 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Sulphate-reducing bacteria (SRB) are known to cause severe corrosion of steel structures in various industries, resulting in significant economic and environmental consequences. This review paper critically examines the impact of SRB-induced corrosion on steel, including the formation of SRB biofilms, the effect on different types of steel, and the various models developed to investigate this phenomenon. The role of environmental factors in SRB-induced corrosion, molecular techniques for studying SRBs, and strategies for mitigating corrosion are discussed. Additionally, the sustainability implications of SRB-induced corrosion and the potential use of alternative materials were explored. By examining the current state of knowledge on this topic, this review aims to provide a comprehensive understanding of the impact of SRB-induced corrosion on steel and identify opportunities for further research and development.
Introduction
The term microbiologically influenced corrosion (MIC) refers to a type of corrosion that occurs when microorganisms interact with metals and alloys (Little and Lee Citation2015). Discovered over 100 years ago (Gaines Citation1910), it is a widespread phenomenon that affects a variety of industries, including oil and gas, marine, aviation, and water treatment. MIC is believed to account for around 10% to 20% of corrosion (Javaherdashti Citation2011), and a study conducted by NACE International estimated it to account for US$2.5 trillion, which is equivalent to 3.4% of the global GDP in 2013 (Bowman et al. Citation2016). Despite its economic impact is significant, estimates of its cost reach billions of dollars annually.
In addition to the direct costs associated with pipeline failures, equipment damage, and safety hazards, MIC can also have indirect economic impacts. The costs of inspection and monitoring programs to detect and prevent MIC can be substantial. Furthermore, the economic impact of MIC can extend beyond individual companies and industries. The environmental consequences of MIC, such as the release of toxic metals and chemicals, can have a significant impact on ecosystems and human health, leading to additional costs related to cleanup and remediation efforts. Despite the economic impact of MIC, there are still limitations in assessing it, and obtaining globalized data in the literature is next to impossible, and most data remains central to industries based in the west, ignoring the global South (Thompson et al. Citation2022).
A bibliometric analysis of MIC in oil and gas engineering systems by Hashemi et al (Citation2018) revealed that the United States and China are the top two countries with MIC research, followed by Canada, France, and the United Kingdom (Hashemi et al. Citation2018). Traditional corrosion testing methods may not accurately detect or quantify the contribution of microbes to the corrosion process (Vinagre et al. Citation2022). Additionally, identifying the specific microbes responsible for MIC can be challenging due to the complexity and diversity of microbial communities in affected environments, and the interdisciplinary nature of the corrosion process (Núñez et al. Citation2023). Addressing these knowledge gaps is critical for effective corrosion control and infrastructure protection, emphasizing the importance of further research in this area. This includes identifying the microorganisms involved in MIC, understanding the environmental factors that influence corrosion rates, and developing methods for detecting and monitoring MIC. Additionally, there is a need for new materials and coatings that are resistant to MIC, as well as improved maintenance and inspection practices to reduce the risk of corrosion-related failures. Sulphate-reducing bacteria (SRB) are one of the important types of microorganisms responsible for corrosion. These are diverse groups of microorganisms with playing key roles in biogeochemical cycles. They significantly influence both environmental and human health. Desulfomonas pigra, recently known as Desulfovibrio piger, was first isolated in 1976 from the intestinal microbiome. Particularly, the sulfide resulting from these bacteria causes impairment of oral mucosa and intestinal cells (Kushkevych et al. Citation2021). Sulphate-reducing bacteria can be grouped into seven phylogenetic lineages, five within the bacteria and two within the Archaea. Most of the sulfate reducers belong to the twenty-three genera within the Deltaproteobacteria, followed by the Gram-positive SRB within the Clostridia (Desulfotomaculum, Desulfosporosinus, and Desulfosporomusa genera). Three lineages, Nitrospirae (Thermodesulfovibrio genus), Thermodesulfobacteria (Thermodesulfobacterium genus) and Thermodesulfobiaceae (Thermodesulfobium genus), only contain thermophilic sulphate reducers. Within the Archaea, SRB belongs to the genus Archaeoglobus in the Euryarchaeota, and to the genera Thermocladium and Caldirvirga in the Crenarchaeota (Muyzer and Stams Citation2008). One of the most notable effects of SRBs is their ability to induce corrosion in metallic structures, including steel pipelines, oil rigs, and other infrastructure. MIC caused by SRB is the most intensively investigated topic in MIC research due to its practical importance and complexity (Anandkumar et al. Citation2016) (Enning and Garrelfs Citation2014). New genus of SRBs were successively isolated and named, with the improvement of biotechnology. The Bergey’s systematic bacteriology manual, published in 1984, updated SRB to 8 genera: Desulfovibrio, Desulfomonas, Desulfobulbus, Desulfotomaculum, Desulfococcus, Desulfobacter, Desulfosarcina and Desulfonema. In 2000, with the rapid development of molecular biology, few researchers suggested that SRB could be divided into six clusters: Desulfotomaculum, Desulfobubus, Desulfobacterium, Desulfobacter, Desulfococcus-Desulfonema-Desulfosarcina, and Desulfovibrio-Desulfomicrobium. Furthermore, based on 16S rRNA gene sequence, Sulphate-reducing bacteria could be divided into four groups: Gram- negative mesophilic SRB; Gram-positive spore-forming SRB; thermophilic bacterial SRB; and thermophilic archaeal SRB (Diao et al. Citation2023).
Sulfate-reducing bacteria (SRB) reduce sulfate to sulfide in sewers, under anaerobic environments which is present in the forms of H2S, HS, and S2 in sewage. In sewers, Sulfide could accumulate to high levels, especially in networks with a long transport time. When transferred to sewer air, hydrogen sulfide causes sewer corrosion via oxidation to sulfuric acid at the inner surface of concrete pipes, odour nuisance and health hazards when emitted to the surrounding environment (Zhang et al. Citation2023). SRB is known to shorten the service life of metal. SRB can accelerate corrosion in steel rebar by producing reduced sulfur that enhances pitting (Etim et al. Citation2021). In oil and gas industries, most SRB comes from auto-tone microbiota via subsurface fluid movement in reservoirs or are introduced by drilling operations and by secondary oil recovery methods. Properties of sulfides affect the oil production, pipelines, and storage tanks, and quality of the gas and oil produced are all affected by the corrosive (Almeida et al. Citation2006). Despite this importance, the mechanisms underlying SRB-induced corrosion are not yet fully understood, and there is a need for continued research to better understand the biological, chemical, and physical factors that contribute to this phenomenon. In recent years, significant progress has been made in developing different scientific approaches (), and mathematical and mechanistic models to predict and quantify the effects of SRBs on metallic surfaces. However, many questions remain, and there is a need for continued interdisciplinary collaborations between biologists, chemists, engineers, and other experts to address the challenges posed by SRB-induced corrosion. Yet, there do not exist a lot of standards for these tests, and a wide range of test conditions are used in existing literature, making it difficult to compare results. This may unintentionally cause the selection of those specific test conditions to directly affect the outcome and interpretation of the test (Wade et al. Citation2017) instead of the actual focus of the work.
Table 1. Various measurement techniques applied to study MIC due to Sulphate reducing bacteria.
In this review, the impact of SRB-induced corrosion on steel has been examined. This is begun by discussing the biology of SRBs and their role in corrosion, followed by examining the different types of steel that are susceptible to SRB-induced corrosion and the mechanisms that underlie this process. The various mathematical and mechanistic models that have been developed to study SRB-induced corrosion along with their strengths and limitations have been discussed. Based on this, the implications of SRB-induced corrosion for sustainability have been considered. By synthesizing the existing literature and identifying gaps in our knowledge, this review aims to provide a comprehensive resource for researchers and practitioners working in the field of SRB-induced corrosion. Previous reports and reviews on SRB have focused on the mechanism of corrosion on metals. Nevertheless, the metabolic pathways and genes involved in metal degradation is not discussed in detail. Therefore, this review attempts to illuminate the metabolic mechanism of SRB, explore molecular techniques used to study SRB. The systematic review gives the important information about the computational modelling of SRB biofilms.
Microbial communities and biofilm formation
A key factor in the corrosion process is the formation of biofilms. Studies on metallic surfaces such as galvanized steel, stainless steel, and copper have confirmed that over time, SRB biofilm formation increases (Ilhan Sungur et al. Citation2010). Biofilms are complex communities of microorganisms that adhere to surfaces and secrete extracellular polymeric substances (EPS), which provide a protective matrix and enable the exchange of nutrients and signals between cells. SRBs are known to form biofilms on steel surfaces, which can lead to localized corrosion and pitting.
Corrosion of iron metals are characteristically covered with biofilms of diverse structures and functions that primarily promote degradation of the metal by oxidizing Fe0 to ferrous ion (Fe2+):
(1)
(1)
For this reaction to proceed, the electrons (e–) released from Fe0 must be moved to potential electron-accepting molecules (Xu et al. Citation2023)
Biofilm formation is generally established through several steps. The growth model used to be considered () that proposed a cyclic process occurring in a five-stage-specific and progressive manner (Aiyer et al. Citation2018). In the initial stage, planktonic cells adhere to the surface through a process called surface attachment. Following this, biofilms are constructed with the aid of EPS during the maturation phase, resulting in a well-defined architecture. In the dispersal phase, the cell exits the biofilm.
Figure 1. The original five-step model of biofilm development (Aiyer et al. Citation2018).
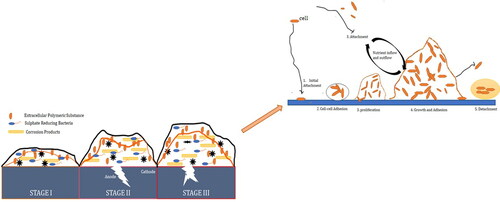
While the schematic conceptual biofilm developmental model is straightforward and has been widely used to explain biofilms, it may not fully capture the intricacies of biofilms in practical scenarios such as industrial, natural, and clinical settings.
The accumulation of biofilm is dynamic, and corrosion is initiated with changes in interface chemistry between the metal and the biofilm. Sauer et al. (Citation2022) proposed an expanded version of the model, to develop an understanding of biofilms and anti-biofilm strategies that can be tailored to the microenvironment under investigation. Once the biofilm is formed, corrosion is affected in several ways: (1) enzymes that can promote cathode reaction are produced due to electrochemical; (2) compounds that can promote (or inhibit) corrosion are produced to microbial metabolic processes; and (3) the biofilm formed on the metal surface changes its thermodynamic state (C. Wu et al. Citation2021). Thus, the dynamic flux of the interaction between various compartments results in MIC (Pal and Lavanya Citation2022). Surface properties of the metal (chemical composition, hydrophobicity, and roughness) and environmental conditions (static magnetic field and shear stress, pH, ionic strength) also play an important role (Lv et al. Citation2017).
Several studies have investigated the composition and structure of SRB biofilms and have identified a range of factors that can influence their formation and stability. These include environmental factors such as temperature, pH, and nutrient availability, as well as the presence of other microorganisms that can interact with SRBs and modify the biofilm structure. In addition, recent research has highlighted the role of quorum sensing in SRB biofilm formation. Quorum sensing is a process by which bacteria communicate with one another and coordinate their behavior through the production and detection of signaling molecules (Dobretsov et al. Citation2009). Studies have shown that SRBs use quorum sensing to regulate the formation and dispersal of biofilms and that this process can be disrupted using targeted inhibitors.
Overall, the formation and stability of SRB biofilms is a complex and dynamic process that is influenced by a range of environmental and biological factors. A better understanding of these processes is critical for developing effective strategies to prevent and mitigate SRB-induced corrosion.
The presence of SRB in biofilm may trigger or accelerate the corrosion process by creating conditions suitable for the same (Rubio et al. Citation2006). SRB on the metal surface affects the dissolution of metal through the SRB metabolites by modifying the local chemistry beneath the biofilm. This leads to intrinsic heterogeneity and causes critical localised corrosion on the metal (Dong, Shi, et al. Citation2011). SRB contribute to corrosion by using released molecular hydrogen as a substrate and inducing further hydrogen dissociation from the metal. According to Hamilton, this depolarises the cathodic region of steel, giving rise to biomineralized iron sulphides as corrosion products (Hamilton Citation2003). Consequently, SRB begin forming colonies while also attracting other molecules to bind to the metal (Dordević et al. Citation2021), inducing more corrosion.
Recent studies done on MIC induced due to SRB do not account a lot for the effects due to SRB biofilm formation. Chiefly, laboratory tests conducted attempt to simulate corrosion by SRB simulate the effects of H2S as the corrosion product and ignore the presence of biofilm and other products. Certainly, H2S plays an important role in producing corrosion products, but there are other organic sources that can enable corrosion (Zhang et al. Citation2015) through biofilm. While literature has many studies with iron as an electron donor, the use of other donors (that is more applicable to natural conditions) is not well investigated (Liu, Gu, et al. Citation2018). Eduok et al (Citation2019) made use of a general organic source instead of the conventional iron source as electron donor to understand MIC when SRB are present in limited carbon sources, as seen in oil fields. Overall, it was observed that 100% carbon source resulted in weaker biofilms, reducing corrosion (Eduok et al. Citation2019). SRB biofilms may also result in MIC pitting under it, on the material surface (Xu and Gu Citation2014). A lot of corrosion studies done on biofilms also ignore the presence of multiple species mixed in the biofilm. While studies are conducted on the mono-species and even individual strains of the bacteria, interspecies interactions are ignored (Lv et al. Citation2022). Madirisha et al. (Citation2022) thus attempted to study SRB-induced corrosion by simulating a biofilm made with materials mimicking the SRB environment (Madirisha et al. Citation2022). However, their results contradicted the role of a biofilm and attributed to the use of simulated materials. Despite of this, the corrosion rates obtained by these authors matched that of experiments that study SRB-induced MIC (Madirisha et al. Citation2022). This has resulted in difficulties elucidating mechanisms for SRB, leading to multiple mechanisms being proposed.
Metabolic pathways and SRB genes involved in corrosion
SRBs are capable of utilizing a variety of organic compounds as electron donors for energy production, including hydrocarbons, fatty acids, and alcohols (Almeida et al. Citation2006). In the absence of oxygen, SRBs utilize sulfate as a terminal electron acceptor, which results in the production of sulfide and protons. The accumulation of sulfide can lead to the formation of corrosive species such as hydrogen sulfide and iron sulfide. illustrates dissimilatory sulfate reduction in SRB. Several key genes and enzymes are involved in the metabolic pathways of SRBs, including the dissimilatory sulfate reduction pathway and the sulfide oxidation pathway (Tran et al. Citation2021). The dissimilatory sulfate reduction pathway involves the reduction of sulfate to sulfide, which is catalyzed by the enzyme adenosine-5′-phosphosulfate reductase (AprA). The sulfide oxidation pathway involves the oxidation of sulfide to sulfate, which is catalyzed by the enzyme sulfide-quinone oxidoreductase (Sqr). In addition to these core metabolic pathways, SRBs possess a range of other genes and enzymes that are involved in stress responses, biofilm formation, and other cellular processes that can impact corrosion (Tran et al. Citation2021). For example, several studies have identified genes involved in the production of extracellular polymeric substances (EPS) that can help to protect biofilms from external stresses.
Figure 2. Schematic representation of dissimilatory sulfate reduction in SRB (Tran et al. Citation2021).
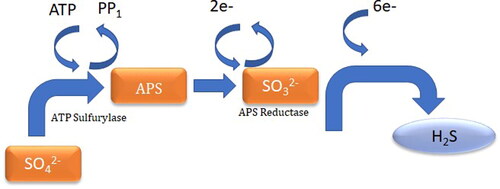
Overall, the metabolic pathways and genes involved in SRB-induced corrosion are complex and highly interconnected and are influenced by a range of environmental and biological factors. A better understanding of these processes is critical for developing effective strategies to prevent and mitigate corrosion in industrial settings.
Molecular techniques used to study SRB
Molecular techniques have played a critical role in advancing our understanding of SRBs and their role in corrosion. These techniques allow researchers to study the microbial community composition, diversity, and activity in situ and provide insight into the molecular mechanisms that underpin SRB-induced corrosion (Dobretsov and Rittschof Citation2023). One of the most commonly used molecular techniques is polymerase chain reaction (PCR), which allows researchers to amplify specific DNA fragments of interest from environmental samples. PCR can be used to detect and quantify the abundance of SRBs in a sample and to track changes in their abundance over time. Another powerful molecular tool is high-throughput sequencing, which enables the sequencing of millions of DNA fragments in a single run. This technique has revolutionized our ability to study complex microbial communities and has been used to identify and characterize the diversity of SRBs in various environments, such as marine sediments, oil reservoirs, and industrial pipelines. Other molecular techniques that have been used to study SRBs include fluorescence in situ hybridization (FISH), which allows researchers to visualize the spatial distribution of specific microorganisms in a sample, and metagenomics, which involves the sequencing of DNA extracted directly from environmental samples without prior culturing (Rao and Feser Citation2023). Overall, molecular techniques have provided valuable insights into the diversity and activity of SRBs in various environments and have helped to unravel the molecular mechanisms that underlie SRB-induced corrosion. These techniques are likely to continue to play a critical role in advancing our understanding of these microorganisms and their impact on industrial infrastructure.
Modelling and mechanisms
Traditional MIC models are based on extending many of the assumptions and principles developed for abiotic corrosion to MIC, failing to predict either the initiation or the propagation of MIC under field conditions, in all but a few cases (Aiyer et al. Citation2018). Over time, various mechanisms have been proposed to understand MIC due to SRB. These include cathodic depolarization theory (CDT), corrosive metabolites theory (CMT), biocatalytic cathodic sulfate reduction (BCSR), and direct electron transfer theory (DETT) (Chen et al. Citation2021). MIC models continue to be a field actively researched. The widely accepted MIC mechanisms of Sulphate Reducing Bacteria (SRB) are the cathodic depolarization theory (CDT) and the biocatalytic cathodic sulfate reduction (BCSR) theory (Gu et al. Citation2019).
Environmental and laboratory conditions used to investigate SRB MIC mechanisms
SRBs are one of the earliest anaerobic bacteria that evolved on the planet. As such, most of them prefer low-grade carbon sources such as volatile fatty acids. A minority of these microorganisms have undergone evolutionary changes that enable them to utilize hexoses such as glucose as both a carbon source and a source of energy. Additionally, certain species have evolved to use O2 as an electron acceptor to oxidize organic carbon (Gu et al. Citation2019). However, no growth is observed as this form of respiration is used to only maintain energy. SRB was found to grow in an open-to-air system due to the presence of aerobes and facultative microbes providing a locally oxygen-free shelter, implying a mixed-culture system (Thauer et al. Citation2007). To encourage the growth of SRBs and prevent microbial contamination, it is suggested that an environment free of oxygen be maintained. Experts in the industrial sector have emphasized the importance of keeping the dissolved O2 level below 40 ppb in the context of steel corrosion caused by CO2, as this ensures that the contribution of O2 to the corrosion process is insignificant (Thauer et al. Citation2007). This standard is generally accepted as a criterion for establishing strictly oxygen-free conditions in studies investigating abiotic CO2 corrosion (Jia et al. Citation2017). Over the years this has been proven a good practice for oxygen-free MIC investigations as well.
Mechanisms of SRB-induced corrosion
SRBs are known to induce corrosion through a complex interplay of biological, chemical, and physical processes. The exact mechanisms by which SRBs cause corrosion are still not fully understood, but several hypotheses have been proposed. One of the most widely accepted mechanisms is the production of hydrogen sulfide (H2S) by SRBs (Tran et al. Citation2021). H2S is a toxic gas that can react with iron to form iron sulfides, which are less stable and more prone to corrosion than the original metal. Additionally, SRBs produce organic acids and other metabolites that can contribute to the corrosion of metal surfaces (Zhang et al. Citation2023). Another proposed mechanism involves the formation of biofilms on metal surfaces. SRBs are known to form thick, complex biofilms that can shield the bacteria from oxygen and other antimicrobial agents, creating a microenvironment that is favorable for corrosion. The biofilm matrix also provides a surface for the accumulation of minerals and other corrosive agents. Other potential mechanisms include the production of extracellular polymeric substances (EPS), which can bind to metal surfaces and facilitate the attachment and growth of SRBs, and the formation of localized electrochemical cells that can accelerate corrosion (Beech et al. Citation2006). It is important to note that the mechanisms of SRB-induced corrosion are likely to be highly dependent on the specific environment and the type of metal involved. Therefore, further research is needed to fully understand the complex interactions between SRBs and metal surfaces and to develop effective strategies for mitigating corrosion in industrial settings.
Mathematical modelling of SRB-induced corrosion
Mathematical modelling is a powerful tool for understanding the complex processes involved in SRB-induced corrosion and for predicting the rate and extent of corrosion under different conditions (Aiyer et al. Citation2018). Several mathematical models have been developed to simulate the corrosion behaviour of metals in the presence of SRBs. One common approach is to model the corrosion process as a combination of electrochemical and biological reactions. These models typically include equations that describe the mass transport of reactants and products, the kinetics of the chemical and biological reactions, and the electrochemical behaviour of the metal surface. The parameters in these models can be adjusted to match experimental data and to predict the effect of different variables such as temperature, pH, and oxygen concentration on corrosion rates.Another approach is to use computational fluid dynamics (CFD) to simulate the fluid flow and mass transport in biofilms and to predict the distribution of corrosive agents on metal surfaces. These models can provide valuable insights into the microscale processes that govern the corrosion behaviour of SRBs and can be used to optimize the design of corrosion-resistant materials and coatings.
More recently, machine learning techniques have been applied to predict the corrosion behaviour of metals in the presence of SRBs. These models use large datasets of experimental data to train algorithms to identify patterns and relationships between different variables and to make predictions about corrosion rates and mechanisms (Anguita et al. Citation2022). Overall, mathematical modelling provides a powerful tool for understanding and predicting the complex processes involved in SRB-induced corrosion. By combining experimental data with computational models, researchers can gain a deeper understanding of the mechanisms of corrosion and develop more effective strategies for preventing and mitigating the effects of SRBs in industrial settings. Anguita et al. (Citation2022) conducted a study and developed a mechanistic mathematical model of microbial corrosion induced by SRB through EET. The research reported the effect of EET on MIC using a hybrid differential-discrete model. The model represents a SRB biofilm’s morphology and the variation in the surface topography due to MIC. The results also illustrated that the maintenance coefficient of SRB is a valuable parameter to quantify the electrons gained from iron oxidation (Anguita et al. Citation2022).
Computational modelling of SRB biofilms
Biofilms are complex, heterogeneous communities of microorganisms that play a critical role in SRB-induced corrosion (Vinagre et al. Citation2022). Computational modelling provides a powerful tool for simulating the behavior of these biofilms and predicting their impact on metal corrosion.
Early efforts on the mathematical modelling of biofilms can be traced back to the1980s. Biofilm formation is overseen by various chemical, physical principles and biological protocols and is a complex dynamical process (Zhang et al. Citation2023). Biofilm development mostly depends on the environmental conditions and properties of different types of bacteria inside the biofilm. Bacteria inside the biofilms are very resistant to antimicrobial agents leading to the limited penetration of antimicrobial agents. Mathematical modelling of biofilms is crucial in attaining a broader and deeper understanding of this complicated microorganism. The mathematical models can not only be used to corroborate experimental findings but also to make qualitative and quantitative predictions that might well serve as guidelines for experimental design (Wang and Zhang Citation2010).
One common approach to modelling biofilms is to use agent-based models (ABMs), which simulate the behavior of individual bacteria within a biofilm and the interactions between them. ABMs can provide insights into the dynamics of biofilm formation, the distribution of bacteria and corrosive agents within the biofilm, and the effect of different environmental conditions on biofilm growth and activity (Wang and Zhang Citation2010). Another approach is to use lattice-based models (LBMs), which represent the biofilm as a grid of discrete cells and simulate the transport of nutrients and metabolites within the biofilm (Aiyer et al. Citation2018). LBMs can be used to investigate the effect of different parameters such as nutrient availability and oxygen concentration on biofilm growth and activity and to predict the distribution of corrosive agents within the biofilm. More recently, multi-scale models have been developed that combine both ABMs and LBMs to simulate the behavior of biofilms at different spatial and temporal scales. These models can provide a more detailed and comprehensive understanding of the complex processes involved in biofilm formation and activity and can be used to optimize the design of materials and coatings to prevent or mitigate SRB-induced corrosion. Overall, computational modelling provides a powerful tool for understanding and predicting the behavior of SRB biofilms and their impact on metal corrosion (Wang and Zhang Citation2010). By combining computational models with experimental data, researchers can gain a deeper understanding of the mechanisms of corrosion and develop more effective strategies for preventing and mitigating the effects of SRBs in industrial settings.
Molecular mechanisms of SRB-induced corrosion
Sulphate-reducing bacteria (SRB) are known to cause significant damage to metallic structures through the process of microbial-induced corrosion (Thauer et al. Citation2007). The molecular mechanisms underlying this process involve a complex interplay of factors, including the production of corrosive metabolites, the formation of biofilms, and the expression of specific genes and proteins. One key factor in SRB-induced corrosion is the production of hydrogen sulphide (H2S) and other sulphur-containing metabolites. H2S is a highly corrosive compound that can react with metal surfaces to form sulphide compounds (Zhang et al. Citation2023), leading to corrosion and degradation of the material. SRBs are also known to produce organic acids, which can further exacerbate the corrosion process by lowering the pH of the surrounding environment. Another important factor is the formation of biofilms, which provide a protective environment for SRBs and promote the growth and activity of these bacteria. Biofilms can also act as a barrier to corrosion inhibitors and other treatment strategies, making it difficult to prevent or mitigate corrosion in industrial settings.
At the molecular level, several genes and proteins have been identified that play a role in SRB-induced corrosion. These include genes involved in sulfur metabolism, such as the dissimilatory sulphite reductase (dsr) gene, as well as genes involved in biofilm formation and quorum sensing, such as the luxI/luxR system (Dobretsov et al. Citation2009). Overall, the molecular mechanisms underlying SRB-induced corrosion are complex and multifaceted. Understanding these mechanisms is essential for developing effective strategies to prevent and mitigate the effects of SRBs in industrial settings. Recent advances in molecular techniques, such as metagenomics and proteomics, have provided new insights into the genetic and protein-based factors involved in SRB-induced corrosion, opening up new avenues for research and treatment. Predicting MIC is a challenging task because the activities of microorganisms are influenced by various biotic and abiotic factors, leading to changes in their interaction and either an increase or decrease in MIC activity over time. A predictive model that can account for the wide range of independent factors affecting the predicted corrosion rate would be more practical.
Cathodic depolarization theory (CDT)
CDT was first initiated by von Wolzogen Kühr and van der Vlugt and was further adopted by several studies on MIC (Xu and Gu Citation2014). The mechanism of cathodic depolarization is depicted in . In 1934, von Wolzogen Kühr and van der Vlugt proposed CDT after observing high corrosion rates due to SRB in the natural environment. They saw that anaerobic corrosion was due to SRB activity and proposed that SRB catalysed a depolarization reaction where atomic hydrogen from the cathode surface reacted with sulfates in the electrolyte to form sulphides per EquationEquation 5(5)
(5) below, resulting in the rate-limiting reaction of corrosion (Costello Citation1974).
Figure 3. Illustration of the cathodic depolarization mechanism of SRB activity (Lv & Du Citation2018).
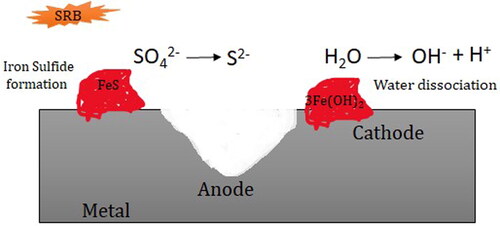
This theory contemplates hydrogen in either atomic or molecular form produced by spontaneous iron dissolution or by water dissociation reactions (reactions 2–4) is consumed by SRB for dissimilatory sulfate reduction pathway (Victoria et al. Citation2021).
(2)
(2)
(3)
(3)
(4)
(4)
The hydrogen that is generated from reaction (4) is utilized by the SRB as shown by reaction 5.
(5)
(5)
In steel, SRB are recognized as promoters of the cathodic depolarization process. This first includes the consumption of the hydrogen formed by the electrons reducing the protons on the metallic surface. Further reduction of protons is then reduced due to the hydrogen film, causing electrostatic isolation (passivation) that can prevent further corrosion. But SRB breaks this balance by the consumption of hydrogen. In recent years, the original theory and interpretation have been highly debated (Little et al. Citation2020), and instead, it seems that SRB plays a more indirect role in the mechanism of steel corrosion. This is due to the following factors (Kakooei et al. Citation2012) not being considered in the classic theory: (a) the effect of the corrosion products on the anodic reaction; (b) the effect of elemental sulfur produced on the further corrosion product; (c) changes in the environment between anaerobic and aerobic conditions; (d) the production of other corrosion products by other elements.
The theory also reports that corrosion rates for hydrogenase− and hydrogenase+ SRB are identical, which is not the case as evidenced by several studies. Further, it does not agree with the known electrochemical mechanism of hydrogen evolution on steel substrates.
EET-MIC
Extracellular Electron Transfer Microbiologically Influenced Corrosion (EET-MIC) has two important types of electron transfer: Direct electron transfer (DET) and Mediated electron transfer (MET) as depicted in (Gu et al. Citation2021).
Figure 4. Schematic representation of DET and MET in EET [Gu et al. Citation2021].
![Figure 4. Schematic representation of DET and MET in EET [Gu et al. Citation2021].](/cms/asset/77c6c396-bef8-452d-ac3d-23d28afe0c05/gbif_a_2284316_f0004_c.jpg)
DET relies on either outer membrane-bound redox-active protein such as c-cytochromes in direct contract with a conductive surface, or conductive pili (conductive nanowires). When sessile cells attach to the iron directly, the c-cytochrome is applied to transfer electrons and when sessile cells are very close to the iron surface, conductive nanowires (pili) will be secreted to link cells to the iron surface only when the SRB culture medium is deficient of organic carbon. MET utilizes soluble electron shuttles (electron mediators or electron carriers) such as H+/H2, ribofavin and favin adenine dinucleotide (FAD), which are redox-active chemicals. EET is a bottleneck for electron transfer in MIC on the macrolevel (Li and Ning Citation2019).
The Extracellular Electron Transfer Microbiologically Influenced Corrosion (EET-MIC) model is a recent approach that has been proposed to explain the underlying mechanisms of MIC. According to this model, MIC occurs when microorganisms use metal as an electron acceptor in their respiratory chain. This process involves extracellular electron transfer (EET) from the metal surface to the microorganisms, which are typically members of biofilms. The EET-MIC model proposes that the electrons are transferred through conductive nanowires or pili, which are specialized appendages that allow the microorganisms to connect with the metal surface (Gu et al. Citation2019). One key aspect of the EET-MIC model is the role of biofilms. The EET-MIC model suggests that biofilms are essential for MIC to occur, as they provide a platform for electron transfer between the metal surface and the microorganisms. The model proposes that the microorganisms in biofilms use EET as a mechanism for energy generation, in addition to traditional metabolic pathways. Another important feature of the EET-MIC model is the role of microbial diversity. The model suggests that MIC is a complex process that involves multiple microorganisms, each with its specific metabolic capabilities. The model proposes that the diversity of microorganisms in biofilms is crucial for MIC to occur, as it allows for a range of metabolic pathways that can contribute to the corrosion process (Gu et al. Citation2019). Additionally, the model suggests that the diversity of microorganisms can influence the formation and composition of biofilms, which can, in turn, impact the corrosion process.
The EET-MIC model provides a new framework for understanding the mechanisms of MIC and has important implications for corrosion control strategies. The model suggests that targeting specific microorganisms or metabolic pathways may be a promising approach for mitigating MIC. Additionally, the model emphasizes the importance of understanding the role of biofilms in MIC and the need to develop methods for controlling biofilm formation and composition. Despite the potential of the EET-MIC model, further research is needed to fully understand the underlying mechanisms of MIC and the factors that influence microbial diversity and biofilm formation. In the last few years, there has been significant progress in comprehending the mechanisms of SRB MIC due to well-planned and executed mechanistic investigations. A recent study on the modification of a gene encoding an electron mediator has provided clarity on EET in aerobic P. aeruginosa MIC against stainless steel (Jia et al. Citation2017). Therefore, it is anticipated that in the future, convincing genetic-level proof will emerge for EET-MIC caused by SRB. However, manipulating the genetics of SRBs is a more challenging task.
Extracellular polymer substance (EPS)
Beech and colleagues suggested that EPS containing iron has a positive effect on promoting the corrosion process (Beech et al. Citation2006). Since SRBs are anaerobes, they require an anaerobic environment to grow. In an open-air system, SRBs can grow beneath an aerobic biofilm that provides a locally anaerobic environment.
Sulphate Reducing Bacteria biofilm consists of proteins with minimal EPSs. Few research works have been conducted on genes and proteins on a molecular level in SRB other than Desulfovibrio vulgaris Hildenborough (DVH) and Desulfovibrio alaskensis G20 (DA-G20) (Tripathi et al. Citation2021)
Dong and colleagues (Dong, Liu, et al. Citation2011) found that low concentrations of EPS secreted by SRB could inhibit, while the high concentration of EPS enhanced the corrosion of carbon steel. The effect of EPS produced by various bacteria and archaea on metal corrosion varies considerably. Some studies have shown that certain functional groups in EPS can complex metal ions like iron and copper, thereby accelerating the anode’s dissolution and corrosion (Jin and Guan Citation2014). Conversely, other studies have found that EPS attachment has an inhibitory effect on metal corrosion. This is because EPS adsorbs onto the material’s surface and forms a protective film. The negatively charged groups in EPS chelate metal cations like Ca2+ and Mg2+ in the solution, create a dense protective film on the material surface that reduces cathodic polarization and, thus, inhibits corrosion (Stadler et al. Citation2008).
Biocatalytic cathodic Sulphate reduction (BCSR)
Traditionally, MIC studies search for a physical anode and a cathode (2009). In opposite, Gu et al. (Citation2009) proposed the BCSR theory, which suggested that the utilization of electrons from iron oxidation by sulfate reduction in SRB cells is the cause for MIC due to SRB. The mechanism is diagrammatically shown in .
Figure 5. Schematic illustration of BCSR microbially influenced corrosion mechanism (Gu et al. Citation2019).
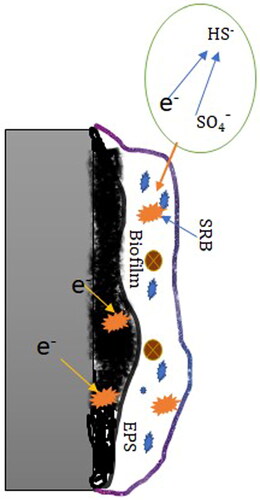
Reactions (2) and (3) make use of sulfate as the terminal electron acceptor to explain the energy production of MIC due to SRB
Anodic: Fe → Fe2+ + 2e- (3)
Cathodic: SO42- + 9H+ + 8e- → HS- + 4H2O (4)
The half-reaction in Reaction (3) shows the consumption of protons. However, there is no net consumption or generation of protons in the overall sulfate reduction pathway with organic carbon donating the electron in D. vulgaris.
It was found that upon being starved of an organic carbon source, SRB biofilms increased corrosion rates despite the starvation leading to reduced sessile cell densities on carbon steel coupons. This was due to the biofilms using to elemental iron as a substitute for organic carbon as an electron donor.
Effect of SRB on various types of steel
Sulphate-reducing bacteria (SRB) are known to cause corrosion and degradation of a wide range of metallic structures, including various types of steel (Wang et al. Citation2014). The specific effects of SRBs on different types of steel can vary depending on factors such as the chemical composition and surface properties of the metal, as well as the growth conditions and metabolic activity of the bacteria. One of the most studied types of steel for SRB-induced corrosion is carbon steel (Zhang et al. Citation2023). Carbon steel is widely used in industrial applications and is particularly susceptible to corrosion by SRBs due to its high iron content and relatively low resistance to environmental factors such as pH and temperature. In addition to carbon steel, other types of steel that are vulnerable to SRB-induced corrosion include stainless steel, low-alloy steel, and galvanized steel. The specific mechanisms underlying the effects of SRBs on different types of steel can vary depending on the type of steel and the conditions of exposure. In general, however, SRBs can cause damage to steel through a combination of direct and indirect mechanisms. Direct mechanisms may include the production of corrosive metabolites such as H2S (Zhang et al. Citation2023), while indirect mechanisms may involve the formation of biofilms and the inhibition of protective coatings and inhibitors. The effects of SRB-induced corrosion on different types of steel can have significant economic and safety implications in industrial settings. In the oil and gas industry, for example, corrosion of pipelines and other equipment due to SRBs can lead to costly repairs and shutdowns, as well as safety hazards such as leaks and explosions. Understanding the specific effects of SRBs on different types of steel is therefore critical for developing effective strategies to prevent and mitigate corrosion in these settings. MIC is a challenge across various materials and metal grades, as seen in example studies conducted on 1010 carbon steel (Wang et al. Citation2014), aluminum alloys (Rosales and Iannuzzi Citation2008), copper and copper alloys (Liu, Xu, et al. Citation2018), API 5LX70 carbon steel (Liu and Cheng Citation2017), 1018 carbon steel (Jia, Tan, et al. Citation2018), etc. In the upcoming sections, the ways MIC caused due to SRB acts on various types and grades of steel were reviewed.
Carbon steel/X70/X80
Carbon steel is one of the most widely used materials in the construction of pipelines and other industrial structures. However, its susceptibility to corrosion by SRBs has led to significant challenges in maintaining the integrity and safety of these structures (Islam and Rashed Citation2019). In recent years, researchers have focused on developing new materials with better resistance to SRB-induced corrosion. One such material is X70 steel, which has better resistance to corrosion than carbon steel. X80 steel, another high-strength steel, has even better resistance to corrosion than X70 steel. Several studies have investigated the effect of SRBs on carbon steel, X70 steel, and X80 steel. These studies have used different techniques, such as electrochemical measurements, weight loss analysis, and surface characterization, to evaluate the extent of corrosion induced by SRBs. The results of these studies have shown that the corrosion rate of carbon steel is higher than that of X70 and X80 steel. Furthermore, X80 steel is the most resistant to SRB-induced corrosion among the three types of steel. In addition to investigating the effect of SRBs on these types of steel, researchers have also developed mathematical and computational models to predict the corrosion rate of these materials under different environmental conditions (Torres and de França Citation2002). These models have been used to optimize the design of pipelines and other industrial structures, taking into account the potential for SRB-induced corrosion. Overall, the studies on carbon steel, X70 steel, and X80 steel have highlighted the importance of developing materials with better resistance to SRB-induced corrosion. The use of X70 and X80 steel has shown promise in mitigating the effects of SRB-induced corrosion in industrial applications. However, further research is needed to fully understand the mechanisms of corrosion and to develop more effective materials and mitigation strategies. presents SRB corrosion in various media and the findings.
Table 2. SRB corrosion in various materials and medium and their findings.
Carbon steel is an important material used in several industries, such as the marine industry and the petroleum industry. This is primarily due to its cost-effectiveness, toughness, strength, and efficiency (Islam and Rashed Citation2019). Despite this, carbon steel is relatively limited in its resistance to MIC, which has led to several failures and economic losses (Rios et al. Citation2015). This is also concerning the environment that carbon steel is usually used in, which is usually facilitator of EPS. Carbon steel is also relatively less resistant to MIC and is susceptible to accelerated corrosion after the initial corrosion products have formed (Javaherdashti Citation2009). Torres and De Franca observed that the concentration of oxygen directly influences the number of SRB formed in biofilms when carbon steel was exposed to seawater (Torres and de França Citation2002). In a different study by Liduino and colleagues, a similar result was observed, where the highest corrosion rates due to SRB were noted in conditions of water with high dissolved oxygen content (Liduino et al. Citation2021). Thus, the presence of SRB biofilms enables different corrosion products and altered the kinetic behavior of the system, while acting as a biocatalyst to increase the corrosion rate significantly (AlAbbas et al. Citation2013). Two popular kinds of carbon steel used are of the X70 and X80 kind. They both undergo different levels of corrosion, and studies have shown that X70 undergoes a higher rate of corrosion as compared to X80 in similar conditions. For example, Cai and colleagues conducted a comparative analysis of the effect of Desulfovibrio desulfuricans-induced corrosion behaviors in pipeline steels and found that the relative pitting severity (RPS) value of X70 was 32.52, a value much larger than the 11.64 RPS value of X80 (Cai et al. Citation2022). Through microstructure analysis, they proposed this to be due to the less uniform structure of X70 when compared to other types of steel. As carbon steel is frequently used in the marine industry, the limitation of organic carbon sources also results in the acceleration of corrosion due to SRB, confirmed by other researchers (Xu and Gu Citation2014; Dou et al. Citation2019). Xu and colleagues conducted a study on MIC of carbon steel by bacterium Desulfotomaculum nigrificans under carbon starvation conditions and found that the corrosion rate due to SRB varied between 6 to 7.7 times as that of the control (Liu et al. Citation2019). In such situations of carbon limitation, SRB may directly use the electrons of the metal for corrosion, causing a synergistic effect of carbon starvation and redox mediation on the corrosion. Guan and colleagues studied the corrosion due to Desulfovibrio singaporenus on X70 pipeline steel and found a corrosion rate increase of up to 1.87 times in case of 100% carbon starvation (Cai et al. Citation2022). Aside from the above, mild steel typically shows pitting corrosion with rates varying from 0.13 mm/year to 0.45 mm/year based on on-site studies (Jiang et al. Citation2020), with under-deposit corrosion being the main cause for further corroding of the metal.
Stainless steel (SS)
SRB has been widely identified as a major bacterial group causing corrosion in SS. Colonization of SRB on SS has been found to cause micro-pitting corrosion, by depleting iron and enriching chromium (Williams et al. Citation2010). The adhesion of SRB onto the SS surface seems to be influenced by the surface free energy of the metal, as observed by Liang et al., where SS showed more affinity towards SRB interaction as compared to hydrophobic interaction. This further leads to the use of inhibitors that decrease the hydrophobic interaction while increasing the surface free energy, to prevent SRB adhesion (Liang et al. Citation2018). Possible forms of corrosion seen in SS may be etching of the metal, pitting, and crevice attack, as reported by Antony and others on investigating the corrosion of 2205 duplex SS in a chloride medium containing SRB (Antony et al. Citation2007). This may also be influenced by pH, with increasing pH slowing down the process of corrosion of metals, as seen in a study on the corrosion behavior of 2205 duplex SS in artificial acidic seawater (Tran et al. Citation2021). Aside from the 2005 duplex SS, other grades of SS such as 316 L (Xu et al. Citation2007, Citation2008; C. Wu et al. Citation2021), 304 SS (Ismail et al. Citation1999), and SS AISI 316 (Sheng et al. Citation2007) have been studied, although to a lesser degree. presents the various types of bacteria affecting the different types of steel and the type of corrosion caused.
Table 3. Different types of corrosion caused by various bacteria on steel.
Crevice corrosion and SRB
Crevice corrosion is one of the most common types of corrosion seen in metals, especially in steel. Crevices have environments that are oxygen-free and of low pH environment, making it vulnerable to corrosion. The pH value, the oxygen content of the environment, temperature (Jakobsen and Maahn Citation2001) and geometric size of the crevice has an impact on the rate of crevice corrosion (Chang et al. Citation2000). Crevice width is also an important factor since the crevice environment changes with differences in crevice width, influencing the activity and reproductive behaviour of SRB (C. Wu et al. Citation2021). Yet, there are only a few studies conducted on the effect of SRB on SS corrosion in crevice conditions.
Mechanistic models developed
Mechanistic models are often a preferred method of predicting MIC rate because they provide straightforward predictions. However, this makes mechanistic models more rigid as they require many assumptions to reduce their complexity. Over the years, the development of mechanistic models to investigate the progression of microbiological corrosion rate has been preferred owing to their straightforward predictions (Dawuda et al. Citation2021). The modelling of corrosion loss and maximum pit depth over the short term and long term has a number of applications, including (i) enabling interpretation of on-site corrosion rates to understand the significance, (ii) estimation and prediction of corrosion rates in the future, and (iii) assessment of acceptable corrosion rates (Melchers and Jeffrey Citation2008). The development of these models would help in mitigating MIC by managing corrosion risk. A numerical model explaining SRB-induced MIC in anaerobic environments of water bodies including marine and freshwater (Al-Darbi et al. Citation2008), with CDT being the model basis was developed by Al-Darbi et al. On a similar note, Xu and others adopted the BCSR theory to develop a mechanistic model of biocorrosion due to biofilms, using charge transfer and mass transfer concepts (Xu et al. Citation2016). These models enable long-term planning and worst-case scenarios after making use of short-term pit depth data caused due to SRB, enabling the simulation of various parameters, and enabling mathematical modelling (Anguita et al. Citation2022).
In most research investigations, the prediction modes for are divided into empirical models, semi-empirical models, and mechanism models based on the type of mechanism involved and the degree of the mechanism involved (Wang et al. Citation2023). Further, with the development of information technology, data-driven models based on Machine Learning (ML) have progressively succeeded. The development history of the models can be explained by . High-throughput computing combined with ML also shows immense potential (Wang et al. Citation2023)
Figure 6. Evolution of various model types (Wang et al. Citation2023).
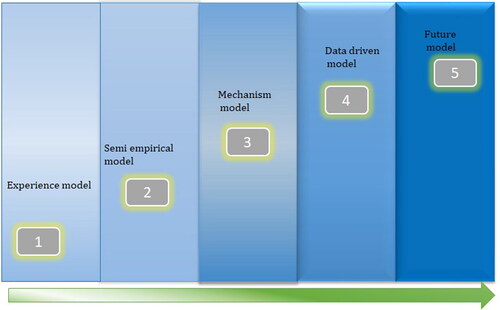
Molecular modelling techniques were also used to capture the presence and activities of microorganisms. Molecular Microbiological Methods (MMM) monitor microorganisms’ distribution and help analyze MIC risk factors and pitting corrosion rates. These calculations estimate the number of MIC microorganisms with reaction stoichiometric and electron flow (Bernt et al. Citation2012). A CDT mathematical model represents the role of SRB in pit development (Wang et al. Citation2000). This model included bacterial consumption of sulfate as a boundary condition and described pit geometry in cylindrical coordinates with rotational symmetry about the longitudinal axis (Wang et al. Citation2000). In the past decade, MIC has been increasingly recognized as a significant form of corrosion. However, detecting and predicting MIC can be challenging due to the complex behaviour of microorganisms involved. Current MIC risk assessment models consider the interdependencies of various parameters and their synergistic interactions. A data-driven approach is needed to utilize available operational and microbiological data and continuously learn as the data changes. In this study, a model is proposed to strengthen the correlation of variables and their features to assess the likelihood of MIC. The model can integrate field and laboratory data into a Learning-based Bayesian network (LBN) model (Kamil et al. Citation2021).
Conclusion
In conclusion, this review has provided a critical analysis of the impact of sulfate-reducing bacteria (SRB) induced corrosion on steel. The study revealed that SRB-induced corrosion has significant consequences for the environment and infrastructure and that the mechanisms underlying this phenomenon are complex and multifaceted. Through an examination of the available literature, it can be found that biofilm formation plays a crucial role in the corrosion process and different types of steel exhibit varying degrees of susceptibility to SRB-induced corrosion. Furthermore, this review has presented several different mathematical and mechanistic models used to study SRB-induced corrosion and discussed their strengths and limitations. Although there is still much to learn about the mechanisms of SRB-induced corrosion, these models have proven to be valuable tools for understanding and predicting the behavior of SRBs in different environments.
Looking ahead, further research is needed to refine our understanding of the biological, chemical, and physical factors that contribute to SRB-induced corrosion, and to develop new methods for detecting and preventing this phenomenon. These efforts will require interdisciplinary collaborations between biologists, chemists, engineers, and other experts, and will be crucial for ensuring the long-term sustainability of our infrastructure and the environment. In summary, this review highlights the importance of continued research into SRB-induced corrosion and provides a valuable resource for researchers and practitioners working in this area. By deepening the understanding of this phenomenon and its underlying mechanisms, the challenges posed by SRB-induced corrosion can be addressed and effective strategies for mitigating its impact can be developed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aiyer KS, Vijayakumar BS, Vishwanathan AS. 2018. The enigma of biofilms. Curr Sci. 115:204–205. doi:10.18520/cs/v115/i2/204-205.
- AlAbbas FM, Williamson C, Bhola SM, Spear JR, Olson DL, Mishra B, Kakpovbia AE. 2013. Influence of sulfate reducing bacterial biofilm on corrosion behavior of low-alloy, high-strength steel (API-5L X80). Int Biodeterior Biodegradation. 78:34–42. doi:10.1016/j.ibiod.2012.10.014.
- Al-Darbi MM, Agha K, Islam MR. 2008. Comprehensive modelling of the pitting biocorrosion of steel. Can J Chem Eng. 83:872–881. doi:10.1002/cjce.5450830509.
- Almeida PF, Almeida RC, Carvalho EB, Ramos-de-Souza E, Carvalho AS, Silva CHTP, Taft CA. 2006. Overview of sulfate-reducing bacteria and strategies to control biosulfide generation in oil waters. In: Taft CA, editor. Modern biotechnology in medicinal chemistry and industry. Research Signpost. pp. 183–195.
- Anandkumar B, George RP, Maruthamuthu S, Parvathavarthini N, Mudali UK. 2016. Corrosion characteristics of sulfate-reducing bacteria (SRB) and the role of molecular biology in SRB studies: an overview. Corr Rev. 34:41–63. doi:10.1515/CORRREV-2015-0055/ASSET/GRAPHIC/J_CORRREV-2015-0055_CV_005.JPG.
- Anguita J, Pizarro G, Vargas IT. 2022. Mathematical modelling of microbial corrosion in carbon steel due to early-biofilm formation of sulfate-reducing bacteria via extracellular electron transfer. Bioelectrochemistry. 145:108058. doi:10.1016/J.BIOELECHEM.2022.108058.
- Antony PJ, Chongdar S, Kumar P, Raman R. 2007. Corrosion of 2205 duplex stainless steel in chloride medium containing sulfate-reducing bacteria. Electrochim Acta. 52:3985–3994. doi:10.1016/j.electacta.2006.11.016.
- Beech IB, Sunner JA, Arciola CR, Cristiani P. 2006. Microbially-influenced corrosion: damage to prostheses, delight for bacteria. Int J Artif Organs. 29:443–452. doi:10.1177/039139880602900415.
- Bernt K, Uffe S, Thomsen S, Juhler S, Larsen J. 2012. Cost efficient MIC management system based on molecular microbiological methods. /NACECORR/proceedings-abstract/CORR12/All-CORR12/119789.
- Bowman E, Thompson N, Gl D, Moghissi O, Gould M, Payer J. 2016. International measure of prevention, application, and economics of corrosion technologies study. http://impact.nace.org/economic-impact.aspx.
- Cai Z, Xu J, Wei B, Sun C. 2022. A comparative study of sulfate-reducing Desulfovibrio desulfuricans induced corrosion behaviours in Q235, X65, X70, and X80 pipeline steels. Int J Press Vessels Pip. 195:104599. doi:10.1016/j.ijpvp.2021.104599.
- Chang HY, Park YS, Hwang WS. 2000. Initiation modeling of crevice corrosion in 316L stainless steels. J Mater Process Technol. 103:206–217. doi:10.1016/S0924-0136(00)00462-3.
- Chen L, Wei B, Xu X. 2021. Effect of Sulfate-Reducing Bacteria (SRB) on the corrosion of buried pipe steel in acidic soil solution. Coatings 2021, Vol 11, Page 625. 11:625. doi:10.3390/coatings11060625.
- Chesnokova MG, Shalaj VV, Kraus JA, Mironov AJ. 2015. Assessment of soil biocorrosion severeness on the pipeline locations. Procedia Eng. 113:57–61. doi:10.1016/j.proeng.2015.07.289.
- Chesnokova MG, Shalaj VV, Kraus YA, Cherkashina NV, Mironov AY. 2016. Analysis of corrosion defects on oil pipeline surface using scanning electron microscopy and soil thionic and sulfate-reducing bacteria quantification. Procedia Eng. 152:247–250. doi:10.1016/j.proeng.2016.07.698.
- Costello JA. 1974. Cathodic depolarization by sulphate-reducing bacteria. S Afr J Sci. doi:10.10520/EJC-15B61CD63D.
- Dawuda AW, Taleb-Berrouane M, Khan F. 2021. A probabilistic model to estimate microbiologically influenced corrosion rate. Process Saf Environ Protec. 148:908–926. doi:10.1016/j.psep.2021.02.006.
- De Andrade JS, Vieira MRS, Oliveira SH, de Melo Santos SK, Urtiga Filho SL. 2020. Study of microbiologically induced corrosion of 5052 aluminum alloy by sulfate-reducing bacteria in seawater. Mater Chem Phys. 241:122296. doi:10.1016/j.matchemphys.2019.122296.
- Diao C, Ye W, Yan J, Hao T, Huang L, Chen Y, Long J, Xiao T, Zhang H. 2023. Application of microbial sulfate-reduction process for sulfate-laden wastewater treatment: a review. J Water Process Eng. 52:103537. doi:10.1016/j.jwpe.2023.103537.
- Dobretsov S, Teplitski M, Paul V. 2009. Mini-review: quorum sensing in the marine environment and its relationship to biofouling. Biofouling. 25:413–427. doi:10.1080/08927010902853516.
- Dobretsov S, Rittschof D. 2023. “Omics" Techniques Used in Marine Biofouling Studies. Int J Mol Sci. 24:10518. doi:10.3390/ijms241310518.
- Dong ZH, Liu T, Liu HF. 2011. Influence of EPS isolated from thermophilic sulphate-reducing bacteria on carbon steel corrosion. Biofouling. 27:487–495. doi:10.1080/08927014.2011.584369.
- Dong ZH, Shi W, Ruan HM, Zhang GA. 2011. Heterogeneous corrosion of mild steel under SRB-biofilm characterised by electrochemical mapping technique. Corros Sci. 53:2978–2987. doi:10.1016/j.corsci.2011.05.041.
- Dordević D, Jančíková S, Vítězová M, Kushkevych I. 2021. Hydrogen sulfide toxicity in the gut environment: meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J Adv Res. 27:55–69. doi:10.1016/J.JARE.2020.03.003.
- Dou W, Liu J, Cai W, Wang D, Jia R, Chen S, Gu T. 2019. Electrochemical investigation of increased carbon steel corrosion via extracellular electron transfer by a sulfate reducing bacterium under carbon source starvation. Corros Sci. 150:258–267. doi:10.1016/j.corsci.2019.02.005.
- Eduok U, Ohaeri E, Szpunar J. 2019. Accelerated corrosion of pipeline steel in the presence of Desulfovibrio desulfuricans biofilm due to carbon source deprivation in CO2 saturated medium. Mater Sci Eng C Mater Biol Appl. 105:110095. doi:10.1016/J.MSEC.2019.110095.
- Enning D, Garrelfs J. 2014. Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl Environ Microbiol. 80:1226–1236. doi:10.1128/AEM.02848-13.
- Etim IIN, Dong J, Wei J, Nan C, Pokharel DB, Umoh AJ, Xu D, Su M, Ke W. 2021. Effect of organic silicon quaternary ammonium salts on mitigating corrosion of reinforced steel induced by SRB in mild alkaline simulated concrete pore solution. J Mater Sci Technol. 64:126–140. doi: 10.1016/j.jmst.2019.10.006.
- Gaines RH. 1910. Bacterial activity as a corrosive influence in the soil. J Ind Eng Chem. 2:128–130. doi:10.1021/IE50016A003/ASSET/IE50016A003.FP.PNG_V03.
- Gu T, Zhao K, Nesic S. 2009. A new mechanistic model for mic based on a biocatalytic cathodic sulfate reduction theory. Corrosion.
- Gu T, Jia R, Unsal T, Xu D. 2019. Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria. J Mater Sci Technol. 35:631–636. doi:10.1016/j.jmst.2018.10.026.
- Gu T, Wang D, Lekbach Y, Xu D. 2021. Extracellular electron transfer in microbial biocorrosion. Curr Opin Electrochem. 29:100763. doi:10.1016/j.coelec.2021.100763.
- Hamilton WA. 2003. Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis. Biofouling. 19:65–76. doi:10.1080/0892701021000041078.
- Hashemi SJ, Bak N, Khan F, Hawboldt K, Lefsrud L, Wolodko J. 2018. Bibliometric analysis of microbiologically influenced corrosion (MIC) of oil and gas engineering systems. Corrosion. 74:468–486. doi:10.5006/2620.
- Ilhan Sungur E, Türetgen I, Javaherdashti R, Çotuk A. 2010. Monitoring and disinfection of biofilm-associated sulfate reducing bacteria on different substrata in a simulated recirculating cooling tower system. Turk J Biol. 34:389–397. doi:10.3906/biy-0902-8.
- Islam T, Rashed HMMA. 2019. Classification and application of plain carbon steels. Module Mater Sci Mater Eng. 1–14. doi:10.1016/B978-0-12-803581-8.10268-1.
- Ismail KM, Jayaraman A, Wood TK, Earthman JC. 1999. The influence of bacteria on the passive film stability of 304 stainless steel. Electrochim Acta. 44:4685–4692. doi:10.1016/S0013-4686(99)00218-2.
- Jakobsen PT, Maahn E. 2001. Temperature and potential dependence of crevice corrosion of AISI 316 stainless steel. Corros Sci. 43:1693–1709. doi:10.1016/S0010-938X(00)00167-0.
- Javaherdashti R. 2009. A brief review of general patterns of MIC of carbon steel and biodegradation of concrete. IUFS J Biol. 65:65–73.
- Javaherdashti R. 2011. Impact of sulphate-reducing bacteria on the performance of engineering materials. Appl Microbiol Biotechnol. 91:1507–1517. doi:10.1007/S00253-011-3455-4/FIGURES/10.
- Jia R, Tan JL, Jin P, Blackwood DJ, Xu D, Gu T. 2018. Effects of biogenic H2S on the microbiologically influenced corrosion of C1018 carbon steel by sulfate reducing Desulfovibrio vulgaris biofilm. Corros Sci. 130:1–11. doi:10.1016/j.corsci.2017.10.023.
- Jia R, Yang D, Xu D, Gu T. 2017. Electron transfer mediators accelerated the microbiologically influence corrosion against carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm. Bioelectrochemistry. 118:38–46. doi:10.1016/J.BIOELECHEM.2017.06.013.
- Jia R, Yang D, Xu D, Gu T. 2018. Carbon steel biocorrosion at 80 °C by a thermophilic sulfate reducing archaeon biofilm provides evidence for its utilization of elemental iron as electron donor through extracellular electron transfer. Corros Sci. 145:47–54. doi:10.1016/j.corsci.2018.09.015.
- Jiang X, Zhang Q, Qu D, Xu K, Song X. 2020. Corrosion behavior of L360 N and L415 N mild steel in a shale gas gathering environment – Laboratory and on-site studies. J Nat Gas Sci Eng. 82:103492. doi:10.1016/j.jngse.2020.103492.
- Jin J, Guan Y. 2014. The mutual co-regulation of extracellular polymeric substances and iron ions in biocorrosion of cast iron pipes. Bioresour Technol. 169:387–394. doi:10.1016/J.BIORTECH.2014.06.059.
- Jin YT, Li JQ, Zhang MX, Zheng BR, Xu DK, Gu TY, Wang FH. 2024. Effect of exogenous flavins on the microbial corrosion by Geobacter sulfurreducens via iron-to-microbe electron transfer. J Mater Sci Technol. 171:129–138. doi:10.1016/j.jmst.2023.06.014.
- Kakooei S, Ismail C, Ariwahjoedi B. 2012. Mechanisms of microbiologically influenced corrosion: a review. World Appl Sci J. 17:524–531.
- Kamil MZ, Taleb-Berrouane M, Khan F, Amyotte P. 2021. Data-driven operational failure likelihood model for microbiologically influenced corrosion. Process. Saf. Environ. Prot. 153:472–485. doi:10.1016/j.psep.2021.07.040.
- Kushkevych I, Dordević D, Vítězová M, Rittmann SK-MR. 2021. Environmental impact of sulfate-reducing bacteria, their role in intestinal bowel diseases, and possible control by bacteriophages. Appl Sci. 11:735. doi: 10.3390/app11020735.
- Li E, Wu J, Zhang D, Wang P, Zhu L, Li C, Sun Z, Gao Y. 2022. Effect of autoinducer-2 on corrosion of Q235 carbon steel caused by sulfate reducing bacteria. Corros Sci. 200:110220. doi:10.1016/j.corsci.2022.110220.
- Li Y, Ning C. 2019. Latest research progress of marine microbiological corrosion and bio-fouling, and new approaches of marine anti-corrosion and anti-fouling. Bioact Mater. 4:189–195. doi:10.1016/j.bioactmat.2019.04.003.
- Liang R, Li J, Liu M, Huang ZY. 2018. Influence of inhibitors on the adhesion of SRB to the stainless steel in circulating cooling water. Colloids Surf B Biointerfaces. 172:1–9. doi:10.1016/J.COLSURFB.2018.08.013.
- Liao W, Yuan J, Wang X, Dai P, Feng W, Zhang Q, Fu A, Li X. 2023. Under-deposit microbial corrosion of X65 pipeline steel in the simulated shale gas production environment. Int. J. Electrochem.Sci. 18:100069. doi:10.1016/j.ijoes.2023.100069.
- Liduino V, Galvão M, Brasil S, Sérvulo E. 2021. SRB-mediated corrosion of marine submerged AISI 1020 steel under impressed current cathodic protection. Colloids Surf B Biointerfaces. 202:111701. doi:10.1016/J.COLSURFB.2021.111701.
- Liduino VS, Payão Filho JC, Cravo-Laureau C, Lutterbach MT, Camporese Sérvulo EF. 2019. Comparison of flow regimes on biocorrosion of steel pipe weldments: fluid characterization and pitting analysis. Int Biodeterior Biodegrad. 144:104750. doi:10.1016/j.ibiod.2019.104750.
- Little BJ, Hinks J, Blackwood DJ. 2020. Microbially influenced corrosion: towards an interdisciplinary perspective on mechanisms. Int Biodeterior Biodegradation. 154:105062. doi:10.1016/j.ibiod.2020.105062.
- Little BJ, Lee JS. 2015. Microbiologically influenced corrosion. In: Oil and gas pipelines: integrity and safety handbook (pp. 387–398). John Wiley & Sons. doi:10.1002/9781119019213.CH27.
- Liu F, Zhang J, Sun C, Yu Z, Hou B. 2014. The corrosion of two aluminium sacrificial anode alloys in SRB-containing sea mud. Corros Sci. 83:375–381. doi:10.1016/j.corsci.2014.03.003.
- Liu H, Meng G, Li W, Gu T, Liu H. 2019. Microbiologically influenced corrosion of carbon steel beneath a deposit in CO2-saturated formation water containing Desulfotomaculum nigrificans. Front Microbiol. 10:1298. doi:10.3389/fmicb.2019.01298.
- Liu H, Chen C, Asif M, Zhao T, Lei B, Meng G, Liu H. 2022. Mechanistic investigations of corrosion and localized corrosion of X80 steel in seawater comprising sulfate-reducing bacteria under continuous carbon starvation. Corros.Commun. 8:70–80. doi:10.1016/j.corcom.2022.08.002.
- Liu H, Gu T, Zhang G, Liu H, Cheng YF. 2018. Corrosion of X80 pipeline steel under sulfate-reducing bacterium biofilms in simulated CO2-saturated oilfield produced water with carbon source starvation. Corros Sci. 136:47–59. doi:10.1016/j.corsci.2018.02.038.
- Liu H, Xu D, Yang K, Liu H, Cheng YF. 2018. Corrosion of antibacterial Cu-bearing 316L stainless steels in the presence of sulfate reducing bacteria. Corros Sci. 132:46–55. doi:10.1016/j.corsci.2017.12.006.
- Liu T, Cheng YF. 2017. The influence of cathodic protection potential on the biofilm formation and corrosion behaviour of an X70 steel pipeline in sulfate reducing bacteria media. J Alloys Compd. 729:180–188. doi:10.1016/j.jallcom.2017.09.181.
- Lu S, He Y, Xu R, Wang N, Chen S, Dou W, Cheng X, Liu G. 2023. Inhibition of microbial extracellular electron transfer corrosion of marine structural steel with multiple alloy elements. Bioelectrochemistry. 151:108377. doi:10.1016/j.bioelechem.2023.108377.
- Lv M, Chen X, Li Z, Du M. 2021. Effect of sulfate-reducing bacteria on hydrogen permeation and stress corrosion cracking behavior of 980 high-strength steel in seawater. J Mater Sci Technol. 92:109–119. doi:10.1016/j.jmst.2021.02.039.
- Lv M, Du M, Li Z. 2022. Investigation of mixed species biofilm on corrosion of X65 steel in seawater environment. Bioelectrochemistry. 143:107951. doi:10.1016/J.BIOELECHEM.2021.107951.
- Lv Y, Zheng B, Liu H, Xiong F, Liu H, Hu Y, Lv Y, Zheng B, Liu H, Xiong F, et al. 2017. Effect of Static Magnetic Field on Adhesion of Sulfate Reducing Bacteria Biofilms on 304 Stainless Steel. J. Chin. Soc. 36:652–658. doi:10.11902/1005.4537.2016.120.
- Lv M, Du M. 2018. A review: microbiologically influenced corrosion and the effect of cathodic polarization on typical bacteria. Rev Environ Sci. 17:431–446.
- Madirisha M, Hack R, van der Meer F. 2022. Simulated microbial corrosion in oil, gas and non-volcanic geothermal energy installations: the role of biofilm on pipeline corrosion. Energy Rep. 8:2964–2975. doi:10.1016/j.egyr.2022.01.221.
- Melchers R, Jeffrey R. 2008. The critical involvement of anaerobic bacterial activity in modelling the corrosion behaviour of mild steel in marine environments. Electrochim Acta. 54:80–85. doi:10.1016/j.electacta.2008.02.107.
- Melchers RE, Wells T. 2006. Models for the anaerobic phases of marine immersion corrosion. Corros Sci. 48:1791–1811. doi:10.1016/j.corsci.2005.05.039.
- Muyzer G, Stams AJ. 2008. The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol. 6:441–454. doi: 10.1038/nrmicro1892.
- Núñez A, García AM, Ranninger C, Moreno DA. 2023. Microbiologically influenced corrosion on naval carbon steel inside the hull of tugboats: a case study of prevention and control. Biofouling. 39:257–270. doi:10.1080/08927014.2023.2209013.
- Pal MK, Lavanya M. 2022. Microbial Influenced Corrosion: understanding Bioadhesion and Biofilm Formation. J Bio Tribocorros. 8:1–13. doi:10.1007/S40735-022-00677-X/FIGURES/5.
- Qin Q, Xu J, Wei B, Fu Q, Gao L, Yu C, Sun C, Wang Z. 2021. Synergistic effect of alternating current and sulfate-reducing bacteria on corrosion behavior of X80 steel in coastal saline soil. Bioelectrochemistry. 142:107911. doi:10.1016/J.BIOELECHEM.2021.107911.
- Rajala P, Nuppunen-Puputti M, Wheat CG, Carpen L. 2022. Fluctuation in deep groundwater chemistry and microbial community and their impact on corrosion of stainless-steels. Sci Total Environ. 824:153965. doi:10.1016/j.scitotenv.2022.153965.
- Rao TS, Feser R. 2023. Biofilm formation by sulphate-reducing bacteria on different metals and their prospective role in titanium corrosion. Environ Technol. 20:1–14. doi:10.1080/09593330.2023.2178976.
- Rios EC, Zimer AM, Mendes PCD, Freitas MBJ, de Castro EVR, Mascaro LH, Pereira EC. 2015. Corrosion of AISI 1020 steel in crude oil studied by the electrochemical noise measurements. Fuel. 150:325–333. doi:10.1016/j.fuel.2015.02.022.
- Rosales BM, Iannuzzi M. 2008. Aluminium AA2024 T351 aeronautical alloy: part 1. Microbial influenced corrosion analysis. Mater. Sci. Eng: A. 472:15–25. doi:10.1016/j.msea.2007.06.079.
- Rubio C, Ott C, Amiel C, Dupont-Moral I, Travert J, Mariey L. 2006. Sulfato/thiosulfato reducing bacteria characterization by FT-IR spectroscopy: a new approach to biocorrosion control. J Microbiol Methods. 64:287–296. doi:10.1016/J.MIMET.2005.05.013.
- Sauer K, Stoodley P, Goeres DM, Hall-Stoodley L, Burmølle M, Stewart PS, Bjarnsholt T. 2022. The biofilm life cycle: expanding the conceptual model of biofilm formation. Nat Rev Microbiol. 20:608–620. doi: 10.1038/s41579-022-00767-0.
- Sheng X, Ting YP, Pehkonen SO. 2007. The influence of sulphate-reducing bacteria biofilm on the corrosion of stainless steel AISI 316. Corros Sci. 49:2159–2176. doi:10.1016/j.corsci.2006.10.040.
- Stadler R, Fuerbeth W, Harneit K, Grooters M, Woellbrink M, Sand W. 2008. First evaluation of the applicability of microbial extracellular polymeric substances for corrosion protection of metal substrates. Electrochim Acta. 54:91–99. doi:10.1016/j.electacta.2008.04.082.
- Sun D, Wang D, Li L, Gong K, Ren S, Xie F, Wu M. 2023. Study on stress corrosion behavior and mechanism of X70 pipeline steel with the combined action of sulfate-reducing bacteria and constant load. Corros Sci. 213:110968. doi:10.1016/j.corsci.2023.110968.
- Sun M, Xu W, Rong H, Chen J, Yu C. 2023. Effects of dissolved oxygen (DO) in seawater on microbial corrosion of concrete: morphology, composition, compression analysis and transportation evaluation. Constr Build Mater. 367:130290. doi:10.1016/j.conbuildmat.2023.130290.
- Thauer RK, Stackebrandt E, Hamilton AW. 2007. Energy metabolism and phylogenetic diversity of sulphate-reducing bacteria. In: Sulphate-reducing bacteria: environmental and engineered systems. Cambridge University Press. pp. 1–38. doi:10.1017/CBO9780511541490.002.
- Thompson AA, Wood JL, Palombo EA, Green WK, Wade SA. 2022. From laboratory tests to field trials: a review of cathodic protection and microbially influenced corrosion. Biofouling. 38:298–320. doi:10.1080/08927014.2022.2058395.
- Torres ES, de França FP. 2002. Kinetics of biofilm formation as a function of dissolved oxygen concentration on Aisi-1020 carbon steel coupons. Corros. Rev. 20:115–128. doi:10.1515/CORRREV.2002.20.1-2.115/MACHINEREADABLECITATION/RIS.
- Tran TTT, Kannoorpatti K, Padovan A, Thennadil S. 2021. Effect of pH regulation by sulfate-reducing bacteria on corrosion behaviour of duplex stainless steel 2205 in acidic artificial seawater. R Soc Open Sci. 8:200639. doi:10.1098/RSOS.200639.
- Tran TTT, Kannoorpatti K, Padovan A, Thennadil S. 2021. Sulphate-reducing bacteria’s response to extreme pH environments and the effect of their activities on microbial orrosion. Appl Sci. 11:2201. doi:10.3390/app11052201.
- Tripathi AK, Thakur P, Saxena P, Rauniyar S, Gopalakrishnan V, Singh RN, Gadhamshetty V, Gnimpieba EZ, Jasthi BK, Sani RK. 2021. Gene sets and mechanisms of sulfate-reducing bacteria biofilm formation and quorum sensing with impact corrosion. Front Microbiol. 12:754140. doi:10.3389/fmicb.2021.754140.
- Victoria SN, Sharma A, Manivannan R. 2021. Metal corrosion induced by microbial activity–Mechanism and control options. J Ind Chem Soc. 98:100083. doi:10.1016/j.jics.2021.100083.
- Vinagre PA, Lindén JB, Mardaras E, Pinori E, Svenson J. 2022. Probing the correlation between corrosion resistance and biofouling of thermally sprayed metallic substrata in the field. Biofouling. 38:147–161. doi:10.1080/08927014.2022.2033736.
- Wade SA, Javed MA, Palombo EA, McArthur SL, Stoddart PR. 2017. On the need for more realistic experimental conditions in laboratory-based microbiologically influenced corrosion testing. Int Biodeterior Biodegrad. 121:97–106. doi:10.1016/j.ibiod.2017.03.027.
- Wan H, Zhang T, Xu Z, Rao Z, Zhang G, Li G, Liu H. 2023. Effect of sulfate reducing bacteria on the galvanic corrosion behavior of X52 carbon steel and 2205 stainless steel bimetallic couple. Corros Sci. 212:110963. doi:10.1016/j.corsci.2023.110963.
- Wang H, Ju LK, Castaneda H, Cheng G, Zhang Newby BM. 2014. Corrosion of carbon steel C1010 in the presence of iron oxidizing bacteria Acidithiobacillus ferrooxidans. Corros Sci. 89:250–257. doi:10.1016/j.corsci.2014.09.005.
- Wang J, Huang CP, Allen HE. 2000. Characteristics of sludge particulates. Water Environ Res. 72:545–553. doi:10.2175/106143000X138120.
- Wang J, Yin S, Lu L, Zhou J, Fu Q. 2022. Characterization of microbial-induced concrete corrosion by combining morphology observation and fluorescence staining. Case Stud Constr Mater. 17:e01586. doi:10.1016/j.cscm.2022.e01586.
- Wang Q, Zhang T. 2010. Review of mathematical models for biofilms. Solid State Commun. 150:1009–1022. doi:10.1016/j.ssc.2010.01.021.
- Wang Q, Song Y, Zhang X, Dong L, Xi Y, Zeng D, Liu Q, Zhang H, Zhang Z, Yan R, et al. 2023. Evolution of corrosion prediction models for oil and gas pipelines: from empirical-driven to data-driven. Eng Fail Anal. 146:107097. doi:10.1016/j.engfailanal.2023.107097.
- Wei B, Xu J, Fu Q, Qin Q, Bai Y, Sun C, Wang C, Wang Z, Ke W. 2021. Effect of sulfate-reducing bacteria on corrosion of X80 pipeline steel under disbonded coating in a red soil solution. J Mater Sci Technol. 87:1–17. doi:10.1016/j.jmst.2020.12.076.
- Williams DE, Kilburn MR, Cliff J, Waterhouse GIN. 2010. Composition changes around sulphide inclusions in stainless steels, and implications for the initiation of pitting corrosion. Corros Sci. 52:3702–3716. doi:10.1016/j.corsci.2010.07.021.
- Wu M, Gong K, Xie F. 2021. Effect of sulfate reducing bacteria and stress on corrosion behavior of X100 steel in sea mud environment. Electroanal Chem. 886:115129. doi:10.1016/j.jelechem.2021.115129.
- Wu C, Wang Z, Zhang Z, Zhang B, Ma G, Yao Q, Gan Z, Wu J, Li X. 2021. Influence of crevice width on sulfate-reducing bacteria (SRB)-induced corrosion of stainless steel 316L. Corrosion Commun. 4:33–44. doi:10.1016/j.corcom.2021.12.001.
- Wu TQ, Yan MC, Zeng DC, Xu J, Yu CK, Sun C, Ke W. 2015. Microbiologically induced corrosion of X80 pipeline steel in a near-neutral pH soil solution. Acta Metall Sin. 28:93–102. doi:10.1007/S40195-014-0173-9/METRICS.
- Xie F, Li J, Zou T, Wang D, Wu M, Sun X. 2021. Stress corrosion cracking behaviour induced by Sulfate-reducing bacteria and cathodic protection on X80 pipeline steel. Constr Build Mater. 308:125093. doi:10.1016/j.conbuildmat.2021.125093.
- Xie F, Wang Y, Wang D, Sun D, Zhou Y, Wang Y. 2023. Influence of anion and sulfate-reducing bacteria on the stress corrosion behaviour and mechanism of X70 steel in a marine mud environment. Eng Fail Anal. 143:106834. doi:10.1016/j.engfailanal.2022.106834.
- Xu C, Zhang Y, Cheng G, Zhu W. 2007. Localized corrosion behavior of 316L stainless steel in the presence of sulfate-reducing and iron-oxidizing bacteria. Mater Sci Eng A. 443:235–241. doi:10.1016/j.msea.2006.08.110.
- Xu C, Zhang Y, Cheng G, Zhu W. 2008. Pitting corrosion behavior of 316L stainless steel in the media of sulphate-reducing and iron-oxidizing bacteria. Mater Charact. 59:245–255. doi:10.1016/j.matchar.2007.01.001.
- Xu D, Gu T. 2014. Carbon source starvation triggered more aggressive corrosion against carbon steel by the Desulfovibrio vulgaris biofilm. Int Biodeterior Biodegrad. 91:74–81. doi:10.1016/j.ibiod.2014.03.014.
- Xu D, Li Y, Gu T. 2016. Mechanistic modeling of biocorrosion caused by biofilms of sulfate reducing bacteria and acid producing bacteria. Bioelectrochemistry. 110:52–58. doi:10.1016/J.BIOELECHEM.2016.03.003.
- Xu D, Gu T, Lovley DR. 2023. Microbially mediated metal corrosion. Nat Rev Microbiol. 21:705–718. doi:10.1038/s41579-023-00920-3.
- Xu Z, Zhang T, Wan H, Liu H, Gu T, Liu H. 2023. Accelerated development of Ti-6Al-4V microbial corrosion triggered by electroactive sulfate-reducing Desulfovibrio ferrophilus biofilm in enriched artificial seawater containing soluble electron shuttle. Corros. Sci. 220:111306. doi:10.1016/j.corsci.2023.111306.
- Yang B, Lu P, An Q, Zhu C. 2023. Protective effect of silicone-modified epoxy resin coating on iron-based materials against sulfate-reducing bacteria-induced corrosion. Prog Org Coat. 174:107241. doi:10.1016/j.porgcoat.2022.107241.
- Yang J, Wang ZB, Qiao YX, Zheng YG. 2022. Synergistic effects of deposits and sulfate reducing bacteria on the corrosion of carbon steel. Corros Sci. 199:110210. doi:10.1016/j.corsci.2022.110210.
- Yang X, Shao J, Liu Z, Zhang D, Cui L, Du C, Li X. 2020. Stress-assisted microbiologically influenced corrosion mechanism of 2205 duplex stainless steel caused by sulfate-reducing bacteria. Corros Sci. 173:108746. doi:10.1016/j.corsci.2020.108746.
- Yang D, Wang N, Wang J, Jing X. 2020. Study on microbial corrosion resistance of Ni-P-Ag coatings in artificial marine environments containing sulphate-reducing bacteria. Int. J.Electrochem. Sci. 15(12): 11779–11790. doi:10.20964/2020.12.18.
- Zhang P, Xu D, Li Y, Yang K, Gu T. 2015. Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry. 101:14–21. doi:10.1016/J.BIOELECHEM.2014.06.010.
- Zhang T, Wang J, Li G, Liu H. 2021. Crevice corrosion of X80 carbon steel induced by sulfate reducing bacteria in simulated seawater. Bioelectrochemistry. 142:107933. doi:10.1016/J.BIOELECHEM.2021.107933.
- Zhang L, Qiu Y-Y, Sharma KR, Shi T, Song Y, Sun J, Liang Z, Yuan Z, Jiang F. 2023. Hydrogen sulfide control in sewer systems: critical review of recent progress. Water Res. 240:120046. doi: 10.1016/j.watres.2023.120046.
- Zhao R, Wang B, Li D, Chen Y, Zhang Q. 2022. Effect of sulfate-reducing bacteria from salt scale of water flooding pipeline on corrosion behavior of X80 steel. Eng Fail Anal. 142:106788. doi:10.1016/j.engfailanal.2022.106788.
- Zhou X, Zhou Z, Wu T, Li C, Li Z. 2021. Effects of non-viable microbial film on corrosion of pipeline steel in soil environment. Corros. Commun. 3:23–33. doi:10.1016/j.corcom.2021.11.003.
