Abstract
Medically relevant biofilms have gained a significant level of interest, in part because of the epidemic rise in obesity and an aging population in the developed world. The associated comorbidities of chronic wounds such as pressure ulcers, venous leg ulcers, and diabetic foot wounds remain recalcitrant to the therapies available currently. Development of chronicity in the wound is due primarily to an inability to complete the wound healing process owing to the presence of a bioburden, specifically bacterial biofilms. New therapies are clearly needed which specifically target biofilms. Lactoferrin is a multifaceted molecule of the innate immune system found primarily in milk. While further investigation is warranted to elucidate mechanisms of action, in vitro analyses of lactoferrin and its derivatives have demonstrated that these complex molecules are structurally and functionally well suited to address the heterogeneity of bacterial biofilms. In addition, use of lactoferrin and its derivatives has proven promising in the clinic.
Biofilms: from the noxious to the nasty
The effect of medically relevant biofilms can vary from the common bacterial film on the surface of teeth to noxious open chronic wounds often found on lower extremities. Biofilms resulting in chronic wounds include, but are not limited to periodontal disease (a chronic wound of the mouth), venous leg ulcers, pressure ulcers, nosocomial (hospital-acquired) infections, and contaminated implanted devices. While medically relevant biofilms have recently received more attention, it is difficult to grasp the socio-economic significance of the escalating biomedical problem of the bacterial bioburden and the chronic wound. In addition, a significant amount of work remains to be done to comprehensively decipher the complexity of the biofilm phenotype, including determining how little is understood about this mode of growth and how biofilms continue to evade every tool in the traditional biomedical toolbox.
In 2002, 1.7 million nosocomial bacterial infections were equivalent to an average of 4.5 infections reported for every 100 people admitted to the hospital and these infections resulted in 99,000 deaths (Klevens et al. Citation2007), and upwards of 65% of nosocomial infections are suspected to originate from bacterial biofilms (Licking Citation1999). The scope of this problem cannot be overemphasized and new, cost-effective approaches need to reach the biomedical pipeline.
The mortality rate resulting from such types of infections is very great, and the morbidity rate is even greater as many of the infections acquired in the medical setting result in long-term, non-healing chronic wounds. Such wounds can present themselves as a broad spectrum of ‘symptoms’ ranging from a ‘spider bite’ that develops into a small, treatment-resistant ulcer on the skin to large, open wounds on the torso with significant tissue necrosis and effluent. Chronicity is not well defined and can vary from 6 months in a minor chronic wound to decades in a persistent chronic wound. Therefore, a chronic wound is broadly characterized as a wound that does not proceed through the normal stages of healing in a predictable period of time. While for most of the healthy population, wounds progress through a normal process of haemostasis, inflammation, granulation, epithelialization, and maturation to healing, chronic wounds most commonly stall at the stage of inflammation and affect moderately immunocompromised individuals such as diabetics and the elderly (McGuckin et al. Citation2003).
Systemic disregulation of immunity, circulation, and the nervous system in diabetics means that preventive interventions against the development of a chronic wound are far less effective. Over the course of a lifetime, up to 15% of diabetics can expect to have at least one amputation of an extremity due to an untreatable chronic wound (James et al. Citation2008); a problem that has been compounded by the development of antibiotic resistance and the absence of new effective methods of treatment (Boucher et al. Citation2009; Peleg & Hooper Citation2010). Current therapeutic options for the care of chronic wounds have proven ineffectual, primarily because treatments have been developed for use against planktonic bacteria with little or no consideration of the biofilm mode of growth.
Though not yet a precisely defined phenotype, biofilms are generally accepted to be structured communities of micro-organisms, adhered to a surface, and exhibiting phenotypic heterogeneity (Costerton et al. Citation1999). In the environment of the chronic wound, bacterial contamination develops into colonization, devitalized tissue provides the surface to which biofilms adhere, and non-healing is dependent on the bacterial cells successfully establishing biofilm growth (Costerton et al. Citation1999; Donlan & Costerton Citation2002; Parsek & Singh Citation2003). It has been well demonstrated that establishment of a bacterial biofilm in the wound is the major reason for treatment failure of acute wounds and development of chronic, non-healing wounds (Harrison-Balestra et al. Citation2003; Bjarnsholt et al. Citation2008; Davis et al. Citation2008; James et al. Citation2008). Therefore, development of a chronic wound is characterized by the persistence of a bacterial infection that is insurmountable by the immune system and inconsistently responsive to antibiotic treatment, characteristics that are particularly relevant to the biofilm mode of growth.
Treatment of bacterial biofilms in the wound is complicated by the character of the biofilm mode of growth, including increased resistance of the biofilm to antimicrobial treatments and the immune defense of the host (Davey & O’Toole Citation2000; Donlan Citation2000; Donlan & Costerton Citation2002; Davies Citation2003; Fux et al. Citation2005). Many characteristics of the biofilm contribute to both types of resistance, including protective shielding by the biofilm matrix, specific expression of microbial proteins for nutrient scavenging, a ramping down of metabolic activity, and induction of a persister state in which bacterial cells are in a phenotypic state and particularly resistant to antimicrobial treatment. While this bacterial population has not acquired antibiotic resistance per se, variations in phenotype result in a recalcitrant population capable of reseeding a wound in which bacterial contamination was thought to have been cleared (Lewis Citation2001). Commonly used antibiotics have been designed against planktonically grown bacteria and to treat metabolically active bacteria. However, bacteria in a biofilm are metabolically different from planktonic bacteria (Davey & O’Toole Citation2000).
Clearly there is a need for novel, biofilm-targeted therapies that are designed to target specific weaknesses in the biofilm mode of growth. However, identification of those weaknesses remains elusive, and is an area in need of more in-depth research. Factors that need to be taken into account in the design of novel chronic wound therapeutics include the need for drugs to penetrate the EPS matrix and the ability to retain efficacy against phenotypic variability within the biofilm including persister cell populations. In this perspective, an example of one such protein, the milk protein lactoferrin, which has been demonstrated recently to show promise as a novel, anti-biofilm therapeutic safe for use in chronic wound will be considered.
Lactoferrin and its antimicrobial peptide derivatives
Lactoferrin is an abundant, iron-binding protein from the innate immune system (reviewed in Valenti et al. Citation2004) found circulating in the blood, as well as in secreted fluids such as tears, semen, vaginal secretions, and most recently sweat (Park et al. Citation2011). Found in greatest abundance in milk, lactoferrin is generally thought of as a milk protein. However, lactoferrin is a multifunctional protein serving many roles. As lactoferrin is an ∼80 kDa member of the transferrin family of non-heme, iron-binding glycoproteins, it was originally thought to function as an iron transport protein in blood, but observation of lactoferrin in neutrophils suggest that this protein might have a greater role in innate immunity (Masson et al. Citation1969). Indeed, lactoferrin plays diverse roles in innate immunology from inhibition of neutrophil priming by bacterial lipopolysaccharide (LPS) (Cohen et al. Citation1992), to enhancing neutrophil adherence to endothelial cells (Oseas et al. Citation1981), and modulating inflammation by amplifying apoptotic signals (Valenti et al. Citation1999). In addition to being anti-inflammatory, lactoferrin has demonstrated anti-tumor, anti-viral, anti-fungal, and anti-bacterial properties (Farnaud & Evans Citation2003).
Structural analysis of lactoferrin and its derivatives has provided some insight into the function of these innate immune molecules (Anderson et al. Citation1987). Lactoferrin can bind two ferric ions (Fe3+) in two similar, but not identical structural lobe regions. In comparison to transferrin, lactoferrin binds iron very tightly in each lobular cleft (Kd = 10−20 M) further suggesting that iron sequestration rather than transportation is the primary function of iron chelation. Within the cleft of each iron-binding lobe, a single ferric ion is surrounded by three side chains of Tyr, Asp, and His residues, in addition to a carbonate ion providing two ligands to form an octahedral complex (Saponja & Vogel Citation1996). Although iron-binding is clearly essential to the function of lactoferrin, the protein can exist in either an open, non-iron-binding form (apo-lactoferrin) or a closed, iron-bound form (halo-lactoferrin) (Baker & Baker Citation2012), and the highly basic (positively charged), N-terminal has been shown to interact with LPS, glycosaminoglycans, and DNA (Appelmelk et al. Citation1994; Kanyshkova et al. Citation1999; El Yazidi-Belkoura et al. Citation2001). The demonstration that lactoferrin binds DNA, suggests that this immune molecule can function as a transcription regulator. Indeed, the Gly–Arg–Arg–Arg–Arg sequence on human lactoferrin resembles a nuclear localization sequence, and has been shown to enable lactoferrin crossing of cellular membranes and regulation of transcription (He & Furmanski Citation1995).
The versatile character of lactoferrin is attributable to a number of peptide derivatives characterized as antimicrobial peptides, including lactoferrampin. Originally identified as residues 268–284 of bovine lactoferrin, lactoferrampin has demonstrated efficacy against Candida albicans, as well as a number of bacterial species including the opportunistic pathogen Pseudomonas aeruginosa (van der Kraan et al. Citation2004). Antimicrobial peptides such as those derived from lactoferrin generally contain alternating positive and uncharged residues that result in a structural motif of a positively charged α-helix. This structure effectively mediates membrane disruption through preferential binding to negatively charged microbial cell membranes in contrast to more neutral eukaryotic cell membranes. However, if the net positive charge of the molecule is too high, the ability of the peptide to permeabilize the bacterial membrane decreases. This is likely to be because electrostatic interactions anchor the peptide too strongly to the lipid head group, and/or because interaction between the positively charged side chains might result in intramolecular and intermolecular repulsion and inhibition of pore formation (Dathe & Wieprecht Citation1999; Matsuzaki Citation1999). Demonstrating the essential link between structure and function in lactoferrampin, successive truncation of lactoferrampin from the N- and C-termini identified the region comprised of residues 265–284 as the peptide derivative most effective as an antimicrobial. N-terminal truncation likely leads to a destabilization of the α-helix, while truncation at the C-terminal likely leads to loss of essential positively charged residues (van der Kraan et al. Citation2005).
Lactoferricin is the most well-characterized peptide derivative of lactoferrin, resulting from pepsin cleavage at residues 17–41 (Bellamy, Takase, Wakabayashi et al. Citation1992) and is found as a breakdown product in the human gut (Kuwata et al. Citation1998). Like lactoferrampin, lactoferrin is multifunctional and has been demonstrated to have anti-fungal, anti-viral, anti-microbial, anti-tumor characteristics as well as the ability to modulate both the inflammatory response and adaptive immunity (Bellamy, Takase, Yamauchi et al. Citation1992; Yamauchi et al. Citation1993; Brock Citation1995; Wakabayashi et al. Citation1999; Andersen et al. Citation2001). Unlike lactoferrampin, lactoferricin does not have an extended α-helical structure, but rather forms a somewhat distorted β-sheet (Hwang et al. Citation1998). There is some variability between different lactoferricin derivatives. However, the most characterized structure is comprised of residues 17–41 of the native protein which are linked through a disulfide bond at residues 19 and 36. The function of this disulfide bond remains to be determined. In all likelihood it plays a structural role, stabilizing the protein, but it is not known whether it has any catalytic activity, although the peptide retains antimicrobial activity when the S–S bond is reduced (Bellamy, Takase, Wakabayashi et al. Citation1992). As with lactoferrampin, lactoferricin primarily mediates antimicrobial activity through disruption of the bacterial membrane. In all likelihood, the mixture of cationic and lipophilic residues that comprise lactoferricin results in an efficient, antimicrobial structural motif. Indeed, antimicrobial activity is enhanced with increased Trp content or insertion of non-native aromatic amino acids with bulkier side chains than Trp (Strom et al. Citation2002).
While by itself, lactoferrampin appears to have mild antimicrobial properties, covalently linked bovine lactoferrampin and lactoferricin display enhanced antimicrobial efficacy (Bolscher et al. Citation2012; Haney et al. Citation2012). On the other hand, lactoferricin is an efficacious antimicrobial lactoferrin derivative and is more efficacious as an antimicrobial than the native protein (Jones et al. Citation1994). Although not shown to have direct antimicrobial activity as observed for lactoferricin and lactoferrampin, the lactoferrin derivative delta-lactoferrin is interesting in that it may have a secondary role in host immune response to pathogens. Delta-lactoferrin is expressed intracellularly and lacks the leader sequence and first 25 N-terminal residues of the native protein (Siebert & Huang Citation1997). Interestingly, delta-lactoferrin is not present in tumors (Klein et al. Citation2007), but in normal cells it acts as a transcription factor (Mariller et al. Citation2012). Therefore, its role in immunity may be more subtle, perhaps through the regulation of genes important to host immunity. Of interest would be an analysis of whether the correlation between cancer and inflammation is related to the intracellular expression of this isoform of the innate immune molecule lactoferrin.
Lactoferrin: a biofilm-targeted antimicrobial
The iron-chelating nature of lactoferrin suggests that this might be the primary mechanism of action of its antimicrobial activity. Iron is an essential nutrient for bacteria and a global regulator of biochemical, cellular, and metabolic functions. Among a large number or reactions, iron participates in DNA synthesis, the electron transport system, formation of heme, as a cofactor for enzymes, in oxygen transport, the synthesis of ATP, and nitrate reduction in the nitrogen cycle. In addition, iron is an essential nutrient for biofilm development and growth (Neilands Citation1981a, Citation1981b; Crosa Citation1989). Under physiological conditions of neutral pH and in the presence of oxygen, iron will undergo rapid oxidation from Fe2+ to Fe3+ to insoluble ferric oxyhydroxide if not bound by an iron-chelating molecule. While only about 10−9–10−18 M iron is bioavailable, most microbes need ∼10−8 M iron for normal cellular functions. Therefore, microbes have evolved a set of iron-chelating molecules called siderophores for scavenging iron from the environment (Neilands Citation1995). Siderophores fall into one of three categories depending on their iron-binding motif: the catecholates (or phenolates) such as the P. aeruginosa pyochelin, hydroxymates (or carboxylates) such as the staphyloferrin of staphylococcal species, and the mixed moieties (Miethke & Marahiel Citation2007). Although biosynthesis of siderophores varies between species, many of the species of biomedical concern such as the opportunistic pathogen P. aeruginosa utilize non-ribosomal peptide synthetases, which do not use an RNA template for biosynthesis (Lamont et al. Citation2006). Once synthesized, siderophores are exported into the extracellular environment through one of three different types of protein families: the major facilitator superfamily; the resistance, nodulation, and cell division (RND) superfamily; and the ABC transporter superfamily. An example of the RND exporter family includes the P. aeruginosa MexA–MexB–OprM complex. Staphylococcus aureus utilizes an ABC transporter for exportation of siderophores (Poole et al. Citation1993; Vettoretti et al. Citation2009; Grigg et al. Citation2010; Beasley et al. Citation2011). Once secreted, siderophores, such as pyoverdine from P. aeruginosa, are examples of molecules with extracytoplasmic functions in that activation of the biosynthesis and export of siderophores is dependent on the detection of iron in the environment by the secreted siderophores. Thus, a feedback mechanism regulates the energy input of the bacteria into scavenging iron based on environmental iron availability (Redly & Poole Citation2005). Iron scavenging is also important to virulence in the chronic wound, particularly for opportunistic pathogens such as P. aeruginosa. For example, siderophore mutants of P. aeruginosa form thin poorly attached biofilms (Banin et al. Citation2005). Additionally, activation of the pyoverdine expression and biosynthesis pathway is linked to expression of other virulence factors such as exotoxin A and the endoprotease Prpl (Wilderman et al. Citation2001). By understanding how wound-populating micro-organisms such as P. aeruginosa acquire iron from the environment, the mechanism by which iron-chelation by lactoferrin affects the viability of bacterial biofilms begins to emerge (Figure ).
Figure 1 Combined treatment of P. aeruginosa biofilms with lactoferrin and xylitol results in membrane disruption. SEM imaging (panels A and B) and LIVE/DEAD staining (panels C and D) demonstrate significant membrane disruption of treated cells (panels B and D) when compared to untreated cells (panels A and C). For more information see Ammons, Ward and James (Citation2011) and Ammons, Ward, Dowd et al. (Citation2011).
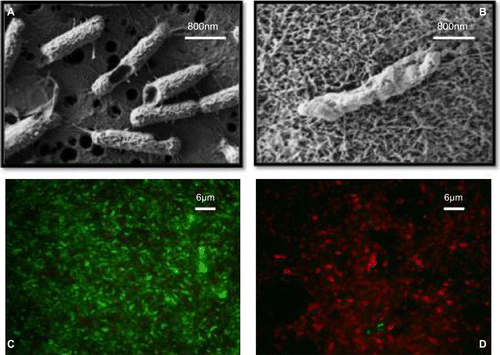
While anti-biofilm efficacy has been demonstrated for lactoferrin and its derivatives, the mechanism of action remains an area of active research (Singh et al. Citation2002; Ammons et al. Citation2009). The most obvious mechanism of lactoferrin action is that by binding and sequestering the iron in the environment, lactoferrin deprives the biofilm of this essential nutrient, thus limits the capacity of the biofilm to survive. However, some evidence suggests that lactoferrin interaction with the biofilm may be more complex, especially considering that bacterial siderophores can strip iron from lactoferrin (Xiao & Kisaalita Citation1997). While iron chelation clearly plays an important role, in vitro analysis with iron saturated lactoferrin does not completely abrogate the anti-biofilm capacity of the native molecule, suggesting that other mechanisms might be at work. Interestingly, iron saturation of lactoferrin significantly decreases the efficacy of the bacteriocidal effect of lactferrin on planktonically grown bacteria in comparison to biofilm grown bacteria, thus indicating that the alternative modes of action of lactoferrin and its derivative may be better suited for treatment of the biofilm mode of growth (Ammons, Ward, Dowd et al. Citation2011).
Essential to the biofilm mode of growth is adherence to a surface. In vitro studies using the opportunistic pathogen P. aeruginosa demonstrated that lactoferrin inhibits biofilm formation and disrupts existing biofilms either by preventing lectin-dependent bacterial adhesion or stimulating bacterial motility (Singh et al. Citation2002; Lesman-Movshovich et al. Citation2003). Lactoferrin appears to inhibit bacterial adhesion to epithelial cells and intestinal mucosa and oral administration of lactoferrin to mice challenged with Escherichia coli resulted in reduced bacterial counts from the lower intestine, suggesting that lactoferrin interferes with adhesion of bacteria in vivo (Giugliano et al. Citation1995; Kawasaki et al. Citation2000; de Araujo & Giugliano Citation2001; de Oliveira et al. Citation2001). While adherence is essential to the development and survival of bacterial biofilms, this is not the only unique feature of the biofilm phenotype and therefore likely not the only target for anti-biofilm therapies (Table ).
Table 1. Antimicrobial efficacy of lactoferrin and peptide derivatives against MRSA in vitro and in vivo.
Another distinctive characteristic of biofilm growth is the production of an extracellular matrix (EPS) in which the cells are embedded. The matrix is composed primarily of polysaccharides and proteins with some species-specific variability such as alginate in P. aeruginosa biofilms. While the biofilm matrix acts as a scavenging system for trapping and concentrating essential minerals and nutrients (Carpentier & Cerf Citation1993), the matrix is also an essential component of antimicrobial resistance (Donlan & Costerton Citation2002). Therefore, the ability of an antimicrobial agent to diffuse through the bacterial biofilm is directly related to the efficacy of the molecule. In vivo, the matrix provides a barrier to immune response, including molecular modifications that interfere with immune response such as hexa-acylated and penta-acylated LPS produced by P. aeruginosa (Hajjar et al. Citation2002). The generally net negative environment of the matrix also interferes with antimicrobial penetration by sequestering net positive compounds such as chlorhexidine or inhibiting the diffusion of net negatively charged molecules such as the aminoglycosides (Costerton et al. Citation1987; Gordon et al. Citation1988; Kumon et al. Citation1994). In P. aeruginosa biofilms, the production of the polyanionic exopolysaccharide alginate concentrates divalent cations, thus reducing the efficacy of aminoglycosides and tetracyclines (Dunne Citation2002). On the other hand, while bacterial biofilms consist of ∼20% cells and ∼80% matrix, the matrix is composed of ∼95% water (Lawrence et al. Citation1991). Therefore, the matrix likely only inhibits molecular diffusion when the molecule interacts directly with some component of the matrix (Stewart et al. Citation1980) or is specifically targeted by the bacteria in the biofilm mode of growth. For example, P. aeruginosa has been demonstrated to sequester antimicrobials in the periplasm through binding to periplasmic glycans, thus interfering with the ability of the antimicrobial to reach the relevant targets (Mah et al. Citation2003). The diffusion characteristics of an antimicrobial are therefore of utmost importance.
Although lactoferrin penetrates into the interior of a P. aeruginosa biofilm with relative ease (Ammons et al. Citation2009), the molecular dynamics of this process are still under investigation. Under conditions of constant temperature, pressure, and viscosity, only the size of the molecule should determine the effective diffusion rate of a solute. Indeed, initial comparison of the diffusion rates of an uncharged bead of similar size to fluorescently labeled lactoferrin indicated that the net charged character of the molecule contributed only minor inhibition of the rate of diffusion into P. aeruginosa biofilms (Tomas et al. personal communication), suggesting that charge is not an effective inhibitor of the antimicrobial activity of lactoferrin in bacterial biofilms. Because lactoferrin shifts from an open to closed structural conformation when binding iron, this likely contributes to the mechanism by which the molecule diffuses through the biofilm. Indeed, a preliminary comparison of iron-free and iron-bound lactoferrin demonstrated unique magnetic resonance NMR relaxation correlation patterns when analyzed by two-dimensional magnetic relaxation and diffusion in water and indicated that protons on the interior of the molecule likely contribute to differences in diffusion rates (Brown et al. personal communication).
Additional insight can be derived from the structure of lactoferrin. Amphipathic in character, lactoferrin contains positively charged, hydrophilic regions that can bind negatively charged segments of microbial membranes. This includes LPS in Gram-negative bacteria and lipoteichoic or teichoic acid in Gram-positive bacteria, and lactoferrin binding (Gonzalez-Chavez et al. Citation2009) and may result in damage to bacterial membranes and disruption of the bacterial biofilm. Indeed, in in vitro studies with P. aeruginosa, permeabilization of the bacterial membranes of biofilm-forming bacteria was observed (Ammons et al. Citation2009). Lactoferrin binding to the bacterial membrane may also serve more than one purpose: lactoferrin has a protease-like activity (Ling & Schryvers Citation2006), including the ability to degrade and impair the expression of surface expressed virulence factors (Ochoa et al. Citation2008) and in E. coli it has been shown to damage proteins of the type III secretory system necessary for bacterial contact and pore formation during infection (Ochoa et al. Citation2003). Whether this protease-like activity is a component of the anti-biofilm formation function of lactoferrin remains to be established, but it persists as an interesting question. While membrane permeabilization by lactoferrin may be a secondary antimicrobial mechanism to iron-chelation utilized by the native protein, derivatives of lactoferrin are likely to primarily utilize the former as mechanism of action to both inhibit and disrupt established biofilms since these molecules do not retain the iron-chelating capacity of the native protein, yet demonstrate greater antimicrobial efficacy than lactoferrin (Wakabayashi et al. Citation2003). Further structural and molecular analysis of both lactoferrin and its derivatives would give significant insight into the mechanisms by which binding to bacterial membranes and secreted factors such as LPS contributes to the antimicrobial capacity of these molecules.
Essential characteristics of the biofilm phenotype include altered metabolic states and phenotypic heterogeneity. Therefore, it is also of interest to consider how lactoferrin may affect the survivability of biofilms through changes in the central metabolism of bacteria. Stoichiometric systems biology modeling utilizing the elementary flux mode analysis of E. coli identified >10 million mathematically unique biochemical pathways when only a single substrate was considered, indicating the vast complexity of the central metabolism of even these simple organisms (Carlson Citation2007, Citation2009). The systems-based approach assesses ecologically competitive trade-offs between nutrient investment into a metabolic pathway and the efficiency of energy extraction from that investment. For example, in E. coli metabolic adaptations can be utilized to minimize the requirement for anabolic resources such as iron. However, this is at the risk of low substrate yields (Carlson & Taffs Citation2010). Biofilms contain significant heterogeneity in nutrient resources including factors that contribute to metabolic adaptation to microniches of aerobic and anaerobic metabolism (Stewart & Franklin Citation2008). This variability in metabolic investment has the potential to alter bacterial virulence and the ability to form robust biofilms (Bowden & Li Citation1997). Indeed, oxygen concentration can be directly related to expression of virulence factors (Fuchs et al. Citation2007). A comprehensive and mathematically robust analysis of how changes in iron availability in the context of the biofilm mode of growth mediate adaptations in the central metabolism of opportunistic pathogens such as P. aeruginosa and S. aureus will provide significant insight into how metabolism and virulence are correlated in the chronic wound environment, including how nutrient scavenging by iron-chelating lactoferrin contributes to these adaptations (Figure ).
Figure 2 SEM image showing an undifferentiated HL60 human monocytic cell which has come up against a wall of P. aeruginosa and S. aureus cells co-cultured in a biofilm grown in vitro.

While the metabolic character of biofilms is still under investigation, recent analyses integrating transcriptomics and proteomics have suggested that biofilms may have unique metabolic signatures when compared to planktonically cultured bacteria (Beenken et al. Citation2004; Resch et al. Citation2005, Citation2006; Gjersing et al. Citation2007; Booth et al. Citation2011), especially with regard to microanaerobic metabolism and amino acid catabolism. In S. aureus, biofilms-secreted metabolites indicate that glucose is being catabolized to pyruvate, which is then catabolized via lactate dehydrogenase, pyruvate formate lyase, and the butanediol pathway resulting in accumulation of fermentation products and acetoin (Zhu et al. Citation2007). In addition, arginine catabolism through induction of the arginine deiminase pathway and coupling of fermentation and glycolysis to both the TCA cycle and cell wall metabolism distinguish S. aureus biofilms from planktonically grown cultures (Beenken et al. Citation2004; Resch et al. Citation2005, Citation2006). The most obvious metabolic effect of lactoferrin is the scavenging of iron, an essential element in bacterial metabolism. However, analysis of P. aeruginosa biofilms suggests that nutrient deprivation is only part of the effect of lactoferrin on metabolism and that treatment of biofilms with lactoferrin results in significant changes in the expression of genes associated with metabolism (Ammons, Ward, Dowd et al. Citation2011). The question of how lactoferrin and its derivatives mediate changes in the central metabolism of bacteria both dependent and independent of iron-chelation is currently under investigation. Further and more comprehensive analyses of the effects of lactoferrin on bacterial biofilm are likely to include investigating the importance and the effect of disrupting amino acid catabolism, especially the arginine–citrulline metabolism important at the site of infection (Sitaram Citation2006). Multiple functions in the host have been well established for lactoferrin. However, an understanding of the versatile nature of this complex and the anti-biofilm property of this protein during bacterial infection is at a very early stage.
Biotherapeutic applications of lactoferrin
Antimicrobial peptides such as lactoferrin and its derivatives have been of great interest as an alternative class of antibiotics (Zasloff Citation2002). Antimicrobial peptides are an area of increasing interest to pharmaceutical companies, as unlike the antibiotics currently in use, bacteria do not seem to be very effective at developing resistance, likely because of co-evolutionary forces in the ongoing struggle between host and pathogen (Yen et al. Citation2011). Indeed, one hypothesis proposes that milk and its associated antimicrobial peptides may have originated as a disinfectant secretion onto the eggs of marsupials (Hayssen Citation1995). While antimicrobial peptides have an intrinsic safety history as innate immune molecules, their commercialization has suffered from difficulty in obtaining FDA approval for therapeutic use (Laverty et al. Citation2011). Antimicrobial peptide wound therapies have not only suffered complications in the FDA approval process, but commercialization has been further complicated by a lack of clarity from the FDA on claims for biofilms on medical devices. While the path to commercialization for these types of therapies is outside the scope of this review, lactoferrin has been granted orphan drug status in combination with hypothiocyanite by both the FDA and the European Medicines Agency for use in the treatment of cystic fibrosis. Regardless of the status of lactoferrin as a wound therapy, a long safety history for lactoferrin has been demonstrated as exemplified by lactoferrin use as a nutritional supplement.
Besides its use as a nutritional supplement, lactoferrin and its antimicrobial peptide derivatives are effective for a number of clinical uses. Lactoferricin therapy is effective against infections of the urinary tract and lactoferrin has been used as an oral treatment for irritable bowel syndrome with a normalizing effect on the gut microbiome (Bellamy et al. Citation1993; Zagulski et al. Citation1998; Ha & Kornbluth Citation2010; Hu et al. Citation2012; Ochoa et al. Citation2012). In treating systemic or internal infections, lactoferrin and its derivatives have promise in part because these antimicrobial peptides retain efficacy when administered through various routes (Brouwer et al. Citation2011). While systemic treatment by lactoferrin and its derivatives is currently under development, it remains to be established clinically whether these antimicrobial peptides are effective for the treatment of localized infections in biofilm-contaminated medical devices such as knee and hip implants, a common outcome of hospital-acquired infections. Complications in designing impregnated or slow release medical devices include the susceptibility of lactoferrin to breakdown within the body. However, because the derivatives of lactoferrin retain antimicrobial efficacy, this characteristic could be advantageous if the cleaving of lactoferrin occurred at the site of the implant and thus provided extra protection from device colonization.
In chronic wounds, progression towards the normal wound healing process is halted at the pro-inflammatory stage. Whether this chronic inflammation is a response of the host to the infection or whether the chronic inflammation aids the colonization of the bacterial biofilm remains an area of intense research. However, clinically, it is of great importance to distinguish between aseptic inflammation and inflammation associated with an infective bacterial bioburden (Gemmel et al. Citation2009). While aseptic inflammation will likely progress towards resolution, the presence of contaminating bacteria in an environment of inflammation inhibits the ability of the immune system to resolve the normal immune response. For example, bacterial biofilm on an implanted device is recognized by phagocytic cells. However, these cells appear unable to engulf the bacteria and exhibit ‘frustrated phagocytosis’ in which the phagocytic cell continuously deposits pro-inflammatory proteins and small molecules into the environment resulting in secondary damage to the host tissue (Stoodley et al. Citation2002; Archer et al. Citation2011).
In the wound, autologous lactoferrin comes from degranulating neutrophils that have migrated to the wound bed and from the exudate within the wound. The concentration of lactoferrin in the wound bed likely varies significantly from patient to patient as multiple factors come into play. Lactoferrin is stored in the specific (or secondary) granules of neutrophils and during degranulation is released at the site of infection. Although neutrophils appear to be recruited to the chronic wound site in disproportionate numbers (Fazli et al. Citation2011), whether degranulation continues to occur and in what concentrations the contents of the granules, including lactoferrin, are released into the environment remains to be established (Table ).
Table 2. Selection of clinical trials using lactoferrin and/or peptide derivatives.
The concentration of lactoferrin in the wound exudate may also vary depending on a number of environmental factors. In primates, lactoferrin concentration in the milk can exceed 200 mg l−1, with concentrations up to 7 g l−1 in human colostrum (Suzuki et al. Citation2005). However, the blood concentration of lactoferrin is much lower and maybe significantly less in wound exudate. Lactoferrin is likely to be elevated in the inflammatory environment of the chronic wound. Indeed, when assaying arthritic exudates, the average concentration of lactoferrin exceeded 9 mg l−1 compared to the non-arthritic, average concentration of 3.3 mg l−1, regardless of the concentration of leukocytes in the exudate (Malmquist et al. Citation1977). However, the chronic wound environment is complicated by co-morbidity issues such as patient age and immunostatus. Regardless of this, the autologous concentration of lactoferrin is significantly less than the therapeutic level of 20 g l−1 used both in vitro and in vivo (Wolcott & Rhoads Citation2008; Ammons, Ward, Dowd et al. 2011; Ammons, Ward, James Citation2011). It is possible that the autologous lactoferrin still plays an important role in the wound environment as subinhibitory concentrations of lactoferrin (20 μg ml−1) induce swarming in cultures of P. aeruginosa and thus might help healing by dispersing the biofilm (Singh et al. Citation2002).
Because lactoferrin binds bacterial membrane components, specifically LPS, lipoteichoic acid, and teichoic acid, technetium-99m-labeled lactoferrin derivatives have been developed for deducing whether an internal site of inflammation is accompanied by bacterial infection (Welling et al. Citation2000). In vivo analysis confirmed that technetium-99m-labeled lactoferrin peptide could localize and visualize methicilin-resistant S. aureus (MRSA) infection in the thigh muscle of immunocompromised mice within 15 min of administration (Brouwer et al. Citation2008). While neither CT nor MRI can detect a localized microbial bioburden in colonized medical devices or pathogenic biofilms adhered to an internal tissue surface, the addition of radiolabeled lactoferrin derivatives to the current set of clinical techniques may well prove to be an effective prognostic tool. Utilizing this tool to successfully identify whether a site of inflammation indicates an aseptic host rejection of a medical device or indicates biofilm contamination of an implanted device can be envisaged. The treatment regime and prognostic outcome could then be appropriately tailored.
Finally, lactoferrin and its antimicrobial peptide derivatives have been shown to enhance the efficacy of clinically relevant agents. Within the current set of antimicrobial tools, none have been completely unaffected by the development of antibiotic resistance. Indeed, 50–60% of hospital-acquired infections are likely the result of microbes that have developed antibiotic resistance, demanding the development of synergistic tools which are not vulnerable to the development of resistance (Jones Citation2001). Lactoferrin has successfully been utilized in combination with antibacterials for treating mammary gland infections originating from antibiotic resistant pathogens (Lacasse et al. Citation2008), and infections with E. coli (Sanchez & Watts Citation1999; Chen et al. Citation2004). Clinically, lactoferrin and peptide derivatives have been successfully used in combination with antibiotic resistant Helicobacter pylori and hepatitis C infections (Kaito et al. Citation2007; Zullo et al. Citation2007). In addition, lactoferrin and its peptide derivatives have been used in combination with anti-fungal agents against clinically relevant Candida species in vitro (Wakabayashi et al. Citation1996; Lupetti et al. Citation2003; Naidu et al. Citation2004; Venkatesh & Rong Citation2008; Harris & Coote Citation2010).
Lactoferrin has been shown to be synergistically efficacious both in vitro and in vivo in combination with another bioinspired, anti-biofilm therapy, xylitol (Ammons et al. Citation2009; Ammons, Ward, Dowd et al. Citation2011; Ammons, Ward, James Citation2011). A rare sugar alcohol, xylitol is taken up by bacteria, but cannot be metabolized (Granstrom et al. Citation2007). In vitro analysis of combined treatment with xylitol/lactoferrin of the opportunistic pathogen P. aeruginosa suggested that xylitol and lactoferrin act synergistically against P. aeruginosa grown as a biofilm. While lactoferrin destabilizes the membrane, allowing xylitol to cross the bacterial membrane more effectively, xylitol inhibits the expression of the pyochelin pathway, which, as described above, directly competes with lactoferrin for bioavailable iron (Ammons, Ward, Dowd et al. Citation2011). Furthermore, in vitro challenge of P. aeruginosa biofilm with a hydrogel formulated with lactoferrin and xylitol demonstrated significantly improved antimicrobial efficacy of commercially available silver wound dressings (Ammons, Ward, James 2011). The efficacy of this multipronged approach to treating bacterial biofilm in vitro has further been shown to be effective in the clinic (Wolcott & Rhoads Citation2008). Such multitargeted approaches are mostly likely to succeed at treating pathogenic biofilms, given the heterogeneous phenotypic character of such biofilms.
With an estimated 2% of the population of the US suffering from chronic, non-healing wounds and estimates of upwards of 350 million people expected to develop diabetes by 2050, the problem of how to target bacterial biofilm effectively cannot be over estimated (Gottrup Citation2004; Wild et al. Citation2004). While significant progress has been made in establishing the characteristics (and thus potential vulnerabilities) of bacterial biofilms, much remains to be investigated, most notably in understanding the metabolic phenotype of the biofilm, including unique and prognostic metabolite biosignatures. With the development of bioinspired therapeutics such as lactoferrin, the evolving set of medical tools will have the ability to combat an expanding problem of biofilm-associated diseases.
Acknowledgments
The authors thank Dr Kelly Kirker at the Center for Biofilm Engineering for her assistance acquiring the SEM images. Dr Ammons is supported by the National Institute of General Medicine Sciences of the National Institutes of Health under award number K01GM103821 as well as an ARRA supplement grant to the CoBRE ‘Center for the Analysis of Cellular Mechanisms and Systems Biology’ (grant number 3P20RR024237-02S1).
References
- Ammons , MC , Ward , LS , Dowd , S and James , GA . 2011 . Combined treatment of Pseudomonas aeruginosa biofilm with lactoferrin and xylitol inhibits the ability of bacteria to respond to damage resulting from lactoferrin iron chelation . Int J Antimicrob Agents , 37 : 316 – 323 .
- Ammons , MC , Ward , LS , Fisher , ST , Wolcott , RD and James , GA . 2009 . In vitro susceptibility of established biofilms composed of a clinical wound isolate of Pseudomonas aeruginosa treated with lactoferrin and xylitol . Int J Antimicrob Agents , 33 : 230 – 236 .
- Ammons , MC , Ward , LS and James , GA . 2011 . Anti-biofilm efficacy of a lactoferrin/xylitol wound hydrogel used in combination with silver wound dressings . Int Wound J , 8 : 268 – 273 .
- Andersen , JH , Osbakk , SA , Vorland , LH , Traavik , T and Gutteberg , TJ . 2001 . Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts . Antiviral Res , 51 : 141 – 149 .
- Anderson , BF , Baker , HM , Dodson , EJ , Norris , GE , Rumball , SV , Waters , JM and Baker , EN . 1987 . Structure of human lactoferrin at 3.2-A resolution . Proc Natl Acad Sci USA , 84 : 1769 – 1773 .
- Appelmelk , BJ , An , YQ , Geerts , M , Thijs , BG , de Boer , HA , MacLaren , DM , de Graaff , J and Nuijens , JH . 1994 . Lactoferrin is a lipid A-binding protein . Infect Immun , 62 : 2628 – 2632 .
- Archer , NK , Mazaitis , MJ , Costerton , JW , Leid , JG , Powers , ME and Shirtliff , ME . 2011 . Staphylococcus aureus biofilms: properties, regulation, and roles in human disease . Virulence , 2 : 445 – 459 .
- Baker , HM and Baker , EN . 2012 . A structural perspective on lactoferrin function . Biochem Cell Biol , 90 : 320 – 328 .
- Banin , E , Vasil , ML and Greenberg , EP . 2005 . Iron and Pseudomonas aeruginosa biofilm formation . Proc Natl Acad Sci USA , 102 : 11076 – 11081 .
- Beasley , FC , Marolda , CL , Cheung , J , Buac , S and Heinrichs , DE . 2011 . Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by Staphyloferrin A, Staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence . Infect Immun , 79 : 2345 – 2355 .
- Beenken , KE , Dunman , PM , McAleese , F , Macapagal , D , Murphy , E , Projan , SJ , Blevins , JS and Smeltzer , MS . 2004 . Global gene expression in Staphylococcus aureus biofilms . J Bacteriol , 186 : 4665 – 4684 .
- Bellamy , W , Takase , M , Wakabayashi , H , Kawase , K and Tomita , M . 1992 . Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin . J Appl Bacteriol , 73 : 472 – 479 .
- Bellamy , W , Takase , M , Yamauchi , K , Wakabayashi , H , Kawase , K and Tomita , M . 1992 . Identification of the bactericidal domain of lactoferrin . Biochem Biophys Acta , 1121 : 130 – 136 .
- Bellamy , W , Wakabayashi , H , Takase , M , Kawase , K , Shimamura , S and Tomita , M . 1993 . Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin . Med Microbiol Immunol , 182 : 97 – 105 .
- Bjarnsholt , T , Kirketerp-Moller , K , Jensen , PO , Madsen , KG , Phipps , R , Krogfelt , K , Hoiby , N and Givskov , M . 2008 . Why chronic wounds will not heal: a novel hypothesis . Wound Repair Regen , 16 : 2 – 10 .
- Bolscher , J , Nazmi , K , van Marle , J , van’t Hof , W and Veerman , E . 2012 . Chimerization of lactoferricin and lactoferrampin peptides strongly potentiates the killing activity against Candida albicans . Biochem Cell Biol , 90 : 378 – 388 .
- Booth , SC , Workentine , ML , Wen , J , Shaykhutdinov , R , Vogel , HJ , Ceri , H , Turner , RJ and Weljie , AM . 2011 . Differences in metabolism between the biofilm and planktonic response to metal stress . J Proteome Res , 10 : 3190 – 3199 .
- Boucher , HW , Talbot , GH , Bradley , JS , Edwards , JE , Gilbert , D , Rice , LB , Scheld , M , Spellberg , B and Bartlett , J . 2009 . Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America . Clin Infect Dis , 48 : 1 – 12 .
- Bowden , GH and Li , YH . 1997 . Nutritional influences on biofilm development . Adv Dent Res , 11 : 81 – 99 .
- Brock , J . 1995 . Lactoferrin: a multifunctional immunoregulatory protein? . Immunol Today , 16 : 417 – 419 .
- Brouwer , CP , Rahman , M and Welling , MM . 2011 . Discovery and development of a synthetic peptide derived from lactoferrin for clinical use . Peptides , 32 : 1953 – 1963 .
- Brouwer , CP , Wulferink , M and Welling , MM . 2008 . The pharmacology of radiolabeled cationic antimicrobial peptides . J Pharm Sci , 97 : 1633 – 1651 .
- Carlson , RP . 2007 . Metabolic systems cost-benefit analysis for interpreting network structure and regulation . Bioinformatics , 23 : 1258 – 1264 .
- Carlson , RP . 2009 . Decomposition of complex microbial behaviors into resource-based stress responses . Bioinformatics , 25 : 90 – 97 .
- Carlson , RP and Taffs , RL . 2010 . Molecular-level tradeoffs and metabolic adaptation to simultaneous stressors . Curr Opin Biotechnol , 21 : 670 – 676 .
- Carpentier , B and Cerf , O . 1993 . Biofilms and their consequences, with particular reference to hygiene in the food industry . J Appl Bacteriol , 75 : 499 – 511 .
- Chen , PW , Ho , SP , Shyu , CL and Mao , FC . 2004 . Effects of bovine lactoferrin hydrolysate on the in vitro antimicrobial susceptibility of Escherichia coli strains isolated from baby pigs . Am J Vet Res , 65 : 131 – 137 .
- Cohen , MS , Mao , J , Rasmussen , GT , Serody , JS and Britigan , BE . 1992 . Interaction of lactoferrin and lipopolysaccharide (LPS): effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation . J Infect Dis , 166 : 1375 – 1378 .
- Costerton , JW , Cheng , KJ , Geesey , GG , Ladd , TI , Nickel , JC , Dasgupta , M and Marrie , TJ . 1987 . Bacterial biofilms in nature and disease . Annu Rev Microbiol , 41 : 435 – 464 .
- Costerton , JW , Stewart , PS and Greenberg , EP . 1999 . Bacterial biofilms: a common cause of persistent infections . Science , 284 : 1318 – 1322 .
- Crosa , JH . 1989 . Genetics and molecular biology of siderophore-mediated iron transport in bacteria . Microbiol Rev , 53 : 517 – 530 .
- Dathe , M and Wieprecht , T . 1999 . Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells . Biochim Biophys Acta , 1462 : 71 – 87 .
- Davey , ME and O’Toole , GA . 2000 . Microbial biofilms: from ecology to molecular genetics . Microbiol Mol Biol Rev , 64 : 847 – 867 .
- Davies , D . 2003 . Understanding biofilm resistance to antibacterial agents . Nat Rev Drug Discov , 2 : 114 – 122 .
- Davis , SC , Ricotti , C , Cazzaniga , A , Welsh , E , Eaglstein , WH and Mertz , PM . 2008 . Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo . Wound Repair Regen , 16 : 23 – 29 .
- de Araujo , AN and Giugliano , LG . 2001 . Lactoferrin and free secretory component of human milk inhibit the adhesion of enteropathogenic Escherichia coli to HeLa cells . BMC Microbiol , 1 : 25
- de Oliveira , IR , de Araujo , AN , Bao , SN and Giugliano , LG . 2001 . Binding of lactoferrin and free secretory component to enterotoxigenic Escherichia coli . FEMS Microbiol Lett , 203 : 29 – 33 .
- Donlan , RM . 2000 . Role of biofilms in antimicrobial resistance . ASAIO J , 46 : S47 – S52 .
- Donlan , RM and Costerton , JW . 2002 . Biofilms: survival mechanisms of clinically relevant microorganisms . Clin Microbiol Rev , 15 : 167 – 193 .
- Dunne , WM Jr . 2002 . Bacterial adhesion: seen any good biofilms lately? . Clin Microbiol Rev , 15 : 155 – 166 .
- El Yazidi-Belkoura , I , Legrand , D , Nuijens , J , Slomianny , MC , van Berkel , P and Spik , G . 2001 . The binding of lactoferrin to glycosaminoglycans on enterocyte-like HT29-18-C1 cells is mediated through basic residues located in the N-terminus . Biochim Biophys Acta , 1568 : 197 – 204 .
- Farnaud , S and Evans , RW . 2003 . Lactoferrin – a multifunctional protein with antimicrobial properties . Mol Immunol , 40 : 395 – 405 .
- Fazli , M , Bjarnsholt , T , Kirketerp-Moller , K , Jorgensen , A , Andersen , CB , Givskov , M and Tolker-Nielsen , T . 2011 . Quantitative analysis of the cellular inflammatory response against biofilm bacteria in chronic wounds . Wound Repair Regen , 19 : 387 – 391 .
- Fuchs , K , Deutz , A , Kofer , J , Wagner , P and Berg , W . 2007 . Analysis of the resistance behaviour of indicator bacteria to several antibiotics in bulk milk using spatial point pattern methodology . Vet Ital , 43 : 635 – 641 .
- Fux , CA , Costerton , JW , Stewart , PS and Stoodley , P . 2005 . Survival strategies of infectious biofilms . Trends Microbiol , 13 : 34 – 40 .
- Gemmel , F , Dumarey , N and Welling , M . 2009 . Future diagnostic agents . Semin Nucl Med , 39 : 11 – 26 .
- Giugliano , LG , Ribeiro , ST , Vainstein , MH and Ulhoa , CJ . 1995 . Free secretory component and lactoferrin of human milk inhibit the adhesion of enterotoxigenic Escherichia coli . J Med Microbiol , 42 : 3 – 9 .
- Gjersing , EL , Herberg , JL , Horn , J , Schaldach , CM and Maxwell , RS . 2007 . NMR metabolomics of planktonic and biofilm modes of growth in Pseudomonas aeruginosa . Anal Chem , 79 : 8037 – 8045 .
- Gonzalez-Chavez , SA , Arevalo-Gallegos , S and Rascon-Cruz , Q . 2009 . Lactoferrin: structure, function and applications . Int J Antimicrob Agents , 33 : e301 – 308 .
- Gordon , CA , Hodges , NA and Marriott , C . 1988 . Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa . J Antimicrob Chemother , 22 : 667 – 674 .
- Gottrup , F . 2004 . A specialized wound-healing center concept: importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds . Am J Surg , 187 : 38S – 43S .
- Granstrom , TB , Izumori , K and Leisola , M . 2007 . A rare sugar xylitol. Part II: biotechnological production and future applications of xylitol . Appl Microbiol Biotechnol , 74 : 273 – 276 .
- Grigg , JC , Cooper , JD , Cheung , J , Heinrichs , DE and Murphy , ME . 2010 . The Staphylococcus aureus siderophore receptor HtsA undergoes localized conformational changes to enclose staphyloferrin A in an arginine-rich binding pocket . J Biol Chem , 285 : 11162 – 11171 .
- Ha C, Kornbluth A. 2010. Mucosal healing in inflammatory bowel disease: where do we stand? Curr Gastroenterol Rep. 12:471–478.
- Hajjar , AM , Ernst , RK , Tsai , JH , Wilson , CB and Miller , SI . 2002 . Human toll-like receptor 4 recognizes host-specific LPS modifications . Nat Immunol , 3 : 354 – 359 .
- Haney , EF , Nazmi , K , Bolscher , JG and Vogel , HJ . 2012 . Structural and biophysical characterization of an antimicrobial peptide chimera comprised of lactoferricin and lactoferrampin . Biochim Biophys Acta , 1818 : 762 – 775 .
- Harris , MR and Coote , PJ . 2010 . Combination of caspofungin or anidulafungin with antimicrobial peptides results in potent synergistic killing of Candida albicans and Candida glabrata in vitro . Int J Antimicrob Agents , 35 : 347 – 356 .
- Harrison-Balestra , C , Cazzaniga , AL , Davis , SC and Mertz , PM . 2003 . A wound-isolated Pseudomonas aeruginosa grows a biofilm in vitro within 10 hours and is visualized by light microscopy . Dermatol Surg , 29 : 631 – 635 .
- Hayssen , V . 1995 . Milk: it does a baby good . Natural History , 104 : 36
- He , J and Furmanski , P . 1995 . Sequence specificity and transcriptional activation in the binding of lactoferrin to DNA . Nature , 373 : 721 – 724 .
- Hu , W , Zhao , J , Wang , J , Yu , T , Wang , J and Li , N . 2012 . Transgenic milk containing recombinant human lactoferrin modulates the intestinal flora in piglets . Biochem Cell Biol , 90 : 485 – 496 .
- Hwang , PM , Zhou , N , Shan , X , Arrowsmith , CH and Vogel , HJ . 1998 . Three-dimensional solution structure of lactoferricin B, an antimicrobial peptide derived from bovine lactoferrin . Biochemistry , 37 : 4288 – 4298 .
- James , GA , Swogger , E , Wolcott , R , Pulcini , E , Secor , P , Sestrich , J , Costerton , JW and Stewart , PS . 2008 . Biofilms in chronic wounds . Wound Repair Regen , 16 : 37 – 44 .
- Jones , EM , Smart , A , Bloomberg , G , Burgess , L and Millar , MR . 1994 . Lactoferricin, a new antimicrobial peptide . J Appl Bacteriol , 77 : 208 – 214 .
- Jones , RN . 2001 . Resistance patterns among nosocomial pathogens: trends over the past few years . Chest , 119 ( Suppl ) : 397S – 404S .
- Kaito , M , Iwasa , M , Fujita , N , Kobayashi , Y , Kojima , Y , Ikoma , J , Imoto , I , Adachi , Y , Hamano , H and Yamauchi , K . 2007 . Effect of lactoferrin in patients with chronic hepatitis C: combination therapy with interferon and ribavirin . J Gastroenterol Hepatol , 22 : 1894 – 1897 .
- Kanyshkova , TG , Semenov , DV , Buneva , VN and Nevinsky , GA . 1999 . Human milk lactoferrin binds two DNA molecules with different affinities . FEBS Lett , 451 : 235 – 237 .
- Kawasaki , Y , Tazume , S , Shimizu , K , Matsuzawa , H , Dosako , S , Isoda , H , Tsukiji , M , Fujimura , R , Muranaka , Y and Isihida , H . 2000 . Inhibitory effects of bovine lactoferrin on the adherence of enterotoxigenic Escherichia coli to host cells . Biosci Biotechnol Biochem , 64 : 348 – 354 .
- Klein , G , Imreh , S and Zabarovsky , ER . 2007 . Why do we not all die of cancer at an early age? . Adv Cancer Res , 98 : 1 – 16 .
- Klevens , RM , Edwards , JR , Richards , CL Jr. , Horan , TC , Gaynes , RP , Pollock , DA and Cardo , DM . 2007 . Estimating health care-associated infections and deaths in US hospitals, 2002 . Public Health Rep , 122 : 160 – 166 .
- Kumon , H , Tomochika , K , Matunaga , T , Ogawa , M and Ohmori , H . 1994 . A sandwich cup method for the penetration assay of antimicrobial agents through Pseudomonas exopolysaccharides . Microbiol Immunol , 38 : 615 – 619 .
- Kuwata , H , Yip , TT , Tomita , M and Hutchens , TW . 1998 . Direct evidence of the generation in human stomach of an antimicrobial peptide domain (lactoferricin) from ingested lactoferrin . Biochim Biophys Acta , 1429 : 129 – 141 .
- Lacasse , P , Lauzon , K , Diarra , MS and Petitclerc , D . 2008 . Utilization of lactoferrin to fight antibiotic-resistant mammary gland pathogens . J Anim Sci , 86 ( Suppl ) : 66 – 71 .
- Lamont , IL , Martin , LW , Sims , T , Scott , A and Wallace , M . 2006 . Characterization of a gene encoding an acetylase required for pyoverdine synthesis in Pseudomonas aeruginosa . J Bacteriol , 188 : 3149 – 3152 .
- Laverty , G , Gorman , SP and Gilmore , BF . 2011 . The potential of antimicrobial peptides as biocides . Int J Mol Sci , 12 : 6566 – 6596 .
- Lawrence , JR , Korber , DR , Hoyle , BD , Costerton , JW and Caldwell , DE . 1991 . Optical sectioning of microbial biofilms . J Bacteriol , 173 : 6558 – 6567 .
- Lesman-Movshovich , E , Lerrer , B and Gilboa-Garber , N . 2003 . Blocking of Pseudomonas aeruginosa lectins by human milk glycans . Can J Microbiol , 49 : 230 – 235 .
- Lewis , K . 2001 . Riddle of biofilm resistance . Antimicrob Agents Chemother , 45 : 999 – 1007 .
- Licking , E . 1999 . Getting a grip on bacterial slime . Business Week , 13 : 98 – 100 .
- Ling , JM and Schryvers , AB . 2006 . Perspectives on interactions between lactoferrin and bacteria . Biochem Cell Biol , 84 : 275 – 281 .
- Lupetti , A , Paulusma-Annema , A , Welling , MM , Dogterom-Ballering , H , Brouwer , CP , Senesi , S , Van Dissel , JT and Nibbering , PH . 2003 . Synergistic activity of the N-terminal peptide of human lactoferrin and fluconazole against Candida species . Antimicrob Agents Chemother , 47 : 262 – 267 .
- Mah , TF , Pitts , B , Pellock , B , Walker , GC , Stewart , PS and O’Toole , GA . 2003 . A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance . Nature , 426 : 306 – 310 .
- Malmquist , J , Thorell , JI and Wolheim , FA . 1977 . Lactoferrin and lysozyme in arthritic exudates . Acta Med Scand , 202 : 313 – 318 .
- Mariller , C , Hardiville , S , Hoedt , E , Huvent , I , Pina-Canseco , S and Pierce , A . 2012 . Delta-lactoferrin, an intracellular lactoferrin isoform that acts as a transcription factor . Biochem Cell Biol , 90 : 307 – 319 .
- Masson , PL , Heremans , JF and Schonne , E . 1969 . Lactoferrin, an iron-binding protein in neutrophilic leukocytes . J Exp Med , 130 : 643 – 658 .
- Matsuzaki , K . 1999 . Why and how are peptide–lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes . Biochim Biophys Acta , 1462 : 1 – 10 .
- McGuckin M, Goldman R, Bolton L, Salcido R. 2003. The clinical relevance of microbiology in acute and chronic wounds. Adv Skin Wound Care. 16:12–23; quiz 24–15.
- Miethke , M and Marahiel , MA . 2007 . Siderophore-based iron acquisition and pathogen control . Microbiol Mol Biol Rev , 71 : 413 – 451 .
- Naidu , AS , Fowler , RS , Martinez , C , Chen , J and Tulpinski , J . 2004 . Activated lactoferrin and fluconazole synergism against Candida albicans and Candida glabrata vaginal isolates . J Reprod Med , 49 : 800 – 807 .
- Neilands , JB . 1981a . Microbial iron compounds . Annu Rev Biochem , 50 : 715 – 731 .
- Neilands , JB . 1981b . Iron absorption and transport in microorganisms . Annu Rev Nutr , 1 : 27 – 46 .
- Neilands , JB . 1995 . Siderophores: structure and function of microbial iron transport compounds . J Biol Chem , 270 : 26723 – 26726 .
- Ochoa , TJ , Chea-Woo , E , Campos , M , Pecho , I , Prada , A , McMahon , RJ and Cleary , TG . 2008 . Impact of lactoferrin supplementation on growth and prevalence of Giardia colonization in children . Clin Infect Dis , 46 : 1881 – 1883 .
- Ochoa , TJ , Noguera-Obenza , M , Ebel , F , Guzman , CA , Gomez , HF and Cleary , TG . 2003 . Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli . Infect Immun , 71 : 5149 – 5155 .
- Ochoa , TJ , Pezo , A , Cruz , K , Chea-Woo , E and Cleary , TG . 2012 . Clinical studies of lactoferrin in children . Biochem Cell Biol , 90 : 457 – 467 .
- Oseas , R , Yang , HH , Baehner , RL and Boxer , LA . 1981 . Lactoferrin: a promoter of polymorphonuclear leukocyte adhesiveness . Blood , 57 : 939 – 945 .
- Park , JH , Park , GT , Cho , IH , Sim , SM , Yang , JM and Lee , DY . 2011 . An antimicrobial protein, lactoferrin exists in the sweat: proteomic analysis of sweat . Exp Dermatol , 20 : 369 – 371 .
- Parsek , MR and Singh , PK . 2003 . Bacterial biofilms: an emerging link to disease pathogenesis . Annu Rev Microbiol , 57 : 677 – 701 .
- Peleg , AY and Hooper , DC . 2010 . Hospital-acquired infections due to gram-negative bacteria . N Engl J Med , 362 : 1804 – 1813 .
- Poole , K , Neshat , S , Krebes , K and Heinrichs , DE . 1993 . Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa . J Bacteriol , 175 : 4597 – 4604 .
- Redly , GA and Poole , K . 2005 . FpvIR control of fpvA ferric pyoverdine receptor gene expression in Pseudomonas aeruginosa: demonstration of an interaction between FpvI and FpvR and identification of mutations in each compromising this interaction . J Bacteriol , 187 : 5648 – 5657 .
- Resch , A , Leicht , S , Saric , M , Pasztor , L , Jakob , A , Gotz , F and Nordheim , A . 2006 . Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling . Proteomics , 6 : 1867 – 1877 .
- Resch , A , Rosenstein , R , Nerz , C and Gotz , F . 2005 . Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions . Appl Environ Microbiol , 71 : 2663 – 2676 .
- Sanchez , MS and Watts , JL . 1999 . Enhancement of the activity of novobiocin against Escherichia coli by lactoferrin . J Dairy Sci , 82 : 494 – 499 .
- Saponja , JA and Vogel , HJ . 1996 . Metal-ion binding properties of the transferrins: a vanadium-51 NMR study . J Inorg Biochem , 62 : 253 – 270 .
- Siebert PD, Huang BC. 1997. Identification of an alternative form of human lactoferrin mRNA that is expressed differentially in normal tissues and tumor-derived cell lines. Proc Natl Acad Sci USA. 94: 2198–2203.
- Singh , PK , Parsek , MR , Greenberg , EP and Welsh , MJ . 2002 . A component of innate immunity prevents bacterial biofilm development . Nature , 417 : 552 – 555 .
- Sitaram , N . 2006 . Antimicrobial peptides with unusual amino acid compositions and unusual structures . Curr Med Chem , 13 : 679 – 696 .
- Stewart , JA , Silimperi , D , Harris , P , Wise , NK , Fraker , TD and Kisslo , JA . 1980 . Echocardiographic documentation of vegetative lesions in infective endocarditis: clinical implications . Circulation , 61 : 374 – 380 .
- Stewart , PS and Franklin , MJ . 2008 . Physiological heterogeneity in biofilms . Nat Rev Microbiol , 6 : 199 – 210 .
- Stoodley , P , Cargo , R , Rupp , CJ , Wilson , S and Klapper , I . 2002 . Biofilm material properties as related to shear-induced deformation and detachment phenomena . J Ind Microbiol Biotechnol , 29 : 361 – 367 .
- Strom , MB , Haug , BE , Rekdal , O , Skar , ML , Stensen , W and Svendsen , JS . 2002 . Important structural features of 15-residue lactoferricin derivatives and methods for improvement of antimicrobial activity . Biochem Cell Biol , 80 : 65 – 74 .
- Suzuki , YA , Lopez , V and Lonnerdal , B . 2005 . Mammalian lactoferrin receptors: structure and function . Cell Mol Life Sci , 62 : 2560 – 2575 .
- Valenti , P , Berlutti , F , Conte , MP , Longhi , C and Seganti , L . 2004 . Lactoferrin functions: current status and perspectives . J Clin Gastroenterol , 38 ( Suppl ) : S127 – S129 .
- Valenti , P , Greco , R , Pitari , G , Rossi , P , Ajello , M , Melino , G and Antonini , G . 1999 . Apoptosis of Caco-2 intestinal cells invaded by Listeria monocytogenes: protective effect of lactoferrin . Exp Cell Res , 250 : 197 – 202 .
- van der Kraan , MI , Groenink , J , Nazmi , K , Veerman , EC , Bolscher , JG and Nieuw Amerongen , AV . 2004 . Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin . Peptides , 25 : 177 – 183 .
- van der Kraan , MI , Nazmi , K , Teeken , A , Groenink , J , van’t Hof , W , Veerman , EC , Bolscher , JG and Nieuw Amerongen , AV . 2005 . Lactoferrampin, an antimicrobial peptide of bovine lactoferrin, exerts its candidacidal activity by a cluster of positively charged residues at the C-terminus in combination with a helix-facilitating N-terminal part . Biol Chem , 386 : 137 – 142 .
- Venkatesh , MP and Rong , L . 2008 . Human recombinant lactoferrin acts synergistically with antimicrobials commonly used in neonatal practice against coagulase-negative staphylococci and Candida albicans causing neonatal sepsis . J Med Microbiol , 57 : 1113 – 1121 .
- Vettoretti , L , Plesiat , P , Muller , C , El Garch , F , Phan , G , Attree , I , Ducruix , A and Llanes , C . 2009 . Efflux unbalance in Pseudomonas aeruginosa isolates from cystic fibrosis patients . Antimicrob Agents Chemother , 53 : 1987 – 1997 .
- Wakabayashi , H , Abe , S , Okutomi , T , Tansho , S , Kawase , K and Yamaguchi , H . 1996 . Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents . Microbiol Immunol , 40 : 821 – 825 .
- Wakabayashi , H , Matsumoto , H , Hashimoto , K , Teraguchi , S , Takase , M and Hayasawa , H . 1999 . N-Acylated and D enantiomer derivatives of a nonamer core peptide of lactoferricin B showing improved antimicrobial activity . Antimicrob Agents Chemother , 43 : 1267 – 1269 .
- Wakabayashi , H , Takase , M and Tomita , M . 2003 . Lactoferricin derived from milk protein lactoferrin . Curr Pharm Des , 9 : 1277 – 1287 .
- Welling , MM , Paulusma-Annema , A , Balter , HS , Pauwels , EK and Nibbering , PH . 2000 . Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations . Eur J Nucl Med , 27 : 292 – 301 .
- Wild , S , Roglic , G , Green , A , Sicree , R and King , H . 2004 . Global prevalence of diabetes: estimates for the year 2000 and projections for 2030 . Diabetes Care , 27 : 1047 – 1053 .
- Wilderman , PJ , Vasil , AI , Johnson , Z , Wilson , MJ , Cunliffe , HE , Lamont , IL and Vasil , ML . 2001 . Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa . Infect Immun , 69 : 5385 – 5394 .
- Wolcott RD, Rhoads DD. 2008. A study of biofilm-based wound management in subjects with critical limb ischaemia. J Wound Care. 17:145–148, 150–142, 154–145.
- Xiao , R and Kisaalita , WS . 1997 . Iron acquisition from transferrin and lactoferrin by Pseudomonas aeruginosa pyoverdin . Microbiology , 143 : 2509 – 2515 .
- Yamauchi , K , Tomita , M , Giehl , TJ and Ellison , RT 3rd . 1993 . Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment . Infect Immun , 61 : 719 – 728 .
- Yen , CC , Shen , CJ , Hsu , WH , Chang , YH , Lin , HT , Chen , HL and Chen , CM . 2011 . Lactoferrin: an iron-binding antimicrobial protein against Escherichia coli infection . Biometals , 24 : 585 – 594 .
- Zagulski , T , Lipinski , P , Zagulska , A and Jarzabek , Z . 1998 . Antibacterial system generated by lactoferrin in mice in vivo is primarily a killing system . Int J Exp Pathol , 79 : 117 – 123 .
- Zasloff , M . 2002 . Antimicrobial peptides of multicellular organisms . Nature , 415 : 389 – 395 .
- Zhu , Y , Weiss , EC , Otto , M , Fey , PD , Smeltzer , MS and Somerville , GA . 2007 . Staphylococcus aureus biofilm metabolism and the influence of arginine on polysaccharide intercellular adhesin synthesis, biofilm formation, and pathogenesis . Infect Immun , 75 : 4219 – 4226 .
- Zullo , A , De Francesco , V , Scaccianoce , G , Manes , G , Efrati , C , Hassan , C , Maconi , G , Piglionica , D , Cannaviello , C Panella , C . 2007 . Helicobacter pylori eradication with either quadruple regimen with lactoferrin or levofloxacin-based triple therapy: a multicentre study . Digest Liver Dis , 39 : 806 – 810 .