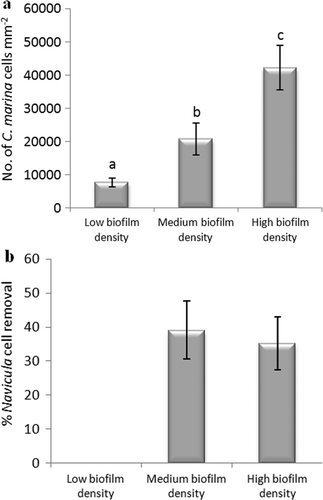
The effect of the density of biofilms formed from C. marina on the adhesion strength of N. incerta was also evaluated on IS700. As observed for the spore assay, significant differences were obtained between the three biofilm densities (low, medium and high density) (ANOVA, Tukey test p < 0.05) (a). However, no significant effect on the adhesion strength of N. incerta was obtained between a medium and a high density of bacterial cells (39 and 36% removal, respectively) (ANOVA, Tukey test p > 0.05). N. incerta was found to adhere strongly when a low biofilm density was present (no removal) (b).
Figure 12. Effect of different biofilm densities of C. marina on the adhesion strength of cells of N. incerta on IS700. (a) Mean density of bacterial cells obtained from the counts of three replicate slides (n = 90). Error bars represent ± 2×SE. Values that are significantly different to each other at p < 0.05, are indicated by different letters above the bars. (b) Mean percentage removal of cells on biofilmed IS700 surfaces, calculated from the counts of three replicate slides exposed to 52 Pa shear stress compared with three unexposed replicate slides. Error bars represent ± 2×SE, calculated from arcsine-transformed data.
Discussion
Although there have been numerous papers published on the effects of bacterial biofilms (single species and natural consortia) on the settlement of marine invertebrate larvae and algal spores (Callow and Callow Citation2006b), to date there is only one report on the effect of biofilms on the adhesion strength of marine species. Zardus et al. (Citation2008) performed laboratory studies on a range of invertebrate species using biofilms formed on glass slides developed from NSW. However, there has not been a comparable study on marine algae. Therefore to rectify this, similar studies to those of Zardus et al. (Citation2008) were performed with two species of marine algae with contrasting adhesion biology, but the scope of the present studies was extended to encompass (a) the quantification of biofilm density, (b) the use of a single-species biofilm in addition to a consortium formed from NSW, (c) the interaction with different surfaces including two commercial FR materials, (d) control treatments in which the influence of surface conditioning from molecules in either NSW or culture medium can be separated from the effect of the bacterial biofilm. While experiments in the laboratory cannot be taken to be indicative of performance in the field, they do allow the control of experimental conditions that is essential if fundamental processes and parameters that influence fouling dynamics in the marine environment are to be understood.
Quantification of biofilms revealed preferences in bacterial attachment between test surfaces, depending on the type of biofilm. From 12-day old biofilms formed from NSW, a much greater bacterial density was found on the three FR silicone elastomer coatings, IS700, IS900 and PDMS than on the hard, hydrophilic glass surface. These results demonstrate that the FR coatings do not deter the initial attachment of bacteria and development of mixed species biofilms, partially confirming the results obtained by Molino et al. (Citation2009) where IS700 coatings formed a substantial bacterial coverage soon after immersion in seawater. There are only limited field studies that describe the colonisation of surfaces by microorganisms. Huggett et al. (Citation2009) showed that the density of 10-day old NSW biofilms at Pearl Harbor, were not strongly influenced by the wettability properties of silanised hard surfaces. However, Dobretsov and Thomason (Citation2011) showed reduced biofilm development on IS900 compared with IS700, 10 days after static immersion in a tropical marina.
In contrast to biofilms formed from NSW, the highest biofilm densities of C. marina were obtained on glass and the lowest on IS900 with intermediate and similar values on IS700 and PDMS, showing that cells of C. marina preferentially attached to hard, hydrophilic surfaces rather than to hydrophobic or amphiphilic silicone coatings. Previous studies using some single bacterial strains (marine, estuarine or medical) have shown that surface hydrophobicity plays an important role, with bacteria preferring hydrophobic surfaces, irrespective of surface chemistries (Samuelsson and Kirchman Citation1990; Wiencek and Fletcher Citation1995; Bakker et al. Citation2004). However, for C. marina specifically, there is a contradictory literature. While Ista et al. (Citation1996, Citation1999, Citation2004) demonstrated greater attachment of C. marina on hydrophobic than on hydrophilic SAMs, regardless of surface chemistry, other studies have reported that C. marina attached preferentially to hydrophilic rather than to hydrophobic surfaces (Shea et al. Citation1991; Ekblad et al. Citation2008). More recently, Pranzetti et al. (Citation2012) demonstrated that the main parameter driving the attachment of cells of C. marina to SAMs was not surface wettability, but surface charge.
Biofilms formed from NSW strongly enhanced the settlement of spores of U. linza as previously demonstrated (Dillon et al. Citation1989; Joint et al. Citation2000) and similar results were obtained for cells of N. incerta, despite the fact that the NSW used to make the biofilms for U. linza and N. incerta assays was not collected at the same time and thus could be slightly different in term of species and abundance (although results for U. linza with both batches of NSW were consistent). This effect was obtained irrespective of coating type and surface wettability. Furthermore, the net effect of the presence of such biofilms for the three FR coatings was to minimise the differences in performance (algal settlement) inherent in the control non-biofilmed surfaces. A similar effect was observed by Huggett et al. (Citation2009) with a similar numbers of tubeworm larvae settled on surfaces of different wettability that had been colonised by 10-day old NSW biofilms.
The adhesion strength of the settled cells, or in the case of U. linza, sporelings formed from the germinated spores, was also strongly influenced by biofilms formed from NSW, but the response was not always in the same direction. Thus, biofilms formed from NSW on some surfaces reduced adhesion strength of spores (ie greater removal under shear) while increasing it on others. However, the inherent differences on spore adhesion strength observed between the control non-biofilmed FR coatings were minimised by the presence of such biofilms. In the case of sporelings, the net effect on all surfaces was to reduce the percentage of removal under shear indicating stronger adhesion to the biofilmed surfaces. On the other hand, for all test surfaces the presence of biofilms formed from NSW significantly reduced the adhesion strength of the diatom N. incerta. On glass the effect was small since diatoms in general do not adhere well to hydrophilic surfaces (Schilp et al. Citation2007; Thompson et al. Citation2008; Finlay et al. Citation2010). But, on the three FR coatings the effect was substantial. As shown by the data, diatoms tend to adhere strongly to hydrophobic materials, especially silicone-based elastomers (Holland et al. Citation2004; Sokolova et al. Citation2012) and the effect of the biofilm on these materials was apparently to convert surfaces that released hardly any cells at the shear stress tested, into more effective FR materials. However, a field study has shown that after a period of immersion of 60 days (East coast of Florida, United States), IS700 and IS900 coatings were highly fouled by diatoms (Zargiel et al. Citation2011).
As a further demonstration of the complexity of these relationships, the results with biofilms formed from C. marina, a species commonly used in marine biology research, were substantially different to those with biofilms formed from NSW. Indeed, the presence of such biofilms induced either enhancement or a reduction in the number of settled algae depending of the test surface used. Gawne et al. (Citation1998) have previously showed that single-species biofilms formed from Pseudomonas atlantica and associated EPS, reduced the settlement of the diatom Achnanthes longipes on polystyrene, enhanced it on glass while no effect was obtained on silicone coatings. Inhibitory effects on diatom settlement of single-species biofilms formed from a Pseudoalteromonas sp. on glass, have also been reported by Wigglesworth-Cooksey and Cooksey (Citation2005).
Biofilms of C. marina either reduced the adhesion strength of spores and sporelings of U. linza, or had no effect. The effects on the adhesion of diatoms were, however, generally similar to those with biofilms formed from NSW, uniformly reducing adhesion on all test surfaces. The effects on reduced adhesion of spores and sporelings may be due to the fact that these biofilms were more weakly attached to the surfaces compared with those formed from NSW (60–80% bacterial cells removed cf. 5–15%), so under shear more of the biofilm/algal composite was removed. Moreover, in experiments where the density of the C. marina biofilm was increased, this had no effect on the adhesion strength of either alga. This suggests that the relationship between alga and this particular biofilm, at least in terms of effects on adhesion strength, may be qualitative rather than quantitative.
The basis for these very different responses obtained, all of which were reproduced in repeat experiments, can only be speculated upon. In the case of spores with the presence of biofilms formed from NSW, the differences observed between coatings are unlikely to be based on differences in biofilm density since all three silicone-based coatings had quite similar biofilm densities. It is possible that removal of spores from biofilmed surfaces is a composite of effects, reflecting a balance between the adhesion of spores to the coating and the biofilm, and observations by confocal microscopy revealed a range of different physical interactions between settled spores and the biofilms. Thus it was observed that spores can settle adjacent to aggregates of bacteria but are also able to settle over bacterial colonies, and, perhaps surprisingly, beneath them, presumably because the biofilm was only weakly adhered to the coating. It is impossible to resolve such complexities in the present paper, but it seems unlikely that the relative adhesion strength of the biofilms formed from NSW to the substrate is responsible since the adhesion of the biofilm per se, under the same shear stress used for spore removal, is relatively strong and the differences that were observed do not correlate with the relative removal of spores. The relationship between the bacterial biofilm and diatom cells is different again. Diatoms are motile on surfaces, secreting EPS for the purposes of adhesion and locomotion. Some intercalation of materials originating from both sources may therefore be expected.
The situation with respect to the effects of biofilms on the adhesion strength of sporelings of U. linza is more complex since during the 7- day period of growth of sporelings, there will have been opportunities for further development of the biofilm beyond that initially present, presumably influenced by nutrients and other molecules including bioadhesives secreted by the developing sporelings. There will also have been extensive intercalation of the colonising filaments of the alga with the biofilm. Any quantitative characterisation of the resulting biofilm/sporeling composite would be a daunting task, well beyond the scope of the present paper. However, it is likely that the differences in adhesion strength of sporelings observed on the different biofilmed coatings reflect the relative adhesion of this composite and the data show that on every substratum, adhesion is enhanced (ie less percentage removal) on the biofilmed surfaces compared with non-biofilmed control surfaces. Enhancement of the adhesion strength of marine organisms by the presence of NSW biofilms formed on glass slides was also observed by Zardus et al. (2008) for some marine invertebrates.
The primary purpose of this paper has been to demonstrate, under controlled conditions, the potential for bacterial biofilms to influence the colonisation and adhesion to different test surfaces of two species of soft-fouling algae. What messages do the results of this paper hold for those interested in testing the AF and FR properties of novel experimental coatings in laboratory-based bioassays? Such tests are routinely performed using pristine (non-biofilmed) surfaces. While such tests may be informative in terms of the intrinsic properties of novel coating materials, it is obvious that they do not reflect the field situation in which test surfaces will be rapidly modified by conditioning macromolecules and primary colonisers including bacteria (Railkin 2004). What the present paper has demonstrated is that the properties of surfaces as perceived by micro- and macrofouling algae, can be substantially modified by the presence of a bacterial biofilm. However, it would be dangerous to assume that routine, laboratory-based performance evaluations should include a biofilm treatment (unless the test organism requires the presence of a biofilm, as in the case of some tubeworm species (Hadfield 2011)) because, as demonstrated in this paper, the interactions between coating type, the stage of development of the organism, and the type of biofilm are highly complex and not unidirectional. When this complexity is further allied to the uncontrolled environmental and biological variability of the natural environment, the dynamics of the coating-biofilm-alga/invertebrate interaction are likely to be quite different to those experienced in laboratory experiments.
Acknowledgements
The authors would like to thank Dr L. Tyson of International Paint Ltd, Felling for advice concerning the preparation and the characterisation of the test surfaces. They thank also Dr S. Thompson for her help with the confocal microscopy. This work was funded by the European Community Framework Programme 7, SEACOAT (Surface Engineering for Antifouling-Coordinated Advanced Training), under Grant Agreement number 237997.
References
- Arahal , D , Castillo , AM , Ludwing , W , Schleifer , KH and Ventosa , A . 2002 . Proposal of Cobetia marina gen. nov., comb. nov., within the family Halomonadaceae, to include the species Halomonas marina . System Appl Microbiol , 25 : 207 – 211 .
- Bakker , DP , Postmus , BR , Busscher , HJ and van der Mei , HC . 2004 . Bacterial strains isolated from different niches can exhibit different patterns of adhesion to substrata . Appl Environ Microbiol , 70 : 3758 – 3760 .
- Brady , RF and Singer , IL . 2000 . Mechanical factors favouring release from fouling release coatings . Biofouling , 15 : 73 – 81 .
- Caldwell , GS and Pagett , HE . 2010 . Marine glycobiology: current status and future perspectives . Mar Biotechnol , 12 : 241 – 252 .
- Callow , JA and Callow , ME . 2006a . “ The Ulva spore adhesive system. In ” . In Biological adhesives , Edited by: Smith , AM and Callow , JA . 63 – 78 . Berlin, Heidelberg , , Germany : Springer-Verlag .
- Callow , JA and Callow , ME . 2006b . “ Biofilms in antifouling compounds. In ” . In Progress in molecular and subcellular biology: marine molecular biotechnology , Edited by: Fusetani , N and Clare , AS . 141 – 169 . Berlin Heidelberg , , Germany : Springer-Verlag .
- Callow , ME , Callow , JA , Pickett-Heaps , JD and Wetherbee , R . 1997 . Primary adhesion of Enteromorpha (Chlorophyta, Ulvales) propagules: quantitative settlement studies and video microscopy . J Phycol , 33 : 938 – 947 .
- Cobet , AB , Wirsen , C and Jones , GE . 1970 . The effect of nickel on a marine bacterium, Arthrobacter marinus sp. nov . J Gen Microbiol , 62 : 159 – 169 .
- Cooper , SP , Finlay , JA , Cone , G , Callow , ME , Callow , JA and Brennan , AB . 2011 . Engineered antifouling microtopographies: kinetic analysis of the attachment of zoospores of the green alga Ulva to silicone elastomers . Biofouling , 27 : 881 – 891 .
- Dillon , PS , Maki , JS and Mitchell , R . 1989 . Adhesion of Enteromorpha swarmers to microbial films . Microb Ecol , 17 : 39 – 47 .
- Dobretsov , S and Thomason , JC . 2011 . The development of marine biofilms on two commercial non-biocidal coatings: a comparison between silicone and fluoropolymer technologies . Biofouling , 27 : 869 – 880 .
- Dobretsov , S , Teplitski , M and Paul , V . 2009 . Mini-review: Quorum sensing in the marine environment and its relationship to biofouling . Biofouling , 25 : 413 – 427 .
- Ekblad , T , Bergström , G , Ederth , T , Conlan , SL , Mutton , R , Clare , AS , Wang , S , Liu , Y , Zhao , Q D'Souza , F . 2008 . Poly(ethylene glycol)-containing hydrogel surfaces for antifouling applications in marine and freshwater environments . Biomacromolecules , 9 : 2775 – 2783 .
- Finlay , JA , Bennett , SM , Brewer , LH , Sokolova , A , Clay , G , Gunari , N , Meyer , AE , Walker , GC , Wendt , DE Callow , ME . 2010 . Barnacle settlement and the adhesion of protein and diatom microfouling to xerogel films with varying surface energy and water wettability . Biofouling , 26 : 657 – 666 .
- Gawne , B , Wang , Y , Hoagland KD and Gretz MR. 1998 . Role of bacteria and bacterial exopolymer in the attachment of Achnanthes longipes (Bacillariophyceae) . Biofouling , 13 : 137 – 156 .
- Hadfield , MG . 2011 . Biofilms and marine invertebrate larvae: what bacteria produce that larvae use to choose settlement sites . Annu Rev Mar Sci , 3 : 453 – 470 .
- Holland , R , Dugdale , TM , Wetherbee , R , Brennan , AB , Finlay , JA , Callow , JA and Callow , ME . 2004 . Adhesion and motility of fouling diatoms on a silicone elastomer . Biofouling , 20
- Holmström , C , James , S , Egan , S and Kjelleberg , S . 1996 . Inhibition of common fouling organisms by marine bacterial isolates with special reference to the role of pigmented bacteria . Biofouling , 10 : 251 – 259 .
- Huggett , MJ , Nedved , BT and Hadfield , MG . 2009 . Effects of initial surface wettability on biofilm formation and subsequent settlement of Hydroides elegans . Biofouling , 25 : 387 – 399 .
- Ista , LK , Perez-Luna , VH and Lopez , GP . 1999 . Surface-grafted, environmentally responsive polymers for biofilm release . Appl Environ Microbiol , 65 : 1603 – 1609 .
- Ista , LK , Fan , H , Baca , O and Lopez , GP . 1996 . Attachment of bacteria to model solid surfaces: oligo(ethylene glycol) surfaces inhibit bacterial attachment . FEMS Microb Lett , 142 : 59 – 63 .
- Ista , LK , Callow , ME , Finlay , JA , Coleman , SA , Nolasco , AC , Simons , RH , Callow , JA and Lopez , GP . 2004 . Effect of substratum surface chemistry and surface energy on attachment of marine bacteria and algal spores . Appl Environ Microbiol , 70 : 4151 – 4157 .
- Joint , I , Callow , ME , Callow , JA and Clarke , KR . 2000 . The attachment of Enteromorpha zoospores to a bacterial biofilm assemblage . Biofouling , 16 : 151 – 158 .
- Joint , I , Tait , K , Callow , ME , Callow , JA , Milton , D , Williams , P and Camara , M . 2002 . Cell-to-cell communication across the prokaryote–eukaryote boundary . Science , 298 : 1207
- Kendall , M , Stuart , A and Ord , JK . 1983 . The advanced theory of statistics. Vol. 3, 4th ed. London & High , Wycombe , , (UK : Charles Griffin & Company. 780 pp .
- Khandeparker , L , Anil , AC and Raghukumar , S . 2006 . Relevance of biofilm bacteria in modulating the larval metamorphosis of Balanus amphitrite . FEMS Microbiol Ecol , 58 : 425 – 438 .
- Kirchman , D , Graham , S , Reish , D and Mitchell , R . 1982 . Lectins may mediate in the settlement and metamorphosis of Janua (Dexiospira) brasiliensis Grube (Polychaeta: Spirorbidae) . Mar Biol Lett , 3 : 131 – 142 .
- Lam , C , Harder , T and Qian , PY . 2005 . Induction of larval settlement in the polychaete Hydroides elegans by extracellular polymers of benthic diatoms . Mar Ecol Progr Ser , 286 : 145 – 154 .
- Lau , SCK , Harder , T and Qian , PY . 2003 . Induction of larval settlement in the serpulid polychaete Hydroides elegans (Haswell): role of bacterial extracellular polymers . Biofouling , 19 : 197 – 204 .
- Molino , PJ and Wetherbee , R . 2008 . The biology of biofouling diatoms and their role in the development of microbial slimes . Biofouling , 24 : 365 – 379 .
- Molino , PJ , Childs , S , Eason , Hubbard MR , Carey , JM , Burgman , MA and Wetherbee , R . 2009 . Development of the primary bacterial microfouling layer on antifouling and fouling release coatings in temperate and tropical environments in Eastern Australia . Biofouling , 25
- Mos , B , Cowden , KL , Nielsen , SJ and Dworjanyn , SA . 2011 . Do cues matter? Highly inductive settlement cues don't ensure high post-settlement survival in sea urchin aquaculture . PLoS ONE , 6 : 1 – 11 .
- Owens , DK and Wendt , RC . 1969 . Estimation of the surface free energy of polymers . J Appl Polym Sci , 13 : 1741 – 1747 .
- Patel , P , Callow , ME , Joint , I and Callow , JA . 2003 . Specificity in the settlement – modifying response of bacterial biofilms towards zoospores of the marine alga Enteromorpha . Environ Microbiol , 5 : 338 – 349 .
- Petrone , L , Di , Fino A , Aldred , N , Sukkaew , P , Ederth , T , Clare , AS and Liedberg , B . 2011 . Effects of surface charge and Gibbs surface energy on the settlement behaviour of barnacle cyprids (Balanus amphitrite) . Biofouling , 27 : 1043 – 1055 .
- Pranzetti , A , Salaün , S , Mieszkin , S , Callow , ME , Callow , JA , Preece , JA and Mendes , PM . 2012 . Model organic surfaces to probe marine bacterial adhesion kinetics by surface plasmon resonance . Advanced Functional Materials , DOI: 10.1002/adfm.201103067
- Railkin , AI . 2004 . Marine biofouling: colonisation processes and defences , 303 Boca Raton , FL : CRC Press .
- Samuelsson , MO and Kirchman , DL . 1990 . Degradation of adsorbed protein by attached bacteria in relationship to surface hydrophobicity . Appl Environ Microbiol , 56 : 3643 – 3648 .
- Scardino , AJ and de , Nys R . 2011 . Mini review: Biomimetic models and bioinspired surfaces for fouling control . Biofouling , 27 : 73 – 86 .
- Schilp , S , Kueller , A , Rosenhahn , A , Grunze , M , Pettitt , ME , Callow , ME and Callow , JA . 2007 . Settlement and adhesion of algal cells to hexa(ethylene glycol)-containing self-assembled monolayers with systematically changed wetting properties . Biointerphases , 2 : 143 – 150 .
- Schultz , MP , Finlay , JA , Callow , ME and Callow , JA . 2000 . A turbulent channel flow apparatus for the determination of the adhesion strength of microfouling organisms . Biofouling , 15 : 243 – 251 .
- Schultz , MP , Finlay , JA , Callow , ME and Callow , JA . 2003 . Three models to relate detachment of low form fouling at laboratory and ship scale . Biofouling , 19 : 17 – 26 .
- Shea , C , Nunley , JW , Williamson , JC and Smithsomerville , HE . 1991 . Comparison of the adhesion properties of Deleya marina and the exopolysaccharide-defective mutant strain DMRt . Appl Environ Microbiol , 57 : 3107 – 3113 .
- Sokolova , A , Cilz , N , Daniels , J , Stafslien , SJ , Brewer , LH , Wendt , DE , Bright , FV and Detty , MR . 2012 . A comparison of the antifouling/foul-release characteristics of non-biocidal xerogel and commercial coatings toward micro- and macrofouling organisms . Biofouling , 28 : 511 – 523 .
- Starr , R and Zeikus , J . 1987 . UTEX – the culture collection of algae at the University of Texas at Austin . J Phycol , 23 : 1 – 47 .
- Sundaram , HS , Cho , Y , Dimitriou , MD , Weinman , CJ , Finlay , JA , Cone , G , Callow , ME , Callow , JA , Kramer , EJ and Ober , CK . 2011 . Fluorine-free mixed amphiphilic polymers based on PDMS and PEG side chains for fouling release applications . Biofouling , 27 : 589 – 602 .
- Thomé , I , Pettitt , ME , Callow , ME , Callow , JA , Grunze , M and Rosenhahn , A . 2012 . Conditioning of surfaces by macromolecules and its implication for the settlement of zoospores of the green alga Ulva linza . Biofouling , 28 : 501 – 510 .
- Thompson , SEM , Taylor , AR , Brownlee , C , Callow , ME and Callow , JA . 2008 . The role of nitric oxide in diatom adhesion in relation to substratum properties . J Phycol , 44 : 967 – 976 .
- Wang , C , Bao , WY , Gu , ZQ , Li , YF , Liang , X , Ling , Y , Cai , LY , Shen , HD and Yang , JL . 2012 . Larval settlement and metamorphosis of the mussel Mytilus coruscus in response to natural biofilms . Biofouling , 28 : 249 – 256 .
- Wheeler , GL , Tait , K , Taylor , A , Brownlee , C and Joint , I . 2006 . Acyl-homoserine lactones modulate the settlement rate of zoospores of the marine alga Ulva intestinalis via a novel chemokinetic mechanism . Plant Cell Environ , 29 : 608 – 618 .
- Wiencek , KM and Fletcher , M . 1995 . Bacterial adhesion to hydroxyl- and methyl-terminated alkanethiol self assembled monolayers . J Bacteriol , 177 : 1959 – 1966 .
- Wigglesworth-Cooksey , B and Cooksey , KE . 2005 . Use of fluorophore-conjugated lectins to study cell-cell interactions in model marine biofilms . Appl Environ Microbiol , 71 : 428 – 435 .
- Zardus , JD , Nedved , BT , Huang , Y , Tran , C and Hadfield , MG . 2008 . Microbial biofilms facilitate adhesion in biofouling invertebrates . Biol Bull , 214 : 91 – 98 .
- Zargiel , KA , Coogan , JS and Swain , GW . 2011 . Diatom community structure on commercially available ship hull coatings . Biofouling , 27 : 955 – 965 .