Abstract
The freshwater zebra mussel (Dreissena polymorpha) is a notorious biofouling organism. It adheres to a variety of substrata underwater by means of a proteinaceous structure called the byssus, which consists of a number of threads with adhesive plaques at the tips. The byssal proteins are difficult to characterize due to extensive cross-linking of 3,4-dihydroxyphenylalanine (DOPA), which renders the mature structure largely resistant to protein extraction and immunolocalization. By inducing secretion of fresh threads and plaques in which cross-linking is minimized, three novel zebra mussel byssal proteins were identified following extraction and separation by gel electrophoresis. Peptide fragment fingerprinting was used to match tryptic digests of several gel bands against a cDNA library of genes expressed uniquely in the mussel foot, the organ which secretes the byssus. This allowed identification of a more complete sequence of Dpfp2 (D. polymorpha foot protein 2), a known DOPA-containing byssal protein, and a partial sequence of Dpfp5, a novel protein with several typical characteristics of mussel adhesive proteins.
Introduction
Zebra mussels (Dreissena polymorpha) are an invasive species that was accidentally introduced into the North American Great Lakes in the late 1980s (Strayer Citation2009). These freshwater bivalves are able to adhere to a variety of surfaces underwater via a secreted proteinaceous structure called the byssus, which anchors the mussel through a series of threads and adhesive plaques (Rzepecki & Waite Citation1993b). They have spread rapidly by anchoring to boat hulls and have had a huge economic impact on water dependent industries and a major ecological impact on native ecosystems in the Great Lakes (Claudi and Mackie Citation1994; Strayer Citation2009). Despite their prevalence and impact, large gaps remain in understanding the mechanism of adhesion, particularly with respect to the identity and distribution of the byssal proteins. Understanding the molecular basis of zebra mussel adhesion will be critical for the development of non-toxic, targeted antifouling strategies and may ultimately also be useful in the design of wet adhesives for medical and dental applications.
Zebra mussels are one of a few freshwater mussels known to produce a byssus and have evolved independently, as a different subclass (Allen Citation1985; Morton Citation1993) from marine mussels, whose byssal adhesion has been the subject of extensive research (Lee et al. 2011). Zebra mussel and marine mussel byssi are superficially similar (Eckroat and Steele Citation1993), and both have proteins containing the rare amino acid 3,4-dihydroxyphenylalanine (DOPA). DOPA is produced by the enzymatic hydroxylation of tyrosine and has been shown in marine mussels to be responsible for both adhesive and cohesive interactions in the byssus (Waite et al. 2005; Nicklisch & Waite Citation2012). Marine and freshwater mussels, however, differ significantly in their overall protein composition and distribution within the byssus (Rzepecki & Waite Citation1993a). In marine mussels, the byssal thread consists of a mixture of three collagenous proteins and the plaque and cuticle contain six different DOPA-containing adhesive, linker and lacquer proteins (Lee et al. 2011). In zebra mussels, on the other hand, amino acid analysis has revealed that the composition of the thread and the plaque is similar, and both contain DOPA, albeit at much lower levels than in marine mussels (Rzepecki & Waite Citation1993a).
Three precursor proteins, Dpfp-1, 2 and 3 (D. polymorpha foot proteins), were identified as byssal proteins based on their DOPA content in an extract from the foot of mussels, the organ that secretes the precursor proteins that form the mature byssus (Rzepecki & Waite Citation1993b) (Table ). Extraction of Dpfp1 and Dpfp2 from mature byssal threads (Rzepecki & Waite Citation1993b) and the immunodetection of Dpfp1 in byssal thread extracts (Anderson & Waite Citation2000) confirmed the presence of these proteins in the byssus. Full sequence information has been determined only for Dpfp1 (Anderson & Waite Citation1998), while consensus sequences from peptide fragments have been identified for Dpfp2 (Rzepecki & Waite Citation1993b). Identification of proteins from foot extracts, however, provides limited information on protein distribution, and no information on non DOPA-containing proteins.
Table 1. Summary of information on the three known D. polymorpha foot proteins (Dpfp).
The zebra mussel adhesive layer is characterized by a 10–20 nm thick region at the plaque–substratum interface that stains distinctly from the bulk plaque matrix in transmission electron microscopy (Farsad & Sone Citation2012). MALDI mass spectrometry (MS) analysis revealed several 5.8–7 kDa proteins in this layer (Gilbert & Sone 2010). Larger proteins were not detected, however, possibly due to DOPA cross-linking. MS also revealed that in spite of similar amino acid compositions between thread and plaque (Rzepecki & Waite Citation1993a), there are differences in protein composition between the thread and plaque bulk and between the plaque and the plaque-substratum interface (Gilbert & Sone Citation2010). However, none of these proteins has been identified.
In this work, to overcome the challenge of extracting proteins from cross-linked byssi, the secretion of fresh threads was induced such that these had minimal DOPA cross-linking and were thus less resistant to extraction. Fresh byssal threads were induced by injecting the foot of the mussel with potassium chloride, a method that has been used extensively in marine mussels (Tamarin et al. 1976; Zhao & Waite Citation2006; Yu et al. 2011), but not in freshwater mussels. In marine mussels, the induced byssal threads were shown to be very similar in composition to natural threads (Sagert & Waite Citation2009; Yu et al. 2011). Using this method it was possible to extract proteins from the byssus after secretion from the foot but before extensive cross-linking, and match some of these by peptide fragment fingerprinting to a recently created cDNA library of genes uniquely expressed in the zebra mussel foot (Xu & Faisal Citation2008).
Methods
Protein extraction from induced byssal threads
Zebra mussels were collected from Round lake, Ontario, Canada, kept for up to 60 days in an aquarium at room temperature in artificial freshwater (Sprung Citation1989) and fed on the alga Chlorella in powdered form. Mussel feet were injected with 0.03 ml of 0.56M potassium chloride (KCl) using an 18G syringe, as first described by Tamarin et al. (1976), leading to the secretion of a byssal thread and plaque. After 3–5 min, the induced thread/plaque was removed from the ventral groove of the foot, washed in a drop of deionized water and saved for protein extraction. The extraction method was adapted from Zhao and Waite (Citation2006) with several changes: 6–14 byssal threads were extracted in 250 μl of a basic extraction buffer (EB) (0.2M sodium borate, 4M urea, 1 mM KCN, 1 mM EDTA, and 10 mM ascorbic acid, pH 8), adapted from Rzepecki and Waite (Citation1993b). Samples were homogenized on ice in a 1 ml ground glass hand-held tissue grinder (Kimble Chase), sonicated with a probe sonicator (15 times, 2 s each) and centrifuged (17,000g, 8 min, 4°C). The supernatant (soluble extract) was stored at −20°C. Where relevant, the induced byssal material was separated into threads and plaques prior to extraction.
Soluble extracts from several extractions were pooled and dialyzed against 0.15 M sodium borate (pH 8.1–8.4) and then against nitrogen bubbled 1% acetic acid (Waite Citation1984), using a 2 kDa cutoff (Slide-A-Lyzer G2 Thermo Scientific). Dialyzed samples were lyophilized and stored at −20°C. Protein quantification was determined according to absorbance at 280 nm (Nanodrop ND-1000 Spectrophotometer, Thermo Scientific) of samples resuspended in deionized water. Resuspended samples were stored in liquid nitrogen prior to use.
Tricine polyacrylamide gel electrophoresis (Tricine-PAGE)
Proteins from lyophilized samples were separated by tricine polyacrylamide gel electrophoresis (Tricine-PAGE) (Invitrogen precast, 16%, 1 mm) at 125 V for 1.5 h using an XCell SureLock Mini-Cell Electrophoresis System (Invitrogen). Silver-staining of proteins in the gel was adapted from Mortz et al. (2001). Briefly, gels were fixed (40% ethanol, 10% acetic acid for 1 h), washed in deionized water (30 min), sensitized in 0.02% sodium thiosulfate (1 min) and washed three times in water (20 s each). Gels were then incubated in 0.1% cold silver nitrate solution containing 0.02% formaldehyde (20 min, 4°C), washed 3 times in water (20 s each), transferred to a new tray and then washed again in water (1 min). Gels were developed in 3% sodium carbonate containing 0.05% formaldehyde until staining was sufficient, at which point staining was terminated with 5% acetic acid.
Enzymatic digestion of protein gel bands
Silver stained protein gel bands were trypsin-digested at the Advanced Protein Technology Centre at the Hospital for Sick Children, Toronto. Briefly, excised gel bands were destained in 15 mM potassium ferricyanide and 50 mM sodium thiosulfate (15 min), washed in deionized water and then 50 mM ammonium bicarbonate, followed by shrinking in 50% acetonitrile/25 mM ammonium bicarbonate. Samples were reduced with 10 mM DTT (30 min, 56°C), alkylated with 100 mM iodoacetamide (15 min, dark, room temperature) followed by shrinking with 50% acetonitrile/25 mM ammonium bicarbonate (15 min). Samples were then digested with 13 ng μl−1 of trypsin (Porcine, Sequencing Grade, Promega) overnight at 37°C and the liquid was collected. Peptides were extracted by vortexing samples separately with 25 mM ammonium bicarbonate, 5% formic acid, 100% acetonitrile, 5% formic acid and 100% acetonitrile and all supernatants were pooled. Extracted peptides were dried by SpeedVac and resuspended in 20 μl of 0.1% formic acid in water for LC-MS/MS analysis.
Liquid chromatography – tandem mass spectrometry (LC-MS/MS)
LC-MS/MS analysis was performed by the Advanced Protein Technology Centre at Hospital for Sick Children Hospital, Toronto. The digested peptides were loaded onto a 150 μm ID pre-column (Magic C18, Michrom Biosciences) at 4 μl min−1 and separated over a 75 μm ID analytical column packed into an emitter tip containing the same packing material. Peptides were eluted over 60 min at 300 nl min−1 using a 0 to 40% acetonitrile gradient in 0.1% formic acid using an EASY n-LC nano-chromatography pump (Proxeon Biosystems, Odense, Denmark). Peptides were then eluted into a LTQ-Orbitrap XL hybrid mass spectrometer (Thermo-Fisher, Bremen, Germany) operated in a data dependent mode. MS was acquired at 60,000 FWHM resolution in the Fourier Transform Mass Spectrometer (FTMS) and MS/MS was carried out in the linear ion trap. Six MS/MS scans were obtained per MS cycle.
Protein identification and sequence analysis
MS/MS data were searched using Mascot (Matrix Sciences, London, UK) by matching against zebra mussel and metazoa protein databases and against a zebra mussel Expressed Sequence Tag (EST) library (Xu & Faisal Citation2008), virtually translated in six different reading frames using Virtual Ribosome 1.1 (http://www.cbs.dtu.dk/services/VirtualRibosome/). This cDNA library, representing genes expressed uniquely in the zebra mussel's foot, was prepared using a BD Clontech PCR-Select cDNA Subtraction Kit and comprises 750 genes with accession numbers AM229723 to AM230448 (downloaded November 2011 from the Genbank Server). During formation of the library, base pairs were removed from the 5’ end of the cDNA to create blunt ends for ligation of adaptor sequences (Xu & Faisal Citation2008). Therefore, in several sequences where base pairs were removed from the 5′ translated region, the virtual protein sequences were incomplete at the N-terminus (Zhu et al. 2001). An incomplete N-terminus also means that the correct reading frame of the cDNA sequence is not known and hence it is necessary to theoretically translate the cDNA in all three positive reading frames. cDNA second strand synthesis during amplification means that it must be theoretically translated from the C-terminus in the three negative reading frames as well.
MS data were visualized and validated using Scaffold 3.3.1 (Proteome Software Inc, Portland, OR). Peptide identifications were accepted at >80.0% probability and protein identifications were accepted at >95.0% probability. Parent ion and fragment ion mass tolerances were set to 20 PPM and 0.40 Da, respectively, and hits were confirmed manually by inspecting the spectra. The data were searched using carbamidomethylation as fixed modification and deamidation of asparagine and glutamine, hydroxylation of lysine and tyrosine, oxidation of methionine, acetylation of the N-terminus and phosphorylation of serine and threonine as variable modifications.
Signal peptides were searched using the SignalP 4.0 (http://www.cbs.dtu.dk/services/SignalP/) and PrediSi (http://www.predisi.de/) online tools and multiple sequence alignments were performed with the Clustal-W2 online tool (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Protein homology searches were done using NCBI Protein BLAST (Basic Local Alignment Search Tool) (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins). The theoretical mass, pI and amino acid composition of virtual EST protein matches were determined using EMBOSS Pepstats on the European Bioinformatics Institute website (http://www.ebi.ac.uk/Tools/seqstats/emboss_pepstats).
Results
Protein extraction and separation
To enhance protein extraction directly from the zebra mussel byssus, the secretion of fresh threads and plaques having minimal DOPA cross-linking was induced. While previous extractions from mature byssi used an acidic extraction buffer (Rzepecki & Waite Citation1993b), extractions of induced threads and plaques (T/P) in acetic acid (5% or 8%) and 8M urea did not reveal clear bands on acetic acid urea PAGE gels (not shown). A basic borate extraction buffer improved yield. While the yield per T/P was still small, several extractions could be pooled and separated by tricine-PAGE. Figure displays the silver-stained protein bands extracted from intact byssal T/P and from separated threads and plaques. Five bands were visible in the byssal T/P extract (Figure ). The apparent molecular weight of two of these correspond to previously identified DOPA-containing proteins that were identified from foot extracts, as well as from acidic extracts of mature byssal threads (Rzepecki & Waite Citation1993b). The doublet band centered around 65 kDa corresponds to the SDS-PAGE determined molecular weights of the two forms of Dpfp1 (76 and 65 kDa), and the thick band between 30 and 20 kDa was tentatively identified as Dpfp2, which has an apparent molecular weight of 26 kDa on SDS-PAGE. It is noted that Dpfp3, a third DOPA-containing protein, also previously seen in the foot extracts at 12–13 kDa, was not observed. In addition to the two known proteins, three bands representing novel byssal proteins were observed. These proteins were named Dpfp0 (>210 kDa), Dpfp4 (>90 kDa) and Dpfp5 (∼30 kDa) (underlined in Figure ).
Figure 1. Electrophoretic separation of zebra mussel byssal proteins extracted from induced, freshly secreted byssal threads. Proteins extracts were loaded on 16% Tricine PAGE gels and silver-stained. (A) Byssal proteins identified in an extract from ∼33 complete byssal threads and plaques (T/P). Left lane: Colorburst molecular weight ladder. (B) Byssal proteins identified in the extracts from ∼57 separated threads and plaques. Arrows indicate bands observed on the gel, some of which are quite faint. Underlined proteins represent novel byssal foot proteins that we have named Dpfp0 (>210 kDa), Dpfp4 (>90 kDa) and Dpfp5 (∼30 kDa). The other protein bands correspond to the molecular weights of previously known DOPA containing foot proteins: Dpfp1 (76, 65 kDa) and Dpfp2 (26 kDa).
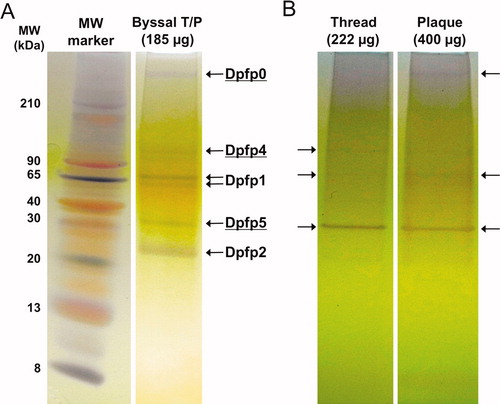
To examine the distribution of proteins in induced byssal material, proteins were extracted from separated threads and plaques. A number of very faint bands and one major band (∼30 kDa) were observed in each lane (Figure ). Although direct comparisons of band densities cannot be made, as the mass loading was not equal (222 μg of thread extract and 400 μg of plaque extract), some observations can be made. The bands corresponding to Dpfp1, Dpfp2, Dpfp5 protein were present both in the thread and in the plaque, with Dpfp5 being the most prominent in both. The faint band corresponding to Dpfp4 (>90 kDa) appeared only in the thread, despite the smaller mass loading, suggesting that this protein is unique to the thread.
Mass spectrometric analysis
The protein gel bands of presumed Dpfp1, Dpfp2, and the ∼30 kDa band corresponding to Dpfp5 in the byssal T/P lane in Figure were subjected to in-gel trypsin digestion and LC-MS/MS analysis. The peptide mass spectra were then matched against known zebra mussel proteins and against virtually translated EST sequences from a cDNA library of genes unique to the zebra mussel foot. The presumed Dpfp1 band indeed matched the known sequence of Dpfp1 (AF265353) (Anderson & Waite Citation1998). The mass spectra of protein bands Dpfp2 and Dpfp5 did not match any known zebra mussel protein sequences, but matched a number of EST sequences virtually translated in any of six reading frames (±1, ±2, ±3). Table shows a sequence match comparison of the three sequenced proteins, Dpfp1, Dpfp5 and Dpfp2. Dpfp1 and Dpfp2 have a protein identification probability of 100%. The derived sequence of Dpfp2 (AM229739) has three matching peptides, and the sequence compares very well to the proteolytic fragments of Dpfp2 sequenced by Rzepecki and Waite (Citation1993b). The Dpfp5 derived sequence (AM230139) has a protein identification probability of 95%, based on a single spectrum match with a high Mascot score (46). While only a single peptide match was found in this study, an additional peptide match (NDVDGNENIVGGQSNAVGGK) was also observed from tryptic digests of the insoluble byssal matrix (Gantayet Citation2012), confirming the protein identification.
Table 2. Summary of peptide mass fingerprinting identification of three gel bands from induced byssal material extract.
The accession numbers listed for Dpfp2 and Dpfp5 in Table represent the sequence match that has the highest molecular weight, being closest to the molecular weight of the protein as identified by Tricine-PAGE. The theoretical protein masses derived from the matched sequence are in each case smaller than the molecular weight seen by gel electrophoresis (Table ). It is notable in this regard that SDS-PAGE significantly overestimated the mass of Dpfp1: 76 and 65 kDa on the gel vs 54.5 and 48.6 kDa by mass spectrometry (Anderson & Waite Citation1998). This may also account for a significant portion of the discrepancy in Dpfp2 and Dpfp5, in addition to post-translational modifications. Only the Dpfp2 peptide matches reveal post-translational modifications in the form of glutamine deamidation (Q) and tyrosine hydroxylation to DOPA (Y). Furthermore, it is noted that N-termini of the sequences are incomplete, owing to limitations in the creation of the cDNA library (see Methods), which would also contribute to a lower theoretical MW.
EST-derived sequence of Dpfp5
The partial sequence of Dpfp5 was determined through EST matching of peptide mass spectra. The multiple sequence alignment of four matches is shown in Figure S1 (Supporting information) [Supplementary material is available via a multimedia link on the online article webpage]. No N-terminal signal peptide or methionine residue (representing start codon) was observed in any of the EST matches, as the N-terminus of the sequences is incomplete due to removal of base pairs from the 5′ end of the cDNA for adaptor ligation for cDNA cloning purposes (Xu & Faisal Citation2008). Premature termination of reverse transcription, due to strong mRNA secondary structure, could also result in an incomplete 5′ cDNA end and therefore in an incomplete N-terminal sequence (Zhu et al. 2001). While the predicted mass of Dpfp5 (20.1 kDa) is much lower than its apparent molecular weight by tricine-PAGE (30 kDa), it is noted that many byssal proteins tend to migrate anomalously on PAGE (Anderson & Waite Citation2002). Such is the case for Dpfp1, for instance, whose actual mass is ∼70% of the apparent mass by SDS-PAGE (Table ).
For further analysis of the Dpfp5 sequence, the N-terminal adaptor sequence was excluded from the longest and what appears to be the most complete EST (AM230139). This sequence is 183 residues long (Figure ), and is rich in proline (18%), glycine (12%), glutamine (12%), asparagine (10%) and tyrosine (10%). Different regions of the Dpfp5 protein display noticeable distinctions in amino acid properties and repeat patterns. The N-terminus (residues 1–68) has a theoretical pI of 9.6 whereas the rest of the protein has a pI of 4.5. Additionally, the N-terminus is quite hydrophilic in contrast to the rest of the protein, which is mostly hydrophobic. While positive residues (K + R) are uniformly distributed through the sequence, negative residues (D + E) are absent at the N-terminus. The N-terminus is rich in triads of proline and glutamine (generally PQQ and PKQ) alternately underlined and highlighted in green. The latter of these repeats are interspersed with consensus repeats of SYP(A/T)YP highlighted in blue. The middle region of the Dpfp5 sequence (residues 69–114) has a pI of 5.9 and has no discernible repeats. It does, however, contain six cysteine residues that are otherwise absent from the rest of the protein. The C-terminus of the sequence (residues 115–183) has a theoretical pI of 4.2 and contains 10 VGG repeats (highlighted in yellow) where the third glycine is occasionally substituted with aspartic acid (D), glutamic acid (E) or tryptophan (W). Five GN(N/D/T) repeats are also observed at the C-terminus (highlighted in grey), preceding the VGG repeats.
Figure 2. Illustration of the pattern of repeats identified in the EST derived sequence of Dpfp5 (AM230139). The adaptor sequence inserted during cDNA cloning has been excluded and the N-terminus of the sequence is incomplete. Alternating underlined and non-underlined green highlighted sequences represent proline and glutamine rich triads. Blue, grey and yellow highlights represent other repeat sequences. Cysteine residues are indicated in red.

EST-derived sequence of Dpfp2
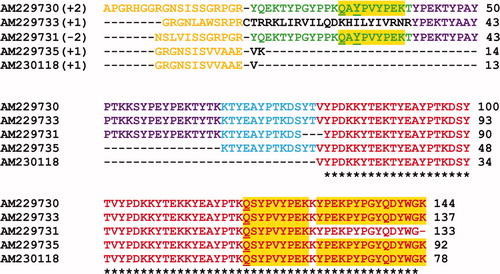
While fragments of the protein sequence of Dpfp2 had previously been determined by automated Edman degradation of proteolytic fragments (Rzepecki & Waite Citation1993b), a more complete sequence of Dpfp2 has been determined through EST matching of its peptide mass spectra. The multiple sequence alignment of five of these matches, which are quite similar, is shown in Figure S2 (Supporting information) [Supplementary material is available via a multimedia link on the online article webpage]. All the peptide sequences, with the exception of the one in black in AM229733, correspond well to the Dpfp2 sequence fragments observed by Rzepecki and Waite (Citation1993b). As with Dpfp5, the N-terminus of the Dpfp2 sequence is incomplete. For further analysis of the Dpfp2 sequence properties, the N-terminal adaptor sequence was excluded from the EST AM229730, representing the longest EST with three peptide matches. This sequence is 125 residues long and has a theoretical mass of 15.3 kDa. It is richest in tyrosine (23%), lysine (19%) and proline (16%).
Analysis of the EST derived sequence of Dpfp2 reveals five full tandem repeats of a 22 residue consensus sequence that make up the bulk of the protein (Figure ). The consensus sequence can be represented by KTY(P/E)AYPTK(Q/D)SYPVYPEKKYTE where non-italicized residues represent highly conserved residues. There are five tyrosines (Y) in every consensus and the position of the tyrosine residue is fully conserved (indicated in bold in Figure ). A previous study (Rzepecki & Waite Citation1993b) had identified two fragments of this Dpfp2 consensus sequence; K-(K/T)-Y-(X/P)-E and *-Y-(P/X)-*-(Y/K)-*-D, where * is any residue, Y is DOPA and X was speculated to be glycosylated threonine. Within the EST match, only one DOPA ( Y ) residue was identified in the first full repeat.
Figure 3. Illustration of the tandem repeat pattern identified in the EST derived sequence of Dpfp2 (AM229730). The sequence consists of five full repeats of a 22 residue consensus sequence KTY(P/E)AYPTK(Q/D)SYPVYPEKKYTE where non-italicized residues represent highly conserved residues. Each full repeat is on a new line and tyrosine residues with conserved positions within the consensus are indicated in bold. The underlined residues indicate post-translational modifications; Q and Y signify glutamine deamidation and tyrosine hydroxylation to DOPA, respectively.

Discussion
Three novel zebra mussel byssal proteins have been identified by performing gel electrophoresis of proteins extracted from freshly secreted, minimally cross-linked byssal threads. These proteins, Dpfp0 (>210 kDa), Dpfp4 (>90 kDa) and Dpfp5 (∼30 kDa) did not previously stain for DOPA in foot extracts (Rzepecki & Waite Citation1993b) and were thus not known to be present in the byssus. Dpfp5 was shown to be present in both threads and plaques, while Dpfp4 appears to be unique to the thread. Further, peptide fragment fingerprinting (LC-MS/MS analysis and cDNA database matching) was used to determine the sequence of Dpfp5 as well as a more complete sequence of Dpfp2, a known DOPA-containing byssal protein (Rzepecki & Waite Citation1993b). It is notable that low molecular weight proteins were not detected in the present extractions, such as Dpfp3, or the species previously observed in mass spectrometry of byssi (Gilbert & Sone Citation2010). Furthermore, although the present study was restricted to the extractable proteins, this clearly does not represent the full complement of byssal proteins as some insoluble material remained.
Dpfp5
Dpfp5, a novel zebra mussel byssal protein, shares several similarities with Dpfp1, a known DOPA-containing byssal protein: both are acidic, in contrast with the basic byssal precursor proteins of marine mussels (Waite et al. 2005); both have a segregated structure of repeated consensus sequences; and both have very different overall charges on the N- and C-terminal portions of the protein. Dpfp1 has diblock copolymer-like structure, with repeats of a basic consensus motif at its N-terminus (pI 9.0), and an acidic consensus sequence repeated at its C-terminus (pI 4.6) (Anderson & Waite Citation1998). In Dpfp5, the N-terminus (residues 1–68) is also basic (pI 9.6) and completely lacks negative residues, whereas the rest of the protein has an acidic pI of 4.5. Charge separation has also been observed in an adhesive protein of the sandcastle worm (Phragmatopoma californica), pc-3A, which has an acidic N-terminus and basic C-terminus (Wang & Stewart Citation2012). Like zebra mussels, sandcastle worms also contain both acidic and basic proteins (Endrizzi & Stewart Citation2009; Stewart et al. 2011). It has been suggested that such segregation of charges indicates a possible role for complex coacervation in the adhesion process of marine mussels and sandcastle worms, which has several advantages for underwater adhesion, as recently reviewed elsewhere (Lee et al. 2011).
The EST derived sequence of Dpfp5 displays several repetitive sequences (Figure ), a common characteristic of adhesive proteins, including those in marine mussels (Waite et al. 1985), and the sandcastle worm (Zhao et al. 2005). The N-terminus is rich in triads of proline and glutamine, most commonly PQQ and PKQ. In the first part of the sequence these triads are tandemly repeated, whereas in residues 30–68 they are interspersed with an SYPAYP consensus sequence. A BLAST search of the first 29 Dpfp5 residues revealed PQQ sequence homologies with a number of extracellular structural proteins, including glycoproteins from the oocyte egg coat of the two different fish species: the winter flounder flatfish (a zona pellucida protein of Pseudopleuronectes americanus, score 56.2) and the mummichog (Choriogenin H minor glycoprotein of Fundulus heteroclitus, score 53.7). Lyons et al. (1993) found PQQ repeats in the winter flounder glycoprotein gene to be part of a longer (PQQ)4PKY repeat. These glycoproteins are believed to be involved in hardening of the egg coat by glutamine-lysine cross-linking following fertilization (Lyons et al. 1993; Lee et al. 2002). The presence of both PQQ and PKQ in Dpfp5 raises the possibility that such cross-linking may also occur in the zebra mussel byssus, in addition to DOPA-mediated cross-linking. More generally, repeats containing a proline at every third residue position interspersed with hydrophilic residues, are a common feature of many extracellular structural proteins (Lyons et al. 1993) supporting the notion of a structural role for the N-terminus of Dpfp5.
The 46 central residues in the Dpfp5 sequence (69–114) display no discernible repeat pattern, but this region is notable in that it contains cysteine residues that are otherwise absent from the rest of the Dpfp5 sequence. Such specific cysteine localization could possibly indicate a role for disulfide bridge interactions by the middle region of the protein. Cysteine residues are also known for their potential roles as antioxidants owing to the hydrogen atom in the thiol group available for donation (Elias et al. 2005). Yu et al. (2011) demonstrated that cysteine containing byssal proteins in marine mussels can reduce oxidized DOPA (dopaquinone) back to DOPA, restoring adhesive function, and can later transform into a cross-linker forming S-cysteinyldopa adducts. The distribution of cysteine residues uniquely within the middle region of Dpfp5 suggests this region could conceivably play similar roles in the zebra mussel byssus, if the cysteines are indeed present as thiols, which remains to be confirmed.
The Dpfp5 C-terminus (residues 115–183) also has distinct composition and repeated sequences. In contrast to the 29% proline present in the rest of the sequence, the C-terminus has no proline, and has only one tyrosine compared to seventeen in the rest of the sequence. It is rich instead in the hydrophobic residues glycine (28%) and valine (16%), which are absent from the N-terminal region. These residues are represented in a series of VGG repeats, which dominate the C-terminal portion of the sequence. The third glycine in VGG is occasionally substituted with positive residues glutamic acid (D) and aspartic acid (E). Interestingly, VGG repeats are also found in the adhesive protein pc-1 of the sandcastle worm (Endrizzi & Stewart Citation2009), although their role is not known. The relative hydrophobicity of middle and C-terminal sections of the sequence compared to the N-terminus, in addition to the segregation of charges, likely has important implications for protein aggregation/assembly.
Dpfp2
Dpfp2 is very different from Dpfp1 and Dpfp5 in that it is basic and does not have a block structure, but rather has the same consensus sequence repeated throughout. The bulk of the protein, from residues 4 to 117, consists of five full repeats of a relatively long 22 residue consensus sequence KTY(P/E)AYPTK(Q/D)SYPVYPEKKYTE, where non-italicized residues represent highly conserved residues (Figure ). Interestingly, the position of the tyrosine residues is conserved in all of the repeats. Tyrosine is often highly conserved in mussel byssal protein repeats (Rzepecki et al. 1991). Since tyrosine can be post-translationally modified to DOPA, this indicates that the positioning of the DOPA residue plays an important role in the structure/function of the protein. It is also notable that the AYPTK(D/Q) and the SYPVYPE regions within the consensus are somewhat similar to the SYPAYP repeat in the N-terminus Dpfp5.
Summary
In addition to providing a more complete sequence for Dpfp2, three novel zebra mussel byssal proteins from induced byssal secretions have been identified. Presumably these contain little or no DOPA, as they were not previously identified from foot extracts. A partial sequence has been determined for one of these, Dpfp5, an acidic protein with distinct repetitive sequences and charge in its N- and C-terminal regions. Similarities to other adhesive and structural proteins give intriguing hints as to possible roles for the different regions of Dpfp5, but a true understanding of the function of this protein awaits more precise localization and direct functional studies.
Acknowledgments
The authors gratefully acknowledge Trevor and Graham Gilbert for zebra mussel collections and Zahra Shahrokh for advice on protocols. This work was supported by grants to EDS from the National Sciences and Engineering Research Council (NSERC) of Canada and the Canadian Foundation for Innovation (CFI). A.G. is grateful to the Ontario Ministry of Training, Colleges and Universities for an Ontario Graduate Scholarship.
References
- Allen , JA . 1985 . “ The recent Bivalvia: their form and evolution ” . In The mollusca , Edited by: Trueman , ER and Clarke , MR . 337 – 403 . Orlando , FL : Academic Press . Volume 10, Evolution
- Anderson , KE and Waite , JH . 1998 . A major protein precursor of zebra mussel (Dreissena polymorpha) byssus: deduced sequence and significance . Biol Bull , 194 : 150 – 160 .
- Anderson , KE and Waite , JH . 2000 . Immunolocalization of Dpfp1, a byssal protein of the zebra mussel Dreissena polymorpha . J Exp Biol , 203 : 3065 – 3076 .
- Anderson , KE and Waite , JH . 2002 . Biochemical characterization of a byssal protein from Dreissena bugensis (Andrusov) . Biofouling , 18 : 37 – 45 .
- Claudi , R and Mackie , GL . 1994 . Practical manual for zebra mussel monitoring and control , Boca Raton , FL : CRC Press .
- Eckroat , LR and Steele , LM . 1993 . Comparative morphology of the byssi of Dreissena polymorpha and Mytilus edulis . Am Malacol Bull , 10 : 103 – 108 .
- Elias , RJ , McClements , DJ and Decker , EA . 2005 . Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase beta-lactoglobulin in oil-in-water emulsions . J Agric Food Chem , 53 : 10248 – 10253 .
- Endrizzi , BJ and Stewart , RJ . 2009 . Glueomics: an expression survey of the adhesive gland of the sandcastle worm . J Adhes , 85 : 546 – 559 .
- Farsad , N and Sone , ED . 2012 . Zebra mussel adhesion: structure of the byssal adhesive apparatus in the freshwater mussel, Dreissena polymorpha . J Struct Biol , 177 : 613 – 620 .
- Gantayet , A . 2012 . Identification and sequence analysis of novel proteins in the zebra mussel adhesive apparatus [M.A.Sc.] , Toronto , ON : University of Toronto .
- Gilbert , TW and Sone , ED . 2010 . The byssus of the zebra mussel (Dreissena polymorpha): spatial variations in protein composition . Biofouling , 26 : 829 – 836 .
- Lee , BP , Messersmith , PB , Israelachvili , JN and Waite , JH . 2011 . Mussel-inspired adhesives and coatings . Annu Rev Mater Res , 41 : 99 – 132 .
- Lee , C , Jeon , SH , Na , JG and Park , K . 2002 . Sequence analysis of choriogenin H gene of medaka (Oryzias latipes) and mRNA expression . Environ Toxicol Chem , 21 : 1709 – 1714 .
- Lyons , CE , Payette , KL , Price , JL and Huang , RC . 1993 . Expression and structural analysis of a teleost homolog of a mammalian zona pellucida gene . J Biol Chem , 268 : 21351 – 21358 .
- Morton , B . 1993 . “ The anatomy of Dreissena polymorpha and the evolution and success of the heteromyarian form in the Dreissenoidea ” . In Zebra mussels: biology, impacts and control , Edited by: Nalepa , TF and Schloesser , D . 185 – 215 . Boca Raton , FL : Lewis Publishers .
- Mortz , E , Krogh , TN , Vorum , H and Gorg , A . 2001 . Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis . Proteomics , 1 : 1359 – 1363 .
- Nicklisch , SCT and Waite , JH . 2012 . Mini-review: the role of redox in DOPA-mediated marine adhesion . Biofouling , 28 : 865 – 877 .
- Rzepecki , LM , Chin , SS , Waite , JH and Lavin , MF . 1991 . Molecular diversity of marine glues: polyphenolic proteins from five mussel species . Mol Mar Biol Biotechnol , 1 : 78 – 88 .
- Rzepecki , LM and Waite , JH . 1993a . The byssus of the zebra mussel, Dreissena polymorpha. I: morphology and in situ protein processing during maturation . Mol Mar Biol Biotechnol , 2 : 255 – 266 .
- Rzepecki , LM and Waite , JH . 1993b . The byssus of the zebra mussel, Dreissena polymorpha. II: structure and polymorphism of byssal polyphenolic protein families . Mol Mar Biol Biotechnol , 2 : 267 – 279 .
- Sagert , J and Waite , JH . 2009 . Hyperunstable matrix proteins in the byssus of Mytilus galloprovincialis . J Exp Biol , 212 : 2224 – 2236 .
- Sprung , M . 1989 . Field and laboratory observations of Dreissena polymorpha larvae: abundance, growth, mortality and food demands . Arch Hydrobiol , 115 : 537 – 561 .
- Stewart , RJ , Ransom , TC and Hlady , V . 2011 . Natural underwater adhesives . J Polym Sci B Polym Phys , 49 : 757 – 771 .
- Strayer , DL . 2009 . Twenty years of zebra mussels: lessons from the mollusk that made headlines . Front Ecol Environ , 7 : 135 – 141 .
- Tamarin , A , Lewis , P and Askey , J . 1976 . The structure and formation of the byssus attachment plaque in Mytilus . J Morphol , 149 : 199 – 221 .
- Waite , JH; University of Connecticut, Farmington, CT, assignee . 1984 . Process for purifying and stabilizing catechol-containing proteins and materials obtained thereby. United States patent US #4496397 , Farmington [CT] : University of Connecticut .
- Waite , JH , Andersen , NH , Jewhurst , S and Sun , C . 2005 . Mussel adhesion: finding the tricks worth mimicking . J Adhes , 81 : 297 – 317 .
- Waite , JH , Housley , TJ and Tanzer , ML . 1985 . Peptide repeats in a mussel glue protein: theme and variations . Biochemistry , 24 : 5010 – 5014 .
- Wang , CS and Stewart , RJ . 2012 . Localization of the bioadhesive precursors of the sandcastle worm, Phragmatopoma californica (Fewkes) . J Exp Biol , 215 : 351 – 361 .
- Xu , W and Faisal , M . 2008 . Putative identification of expressed genes associated with attachment of the zebra mussel (Dreissena polymorpha) . Biofouling , 24 : 157 – 161 .
- Yu , J , Wei , W , Danner , E , Ashley , RK , Israelachvili , JN and Waite , JH . 2011 . Mussel protein adhesion depends on interprotein thiol-mediated redox modulation . Nat Chem Biol , 7 : 588 – 590 .
- Zhao , H , Sun , C , Stewart , RJ and Waite , JH . 2005 . Cement proteins of the tube-building polychaete Phragmatopoma californica . J Biol Chem , 280 : 42938 – 42944 .
- Zhao , H and Waite , JH . 2006 . Proteins in load-bearing junctions: the histidine-rich metal-binding protein of mussel byssus . Biochemistry , 45 : 14223 – 14231 .
- Zhu , YY , Machleder , EM , Chenchik , A , Li , R and Siebert , PD . 2001 . Reverse transcriptase template switching: a SMART approach for full-length cDNA library construction . Biotechniques , 30 : 892 – 897 .
Supplementary data
Figure S1. Alignment of the multiple EST sequence matches for the Dpfp5 gel band in Figure 1A. Bracketed numbers represent the reading frame of the virtually translated EST sequence. The peptide matches are aligned and color-coded to show regions of sequence similarity between matches. The orange code represents adaptor sequences added during cDNA amplification. The colors red (100%), blue (75%) and purple (50%) represent the percent sequence similarity between different EST matches. The yellow highlight represents peptide sequences that matched from the tryptic fragments. * = residues that are conserved between all EST matches. The first accession number is the sequence that was used for further analysis.
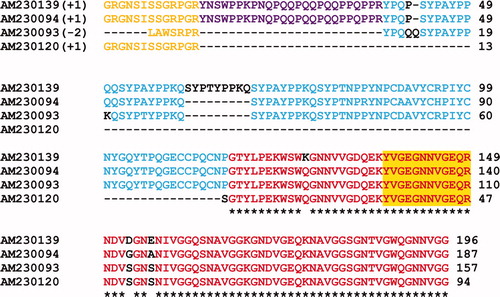
Figure S2. Alignment of the multiple EST sequence matches derived for the Dpfp2 (26 kDa) gel band in Figure 1A. Bracketed numbers represent the reading frame in which the EST sequence was virtually translated. The peptide matches are aligned and color-coded to show regions of similarity between matches. The orange sequence at the N-terminus represents adaptor sequences added during cDNA amplification. The colors red (100%), blue (80%), purple (60%) and green (40%) represent the percent sequence similarity among the EST matches. The yellow highlight represents peptide sequences that matched directly from the tryptic digest. Q and Y signify glutamine deamidation and tyrosine hydroxylation to DOPA, respectively. * = residues that are conserved between all EST matches. The first accession number is the sequence that was used for further analysis.