ABSTRACT
Recent studies indicate a positive correlation between canine-assisted interventions and social and communicative abilities in people with autism. These benefits could be due to more efficient processing of socially informative areas when dog faces are processed. Using an eye tracker, this study aimed to assess the visual processing of faces in 13 children with autism spectrum disorder (ASD) and 13 neurotypical (NT) children when gazing at the faces of humans and of dogs. We divided the faces into two categories. First, individual faces of adult humans, children, and dogs were used in a free-viewing paradigm, where the area of interest was the eyes. We recorded the total time spent gazing at the eyes (dwell time), latency to the first look, and continuous gaze time. Second, pairs of faces were presented at the same time in a pair paradigm, and preferences in terms of face position (left/right) and type (dog/human), and the number of transitions between faces, were measured. When presented with pairs of faces, ASD children gazed for longer at the dog’s face, regardless of its position, and showed a higher number of shifts between pictures when the face of a dog was present. However, the NT group did not discriminate between the two faces. The results for individual faces showed significant differences in how ASD children look at the eyes of faces rather than differences in the total duration of the gaze; they are faster in terms of their first gaze and exhibit a longer average fixation time when gazing at the eyes of dogs compared with those of humans. Both human and dog faces were processed atypically in children with ASD, who seemed to engage with dogs more rapidly and for extended periods. This suggests possible socio-communicative benefits of human–dog interactions for people with autism, from a visual processing point of view.
Autism spectrum disorder (ASD) is a developmental condition with symptom onset in early childhood. A core characteristic of ASD diagnosis is a persistent deficit of socio-communicative abilities (DSM-5; APA, Citation2013). One possible explanation for these difficulties is that the processing of highly socially informative areas of others is affected, which impacts the understanding of and access to social interactions.
Visual processing of human faces in children with ASD is characterized by a lower total time and number of fixations to the eyes compared with neurotypical (NT) children (Chawarska & Shic, Citation2009; Dalton et al., 2005; Jones et al., Citation2008; Noris et al., Citation2011; Rice et al., Citation2012). These children also pay more attention to an image’s background and socially non-relevant stimuli, such as geometrical patterns and stimuli related to their specific interests, than NT children (Celani, Citation2002; Mo et al., Citation2019; Moore et al., Citation2018; Shic et al., Citation2011). Additionally, children with ASD show higher intra-subject variability in gaze patterns compared with NT children, who show stereotyped and predictable patterns (Nakano et al., Citation2010; Noris et al., Citation2012). These divergences from normal gaze development are exhibited in children as young as 6 months of age and can therefore serve as a marker of autism in early childhood (Jones & Klin, Citation2013; Klin et al., Citation2002).
The atypicality of gaze in children with ASD could negatively affect the development of social cognition, thereby hindering the interpretation of others’ emotional states and intentions, as well as complex situations and non-verbal communicative cues (Madipakkam et al., Citation2017; Matsuda et al., Citation2015; Papagiannopoulou et al., Citation2014). However, several studies have reported a positive correlation between canine-assisted interventions and improved socio-communicative skills in people with autism (Hill et al., Citation2020; London et al., Citation2020; Nieforth et al., Citation2021). It has been reported that children with ASD display more social and prosocial behaviors in the presence of an animal compared with other stimuli (Germone et al., Citation2012; Grandgeorge et al., Citation2012; Grandgeorge et al., Citation2015; O'Haire et al., Citation2013; Prothmann et al., Citation2009). Grigore and Rusu (Citation2014) found that verbal and non-verbal communication, social smiling, and visual contact skills all improved after these interventions, which were performed in conjunction with, or instead of, more traditional approaches. Against this background, we wondered whether adaptive advantages associated with the visual processing of dog faces could explain these results and therefore serve as a useful adjunct to other interventions.
Several studies found a preference for interactions with, and greater social orienting toward, an animal compared with toys and human faces (Prothmann et al., Citation2009; Valiyamattam et al., Citation2020). This could be explained by the lower complexity of animal expressions, as well as the lower demand for social and verbal interaction. It is also possible that the hormone oxytocin plays a role in this preference. Interactions with animals, especially pets, can increase the level of oxytocin in both humans and animals (Beetz et al., Citation2012; Nagasawa et al., Citation2009; Odendaal, Citation2000; Odendaal & Meintjes, Citation2003). Among other positive social effects, oxytocin increases eye contact and social visual orientation in people with autism (Domes et al., Citation2013; Strathearn et al., Citation2018). Therefore, the preference to look at animals, including at the eyes, might be due to an increase in oxytocin levels.
Another explanation for this preference is the increased attention paid to anthropomorphized nonhuman stimuli by individuals with ASD, including animals. Anthropomorphizing objects and animals increase self-efficacy in social areas and allow access to social stimuli in a less direct way than gazing directly at faces, for example (Atherton & Cross, Citation2018; Cross et al., Citation2019) Greater orientation toward the face of animals translates to increased recognition of emotions in animals (Cross et al., Citation2019; Davidson et al., Citation2019) and increased success in the theory of mind tasks (Atherton & Cross, Citation2018).
How do children look at the faces of animals? Research on gaze patterns toward nonhuman faces shows a higher total gazing time to dog eyes in both ASD and NT groups. ASD children also show a higher number of fixations to dog eyes compared with dog mouths and human faces (Grandgeorge et al., Citation2016; Muszkat et al., Citation2015; Valiyamattam et al., Citation2020; Whyte et al., Citation2016). A positive social response is also seen (i.e., more fixations in children with ASD when the eyes of the animal were gazing directly) compared with the direct human gaze, suggesting less aversion to animal eyes (Valiyamattam et al., Citation2020). However, not all animals elicit the same facial processing. Children with ASD seem to look at their pets’ faces more than do NT children and more toward their cats’ than their dogs’ faces. The higher number of fixations to their cat could be explained by the shorter gaze of cats compared with dogs toward people, which may be experienced as less invasive by children with ASD (Grandgeorge et al., Citation2020).
This study aimed to collect data about the automatic gaze patterns of children with ASD when looking at images of humans and dogs, both when they appeared alone and when they were in competition with another social stimulus. We presented two categories of faces on the screen to be recorded by an eye tracker: individual faces (human adult, child, or dog) and pairs of faces (adult and dog).
This study explored, in individual faces, the total time spent gazing at the area of the eyes, the time taken to orient the gaze toward the eyes (latency), and the average time spent exploring the area of the eyes (average continuous gaze time). For the pairs of faces presented at the same time, we were interested in how each group explored the faces overall, as well as the stimuli that they oriented toward the most. We analyzed the total gaze time according to the location of the face on the screen and the type of stimulus (human or dog). We also assessed the number of times each group shifted their visual attention from one face to another (transitions).
In line with previous research, we expected a higher gaze time (dwell time) on the eyes of dog faces in the NT group compared with the ASD group. Within the ASD group, we hypothesized that there would be a higher gaze time for dog faces compared with human faces, for both adults and children (Grandgeorge et al., Citation2016; Valiyamattam et al., Citation2020).
As children with ASD show a higher gaze time and number of fixations on animal eyes even when the gaze is direct, we expected a faster first gaze (shorter latency) in the ASD group and a longer continuous gaze on the eyes when looking at dog faces compared with human faces. We also expected differences in the average continuous gaze time between groups, with a longer gaze without leaving the area of interest of the eyes anticipated in the NT group across all categories of faces. We also expected group differences in the latency of the first gaze toward the eyes, with a shorter latency anticipated for the first gaze in the NT group (Falck-Ytter et al., Citation2015).
We hypothesized that when presented with pairs of faces, a left visual bias would not be present in children with autism, regardless of the combination of faces (Guillon et al., Citation2014). We expected to encounter a higher number of attentional disengagements or transitions between faces when one face in the pair was a dog owing to their nonhuman qualities and greater attention being paid toward them (Valiyamattam et al., Citation2020).
Methods
Ethics Statement
This study was reviewed and approved by the Committee of Ethics of the University of Jaén (reference no. ABR.16/9). Written informed consent for all participants was obtained from the parents/guardians of the children. The participants were informed about the stimuli they were going to see and verbal consent for their participation was requested. Participants could withdraw from the experiment if they experienced any discomfort.
Participants
In total, 26 children participated in the study: 13 NT children (10 girls, 3 boys) and 13 children diagnosed with ASD (3 girls, 10 boys) (). The NT children attended a local school in northern Spain and had not been diagnosed with any developmental disorders or learning disabilities. The ASD children were recruited from local associations and therapy centers within the same geographical area. The children in the ASD group had been diagnosed by a neuro-pediatrician according to the Diagnostic and statistical manual of mental disorders (DSM-5; APA, Citation2013).
Table 1. Participants’ characteristics.
The age of the NT children ranged from 5 to 8.91 years (M = 6.21 years, SD = 1.41) and that of the ASD group ranged from 5 to 11.5 years (M = 7.72 years, SD = 2.36). The groups were matched in terms of their non-verbal level using the Test of Nonverbal Intelligence, 2nd Edition (TONI-2) (Brown et al., Citation2009). The groups were also matched in terms of their verbal level using the verbal subscale of Reynolds Intellectual Assessment Scales (Reynolds & Kamphaus, Citation2009) to control for the possible effect of excessive gaze to the mouth/decreased gaze to the eyes, as this seems to be linked with language development in children with ASD (Falck-Ytter & von Hofsten, Citation2011). In addition to a diagnosis by a physician, children with ASD were assessed using the Childhood Autism Rating Scale (Schopler et al., Citation1980) to determine the degree of autistic traits.
Visual difficulties that would affect the calibration or validation of the apparatus and fear toward dogs were exclusion criteria for participation. This information was provided by the parents of each child. Trials characterized by difficulties during calibration, excessive movement of the head, or a missing data rate of 25% were considered invalid and excluded from the analysis.
Information about current treatments was collected from the participants in the ASD group (see ). At the time of the study, all children attended mainstream schools. The native language of all participants was Spanish.
Table 2. Treatments and support for children with autism spectrum disorder (n = 13)Table Footnotea.
Setting
The study was conducted in settings that were familiar to the children: in their school (for NT children) or clinical center (for ASD children).
Apparatus
An eye tracker (iView RED 250 mobile 60 Hz system) was used in conjunction with a 19-inch screen; participants were positioned 70 cm from the screen. Before the images were shown, a 5-point calibration process was applied. The stimulus for the calibration was an animated circle 0.7 cm in diameter that changed color as it moved between the five points. The accuracy of the calibration process was established; a maximum positional error of 0.5° was accepted for the stimuli.
Procedure
The children met the evaluator prior to the experiment and their verbal and non-verbal intelligence were assessed. For the eye-tracker portion of the study, the children were given the following instructions (in Spanish) by the evaluator: “We are going to play the face game. On this screen, you will see faces of people and dogs; the only thing you have to do is look at them, without moving your head or your body. Remember, you can move your eyes, but not your head or body.”
Images
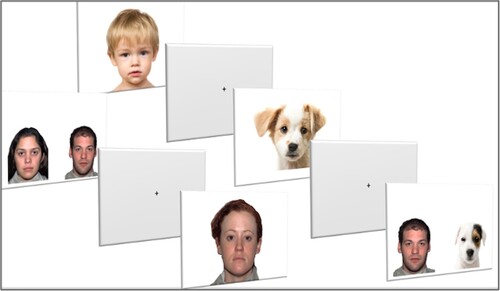
A total of 13 static slides were presented to the participants. Six of the slides were presented individually: two adult faces (male and female), two child faces (male and female), and two dog faces (an adult dog face [Dalmatian] and a puppy face [white and brown mixed breed dog]). The other seven slides were presented as pairs of faces (face-pair paradigm) using a selection of two adult human faces (female and male) and two adult dog faces (Golden retriever and Jack Russel terrier), rotating in pairs presented at the same time (14 images).
All faces were frontal and had a neutral expressions. The background was white and the pictures were 1920 × 1080 pixels. Adult faces were taken from the NimStim database of facial expressions (Tottenham et al., Citation2009), while child and dog faces were retrieved from the internet. The images from the internet were matched in terms of definition (pixels), facial orientation (frontal), expression (neutral with a closed mouth), and view of the eyes (which was required to be clear). Faces were displayed in the original color without any cropping and measured 11 cm horizontally by 14.4 cm vertically (8.9° × 11.7° visual angle). The area of interest (AOI) of the eyes was of the same dimensions for all individual images, measuring 6.3 cm horizontally by 1.5 cm vertically (5.2° × 1.2° visual angle). The faces in the pair paradigm all measured 7 cm horizontally and 9 cm vertically (5.7° × 7.4° visual angle).
The individual and paired faces were randomized and presented during a single experiment. The positions of the human–animal/female–male faces were balanced to avoid left visual bias and habituation effects in the face-pair paradigm. In the individual images, the gender and age of the individual human faces were taken into consideration. Owing to the similar appearance of dogs of different genders, the dog images were not distinguished on the basis of gender, such that there were only two categories of dog faces (adult and puppy). This led to the possibility of greater visual orientation toward neotenized facial features (Borgi et al., Citation2014). We tested for such an effect by plotting the gaze time toward adults (human male, human female, and adult dog) and infants (male child, female child, and puppy). Independent t-tests showed a significant difference between adult faces (M = 6,337.15, SD = 2,594.20) and infant faces (M = 8,127, SD = 3,292.38) in the NT group (p = 0.02): visual orientation was greater toward neotenized features. Interestingly, the ASD group showed no such orientation difference (p = 0.4) between adult faces (M = 4,303.38, SD = 3,165.87) and infant faces (M = 5,229, SD = 2,328.07): that is, there was no neotenization effect. Owing to the retention of neotenized characteristics in some dog breeds until adulthood (Borgi & Cirulli, Citation2016), we performed a 2 × 2 ANOVA between the adult dog and puppy faces: no significant differences between the adult and puppy faces (p = 0.19) were found and there was no effect of combining the adult and puppy faces.
Each stimulus presentation lasted for 3 s and was followed by a gray screen with a cross in the center that was presented for 1 s (). The total presentation time was 90 s per child, including the calibration of the apparatus, and it took 45 min in total for the verbal and non-verbal assessments. Out of a possible 91,000 gaze point observations (45,500 gaze points per group), approximately 84,500 were recorded successfully.
Variables
Individual Face Variables
Dwell time on the eyes according to the type of stimulus: the total time spent (ms) gazing at the AOI of the eyes in ASD and NT children. The images shown were human adult (male and female), child (male and female), and dog (adult and puppy) faces.
Latency of first gaze to the eyes: the latency (ms) to the first gaze to the AOI of the eyes in each group according to face category (adult, child, and dog).
Average duration of continuous gaze to the eyes: the average time (ms) spent gazing at the eyes, without leaving the AOI, for each face category (adult, child, and dog).
Pair Paradigm Variables
Dwell time according to the side of the screen in which the pair of faces appeared: the total time (ms) spent gazing at the face was analyzed according to the side of the screen on which the stimulus appeared (left or right side) regardless of the type of stimulus.
Dwell time according to the type of stimulus: the total time (ms) spent gazing at the face was analyzed according to the type of stimulus (human or dog), regardless of position on the screen.
Transitions between faces: the average number of gaze shifts between left and right complete faces. A transition was considered to have occurred when the gaze shifted from the left to right face, and vice versa, with an interval of < 100 ms. Transitions were classified for human–dog, dog–human, human–human, and dog–dog face pairs.
Analysis
Using the Shapiro-Wilk test, independent variables were tested for normality across the 26 participants: all variables were normally distributed. All gaze points were measured using raw data provided by the eye tracker. A 2 (group) × 3 (type of stimuli) mixed factorial ANOVA was used to analyze individual images, and a 2 (group) × 2 (type of stimulus) / 2 (group) × 4 (pair of faces) ANOVA was used for analyzing the pair paradigm images. Hedges’ g was calculated to determine the effect size of the group differences. Grimp software (version 2.99.4) was used to set the coordinates of the AOI for the eyes and complete faces. SPSS software (version 27.0) was used for all statistical analyses.
Results
The descriptive data of the variables in both groups are in and .
Table 3. Results for individual pictures by group.
Table 4. Pair paradigm results by group.
Individual Pictures
Dwell Time on the Eyes According to the Type of Stimulus
The descriptive statistics showed that both groups looked at dogs’ eyes for the longest time, followed by children’s eyes and adults’ eyes (see ). The t-test analysis showed a significant group difference in the total time spent looking at the dogs’ eyes (p = 0.02), with a longer dwell time seen in the NT group (M = 6,072.23, SD = 2,253.86) compared with the ASD group (M = 3,683.31, SD = 1,472.69).
The results of the two-way mixed ANOVA of dwell time on the eyes according to the type of stimulus () showed a significant main effect of type of face (F(2, 48) = 18.87, p < 0.001, η2 = 0.44) and a significant interaction between type of face and group (F(1,24) = 6.24, p = 0.02, η2 = 0.21). Post-hoc Bonferroni tests showed a significant difference in the NT group between dog and adult faces (p < 0.001) and children’s and adults’ faces (p < 0.001). In other words, within the NT group, there were differences in the time spent looking at the eyes among the three types of face. However, there were no such differences in the ASD group.
Figure 2. Dwell time on the area of interest of the eyes in the neurotypical (NT) and autism spectrum disorder (ASD) groups while gazing at faces of adults, children, and dogs. *p < 0.05, **p < 0.01, ***p < 0.001.
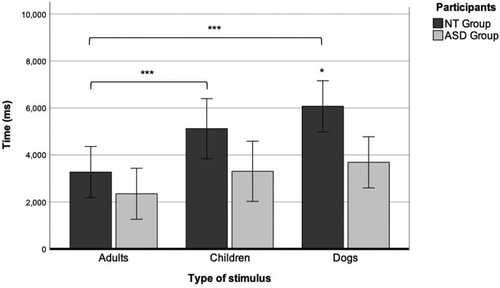
Overall, while more time was spent gazing at the area of the eyes in the images of dog faces in the NT group, the ASD children did not discriminate among the image categories.
Latency of First Gaze to the Eyes According to the Type of Stimulus
Overall, NT children showed a shorter latency in the first gaze to the eyes than ASD children (); significant differences between groups were found for adult faces (p < 0.001) and child faces (p < 0.001), but not for dog faces (p = 0.13). No differences in the latency of the first gaze were found in the NT group according to face category. However, in the ASD group, there was a shorter latency for the first gaze to the eyes for dog faces (M = 384.50, SD = 266.25), followed by child faces (M = 937.92, SD = 992.70) and adult faces (M = 1,200.31, SD = 869.04).
A 2 × 3 mixed ANOVA () revealed a main effect of the group on the latency of the first gaze to the AOI of the eyes (F(1, 24) = 32.65, p < 0.001, η2 = 0.57). The effect of group, according to partial eta squared, was large (0.57), indicating that group had a major influence on the latency of the first gaze toward the face. An interaction effect between participant and face category was also found (F(2, 48) = 5.7, p < 0.04, ηp2 = 0.006).
Figure 3. Latency of the first gaze to the eyes in individual faces of adults, children, and dogs. *p < 0.05, **p < 0.01, ***p < 0.001.
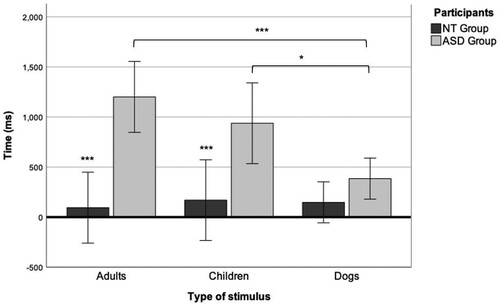
The post-hoc Bonferroni test showed significant differences within the ASD group in the latency for the first gaze between adult and dog faces (p < 0.001) and child and dog faces (p = 0.02). There was a large effect size (g = –1.06) for the group comparison for adult faces and a medium effect size for child faces (g = –0.64). These results indicate that it took longer for children with ASD to look at the eyes than NT children, except at dogs’ eyes. When we compared categories of faces within the ASD group, we found that they gazed significantly faster toward the eyes of dogs than at those of children or adults. Conversely, NT children did not show differences in the latency of the first gaze to the eyes between faces.
Average Continuous Gaze Time on the Eyes According to the Type of Stimulus
We measured the average time that the groups gazed at the eyes without leaving the AOI (see ). No main effect of group or type of stimulus was found (see ). A moderate effect of the type of stimulus and average continuous gaze time was found only in the ASD group (F(2, 48) = 5.52, p = 0.03, η2 = 0.19).
Figure 4. Average continuous gaze time on the eyes in individual faces of adults, children, and dogs. *p < 0.05, **p < 0.01, ***p < 0.001.
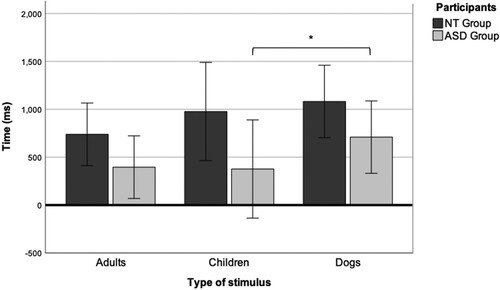
Post-hoc Bonferroni tests showed a higher (p = 0.03) average gaze time at the eyes of dogs in the ASD group (M = 709.39, SD = 493.39) compared with children’s eyes (M = 376.16, SD = 493.49) and, to a lesser extent (p = 0.08), adults’ eyes (M = 395.53, SD = 376.04).
These results suggest that the ASD group gazed longer at the eyes of dogs without leaving the AOI than at any other type of stimulus.
Pair Paradigm
Dwell Time According to the Side of the Screen on Which the Faces Appear and Type of Stimulus
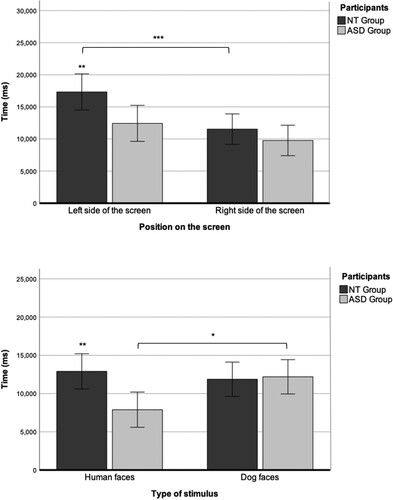
A 2 × 2 ANOVA showed a significant effect of the side of the screen on dwell time but there was no side × group interaction (F(1, 24) = 9.95, p = 0.004, η2 = 0.29). The post-hoc Bonferroni test revealed a significant difference in dwell time between the stimulus shown on the left and right sides of the screen in the NT group (p = 0.004) but not in the ASD group. A t-test showed a significant group difference in dwell time for the stimulus on the left side of the screen (p = 0.02) but not for the stimulus on the right side of the screen (p = 0.29). Both Hedges’ g (0.97) and partial eta squared (η2 = 0.29) were large (see ).
Regarding dwell time according to the type of stimulus, a 2 × 2 ANOVA showed an interaction between the type of stimulus and group (F(1,24) = 5.06, p = 0.03, η2 = 0.17). Post-hoc Bonferroni tests revealed a significant group difference (p = 0.005) for human stimulus, with a longer dwell time seen in the NT group for human faces. Within the ASD group, there was a significant difference (p = 0.02) in dwell time between human and dog stimuli: the dwell time was higher for dog faces in the ASD group.
Taken together, we observed a tendency in the NT group to look longer at the faces on the left, regardless of type, whereas the ASD group preferred to look at dog faces regardless of the position in which they appeared on the screen ().
Number of Transitions between Visual Images
The NT group showed the same number of transitions in all pairs except the dog–dog pairs, for which there were fewer transitions. There was a significant group difference in the number of transitions in the human–human face-pair paradigm (t(21) = 5.37, p < 0.01), with a large effect size (g = 2.89). Conversely, the ASD group showed a higher number of transitions for the dog–dog face pair, followed by human–dog visual face pair and dog–human face pair, and a much lower number of transitions for the human–human face pair (see ).
The results of an ANOVA () showed no main effect of participant or face pair, but there was an interaction effect between the participant and face pair (F(3, 72) = 6.81, p < 0.001, η2 = 0.22).
Figure 6. Total transitions between the left and right picture for pairs of stimuli (human–dog, dog–human, human–human, or dog–dog) in neurotypical (NT) and autism spectrum disorder (ASD) children. *p < 0.05, **p < 0.01, ***p < 0.001.
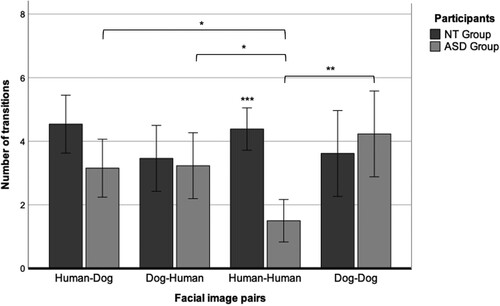
Post-hoc comparisons using Bonferroni's correction indicated a significant difference (p < 0.001) in the number of transitions for human–human face pairs between the NT group (M = 4.39, SD = 1.16) and the ASD group (M = 1.5, SD = 1.17). Within the ASD group, t-tests showed a significant difference in transitions between dog–dog and human–human face pairs (p = 0.003); human–dog and human–human face pairs (p = 0.02); and dog–human and human–human face pairs (p = 0.01).
Discussion
The present study aimed to assess the gaze patterns of children with ASD when looking at human and dog faces, as a basis for achieving positive socio-communicative outcomes in animal-assisted interventions. Gaze patterns were assessed when an individual face (adult, child, or dog) appeared on screen (free viewing) and when two faces appeared simultaneously and were thus in competition (pair paradigm).
For the individual faces, we measured the total time spent gazing at the eyes (dwell time), the latency of the first gaze to the eyes, and the average time spent continuously gazing at the eyes. The results showed a higher total gaze time toward the eyes of dogs compared with adult and child faces in the NT group and compared with the ASD group. These results are consistent with previous research (Grandgeorge et al., Citation2016; Muszkat et al., Citation2015; Valiyamattam et al., Citation2020) and our hypothesis.
However, contrary to the cited research and our preliminary hypothesis, we did not observe differences in total dwell time between categories of faces within the ASD group. In addition, there were no significant group differences in total time spent gazing at adult and child eyes. These results suggest a lack of specificity of the gaze in ASD children; there were no differences between categories of faces, similar to other research reporting no differences among types of stimuli (Wilson et al., Citation2010).
The differences between this study and previous ones could be explained by the sample size. Within the ASD group, the total time spent gazing at dog eyes was slightly, but not significantly, longer compared with other faces. A larger sample might have exacerbated these differences in the ASD group. Another reason could be the type and number of images. We used the faces of dogs, whereas other studies used faces of other species in addition to dogs, such as horses or cats (Grandgeorge et al., Citation2016; Valiyamattam et al., Citation2020). Previous research points to greater visual attention toward cat eyes compared with those of humans and other animal species (Grandgeorge et al., Citation2016; Grandgeorge et al., Citation2020). Utilizing the faces of cats, among other images, may have increased the total gaze time in children with ASD.
Regardless of the total dwell time on the eyes, we observed clear differences between categories of faces in terms of how children with ASD gazed at them. The latency data for the first gaze to the AOI of the eyes showed that the ASD group took longer to gaze at the eyes, which is an informative part of the face, compared with the NT group (Dalton et al., 2005), except in the case of dog eyes. Dog eyes seemed to elicit a faster automatic response toward the eyes that were more similar to typical patterns (Falck-Ytter & von Hofsten, Citation2011; Thompson et al., Citation2019).
Additionally, the ASD group showed a higher average gaze time without leaving the area of the eyes when looking at dog faces compared with those of children and, to a lesser extent, adults. This suggests that dog eyes elicited a longer and more detailed exploration of this region in children with ASD compared with human eyes. A more detailed visual exploration of dog faces could explain the preference therefore seen in previous research (Grandgeorge et al., Citation2015; Guérin et al., Citation2017; Prothmann et al., Citation2009). A longer exploration of dog eyes could also indicate that children with ASD feel more comfortable with the gaze of dogs compared with that of humans; additionally, this longer exploration could allow children with ASD to gather more cues about referential gaze and emotional recognition (Davidson et al., Citation2019), thereby improving self-efficacy in social tasks (Atherton & Cross, Citation2018; Miralles et al., Citation2022). Consequently, improvements in social interactions would likely be observed in the presence of an animal in animal-assisted interventions (AAIs) (Nieforth et al., Citation2021; O'Haire et al., Citation2013).
In our pair paradigm, the NT group showed a bias toward the face on the left side of the screen, regardless of type (dog or human). Conversely, the ASD group showed a lack of bias toward the left picture, unlike what was seen previously for individual faces (Guillon et al., Citation2014); however, they showed a preference for dog faces regardless of the position of the faces on the screen.
The results also showed a higher number of transitions and, therefore, a greater capacity to disengage attentionally, when one or both of the face stimuli were dogs. Some studies have addressed the difficulties in attentional disengagement displayed by ASD children and the associated potential loss of social information (Thorup et al., Citation2018). In this study, there was some evidence that dogs may engage the attention of children with ASD, especially when in competition with other social stimuli, while also allowing them to disengage their attention more rapidly to attend to other highly salient stimuli. These results are consistent with studies that showed a preference for dog, compared with human, stimuli; these results could also explain the results of studies that explored social rivalry (Grandgeorge et al., Citation2017) when a dog and human were present and reported increasing shifts in visual attention between them.
The visual processing of animal and even human faces in children with ASD is not fully understood, although it seems that nonhuman animals could promote social engagement in such children. In this study, we observed increased interest in informative areas of the face in children with ASD and a tendency for longer and more detailed exploration of dog eyes. In addition, we observed a preference for exploring dog faces versus other faces when paired together (i.e., when in competition), but easy disengagement from one face to attend to another was also apparent. Overall, this could explain the positive socio-communicative outcomes of human–animal interactions in children with ASD and by extension, the efficacy of canine-assisted interventions.
Limitations and Future Avenues for Research
This study had some limitations. Although we aimed to represent children on different parts of the spectrum, a larger sample, especially of children with ASD, may have allowed for more nuanced results regarding the particular types of gaze; unfortunately, COVID-19-related restrictions prevented the recruitment of a larger sample. A more diverse sample is needed; specifically, language-delayed and non-verbal children with ASD should be assessed, as the gaze to the mouth and eyes may vary depending on language development (Falck-Ytter & von Hofsten, Citation2011). Additionally, comparing gaze patterns toward animals between female and male children on the spectrum could be a compelling line of research. Finally, it would be interesting to analyze the type and number of treatments that children with ASD receive and their ability to modulate the gaze. As observed in this study, there is high variability in the treatments received by children with ASD, including training in social skills, which could influence performance on the types of tests employed herein. In a larger sample, the correlations between treatments and gaze patterns could be interesting to explore.
Other stimuli such as dynamic scenes, as well as assessing live interactions with animals, could improve our understanding of how children with ASD visually process animal faces. Some research involving animals in live settings showed high levels of visual interaction, and social and prosocial behaviors, in children with ASD (Dollion et al., Citation2021). Furthermore, greater difficulty in looking at stimuli with ecological validity was reported in children with ASD (Noris et al., Citation2012) owing to context blindness (Vermeulen, Citation2015). Exploring visual patterns in natural contexts involving a real dog, instead of an image thereof, could improve our understanding of the mechanisms underlying the efficacy of canine-assisted interventions.
We believe that further exploration of the relationship between the interactions of children with ASD and animals and the exploratory gaze toward animal faces is merited.
Conclusions
Despite some limitations, this study improves our understanding of visual processing in children with ASD when gazing at nonhuman animals. Overall, children with ASD seem more attentionally engaged when looking at dog faces, especially when they appear together (i.e., in competition) with human ones. When looking at the eyes, children with ASD gaze faster and more continuously at the eyes of dogs compared with those of humans. Taken together, the results suggest that dog faces confer advantages with respect to visual exploration of socially informative areas in children with ASD.
Acknowledgements
The authors thank the families of all participants, local schools, and autism centers in A Coruña, Spain for their participation in this study. The authors also thank James Stone and Elizabeth Walsh for proofreading this article and Lieve Meers for providing relevant reviews.
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- APA. (2013). Diagnostic and statistical manual of mental disorders (DSM-5). American Psychiatric Association.
- Atherton, G., & Cross, L. (2018). Seeing more than human: Autism and anthropomorphic theory of mind. Frontiers in Psychology, 9, 528. https://doi.org/10.3389/fpsyg.2018.00528
- Beetz, A., Uvnäs-Moberg, K., Julius, H., & Kotrschal, K. (2012). Psychosocial and psychophysiological effects of human–animal interactions: The possible role of oxytocin. Frontiers in Psychology, 3, 234. https://doi.org/10.3389/fpsyg.2012.00234
- Borgi, M., & Cirulli, F. (2016). Pet face: Mechanisms underlying human–animal relationships. Frontiers in Psychology, 298. https://doi.org/10.3389/fpsyg.2016.00298
- Borgi, M., Cogliati-Dezza, I., Brelsford, V., Meints, K., & Cirulli, F. (2014). Baby schema in human and animal faces induces cuteness perception and gaze allocation in children. Frontiers in Psychology, 5, 411. https://doi.org/10.3389/fpsyg.2014.00411
- Brown, L., Sherbenou, R. J., & Johnsen, S. K. (2009). Toni 2: Test de inteligencia no verbal: Apreciación de la habilidad cognitiva sin influencia del lenguaje: Manual. TEA Ediciones.
- Celani, G. (2002). Human beings, animals, and inanimate objects: What do people with autism like? Autism, 6(1), 93–102. https://doi.org/10.1177/1362361302006001007
- Chawarska, K., & Shic, F. (2009). Looking but not seeing: Atypical visual scanning and recognition of faces in 2 and 4-year-old children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 39(12), 1663. https://doi.org/10.1007/s10803-009-0803-7
- Cross, L., Farha, M., & Atherton, G. (2019). The animal in me: Enhancing emotion recognition in adolescents with autism using animal filters. Journal of Autism and Developmental Disorders, 49(11), 4482–4487. https://doi.org/10.1007/s10803-019-04179-7
- Dalton, K. M., Nacewicz, B. M., Alexander, A. L., & Davidson, R. J. (2007). Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biological Psychiatry, 61(4), 512–520. https://doi.org/10.1016/j.biopsych.2006.05.019
- Davidson, D., Hilvert, E., Misiunaite, I., Kerby, K., & Giordano, M. (2019). Recognition of facial emotions on human and canine faces in children with and without autism spectrum disorders. Motivation and Emotion, 43(1), 191–202. https://doi.org/10.1007/s11031-018-9736-9
- Dollion, N., Toutain, M., François, N., Champagne, N., Plusquellec, P., & Grandgeorge, M. (2021). Visual exploration and observation of real-life interactions between children with ASD and service dogs. Journal of Autism and Developmental Disorders, 51(11), 3785–3805. https://doi.org/10.1007/s10803-021-05293-1
- Domes, G., Sibold, M., Schulze, L., Lischke, A., Herpertz, S. C., & Heinrichs, M. (2013). Intranasal oxytocin increases covert attention to positive social cues. Psychological Medicine, 43(8), 1747–1753. https://doi.org/10.1017/S0033291712002565
- Falck-Ytter, T., Thorup, E., & Bölte, S. (2015). Brief report: Lack of processing bias for the objects other people attend to in 3-year-olds with autism. Journal of Autism and Developmental Disorders, 45(6), 1897–1904. https://doi.org/10.1007/s10803-014-2278-4
- Falck-Ytter, T., & von Hofsten, C. (2011). How special is social looking in ASD: A review. In O. Braddick, J. Atkinson, & G. M. Innocenti (Eds.), Gene expression to neurobiology and behavior: Human brain development and developmental disorders (Vol. 189, pp. 209–222). Elsevier.
- Germone, M. M., Gabriels, R. L., Guérin, N. A., Pan, Z., Banks, T., & O’Haire, M. E. (2019). Animal-assisted activity improves social behaviors in psychiatrically hospitalized youth with autism. Autism, 23(7), 1740–1751. https://doi.org/10.1177/1362361319827411
- Grandgeorge, M., Bourreau, Y., Alavi, Z., Lemonnier, E., Tordjman, S., Deleau, M., & Hausberger, M. (2015). Interest towards human, animal and object in children with autism spectrum disorders: An ethological approach at home. European Child & Adolescent Psychiatry, 24(1), 83–93. https://doi.org/10.1007/s00787-014-0528-9
- Grandgeorge, M., Degrez, C., Alavi, Z., & Lemonnier, E. (2016). Face processing of animal and human static stimuli by children with autism spectrum disorder: A pilot study. Human–Animal Interaction Bulletin, 4(2), 39–53. https://doi.org/10.1079/hai.2016.0005
- Grandgeorge, M., Gautier, Y., Bourreau, Y., Mossu, H., & Hausberger, M. (2020). Visual attention patterns differ in dog vs. cat interactions with children with typical development or autism spectrum disorders. Frontiers in Psychology, 2047. https://doi.org/10.3389/fpsyg.2020.02047
- Grandgeorge, M., Gautier, Y., Brugaillères, P., Tiercelin, I., Jacq, C., Lebret, M. C., & Hausberger, M. (2017). Social rivalry triggers visual attention in children with autism spectrum disorders. Nature Scientific Reports, 7(1), 1–8. https://doi.org/10.1038/s41598-017-09745-6
- Grandgeorge, M., Tordjman, S., Lazartigues, A., Lemonnier, E., Deleau, M., & Hausberger, M. (2012). Does pet arrival trigger prosocial behaviors in individuals with autism? PLoS ONE, 7(8). https://doi.org/10.1371/journal.pone.0041739
- Grigore, A. A., & Rusu, A. S. (2014). Interaction with a therapy dog enhances the effects of social story method in autistic children. Society & Animals, 22(3), 241–261. https://doi.org/10.1163/15685306-12341326
- Guérin, N. A., Rodriguez, K. E., Brodhead, M. T., & O’Haire, M. E. (2017). Assessing preferences for animals in children with autism: A new use for video-based preference assessment. Frontiers in Veterinary Science, 4, 29. https://doi.org/10.3389/fvets.2017.00029
- Guillon, Q., Hadjikhani, N., Baduel, S., Kruck, J., Arnaud, M., & Rogé, B. (2014). Both dog and human faces are explored abnormally by young children with autism spectrum disorders. Neuroreport, 25(15), 1237–1241. https://doi.org/10.1097/WNR.0000000000000257
- Hill, J. R., Ziviani, J., & Driscoll, C. (2020). “The connection just happens”: Therapists’ perspectives of canine-assisted occupational therapy for children on the autism spectrum. Australian Occupational Therapy Journal, 67(6), 550–562. https://doi.org/10.1111/1440-1630.12680
- Jones, W., Carr, K., & Klin, A. (2008). Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives of General Psychiatry, 65(8), 946–954. https://doi.org/10.1001/archpsyc.65.8.946
- Jones, W., & Klin, A. (2013). Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature, 504(7480), 427–431. https://doi.org/10.101038/nature12715
- Klin, A., Jones, W., Schultz, R., Volkmar, F., & Cohen, D. (2002). Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry, 59(9), 809–816. https://doi.org/10.1001/archpsyc.59.9.809
- London, M. D., Mackenzie, L., Lovarini, M., Dickson, C., & Alvarez-Campos, A. (2020). Animal assisted therapy for children and adolescents with autism spectrum disorder: Parent perspectives. Journal of Autism and Developmental Disorders, 50(12), 4492–4503. https://doi.org/10.1007/s10803-020-04512-5
- Madipakkam, A. R., Rothkirch, M., Dziobek, I., & Sterzer, P. (2017). Unconscious avoidance of eye contact in autism spectrum disorder. Scientific Reports, 7(1), 1–6. https://doi.org/10.1038/s41598-017-13945-5
- Matsuda, S., Minagawa, Y., & Yamamoto, J. (2015). Gaze behavior of children with ASD toward pictures of facial expressions. Autism Research and Treatment, 2015, 617190. https://doi.org/10.1155/2015/617190
- Miralles, A., Grandgeorge, M., & Raymond, M. (2022). Self-perceived empathic abilities of people with autism towards living beings mostly differs for humans. Scientific Reports, 12(1), 1–9. https://doi.org/10.1038/s41598-022-10353-2
- Mo, S., Liang, L., Bardikoff, N., & Sabbagh, M. A. (2019). Shifting visual attention to social and non-social stimuli in autism spectrum disorders. Research in Autism Spectrum Disorders, 65, 56–64. https://doi.org/10.1016/j.rasd.2019.05.006
- Moore, A., Wozniak, M., Yousef, A., Barnes, C. C., Cha, D., Courchesne, E., & Pierce, K. (2018). The geometric preference subtype in ASD: Identifying a consistent, early-emerging phenomenon through eye tracking. Molecular Autism, 9(1), 1–13. https://doi.org/10.1186/s13229-018-0202-z
- Muszkat, M., de Mello, C. B., Muñoz, P. d. O. L., Lucci, T. K., David, V. F., de Oliveira Siqueira, J., & Otta, E. (2015). Face scanning in autism spectrum disorder and attention deficit/hyperactivity disorder: Human versus dog face scanning. Frontiers in Psychiatry, 6, 150. https://doi.org/10.3389/fpsyt.2015.00150
- Nagasawa, M., Kikusui, T., Onaka, T., & Ohta, M. (2009). Dog's gaze at its owner increases owner's urinary oxytocin during social interaction. Hormones and Behavior, 55(3), 434–441. https://doi.org/10.1016/j.yhbeh.2008.12.002
- Nakano, T., Tanaka, K., Endo, Y., Yamane, Y., Yamamoto, T., Nakano, Y., Ohta, H., Kato, N., & Kitazawa, S. (2010). Atypical gaze patterns in children and adults with autism spectrum disorders dissociated from developmental changes in gaze behaviour. Proceedings of the Royal Society B: Biological Sciences, 277(1696), 2935–2943. https://doi.org/10.1098/rspb.2010.0587
- Nieforth, L. O., Schwichtenberg, A. J., & O’Haire, M. E. (2021). Animal-assisted interventions for autism spectrum disorder: A systematic review of the literature from 2016 to 2020. Review Journal of Autism and Developmental Disorders. https://doi.org/10.1007/s40489-021-00291-6
- Noris, B., Barker, M., Nadel, J., Hentsch, F., Ansermet, F., & Billard, A. (2011). Measuring gaze of children with autism spectrum disorders in naturalistic interactions. 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 5356–5359. https://doi.org/10.1109/IEMBS.2011.6091325.
- Noris, B., Nadel, J., Barker, M., Hadjikhani, N., & Billard, A. (2012). Investigating gaze of children with ASD in naturalistic settings. PLoS ONE, 7(9), e44144. https://doi.org/10.1371/journal.pone.0044144
- Odendaal, J. S. (2000). Animal-assisted therapy—magic or medicine? Journal of Psychosomatic Research, 49(4), 275–280. https://doi.org/10.1016/S0022-3999(00)00183-5
- Odendaal, J. S., & Meintjes, R. A. (2003). Neurophysiological correlates of affiliative behaviour between humans and dogs. The Veterinary Journal, 165(3), 296–301. https://doi.org/10.1016/S1090-0233(02)00237-X
- O'Haire, M. E., McKenzie, S. J., Beck, A. M., & Slaughter, V. (2013). Social behaviors increase in children with autism in the presence of animals compared to toys. PLoS ONE, 8(2), e57010. https://doi.org/10.1371/journal.pone.0057010
- Papagiannopoulou, E. A., Chitty, K. M., Hermens, D. F., Hickie, I. B., & Lagopoulos, J. (2014). A systematic review and meta-analysis of eye-tracking studies in children with autism spectrum disorders. Social Neuroscience, 9(6), 610–632. https://doi.org/10.1080/17470919.2014.934966
- Prothmann, A., Ettrich, C., & Prothmann, S. (2009). Preference for and responsiveness to people, dogs, and objects in children with autism. Anthrozoös, 22(2), 161–171. https://doi.org/10.2752/175303709X434185
- Reynolds, C. R., & Kamphaus, R. W. (2009). RIAS. Escala de Inteligencia de Reynolds. TEA Ediciones.
- Rice, K., Moriuchi, J. M., Jones, W., & Klin, A. (2012). Parsing heterogeneity in autism spectrum disorders: Visual scanning of dynamic social scenes in school-aged children. Journal of the American Academy of Child & Adolescent Psychiatry, 51(3), 238–248. https://doi.org/10.1016/j.jaac.2011.12.017
- Schopler, E., Reichler, R. J., DeVellis, R. F., & Daly, K. (1980). Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). Journal of Autism and Developmental Disorders, 10(1), 91–103. https://doi.org/10.1007/BF02408436
- Shic, F., Bradshaw, J., Klin, A., Scassellati, B., & Chawarska, K. (2011). Limited activity monitoring in toddlers with autism spectrum disorder. Brain Research, 1380, 246–254. https://doi.org/10.1016/j.brainres.2010.11.074
- Strathearn, L., Kim, S., Bastian, D. A., Jung, J., Iyengar, U., Martinez, S., Goin-Kochel, R., & Fonagy, P. (2018). Visual systemizing preference in children with autism: A randomized controlled trial of intranasal oxytocin. Development and Psychopathology, 30(2), 511–521. https://doi.org/10.1017/S0954579417001018
- Thompson, S. J., Foulsham, T., Leekam, S. R., & Jones, C. R. G. (2019). Attention to the face is characterized by a difficult to inhibit first fixation to the eyes. Acta Psychologica, 193, 229–238. https://doi.org/10.1016/j.actpsy.2019.01.006
- Thorup, E., Nyström, P., Gredebäck, G., Bölte, S., & Falck-Ytter, T. (2018). Reduced alternating gaze during social interaction in infancy is associated with elevated symptoms of autism in toddlerhood. Journal of Abnormal Child Psychology, 46(7), 1547–1561. https://doi.org/10.1007/s10802-017-0388-0
- Tottenham, N., Tanaka, J. W., Leon, A. C., McCarry, T., Nurse, M., Hare, T. A., Marcus, D. J., Westerlund, A., Casey, B. J., & Nelson, C. (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. https://doi.org/10.1016/j.psychres.2008.05.006
- Valiyamattam, G. J., Katti, H., Chaganti, V. K., O'Haire, M. E., & Sachdeva, V. (2020). Do animals engage greater social attention in autism? An eye tracking analysis. Frontiers in Psychology, 11, 727. https://doi.org/10.3389/fpsyg.2020.00727
- Vermeulen, P. (2015). Context blindness in autism spectrum disorder: Not using the forest to see the trees as trees. Focus on Autism and Other Developmental Disabilities, 30(3), 182–192. https://doi.org/10.1177/1088357614528799
- Whyte, E. M., Behrmann, M., Minshew, N. J., Garcia, N. v., & Scherf, K. S. (2016). Animal, but not human, faces engage the distributed face network in adolescents with autism. Developmental Science, 19(2), 306–317. https://doi.org/10.1111/desc.12305
- Wilson, C. E., Brock, J., & Palermo, R. (2010). Attention to social stimuli and facial identity recognition skills in autism spectrum disorder. Journal of Intellectual Disability Research, 54(12), 1104–1115. https://doi.org/10.1111/j.1365-2788.2010.01340.x