Abstract
This study assessed the anti-biofouling performance of an experimental adhesive system containing a naturally occurring essential vegetable oil and examined the following physical and mechanical properties: water sorption (WS) and solubility (SL), microtensile bond strength to dentin (μTBS), and degree of conversion. The following six groups were tested: a self-etching experimental adhesive containing refined essential oil from the seeds of the Butia capitata tree (EAO); an oil-free version of the experimental adhesive (EANO); one group without adhesive as the control (C); and the three following commercial self-etching adhesives: Clearfil Protect Bond (CPB), Clearfil SE Bond, and Adper SE Plus. The antibacterial effect was estimated by microbiological culture on selective/non-selective media, and the results expressed as colony-forming units per unit weight of dry biofilm (CFU mg−1). The data were submitted to ANOVA and Tukey’s post hoc test (α = 0.05). After 24 h, pH changes were similar in the storage medium of all tested adhesive systems. EAO showed similar levels of antimicrobial activity in a model biofilm microcosm as the commercial self-etching adhesive CPB. Both were effective against total microorganisms, aciduric bacteria, lactobacilli, and Streptococcus mutans. WS and SL were not affected by the presence of the essential oil; the values of EAO were similar to or less than those of commercial equivalents. The incorporation of an essential oil into an experimental adhesive did not influence its monomer conversion result. Immediate μTBS values of EAO and EANO were similar and were greater than those of commercial equivalents. After storage for 6 months, the μTBS of the EAO decreased significantly and became similar to the values of commercial equivalents, while the strength of the EANO was not affected.
Introduction
Self-etching adhesives became a popular alternative to total-etching adhesive systems due to their simplicity of use, decreased technique sensitivity, and lowered post-operative complaints (Lima et al. Citation2008). The bonding strategy behind these adhesive systems is based on acidic ionization in water (Lima et al. Citation2008), which promotes solubilizing demineralized dentin while concurrently forming a hybridization layer with exposed collagen fibrils. Unlike total etching, self-etching incorporates the smear layer into the polymerized resin matrix, thereby resulting in some residual microorganisms remaining in the hybrid layer (Cehreli et al. Citation2003). Ongoing implications from these remnant microorganisms remain a clinical concern.
Some studies associate the antimicrobial capacity of these resins with the low pH of the self-etching primers (Emilson & Bergenholtz Citation1993; Meiers & Miller Citation1996), which can be compared to the antibacterial effect of the phosphoric acid used in total etching systems (Settembrini et al. Citation1997). Examples of antibacterial components added to contemporary adhesive systems include glutaraldehyde (Felton et al. Citation1989; Schmidlin et al. Citation2004), 12-methacryloyloxy dodecylpyridinium bromide (MDPB), and most recently, methacrylate dimethyl ammonium chloride (Imazato et al. Citation1998; Xiao et al. Citation2009; Henn et al. Citation2011). Given the incidence of dental caries, increased resistance by bacteria to antibiotics, the adverse effects of some antibacterial agents currently used in dentistry and the financial considerations in developing countries, there is a need for alternative prevention and treatment options that are safe, effective, and economical. While several agents are commercially available, some concern has arisen regarding the biocompatibility of these substances and their toxic, allergenic, and even mutagenic effects. Hence, the search for alternative products continues, and natural phytochemicals isolated from plants are considered good alternatives. However, major gaps exist in knowledge regarding the inclusion of antibacterial components in currently available self-etching adhesives. These types of adhesives are emerging at an ever-increasing rate, thereby motivating the search for new antimicrobial strategies.
Essential oils derived from aromatic and medicinal plants are potentially useful as antimicrobial agents, and their use as medicines has been widely recognized (Burt Citation2004; Holley & Patel Citation2005). The tree Butia capitata (Butiá) belongs to the family Arecaceae and produces an aromatic fruit consumed in natura and as a component in beverages, juices, and ice creams in Brazil (Magalhaes et al. Citation2008). Long- and medium-chain fatty acids exhibit significant antimicrobial activity against oral bacteria (Sun et al. Citation1988). As the essential oil from this tree contains fatty acids in its composition (Faria et al. Citation2008), it has potential as an antimicrobial agent.
The purpose of this study was to investigate and compare the antimicrobial effect of an experimental self-adhesive resin containing an essential oil derived from B. capitata seeds to commercial self-etching adhesives, each of which has been claimed to have an antimicrobial component in its formulation, using dental biofilm microcosms. In addition, specific physical and mechanical properties (water sorption (WS) and solubility (SL), microtensile bond strength, and degree of conversion (DC)) were compared between the experimental resin and the commercial equivalents. As a control, the experimental resin was tested without the essential oil, and bacterial growth on the enamel disc surface free of interference was examined. The null hypotheses tested were (1) that there would be no significant difference among microbiological activities of the experimental resin, its control, and that of the commercially available self-etching resins and (2) that the physical and mechanical properties of the resins produced using the experimental adhesive with the added essential oil would not be significantly different from those of the commercial equivalents or from the experimental resin without oil.
Materials and methods
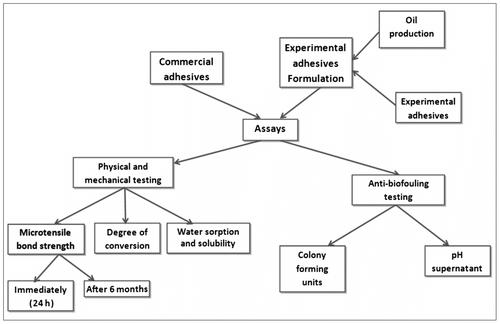
All adhesives tested are listed in Table . The formulation of the experimental adhesive systems comprised a mixture of reagents that were used as received, without further purification. The experimental design is shown in Figure .
Formulation of self-etching adhesive
The experimental primer was formulated by mixing methacrylate and acidic monomers, using water and ethanol as solvents, as described previously (Lima et al. Citation2008). The pH upon mixing was 1.5 (Analion PM pH meter, Ribeirão Preto, SP, Brazil).
Table 1. Materials, compounds, and the components used in the present study.
The experimental adhesive resin was formulated by intensive mixing of Bis-diglycidyl methacrylate (GMA) (50 wt%), triethylene glycol dimethacrylate (25 wt%), and 2-hydroxyethylmethacrylate (HEMA) (25 wt%), which was rendered photocurable using camphorquinone and ethyl 4-dimethylaminobenzoate (Table ). The experimental adhesive resin was given antimicrobial properties by the addition of 1 mol% of the essential oil from B. capitata seeds.
Extraction of essential oil from B. capitata seeds
B. capitata fruits were freshly collected in May 2009, after which the nuts were immediately isolated from the fruit. The nuts were dried at 40 °C for 7 days. The nuts were then ground in a milling machine to powder sizes of ∼60–75 cm. The resultant powder (25 g) was placed in a thimble and continuously extracted with 180 ml of hexane in a Soxhlet extraction apparatus (model no. 9991952, Laborglas® Ind. e Com. de Materiais de Laboratório Ltda, São Paulo, Brazil) at 40 °C for 8 h. The resulting essential oil was washed with dichloromethane, and the remaining solvent was removed using a rotary evaporator under vacuum. Using these methods, the oil yield was approximately 30 wt% with ca 11.45 wt% moisture. The final product was stored in a dark-colored container at 20 °C to prevent potential photo-degradation and was incorporated into the experimental adhesive (EAO) within 1 week of its extraction. The material was ground and the essential oil was extracted in accordance with published protocols (Brazilian Pharmacopoeia Citation2000).
Chromatographic analysis of the B. capitata oil
All solvents and chemicals were analytical grade and used as received from Aldrich (Milwaukee, WI, USA). The progress of the extraction was monitored on a Shimadzu 2010 Gas Chromatograph equipped with a Rtx-Wax polyethylene glycol capillary column (0.32 mm × 30 m).
The essential oil components were identified by comparing with FAME Chemical Standards (Sigma-Aldrich, St Louis, MO, USA). B. capitata fatty acids were submitted to an esterification reaction with BF3-Methanol prior to analysis.
Anti-biofouling testing – the experimental model
The model consisted of microcosm biofilms grown in polypropylene 24-well microplates, where plaque-enriched human saliva was used as the inoculum (Wimpenny Citation1997; Filoche et al. Citation2007; van de Sande et al. Citation2011). Enamel discs obtained from bovine teeth were used as substrata (n = 10), and a defined medium enriched with mucin (DMM) (Wong et al. Citation2001) was supplemented intermittently with 1% sucrose for 3 days.
Enamel discs (6.0 mm in diameter and 2.0 mm in thickness) were fabricated from recently extracted bovine central incisors. A cylindrical, diamond-coated trephine drill (Tyrolit Schleifmittelwerke Swarovski KG, Schwaz, Austria) was used perpendicularly to each buccal tooth surface to obtain a core section of tooth. Dentin and enamel surfaces were ground with 400- and 800-grit SiC papers, respectively, until both surfaces were positioned parallel to each other. The dentin surface was polished to effect total removal, leaving only enamel on both sides. All procedures were performed under constant water cooling. The discs were fixed within a holder that was fabricated with orthodontic wire and kept in a horizontal position during the experimental procedures.
Anti-biofouling testing – biofilm preparation
Approval of this research was granted by the Ethics Committee, School of Dentistry, Federal University of Pelotas, Pelotas, RS, Brazil (protocol No. 180/2010).
Fresh stimulated saliva was collected from a healthy human subject (male, age 31) who had not been under antibiotic therapy for at least 1 year. Fifteen milliliters of saliva were collected in the morning during fasting, prior to which the volunteer abstained from oral hygiene for 24 h prior to collection. One aliquot of the saliva was taken to determine the baseline microbial composition, measured in colony-forming units per unit volume (CFU ml−1).
The prepared enamel discs were covered with the primer for each adhesive system and adhesive component before they were light-activated for 20 s using a halogen-based curing light (LCU) (XL 3000, 3M ESPE, St Paul, MN) emitting ∼450 mW cm2, as measured by a hand-held dental curing radiometer (Model 100, Emetron Research, Danbury, CT, USA). Following resin placement and curing, the discs were transferred into sterile wells (24-well tissue culture plate; TPP – Techno Plastic Products, Trasadingen, SU), and 400 μl of fresh, homogenized saliva were dispensed onto each disc. After storage for 1 h at 37 ± 1 °C, the saliva was aspirated, and 1.8 ml of the DMM solution with 1% sucrose added. After incubation for 6 h with the enriched DMM solution, the specimens were washed for 10 s with sterile saline and transferred to a new plate with fresh DMM for 18 h. This procedure was repeated for 3 days. All plates were incubated at 37 °C in an environment of 5–10% CO2 (Anaerobac – Probac do Brasil products Bacteriological Ltda, Santa Cecília, SP, Brazil) in anaerobic jars (Probac do Brasil Produtos Bacteriológicos Ltda).
After removal of the resin disc from each well, the pH of the contents was determined (Quimis 50w – Quimis Aparelhos Científicos Ltda, Diadema, SP, Brazil; V621 electrode – Analion, Ribeirão Preto, SP, Brazil).
After the time allowed for biofilm growth, the enamel discs containing the biofilms were washed three times with 0.9% NaCl and individually transferred to microcentrifuge tubes containing 1 ml of 0.9% NaCl. The biofilm was detached from the enamel surfaces and solubilized using vortex agitation for 30 s, followed by 30 s of sonication at 30 W (Sonicator DE S500, R2D091109 Brazil). The discs were then carefully removed from the suspension before aliquots of the suspension were used to determine bacterial viability and the dry weight of the biofilm.
Anti-biofouling testing – bacterial viability
One hundred microliters aliquots of the initial biofilm suspension were serially diluted in 0.9% NaCl up to 10−7, after which 2 and 20 μl drops of each dilution were inoculated on brain–heart infusion and blood agar adjusted to pH 7.2 to obtain total microorganism counts. To determine the total aciduric counts, the pH of the brain–heart infusion was adjusted to 4.8 via the dropwise addition of HCl solution. Mitis salivarius agar was supplemented with 0.2 units of bacitracin ml−1 to grow Streptococcus mutans, while total lactobacilli counts were determined using Rogosa agar (Herigstad et al. Citation2001). The plates were incubated at 37 °C for 72 h under 5–10% CO2 (Anaerobac – Probac do Brasil produtos Bacteriológicos Ltda) in anaerobic jars (Probac do Brasil produtos Bacteriológicos Ltda). The number of CFU was counted in a stereomicroscope (×40) with external halogen illumination, and the results expressed as CFU mg−1 of biofilm dry weight (Aires et al. Citation2008). Counts for the selective plates were based on colony morphology and verified by Gram-stain and cell appearance using light microscopy.
Biofilm dry weight
To determine the biofilm dry weight, 200 μl aliquots of the initial biofilm suspension were transferred to pre-weighed tubes and dehydrated with ethanol solutions (75 and 99%). The tubes were centrifuged, and the supernatants were discarded before the pellet was dried under a desiccator (P2O5) for 24 h and weighed (±0.00001 mg). The dry weight of the biofilm was determined by calculating the weight in the tube (initial weight − final weight) and in the original suspension (dry weight in 1 ml = dry weight in 200 μl × 5).
Microtensile bond strength (μTBS)
Fifty freshly extracted bovine incisors were obtained, cleaned of soft tissue, and inspected for the presence of fractures. Non-fractured teeth were stored in an aqueous solution of 0.5% chloramine-T for 7 days. The teeth were randomly assigned into five groups according to the adhesive system tested. At least, 10 teeth were used per group. The buccal enamel was removed to expose the middle dentin layer. The exposed dentin surface was successively wet-ground with 400- and 600-grit SiC abrasive papers to create a standardized flat surface with consistent smear layer formation. After water-rinsing, the dentin substratum was dried with absorbent paper and then gently air-dried for 10 s, after which the self-etching primer component of a selected bonding system was actively applied using agitation over prepared surfaces for 20 s. The bonding systems were applied according to the manufacturers’ recommendations for use and light-activated for 20 s using the halogen LCU, as described previously.
A restoration was fabricated over the top of the cured bonding agent using an incremental technique with a resin composite material (Charisma, Heraeus-Kulzer, Hanau, Germany). Specimens were then stored in distilled water at 37 °C for 24 h, after which they were sectioned perpendicularly to the bonding interfaces using a water-cooled, diamond saw at low speed (Isomet 1000, Buehler; Lake Bluff, IL, USA). This process was reproduced after turning the cut sections 90°, resulting in beams of dentin bonded to composite with cross-sectional areas of ∼0.7 mm2. At least, eight beams per tooth were produced and randomly assigned into four groups for the evaluation of μTBS, both immediately and after six months (n = 20 per group, per time). Beam dimensions were precisely measured using a digital caliper (Mitutoyo, Tokyo, Japan), after which they were attached to the tensile testing device using a special cyanoacrylate glue (Super Bonder Gel, Henkel Loctite, São Paulo, SP, Brazil); the dentin portion was attached to a fixed platform, and the composite side was attached to the upper, movable crosshead. The attached specimen was subjected to tensile vertical loading in a mechanical testing machine (DL500; EMIC, São José dos Pinhais, PR, Brazil) at a crosshead speed of 0.5 mm min−1, and the load recorded at specimen failure was recorded. Bond strength values (MPa) were calculated by dividing the maximal load at failure by the cross-sectional area of the bond interface. The fractured specimens were evaluated under a light microscope at ×100 and ×500 magnifications by a single observer. Failure modes were classified as: (1) adhesive, (2) cohesive within the substratum (dentin), (3) cohesive within the adhesive resin, or (4) mixed failure.
Degree of conversion analysis
The extent to which the original methacrylate monomeric C=C converted into polymeric C–C was determined using Fourier transform infrared spectroscopy (Prestige 21 spectrometer Shimadzu Corporation, Kyoto, Japan), equipped with an attenuated total reflectance attachment incorporating a horizontal ZnSe crystal with a 45° mirror angle (PIKE Technologies, Madison, WI, USA). The halogen LCU was rigidly held in position, enabling standardization of the distance between the fiber tip and the top of the sample at 5 mm. Infrared analysis was performed at a controlled room temperature of 23 ± 2 °C and 60 ± 5% relative humidity. Approximately 5 μl of each ‘bond’, a component of all of the tested adhesive systems, was dispensed directly onto the ZnSe crystal and covered with clear polyester, after which the DC was evaluated (Dentsply Caulk). The spectra of the uncured and cured (after photo-activation for 20 s) adhesive resin were acquired between 1690 and 1575 cm−1, averaging 24 scans at the 4 cm−1 resolution transmission mode, to provide a single spectrum. The spectra of each unpolymerized resin were also captured (ie a single scan was also collected immediately prior to light curing). The extent of the unreacted aliphatic carbon double bonds (% C=C) was determined from the ratio of the absorbance intensities of the aliphatic C=C (peak height at 1637 cm−1) to that of an aromatic C=C absorbance of the internal standard component (1608 cm−1), both before and after curing the specimen. The baseline method used to determine peak height absorbance has been described previously (Ogliari et al. Citation2006). The DC was determined by subtracting the % C=C from 100%. Analyses were conducted in triplicate.
Water sorption and solubility
WS and SL were determined according to the International Organization for Standardization specification no. 4049 (ISO Citation2000), with adaptations made regarding the sample dimension (6.0 mm in diameter and 1.0 mm in thickness, equal to the diameter of the LCU light guide tip), which was designed to standardize the produced light irradiance to the LCU in the curing process of the composite. Ten disc-shaped specimens were fabricated in a plastic mold between two glass slides covered with polyethylene film. They were irradiated with the same LCU as described previously. Immediately after polymerization, the specimens were removed to a desiccator containing freshly dried silica gel and calcium chloride. After 24 h, the specimens were removed, stored in a desiccator at 23 °C for 1 h, and weighed with a precision balance of 0.01 mg (model Gehaka AG2000, Dehaka Ltda, São Paulo, Brazil). This cycle was repeated until a constant mass (m 1) was obtained. The thickness and diameter values of the specimens were randomly measured at five points using the digital caliper as described above, with the values rounded to the nearest 0.01 mm. From these measurements, the volume (V) of each specimen was expressed in mm3. The discs were immersed in distilled water at 37 °C for 7 days, removed, blotted dry, and re-weighed (m 2). Thereafter, the specimens were again dried inside a desiccator and weighed daily to record a third constant mass (m 3). For each disc, the data for WS and SL were calculated using the following formula (ISO Citation2000):
Statistical analysis
The equality of the variances and the normal distribution of the errors were checked for all tested response variables. Those that did not satisfy these conditions were submitted to transformations as an attempt to fulfill parametric assumptions. The CFU count data were non-normal and log transformed. Subsequently, statistical analyses were performed with the transformed data. A log10 transformation of each CFU count was performed to normalize the data before statistical evaluation due to the high range of bacterial numbers. Then, to determine viable bacteria counts, statistical analyses were performed using one-way ANOVA and the Fisher’s least significance difference (LSD) post hoc test for pair-wise means comparisons. Two-way ANOVA (time and adhesive) was used to assess the pH change of the supernatant storage medium for each adhesive system; similar tests were used for the mechanical properties. All statistical testing was performed using Sigma Stat® for Windows Software®, Version 3.5 (Systat Software, Inc., Point Richmond, CA, USA), using a pre-set alpha of 0.05.
Results and discussion
Null hypothesis #1
Hypothesis #1 was rejected; it was assumed that there were no differences in the antimicrobial effect between the experimental adhesive with B. capitata seed essential oil and other commercial adhesive systems. However, there were differences between EAO and EANO, (Figure ).
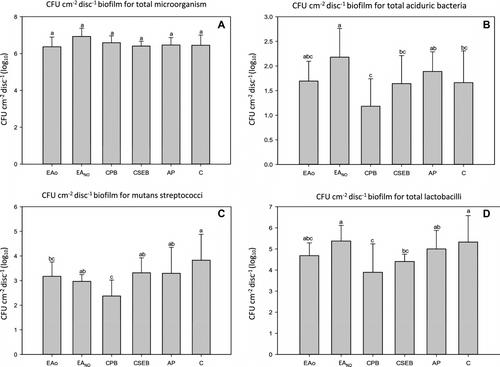
Figure 2 Mean viable bacteria (CFU cm−2 dry biofilm weight) in biofilms grown for 72 h (n = 10). The data were normalized by transforming by log10. Within a panel, group values that were identified using similar lower case letters were not significantly different (p > 0.05). A = total microorganisms; B = total aciduric bacteria; C = S. mutans; D = total lactobacilli bacteria.
Null hypothesis #2
Hypothesis #2 was accepted; the experimental adhesive with oil had physical and mechanical properties similar to those of other adhesives, including the group with the antibacterial monomer in its composition ().
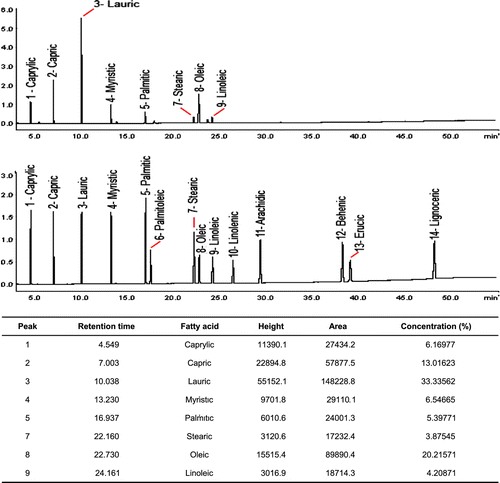
GC analysis of B. capitata oil
Analysis of the essential oil of B. capitata indicated that it is composed mainly of 24% of the unsaturated fatty acids (linoleic and oleic acids) and 76% saturated fatty acids (caprylic, capric, lauric, myristic, palmitic, and stearic acids) (Figure ).
Anti-biofouling testing: the microcosm technique
The antibacterial activity of the experimental adhesive was compared to three commercial adhesives. Several studies have investigated the antibacterial activity of adhesive systems (Feuerstein et al. Citation2007; Paradella et al. Citation2009; Esteves et al. Citation2010). However, few studies have evaluated the antibacterial activity of adhesive systems in biofilms or multiple species models (Imazato et al. Citation2006; Li et al. Citation2009). The microcosm technique is employed as an ecosystem that mimics naturally formed biofilms (Imazato et al. Citation2006; Cenci et al. Citation2009; McBain Citation2009). In studies focusing on supragingival plaque, a microcosm implies the use of dental plaque or human saliva as a source of inoculum for an in vitro device (Wimpenny Citation1997). This study used an in vitro microcosm dental biofilm model, with a semi-continuous sucrose exposure, to evaluate the anti-biofouling effect of self-etching adhesive systems. The microcosm methodology is more complex and more precise than those in vitro biofilm assays with single species because the proportions of species detected in the microcosm community were similar to those observed in supragingival plaque.
Anti-biofouling testing: changes in the pH of the media
The mean values and standard deviations (SDs) of the pH readings for the different dental adhesives are given in Table . A significant effect was observed for the adhesive, medium time, and the interaction factor (α < 0.001). The pH of the Clearfil Protect Bond (CPB) was significantly higher than those of the other groups after f sucrose exposure for 6 h.
Table 2. Supernatant pH (mean ± SD) of the storage medium of each adhesive system.
In relation to the acidogenicity of the biofilm grown in the culture media, the fluctuating values could be explained by the use of a semi-continuous model, which has periods with and without sucrose exposure. After 6 h, the pH levels were decreased in the different groups. However, CPB showed the highest pH values, which may have been due to the presence of fluoride in the formulation (Moszner et al. Citation2005; Van Landuyt et al. Citation2007), which has been shown to influence pH (Lammers et al. Citation1992). However, after 24 h, CPB displayed lower pH values. After 24 h, the pH levels were similar in the different groups. These results were inconsistent with some hypotheses stated in the literature, which suggest that the antibacterial properties of self-etching adhesive are due to the low pH of the materials (Meiers & Miller Citation1996; Cehreli et al. Citation2003). These results also demonstrated that the low pH of the adhesive systems might have limited influence on their antibacterial properties (Imazato Citation2003; Feuerstein et al. Citation2007).
Anti-biofouling testing: total microorganism growth
Figure shows the numbers of viable bacteria. All tested groups had statistically similar antimicrobial activity (α = 0.068), with the highest values of total viable microorganisms being from EANO.
Anti-biofouling testing: aciduric bacteria
EAO demonstrated similar behavior to CPB and Clearfil SE Bond (CSEB), with respect to total viable bacteria within the biofilm, including aciduric bacteria. This is of note as it suggests that the experimental adhesive had similar antibacterial properties to commercial antimicrobial self-etching adhesives (Figure ).
For aciduric bacteria, the EAO group showed lower mean CFU than did EANO, although differences were not statistically significant. Mean CFU values for the two experimental groups were similar to those of the commercial references tested.
Anti-biofouling testing: S. mutans
Viable counts of S. mutans grown in biofilms on the different groups were lower for EAO, EANO and CPB, with the growth of bacteria being statistically similar between EAO and CPB and between EAO and EANO. This suggests that the antimicrobial monomer (MDPB) in the CPB system and the essential oil in EAO have an effect on bacterial viability. The highest means are showed in the other groups and they showed similar levels in their biofilms (α = 0.007) (Figure ).
Anti-biofouling testing: total lactobacilli
For the analysis of the total lactobacilli load in the biofilms, EAO showed an antibacterial effect similar to those of CSEB, CPB, and EANO (α = 0.020) (Figure ). The similarity in the results may be due to CPB and its antibacterial monomer MDPB (Imazato et al. Citation1998). CSEB contains 10-MDP, which could have exerted an antibacterial effect (Paradella et al. Citation2009), while the EAO tested in this study has an essential oil rich in fatty acids (Nakatsuji et al. Citation2009; Huang et al. Citation2010). Unpublished data showed that AP had a cytotoxic effect on fibroblasts, which could explain the antimicrobial effect observed in this study.
The antibacterial effect of the EAO compared with its control (EANO) may be explained by the presence of essential oil from the seeds of B. capitata, which contains different antimicrobial fatty acids such as lauric acid, oleic acid, linoleic acid, and palmitoleic acid (Magalhaes et al. Citation2008; Nakatsuji et al. Citation2009; Huang et al. Citation2010). Lauric acid is one of the most active saturated fatty acids present in the essential oil of B. capitata, and palmitoleic acid is the main unsaturated fatty acid (Wille & Kydonieus Citation2003). The antimicrobial effect of EAO containing this essential oil may be due to this extract, which when mixed with the other monomers for the formulation of EAO cannot be polymerized into the adhesive matrix. Hence, the antibacterial effect can persist even after polymerization because the fatty acid molecules are leached. Typically, the absorbed water induces the leaching process, which, in turn, produces the release of residual unpolymerized monomers and low-polymerized molecules. In this study, the EAO showed WS levels similar to that of CPB (α = 0.063), an adhesive that contains 12-MDPB, although it displayed statistically lower sorption than did the AP adhesive system (α = 0.025), which showed the highest WS and SL.
Several studies have shown higher antibacterial activity for the 12-MDPB monomer (Imazato et al. Citation1997, Citation1998). The results presented here differ from those in the literature. This may result from methodological differences. This in vitro study employed simulated natural biofilms whilst other studies that tested adhesive systems used planktonic models or single-species biofilms. The formation of oral biofilms on hard surfaces is a complex process, involving salivary pellicle formation and adsorption to the surface, as well as the passive transport of bacteria to the pellicle surface, co-adhesion, and multiplication. Bacterial cells in biofilms are known to be less susceptible to antimicrobials than to planktonic microorganisms (Hope & Wilson Citation2004).
Physical–mechanical testing: μTBS
The values of microtensile bond strengths are shown in Table . The adhesive type (α < 0.001), storage time (α < 0.001), and the interaction term (α < 0.001), were statistically significant. The μTBS values after 6 months showed a statistically significant decrease in the EAO and AP groups. Experimental adhesives EAO and EANO showed statistically higher μTBS levels than did their commercial counterparts. However, after 6 months, EAO showed a level of μTBS that was significantly lower than other adhesive systems, with the exception of AP.
Table 3. Microtensile bond strength (MPa), at different times of storage (mean ± SD).
Most dental adhesive systems currently available on the market possess favorable immediate bonding, although the stability of the bonded interfaces still causes major concerns (Breschi et al. Citation2008). The present results show that at 24 h, the performance of EAO was similar to that of EANO, although after 6 months the bond strength values showed a significant reduction. This may be due to the lixiviation process that occurs via the loss of un-reacted solid molecules, in this case a polymer, in the presence of water (Ferracane Citation2006). However, it is common for self-etching simplified adhesive systems to show this reduction in μTBS value with time.
The μTBS values of CPB after 24 h and 6 months were statistically similar (α > 0.05). However, a small increase in the value of MPa was observed, perhaps due to the incorporation of fluoride in the adhesive resins, which increased the durability of the dentin bond. Several studies have shown that the bond strength of fluoride-containing systems did not decrease after long-term water storage, whereas the bond strengths of comparable resins without fluoride decreased during the same time of immersion (Nakajima et al. Citation2003).
AP presented lower values after 24 h and 6 months, which may be due to the material composition of the adhesive. AP also does not contain Bis-GMA, although the exact amounts of monomer and other components are not provided by the manufacturers (Table ). Thus, AP may possess the highest levels of HEMA, which may decrease the ultimate tensile strength compared to agents with lower HEMA content in co-monomer blends (Collares et al. Citation2011).
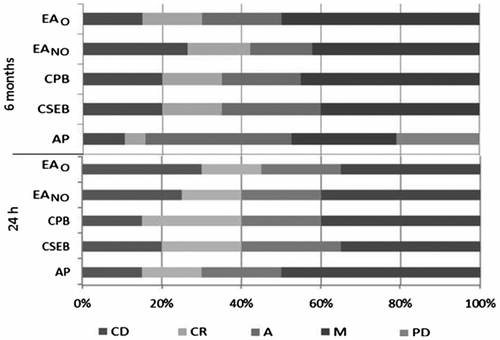
Figure shows the adhesive failures and prematurely debonded sticks found in the AP group, which may explain the low values in μTBS. There was no premature specimen debonding during preparation when the experiments were conducted after 24 h. Conversely, 2 premature failures were observed after 6 months of storage in water.
Physical–mechanical testing: monomer conversion
The incorporation of an essential oil into an experimental adhesive did not influence the DC. The small amount of essential oil that was added, as well as the characteristics of the fatty acids, which contain long hydrophobic chains and hydrophilic carboxyl groups may have contributed to this outcome. As was described previously, CPB, with its incorporation of the antibacterial monomer MDPB, has no effect on the bond strength of the adhesive to dentin or on the DC of this material (Imazato et al. Citation1997).
Further investigations should be performed (ie thermogravimetric analysis) to investigate the thermal degradation process, and to identify and quantify the individual components lixiviated from the monomer blend.
Physical–mechanical testing: water sorption and solubility
The results of WS (Equation (1)) and SL (Equation (2)) are shown in Table . CSEB showed the lowest levels of WS, whereas AP showed the highest (α < 0.001). AP also showed significantly higher levels of SL (α = 0.001).
Table 4. DC, WS, and SL for each self-etch adhesive system (means ± SD).
EAO and CPB performed similarly in the WS assay. For SL, however, the experimental adhesives (EAO and EANO) had lower values. Based on these results, incorporation of the essential oil as tested did not interfere with immediate material deterioration.
Higher WS and SL values were obtained with increased HEMA contents in co-monomer blends that increased plasticization, possibly because macromolecular polymer chains underwent a relaxation process as they swelled to absorb the water. Initially, the presence of water would soften the polymer by swelling the network and reducing the frictional forces between the polymer chains (Ferracane et al. Citation1998). After the relaxation process, un-reacted monomers trapped in the polymer network would be released to the surrounding area at a rate that is controlled by the swelling and relaxation capacities of the polymer. More hydrophilic polymer networks, which have a superior relaxation capacity, induce a faster release of un-reacted monomers through nanovoids in the material (Brazel & Peppas Citation1999).
The presence of both positive and negative values of adhesive SL could be related to the network structure of the polymers. Simple structures without crosslinks comply with the free volume theory that presupposes easier WS. Otherwise, most complex networks have smaller free volume spaces and a longer water uptake time (Malacarne et al. Citation2006).
The global impact of the findings
This study assessed the anti-biofouling performance of an experimental adhesive system containing a naturally occurring essential vegetable oil and embedded experimental controls, as well as comparing the findings to existing commercial products known to have antimicrobial activity. In summary, this study showed that adhesive containing seed essential oil of B. capitata presented anti-biofouling, and physical and mechanical properties similar to those of equivalent commercial adhesive systems.
Conclusion
The experimental self-etching adhesive that includes the essential oil from B. capitata showed an antimicrobial effect in a microcosm biofilm that was similar to that of an antimicrobial adhesive. The physical and mechanical properties of EAO were not significantly different to commercial references, except for the μTBS test, which showed that the tensile strength decreased after 6 months compared to the control. However, this decrease in μTBS was similar to the other adhesives tested.
References
- Aires , CP , Del Bel Cury , AA , Tenuta , LM , Klein , MI , Koo , H , Duarte , S and Cury , JA . 2008 . Effect of starch and sucrose on dental biofilm formation and on root dentine demineralization . Caries Res , 42 : 380 – 386 .
- Brazel , CS and Peppas , NA . 1999 . Mechanisms of solute and drug transport in relaxing, swellable, hydrophilic glassy polymers . Polymer , 40 : 3383 – 3398 .
- Brazilian Pharmacopoeia . 2000 . 4th ed. Part II , São Paulo : Atheneu .
- Breschi , L , Mazzoni , A , Ruggeri , A , Cadenaro , M , Di Lenarda , R and Dorigo , E . 2008 . Dental adhesion review: aging and stability of the bonded interface . Dent Mater , 24 : 90 – 101 .
- Burt , S . 2004 . Essential oils: their antibacterial properties and potential applications in foods – a review . Int J Food Microbiol , 94 : 223 – 253 .
- Cehreli , ZC , Stephan , A and Sener , B . 2003 . Antimicrobial properties of self-etching primer-bonding systems . Oper Dent , 28 : 143 – 148 .
- Cenci , MS , Pereira-Cenci , T , Cury , JA and ten Cate , JM . 2009 . Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model . Caries Res , 43 : 97 – 102 .
- Collares , FM , Ogliari , FA , Zanchi , CH , Petzhold , CL , Piva , E and Werner Samuel , SM . 2011 . Influence of 2-hydroxyethyl methacrylate concentration on polymer network of adhesive resin . J Adhes Dent , 13 : 125 – 129 .
- Emilson , CG and Bergenholtz , G . 1993 . Antibacterial activity of dentinal bonding agents . Quintessence Int , 24 : 511 – 515 .
- Esteves , CM , Ota-Tsuzuki , C , Reis , AF and Rodrigues , JA . 2010 . Antibacterial activity of various self-etching adhesive systems against oral streptococci . Oper Dent , 35 : 448 – 453 .
- Faria , J , Arellano , D , Grimaldi , R , Da Silva , L , Vieira , R , Da Silva , D and Agostini-Costa , T . 2008 . Chemical characterization of nut of Butia capita var capita . Revista Brasileira De Fruticultura , 30 : 549 – 552 .
- Felton , D , Bergenholtz , G and Cox , CF . 1989 . Inhibition of bacterial growth under composite restorations following GLUMA pretreatment . J Dent Res , 68 : 491 – 495 .
- Ferracane , JL . 2006 . Hygroscopic and hydrolytic effects in dental polymer networks . Dent Mater , 22 : 211 – 222 .
- Ferracane , JL , Berge , HX and Condon , JR . 1998 . In vitro aging of dental composites in water – effect of degree of conversion, filler volume, and filler/matrix coupling . J Biomed Mater Res , 42 : 465 – 472 .
- Feuerstein O, Matalon S, Slutzky H, Weiss EI. 2007. Antibacterial properties of self-etching dental adhesive systems. J Am Dent Assoc 138: 349–354; quiz 396–8.
- Filoche , SK , Soma , KJ and Sissons , CH . 2007 . Caries-related plaque microcosm biofilms developed in microplates . Oral Microbiol Immunol , 22 : 73 – 79 .
- Henn , S , Nedel , F , de Carvalho , RV , Lund , RG , Cenci , MS , Pereira-Cenci , T , Demarco , FF and Piva , E . 2011 . Characterization of an antimicrobial dental resin adhesive containing zinc methacrylate . J Mater Sci-Mater Med , 22 : 1797 – 1802 .
- Herigstad , B , Hamilton , M and Heersink , J . 2001 . How to optimize the drop plate method for enumerating bacteria . J Microbiol Methods , 44 : 121 – 129 .
- Holley , R and Patel , D . 2005 . Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials . Food Microbiol , 22 : 273 – 292 .
- Hope , C and Wilson , M . 2004 . Analysis of the effects of chlorhexidine on oral biofilm vitality and structure based on viability profiling and an indicator of membrane integrity . Antimicrob Agents Chemother , 48 : 1461 – 1468 .
- Huang , CB , George , B and Ebersole , JL . 2010 . Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms . Arch Oral Biol , 55 : 555 – 560 .
- Imazato , S . 2003 . Antibacterial properties of resin composites and dentin bonding systems . Dent Mater , 19 : 449 – 457 .
- Imazato , S , Imai , T , Russell , RRB , Torii , M and Ebisu , S . 1998 . Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer . J Biomed Mater Res , 39 : 511 – 515 .
- Imazato , S , Kinomoto , Y , Tarumi , H , Torii , M , Russell , RR and McCabe , JF . 1997 . Incorporation of antibacterial monomer MDPB into dentin primer . J Dent Res , 76 : 768 – 772 .
- Imazato , S , Kuramoto , A , Takahashi , Y , Ebisu , S and Peters , MC . 2006 . In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond . Dent Mater , 22 : 527 – 532 .
- International Organization for Standardization (ISO) (CH) . 2000 . Dentistry – polymer-based filling, restorative and luting materials , Geneve : International Organization for Standardization .
- Lammers , P , Borggreven , J and Driessens , F . 1992 . Influence of fluoride and pH on invitro remineralization of bovine enamel . Caries Res , 26 : 8 – 13 .
- Li , F , Chai , ZG , Sun , MN , Wang , F , Ma , S , Zhang , L , Fang , M and Chen , JH . 2009 . Anti-biofilm effect of dental adhesive with cationic monomer . J Dent Res , 88 : 372 – 376 .
- Lima , Gda S , Ogliari , FA , da Silva , EO , Ely , C , Demarco , FF , Carreño , NL , Petzhold , CL and Piva , E . 2008 . Influence of water concentration in an experimental self-etching primer on the bond strength to dentin . J Adhes Dent , 10 : 167 – 172 .
- Magalhaes , H , Catao , H , Sales , N , de Lima , N and Lopes , P . 2008 . Health quality of Butia capitata seeds in the north of Minas Gerais . Brazil Ciencia Rural , 38 : 2371 – 2374 .
- Malacarne , J , Carvalho , RM , de Goes , MF , Svizero , N , Pashley , DH , Tay , FR , Yiu , CK and de Oliveira Carrilho , MR . 2006 . Water sorption/solubility of dental adhesive resins . Dent Mater , 22 : 973 – 980 .
- McBain AJ. 2009. Chapter 4: In vitro biofilm models: an overview. Adv Appl Microbiol. 69: 99–132.
- Meiers , JC and Miller , GA . 1996 . Antibacterial activity of dentin bonding systems, resin-modified glass ionomers, and polyacid-modified composite resins . Oper Dent , 21 : 257 – 264 .
- Moszner , N , Salz , U and Zimmermann , J . 2005 . Chemical aspects of self-etching enamel-dentin adhesives: a systematic review . Dent Mater , 21 : 895 – 910 .
- Nakajima , M , Okuda , M , Ogata , M , Pereira , P , Tagami , J and Pashley , D . 2003 . The durability of a fluoride-releasing resin adhesive system to dentin . Oper Dent , 28 : 186 – 192 .
- Nakatsuji , T , Kao , MC , Fang , JY , Zouboulis , CC , Zhang , L , Gallo , RL and Huang , CM . 2009 . Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris . J Invest Dermatol , 129 : 2480 – 2488 .
- Ogliari , FA , de Sordi , ML , Ceschi , MA , Petzhold , CL , Demarco , FF and Piva , E . 2006 . 2,3-Epithiopropyl methacrylate as functionalized monomer in a dental adhesive . J Dent , 34 : 472 – 477 .
- Paradella , TC , Koga-Ito , CY and Jorge , AO . 2009 . In vitro antibacterial activity of adhesive systems on Streptococcus mutans . J Adhes Dent , 11 : 95 – 99 .
- Schmidlin , PR , Zehnder , M , Göhring , TN and Waltimo , TM . 2004 . Glutaraldehyde in bonding systems disinfects dentin in vitro . J Adhes Dent , 6 : 61 – 64 .
- Settembrini , L , Boylan , R , Strassler , H and Scherer , W . 1997 . A comparison of antimicrobial activity of etchants used for a total etch technique . Oper Dent , 22 : 84 – 88 .
- Sun , C , Cao , Y and Huang , A . 1988 . Acyl coenzyme-A preference of the glycerol phosphate-pathway in the microsomes from the maturing seeds of palm, maize, and rapeseed . Plant Physiol , 88 : 56 – 60 .
- Van de Sande , FH , Azevedo , MS , Lund , RG , Huysmans , MC and Cenci , MS . 2011 . An in vitro biofilm model for enamel demineralization and antimicrobial dose-response studies . Biofouling , 27 : 1057 – 1063 .
- Van Landuyt , K , Snauwaert , J , De Munck , J , Peurnans , M , Yoshida , Y , Poitevin , A , Coutinho , E , Suzuki , K , Lambrechtsa , P and Van Meerbeek , B . 2007 . Systematic review of the chemical composition of contemporary dental adhesives . Biomaterials , 28 : 3757 – 3785 .
- Wille , JJ and Kydonieus , A . 2003 . Palmitoleic acid isomer (C16:1delta6) in human skin sebum is effective against gram-positive bacteria . Skin Pharmacol Appl Skin Physiol , 16 : 176 – 187 .
- Wimpenny , JW . 1997 . The validity of models . Adv Dent Res , 11 : 150 – 159 .
- Wong , L , Sissons , C and Sissions , CH . 2001 . A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva . Arch Oral Biol , 46 : 477 – 486 .
- Xiao , YH , Ma , S , Chen , JH , Chai , ZG , Li , F and Wang , YJ . 2009 . Antibacterial activity and bonding ability of an adhesive incorporating an antibacterial monomer DMAE-CB . J Biomed Mater Res B Appl Biomater , 90 : 813 – 817 .