Abstract
Studies on human perceptions of Lantana camara invasion are needed to inform management. This study used a two-pronged approach to assess perceptions, knowledge, and uses of L. camara in rural communities as well as evaluate its invasion extent in household yards of the Vhembe Biosphere Reserve, Limpopo province of South Africa. Results from 300 face-to-face household interviews and household ecological surveys showed that most villagers know of L. camara but are not aware that it is an invasive alien plant. Both people’s responses and ecological household yard surveys show that the plant is on properties and is expanding. Few benefits compared to negative impacts were mentioned. Local communities are implementing control measures; however, they need assistance from the government. The results highlight the need to incorporate local and individual clearing initiatives into the current broader national control programmes, such as the Working for Water clearing programme.
Introduction
Invasive alien plants (IAPS) are one of the drivers of environmental change (Rai and Singh Citation2020) and invasion of natural ecosystems has been reported to have detrimental impacts on human well-being (Vardien et al. Citation2012; van Wilgen et al. Citation2020a). For most IAPS, invasion success in natural ecosystem is driven by socio-economic and environmental factors, such as globalization, increased human travel, absence of competitors in invaded ecosystems, and climate change (Leishman and Gallagher Citation2016; Dutta Citation2018; Shackleton et al. Citation2020; van Wilgen et al. Citation2020b). Invasive alien plants are regarded as ecosystem transformers because of their ability to change ecosystem processes and functions (Richardson et al. Citation2020), through soil manipulation and the creation of monostands that result in the displacement of native species (Simberloff et al. Citation2013; Richardson et al. Citation2020). For example, the introduction of Australian Acacias in South Africa is associated with negative effects on soil physio-chemical properties resulting in displacement of native vegetation (Le Maitre et al. Citation2011), whereas Eucalyptus invasions are linked with the creation of monospecific stands with little understory native vegetation (Hirsch et al. Citation2020). The proliferation of IAPS, such as Pinus, Acacias, and Eucalyptus along South African riparian zones is associated with significant loss of river water (Richardson et al. Citation2020; van Wilgen et al. Citation2020a). Recent studies (e.g., Haubrock et al. Citation2021; Bang et al. Citation2022; Eschen et al. Citation2021; Fantle-Lepczyk et al. Citation2022) have shown that invasion by IAPS causes significant effects on global economies, with effects likely to be substantial in the agricultural sector. For example, Eschen et al. (Citation2021) reported that the total annual cost of invasive alien species (IAS) to Africa’s agricultural sector is ∼ US$65.58 billion. Although social effects linked to IAS are difficult to quantify due to potential benefits, some studies (e.g., Ngorima and Shackleton Citation2019; Shackleton et al. Citation2020) have reported that poor rural households are the most affected due to their dependence on natural ecosystems for livelihoods. Therefore, more research is needed on socio-economic impacts of IAS in rural communities (Shackleton et al. Citation2020).
Research on invasion pathways and traits is instrumental in developing adaptive management strategies aimed at reducing invasion impacts and costs (Faulkner et al. Citation2020). However, most research on biological invasion is ecological, thus omitting understanding of the social dimension that has the potential to inform management interventions since most IAPS are moved by humans (Sundaram et al. Citation2012; Shackleton et al. Citation2017a, Citation2017b). Studies (e.g., Shackleton et al. Citation2007; Rai et al. Citation2012; Shackleton et al. Citation2017a, Citation2017b; Ngorima and Shackleton Citation2019) that assess perceptions, knowledge, and practices related to IAPS often highlight key aspects, such as benefits, costs, invasion drivers, and attitudes toward these species. These studies (e.g., Shackleton et al. Citation2017a, Citation2017b; Zengeya et al. Citation2017; Ngorima and Shackleton Citation2019) can provide important information on costs and benefits that can be used to manage IAPS, especially conflict generating species—which are viewed as having both costs and benefits to humans. For example, social studies (e.g., Jevon and Shackleton Citation2015; Shackleton, Le Maitre, and Richardson Citation2015; Shackleton et al. Citation2017a, Citation2017b) on Prosopis (mesquite), Lantana, and Chromolaena invasion in Africa have shown that these species provide important rural livelihood benefits, such as fodder and fuelwood to the extent that removal can cause conflict with users, who are also aware of the negative impacts of the species. Similarly, Ruwanza and Thondhlana (Citation2022) highlighted that Psidium guajava is extensively used by villagers and has several benefits, so its removal needs stakeholder engagement to avoid conflicts. Given that some IAPS offer both costs and benefits there is a need to gather data on human perceptions to develop effective management strategies. Therefore, social studies can provide evidence that invasion biologists need to understand invasion science from a socio-ecological perspective if interventions to manage IAPS are to yield positive outcomes.
Human perceptions toward IAS are shaped by several factors that include individual human attributes, characteristics of the IAS, accrued costs and benefits, social, cultural, landscape, institutional, and policy context (Shackleton et al. Citation2019a). For example, Opuntia ficus-indica has several economic benefits to rural villagers in Eastern Cape Province of South Africa, thus local people view it positively with little efforts to remove the plant (Shackleton, Kirby, and Gambiza Citation2011). Similarly, Eucalyptus camaldulensis generates a lot of income for the bee industry in South Africa to the extent that removal is mainly along sensitive ecosystems like riparian zones, rather than in bee industry hotspot farms (Hirsch et al. Citation2020). Besides L. camara being one of the main invaders in South Africa, little is known regarding human perceptions toward its invasion. The few studies (e.g., Jevon and Shackleton Citation2015) conducted on L. camara in South Africa have indicated that local villagers know about the plant and are aware of its costs and benefits. However, human perceptions vary depending on the landscape, demographic, and socio-cultural changes, thus the need to conduct more research on L. camara to develop a holistic picture that can be used for developing adaptive interventions. Therefore, understanding L. camara invasion from a social standpoint can help explain some difficulties linked to its management as well as generate relevant information that can be used to manage the plant effectively.
The control and management of IAS in South Africa are mostly done by the Working for Water (WfW) programme (van Wilgen et al. Citation2012, Citation2020b). The WfW programme is a government funded programme, which was established in 1995 with the aim of controlling IAS through employing previously disadvantaged communities to remove IAS, thus creating employment (van Wilgen et al. Citation2012, Citation2020b). The programme spends ∼2 billion Rands per year on IAPS clearing and the most used clearing method is slash and stack burning (van Wilgen et al. Citation2020b). In spite of achieving some clearing success (e.g., clearing around 200 000 consolidated hectares per year), the programme has faced challenges, such as conflicts related to removing species that are perceived to be beneficial to some sectors of society (van Wilgen and Wannenburgh Citation2016; van Wilgen et al. Citation2020b), such as attempts to clear E. camaldulensis in bee industry hotspots which have triggered conflicts as the plant is perceived beneficial (Hirsch et al. Citation2020). It is not clear how society perceives the removal of L. camara by WfW, yet its successful management needs to be shaped by human perceptions, thus the need to conduct this study on the human dimension of biological invasion.
Lantana camara (commonly known as Lantana) is one of the world’s worst invasive shrubs ranked among the top 100 invaders (Taylor and Kumar Citation2013). Similar to other aggressive IAPS, L. camara has adverse impacts on biodiversity, ecosystem functions, and human well-being (Day and Neser Citation2000; Vardien et al. Citation2012; Bang et al. Citation2022). Lantana camara is a thorny, multi-stemmed shrub that consists of many hybrids (Vardien et al. Citation2012). In conducive environments, the plant flowers throughout the year (Sharma, Raghubanshi, and Singh Citation2005) and can reach an average height of 1.8 m (Vardien et al. Citation2012). The plant thrives in the tropics and subtropics region as well as the temperate region and invades various habitats, such as disturbed habitats (e.g., old fields and road networks), moist habitats (e.g., riparian zones), and less disturbed habitats (e.g., protected national parks) (Sharma, Raghubanshi, and Singh Citation2005; Barahukwa et al. Citation2023). Its introduction across many global regions has been human-aided, mostly for ornamental purposes, although the dispersal of seeds by birds has been noted (Sharma, Raghubanshi, and Singh Citation2005). In South Africa, the species was introduced around the 1850s and it is currently listed as a category 1b invader, meaning trade, planting, and possession of L. camara is prohibited and where possible the plant should be removed (NEMBA Act 10 of 2004). To date, L. camara has invaded over 2 million hectares of land in South Africa, and impacts include the loss of native biodiversity and agricultural land resulting in reduced crop yields and income (Jevon and Shackleton Citation2015; Vardien et al. Citation2012). Although L. camara has several benefits, such as medicinal, making baskets, furniture, ornamental and use as a hedge (Kannan, Shackleton, and Shaanker Citation2014; Vardien et al. Citation2012), some studies (e.g., Shackleton et al. Citation2017a; Barahukwa et al. Citation2023) have acknowledged that the negative impacts associated with L. camara invasion seem to outweigh these benefits.
The integration of human perceptions and ecological surveys is important as it balances social, economic, and environmental concerns to try and understand global environmental problems, such as invasion by IAPS like L. camara. Such integration can result in a better understanding of ecosystem regime shifts and systems dynamics linked to IAPS at the landscape level. Knowledge generated from such integrative studies is crucial in developing appropriate IAPS response strategies. Considering this context, we aimed to assess human perceptions, knowledge, and uses of L. camara in rural communities in Vhembe Biosphere Reserve, Limpopo Province of South Africa. Specifically, we used a two-pronged approach to examine invasion perceptions and extent on household yards. The two-pronged approach included (i) face-to-face household interviews to solicit local knowledge, perceptions, and uses of L. camara by the rural communities, and (ii) detailed vegetation surveys on all household yards to gather information on invasion extent within each household yard.
Methods
Human Ethics
The research was in accordance with Rhodes University Ethical Standards guidelines and only commenced after it was approved by the university human ethics committee (Rhodes University Ethics Number 2019-0795-799). Before the research study commencement, permission was sought from all six village leaders and ward councilors. Before conducting household interviews, informed consent was obtained following a detailed explanation of the study objectives to the respondent. Confidentiality assurance and response anonymity were explained to all respondents before conducting the interview. Participation was voluntary and the right to participation withdraw at any given time without penalty was granted. Also, participants had the right to decline responses to a particular question that they were not comfortable with. In this study, no personal information was collected, and data were anonymized through assigning numbers to every questionnaire.
Study Area
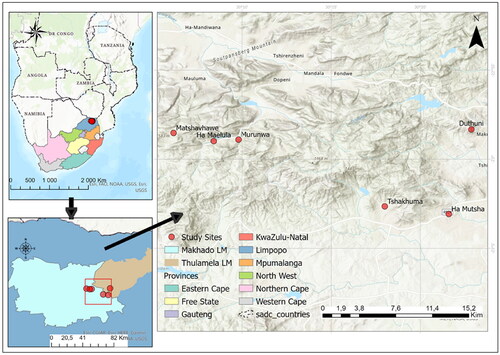
Household interviews were conducted in six villages that are in the Vhembe Biosphere Reserve, Limpopo Province of South Africa (). The six villages are Duthuni (22.9722°S, 30.3810°E), Tshakhuma (23.0441°S, 30.3001°E), Murunwa (22.9816°S, 30.1633°E), Matshavhawe (22.9755°S, 30.1026°E), Ha Mutsha (23.0512°S, 30.3599°E), and Ha Maelula (22.9831°S, 30.1402°E) (Mhlongo Citation2021). Besides Duthuni which is in the Thulamela Municipality, all other villages are in Makhado Municipality. The population sizes of the villages range from 207 (Ha Mutsha) to 6 345 (Duthuni), with most people heavily dependent on subsistence farming and government social grants (Stats SA (Statistics South Africa) Citation2011). The villages are in Soutpansberg Mountain Bushveld vegetation (Mucina and Rutherford Citation2006) comprising of dense trees and shrubs, such as Vachelia karoo, Catha edulis, Berchemia zeyheri, Bridelia mollis, Searsia magalismontana, and Helichrysum kraussii with a poorly developed grassy layer understory (Mucina and Rutherford Citation2006). The mean annual precipitation is ∼1050 mm and most of the rain falls in austral summer (October to March). Temperatures are hot in summer (averaging 28 °C) and mild in winter (averaging 18 °C), whereas soils are generally clay with high acidic content (Mucina and Rutherford Citation2006).
Household Interview Surveys
The six villages were selected because observations during a 2019 reconnaissance study showed that most household yards were dominated by L. camara. Within each village, 50 face-to-face household interview surveys were conducted in 2019, with purposive sampling being used to select the households (Mhlongo Citation2021). A household was selected for interview if L. camara was present in the homestead and if the household had knowledge of the plant. Knowledge of the plant was assessed by showing the respondent a picture panel of the plant and a plant sample in their yard. Although purposive sampling has selection bias, it was preferred to limit the respondents to those who have the plant in their household yards and have knowledge of the plant. Also, by purposively selecting respondents who have the plant in their household yard, it created conditions for ecological data to be collected. At each selected household one adult member was interviewed, and interviews were conducted during weekdays between 08h00 and 16h00 using the local Tshivenda language. Each interview took approximately an hour, and interviews were conducted with the assistance of a local researcher who could speak the language (Mhlongo Citation2021).
The questionnaire consisted of four sections, with the first section gathering information on knowledge and perceptions regarding L. camara. The questions on knowledge and perceptions gathered information on invasion introduction and extent of properties. The second section gathered information on L. camara benefits and negative impacts that villagers experienced. Perceived benefits and negative impacts were identified by asking each respondent to indicate either L. camara is harmful, beneficial, has no impact, or both beneficial and harmful (Shackleton, Le Maitre, and Richardson Citation2015). The third section gathered information on the management of the plant, with questions gathering information on methods of controlling L. camara at the household level as well as the type of management support that respondents are receiving. The last section gathered information on the demographics of the respondents (see Supplementary Appendix S1 for interview guide).
Household Yard Ecological Surveys
Ecological surveys were conducted at each household yard were face-to-face interviews were conducted by counting all the L. camara shrubs present in the yard (Mhlongo Citation2021). For each counted L. camara, plant height and canopy cover were measured using a tape measure, whilst plant basal diameter was measured using a Vernier caliper. Canopy cover was measured using the plant’s widest canopy area of influence, by placing a tape measure from one edge of the tree crown to another edge through the crown center. For multi-stemmed plants, the diameter of all stems was measured and averaged to represent the plant’s diameter (Mhlongo Citation2021).
Data Analysis
Responses from all household interviews were grouped into emerging themes aimed at describing villagers’ knowledge, perceptions, impacts (both positive and negative), and management interventions. Thematic analysis was used to identify the perceived impacts (both positive and negative) and management options of L. camara. Descriptive statistics were used to show the distribution of data and the proportion of responses across villages using tables and figures. Chi-Square tests (χ2) were conducted to assess responses on L. camara knowledge, perceptions, impacts (both positive and negative), and management across the different villages.
The ecological data from household yard surveys were analyzed using one-way analysis of variance (ANOVA) to compare vegetation measurements across the six villages. Where significant differences were observed, a Tukey-HSD homogenous post-hoc test was used to assess differences across villages. Proof of normality and homogeneity of variances were tested using the Kolmogorov-Smirnov tests and Levene’s tests, respectively, and data were normally distributed. Multiple regression analysis was conducted to assess the relationship between some social factors (i.e., age, gender, education level, household income, L. camara occurrence levels, L. camara impact levels, spread at the household level, spread in the area, and management responses) on ecological household yard data (L. camara abundance, height, diameter, and canopy cover). Data for these social factors was obtained from household interview responses. We acknowledge that the above-mentioned social data was collected using purposive sampling, therefore regression analysis results could limit our understanding of the patterns within the responses since our social data was not a reflection of the entire community. Regardless of the above shortcomings, the social data gathered from interview responses was used because previous studies have shown that these indicators can influence species abundance and spread (Thondhlana and Ruwanza Citation2020; Choksi et al. Citation2023). All statistical analyses were performed using STATISTICA version 14.0 software (TIBCO Software Inc Citation2019).
Results
Demographics of the Sample Population
Most of the respondents across all villages were female (70%), with Matshavhawe village having a high number of females (88%) (). The average number of people per household across all villages was seven, being high in Matshavhawe and low in Duthuni and Tshakhuma villages (). The dominant age range across all villages was 61–70 years. The age range of 21–30 years had the least number of respondents (3%) across all villages. Like most rural communities in South Africa, most respondents (36%) across all villages had a primary education level, with more than half of them being in Matshavhawe village (). Across all villages, only a handful of the respondents had a university education. Across all villages, most respondents were dependent on social grants and formal employment ().
Table 1. Demographics of the sampled population in six villages located in Vhembe Biosphere Reserve, Limpopo Province of South Africa.
Knowledge and Perceptions of L. camara
All the respondents (100%) in Tshakhuma, Matshavhawe, and Ha Mutsha did not plant L. camara on their properties, however, few respondents in Duthuni (4%), Murunwa (2%), and Ha Maelula (2%) planted L. camara on their properties, though these results showed no significant differences (χ2 = 23.1, p > 0.05; ). Most of the villagers who did not plant L. camara said it germinated naturally on the property (82%), with the remainder stating that they found it present when they moved onto the property. Although few respondents (26%) across all villages reported that L. camara was spreading on their properties, most respondents (46%) noted that the plant was spreading in the area (). Of those who noted L. camara spread in the area, more than half were in Tshakhuma (64%), Murunwa (54%), and Ha Mutsha (56%), and statistical comparisons across villages showed significant differences (χ2 = 47.4, p < 0.001; ). Few respondents (35%) across all villages knew that L. camara is an IAPS, with most people being in Matshavhawe (64%) and Tshakhuma (40%). Comparisons across all villages showed significant differences for those who knew L. camara invasion status (χ2 = 47.4, p < 0.001; ). Respondents were asked about the level of L. camara occurrence on their property and most of them reported scarce (47%), very scarce (29%), and moderate (21%) occurrences across all villages (). The bulk of the respondents who reported very scarce occurrences were in Duthuni (50%), whereas those who recorded scarce occurrences were in Murunwa (63%) and Matshavhawe (54%; ). Only a few respondents in Tshakuma (4%) reported very common occurrences on their properties ().
Figure 2. Community responses to questions related to Lantana camara occurrence on household properties located in Vhembe Biosphere Reserve. Bars are average percentages of respondents and n = 50 per village.
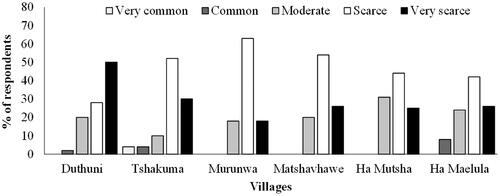
Table 2. Respondents’ responses to questions relating to knowledge and perceptions of Lantana camara across the six villages in Vhembe Biosphere Reserve.
Benefits and Negative Impacts of L. camara
Most respondents in Duthuni (60%), Tshakhuma (58%), Ha Maelula (64%), Murunwa (69%), Matshavhawe (60%), and Ha Mutsha (71%) were of the view that L. camara had no impact on their livelihoods. No significant differences (χ2 = 6.1, p > 0.05) were observed in their responses with regard to impacts on livelihoods. Across all villages, only 25% of the respondents thought that L. camara is harmful and only 9% of the respondents highlighted it was beneficial (). Most of the respondents who view L. camara as harmful were in Ha Maelula (34%), whereas those who view it as beneficial were in Matshavhawe (18%; ). Respondents were asked to list L. camara benefits and few respondents listed medicinal (6%; χ2 = 11.9, p < 0.05) and fruit consumption (9%; χ2 = 21.6, p < 0.05), and these benefits showed significant differences across villages. The most common medicinal benefits stated by respondents were the treatment of sore eyes and teeth as well as the treatment of colds and fevers. The remainder of the benefits, such as fence/hedge, ornamental, and mosquito repellent were mentioned by <5% of the respondents across all the villages, and these showed no significant differences (p > 0.05; see for χ2 values). A total of eight negative impacts associated with L. camara were listed by the respondents, with the plants pricking thorns, toxic to livestock when grazed, and the ability to outcompete native vegetation for resources being listed by more than 10% of the respondents across all villages (). All these negative impacts showed significant differences across villages (p < 0.001; see for χ2 values). Negative impacts, such as causing skin irritation, the proliferation of L. camara as a weed everywhere, its bad odor, water extraction, and ability to harbor snakes were mentioned by <10% of the respondents across all villages (). Statistical comparisons of the above-mentioned negative impacts across all villages showed significant differences (p < 0.05; see for χ2 values).
Figure 3. Community responses to questions related to Lantana camara impacts in Vhembe Biosphere Reserve. Bars are average percentages of respondents and n = 50 per village.
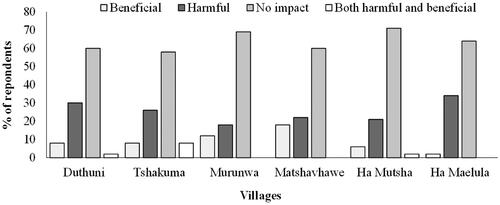
Table 3. Respondent’s views of the benefits and negative impacts of Lantana camara across the six villages in Vhembe Biosphere Reserve.
Management of L. camara
The majority of the respondents (76%) across all the villages would be happy to see a decrease in L. camara in the area, although results showed no statistical differences (χ2 = 18.5, p > 0.05; ). Similarly, most of the respondents across all the villages manage the plant on their properties, and results showed significant differences across villages (χ2 = 25.8, p < 0.001; ). Respondents were asked to elaborate on how they manage the plant on their property and most of the villagers (98%) across all villages were cutting the plant near the base using axes and burning the plant residues, with only a few villagers (2%) using chemicals to control L. camara. Those who do not use chemicals to control the plants listed cost as a barrier to adopting the chemical control method. Only a handful of respondents (5%) across all villages have received information regarding the management of IAPS from tribal gatherings and through word of mouth from fellow villages who mostly interact with the WfW programme, and results showed significant differences (χ2 = 20.1, p < 0.001) across villages (). The bulk of those who received management information are in Tshakhuma (18%), with those in Matshavhawe (0%) highlighting that they received no information at all. A few of the respondents in Tshakhuma (10%), Murunwa (6%), Ha Maelula (6%), and Ha Mutsha (2%) stated that they have received assistance from government to manage L. camara (). The WfW programme was cited as the main organization responsible for assisting in managing L. camara, with assistance mostly being information on how to manage the plant. Most respondents (67%) across all villages highlighted that they want assistance (i.e., tools, chemicals, and clearing employment contracts) from the government (). Although results showed no significant differences across villages (χ2 = 7.3, p > 0.05), most respondents who wanted assistance were in Duthuni (72%), Ha Maelula (72%), and Murunwa (61%).
Table 4. Perceptions on management of lantana camara across the six villages in Vhembe Biosphere Reserve.
The Influence of Social Factors on L. Camara Invasion Extent
The average abundance of L. camara at each household property ranged from 2.1 ± 0.39 trees (Tshakuma) to 2.6 ± 0.19 trees (Ha Mutsha), and there were no significant differences across all villages (ANOVA, F = 0.737, p = 0.596). The height of L. camara in all properties across all villages averaged 1.31 ± 0.06 m. Although average plant height was slightly high in Ha Mutsha (1.36 ± 0.04 m) compared to Duthuni (1.25 ± 0.05 m), no significant differences (ANOVA, F = 1.114, p = 0.351) were observed across villages. In contrast, both L. camara diameter (ANOVA, F = 4.956, p < 0.001) and cover (ANOVA, F = 14.045, p < 0.001) were significantly different across all the villages, with large and tall trees recorded in Tshakhuma compared to Ha Mutsha. Overall, the regression model explained <10% of the variance in L. camara abundance (8%), height (2%), diameter (3%), and canopy cover (3%; ), an indication that the social factors had little influence on L. camara ecological data. However, a closer examination of the regression model shows that only household income, L. camara occurrence, and impact levels showed significant negative regression coefficients with L. camara abundance (). This implies that respondents who received social grants recognized the increase in L. camara abundances as compared to pensioners. Also, respondents who said L. camara is very common and beneficial noted an increase in its abundance. Only, one factor (i.e., L. camara impact levels) showed a significantly negative relationship with species height, meaning respondents who said L. camara is beneficial noticed its increase in height. From a diameter standpoint, households who reported L. camara as a commonly occurring species with benefits tend to notice its increase in diameter (). Only respondent age and income showed a significantly negative regression coefficient with L. camara cover. The above-mentioned result implies that young respondents were likely to notice an increase in L. camara cover compared to older respondents who noticed an inverse relationship. Also, social grant recipients noticed an increase in plant cover compared to pensioners.
Table 5. Multiple regression results showing the influence of social responses on ecological data, namely Lantana camara abundance, height, diameter, and cover.
Discussion
We used a two-pronged approach to assess perceptions, knowledge, uses, and L. camara invasion extent in six rural communities located in the Vhembe Biosphere Reserve, Limpopo Province of South Africa. Most of the villagers are familiar with L. camara but do not know that it is an IAPS that can transform the ecosystem. Previous studies (e.g., Shackleton et al. Citation2019a; Ruwanza and Thondhlana Citation2022) have reported that knowledge about IAPS by local rural communities is linked to numerous factors like benefits, invasion extent, and history. For example, it has been reported that IAPS, such as Acacia dealbata (Ngorima and Shackleton Citation2019) and P. guajava (Ruwanza and Thondhlana Citation2022) are perceived as native species by rural communities due to livelihood benefits. In other studies, IAPS with a long duration in an area is regarded as native by local communities (Shackleton et al. Citation2019a). This is more applicable in areas with harsh climatic conditions, such as semi-arid regions where IAPS that were introduced a long time ago now dominate the landscape compared to native species which naturally have low densities (Shackleton and Shackleton Citation2018). Our study species (i.e., L. camara) was introduced in South Africa around 1850s and now invades more than 2 million hectares (Vardien et al. Citation2012), thus its long history in the environment could result in most villagers viewing it as native since it is now integrated within the natural environment. This observation is consistent with Ngorima and Shackleton (Citation2019) who reported that A. dealbata long history in the Eastern Cape Province of South Africa has resulted in local people regarding it as native.
A third of the respondents reported that L. camara is spreading both within household properties and in the greater region. The view that L. camara is spreading has been reported in previous studies in South Africa (e.g., Jevon and Shackleton Citation2015) and globally (e.g., Sundaram et al. Citation2012; Shackleton et al. Citation2017a). Jevon and Shackleton (Citation2015) reported that respondents in Mazeppa Bay, Eastern Cape Province of South Africa reported low abundances of L. camara in the 1960s but noted increased spread over time. Also, villagers in the above-mentioned study reported that L. camara spread was associated with increased loss of non-timber forest products and agricultural land which subsequently resulted in loss of livelihoods. In India, Sundaram et al. (Citation2012) reported that L. camara was spreading in the Soliga village and community members attributed the spread to the plants prolific fruit output, seed dispersal, and changes in fire regimes. Indeed, several studies have shown that L. camara spread is linked to both species and ecosystem traits. For example, Ruwanza and Shackleton (Citation2016) reported that L. camara has an ability to manipulate some soil properties, such as total carbon and phosphorus to enhance its spread. A bioclimatic modeling study by Vardien et al. (Citation2012) showed that Limpopo province, where this study was conducted, provides suitable environmental conditions for L. camara spread. The above-mentioned observation was also reported by Subhashni and Lalit (Citation2014) who concluded that current and future climatic conditions in Limpopo province are suitable for L. camara spread. Therefore, views by local communities in this study that suggested that L. camara is spreading concur with the above-mentioned climatic modeling research.
The benefits associated with L. camara were reported by few villages. Benefits were more around provisional services, such as medicinal, fruit consumption, fence, and mosquito repellent. Jevon and Shackleton (Citation2015) in the Eastern Cape Province of South Africa confirmed that L. camara has no substantial uses amongst rural communities, with few uses, such as eating ripe fruits and the addition of leaves to food to improve taste. The above-mentioned study suggested that the growing of L. camara as a fence/hedge seems to be by chance and people hardly plant it on their property as was observed in this study. In other areas, such as India, L. camara has many benefits and economic uses, e.g. medicinal, ornamental, mulching, and biomonitoring indicators for air quality (Negi et al. Citation2019).
Although respondents listed several negative impacts associated with L. camara, the proportion of people who mentioned the negative impacts was relatively small with impacts, such as pricking thorns, toxic to livestock, and ability to outcompete native vegetation being mentioned by more than 10% of the respondents across all villages. In Uganda, Shackleton et al. (Citation2017a) reported that L. camara has negative effects on livestock by hindering their movement, whereas Machado et al. (Citation2023) reported that direct consumption of the plant by animals may result in death, reduced fecundity, and animal sickness. Effects on livestock have a significant bearing on rural people’s livelihoods as most people are dependent on livestock production and use cattle for cultivation purposes. It has been noted that L. camara invasion can reduce livestock forage by 50% and crop yields by 26–50%, resulting in substantial household income losses (Shackleton et al. Citation2017a). The ability of L. camara to outcompete native species for resources has been reported in the past. For example, L. camara’s ability to outcompete native plants has been attributed to several direct and indirect factors, such as its ability to alter soil nutrient content to its advantage, creation of monostands, altering fire intensity, and high-water uptake at the expense of neighboring plants (Turner and Downey Citation2010). Also, L. camara is allelopathic and releases allelochemicals (e.g. phenolic compounds and triterpenes) that suppress germination and growth of other plants (Kato-Noguchi and Kurniadie Citation2021). It is important to note that perceptions regarding the negative impacts of plant invasion differ between communities and managers/ecologists, an indication that management could be difficult in cases where views are conflicting, this is also suggested by Zengeya et al. (Citation2017) who forged the term “conflict generating species” to refer to species that have both costs and benefits.
Although both benefits and negative impacts were mentioned by some villagers, a closer look at our results seems to suggest that L. camara benefits are few compared to negative impacts an indication that negative impacts might be outweighing benefits. If our above observation based on these results is correct, then management of L. camara should be relatively easy given that the plant has few benefits to the community. In any case, we reported that villagers are cutting the plant to control it. Lantana camara seems to have more costs than benefits and it is spreading, implying that the costs are likely to increase in the future, thus effects on human livelihoods could increase. From a management standpoint, some studies have shown that IAPS with few benefits are easy to manage because there will be less conflicts among stakeholders (Zengeya et al. Citation2017; Shackleton et al. Citation2019a).
Local people are trying to manage L. camara, however our results showed that they lack support. Most of the interviewed respondents were controlling L. camara, a result that is similar to efforts by community members in Uganda who are managing the plant at the local level (Shackleton et al. Citation2017a). Local people are managing L. camara by cutting it near the base using an ax and burning the residual once dry (Mhlongo Citation2021), a popular and cheap method used globally (Sharma, Raghubanshi, and Singh Citation2005; Sundaram et al. Citation2012; Shackleton et al. Citation2017a). However, if cut and burn is not done properly there exists a potential that the plant will resprout and grow back, therefore this speaks to the need to educate local people on effective methods to manage the plant. On the other hand, our results showed that villages hardly get the much-needed knowledge and information about the plant, as this is hardly given to them. Lack of information, knowledge, or any form of community assistance to manage IAPS has been noted in the past (e.g., Ngorima and Shackleton Citation2019). Respondents indicated the need for government assistance and support (e.g. clearing tools) to help them manage L. camara. Indeed, the WfW programme does most of the clearing, however, it is not possible for them to clear all the areas thus the need to formulate a management strategy that supports local people who are willing to clear the plant on their properties. Given that controlling IAPS is costly and labor intensive, developing a model that supports local clearing initiatives is likely to cut the WfW costs through travel budgets to the clearing sites. Biocontrol for L. camara in South Africa’s inland regions has been reported to be less effective (Simelane, Katembo, and Mawela Citation2021), thus other initiatives, such as supporting clearing by local villagers need to be considered. Such support to local villagers could be in the form of cutting equipment, such as axes and chain saws, brochures with detailed information on how to properly cut or remove the plant, and chemicals for spraying the cut stumps.
Vegetation surveys at the household yard level revealed that L. camara is present in most properties. Although we recorded few abundances (averaging 2–3 plants per household yard), it is possible that household clearing that was mentioned by respondents is reducing L. camara abundances. However, of concern is that future distribution models predict that the plant will expand in Limpopo (Vardien et al. Citation2012), and given L. camara’s invasion aggressiveness it is possible that the plant will proliferate on household yards in the future. Besides that, the high rainfall received in this area creates favorable conditions for L. camara to grow and expand on these properties (Vardien et al. Citation2012). In addition, L. camara tends to thrive in disturbed areas (Vardien et al. Citation2012), and household yards are highly disturbed. For example, most households in the study area practice home gardening (Semenya and Maroyi Citation2020) and clear land for construction, therefore, there exists a huge opportunity for L. camara to take advantage of the local household disturbance to spread.
Although our regression analysis had shortfalls, i.e., using purposively sampled household social data which is not a representation of the entire community, our regression model results are explanatory and offer the potential to explain the observed respondent patterns. Using the regression model results, L. camara abundance and increase was widely observed by social grant holders and respondents who perceive it to be commonly occurring and beneficial. Indeed, a previous study (e.g., Shackleton et al. Citation2019b) highlighted that IAPS with high occurrences and are beneficial to communities tend to shape people’s perceptions. However, it was the young and those dependent on social grants who observed L. camara abundance and cover increase at household and area levels. This could be because the young and poor members of the community who depend on social grants are the most common users of L. camara, thus the tendency to notice its abundance and occurrence. Indeed, some studies (e.g., Ngorima and Shackleton Citation2019; Reynolds et al. Citation2020) have reported that poor members of the community are dependent on natural resources including IAPS. Our result contradicts the notion that older people (in this case pensioners) who are perceived to have stayed in an area for a long time tend to notice IAPS occurrence changes. For example, Jevon and Shackleton (Citation2015) reported that only older people in Mazeppa Bay, Eastern Cape Province of South Africa could relate to the introduction and expansion of L. camara in the area as compared to the younger population and those who are new in the area. Overall, our regression model showed that the selected social factors hardly explained L. camara ecological data. Although this result cannot be generalized both at the species and landscape level, this could be because most respondents dislike L. camara and hardly see its benefits, therefore they hardly pay attention to the plant.
Conclusion
Despite its abundance in household yards and in the area, most of the respondents were not benefiting from the plant. Most respondents did not know that L. camara is an IAPS and this indicates that education and awareness campaigns on IAPS may play a role in helping communities navigate the tradeoffs between managing invasive versus native plants and to successfully implement an effective invasive species management plan. It is possible that knowledge and perceptions on L. camara in these villages are shaped by the plant’s invasion history and extent in the area rather than its benefits since few benefits were mentioned. Local people are taking the initiative to control the plant in household yards, but they need financial support, information on plant management, and tools to enhance their current control initiatives. Local initiatives to manage L. camara at the household level need to be supported and incorporated into the national and regional plans to manage IAPS. Supporting current community and individual clearing initiatives could substantially reduce L. camara invasion extent as well as reduce clearing costs by management agencies since they will no longer need to contract people to clear the plant. Education and awareness campaigns on IAPS are important if the management of IAPS by local communities is to yield positive results. Local people possess important indigenous knowledge that can be used to manage IAPS, therefore clearing programme, such as WfW should tap into this knowledge to develop effective community-based alien plant control plans.
usnr_a_2338773_sm4857.docx
Download MS Word (25 KB)Acknowledgments
This paper has resulted from research work completed for Master’s Thesis at Rhodes University in 2021, entitled “Perceptions of the role of Lantana camara on human well-being and rural livelihoods in Vhembe Biosphere Reserve, South Africa.” Thanks to the National Research Foundation (NRF: research grant UID number 137789) for funding to conduct fieldwork. Also, thanks to Rhodes University and University of Venda for providing transport for fieldwork. We also thank Lily Munzhedzi for assisting with fieldwork. We are grateful to the village leaders for permission to conduct this study in the villages. We thank all the villagers who participated in this study. TD acknowledges funding from the NRF (UID number 138206).
Data Availability Statement
The data that support our research findings are available from the corresponding author on request.
Additional information
Funding
References
- Bang, A., R. N. Cuthbert, P. J. Haubrock, R. D. Fernandez, D. Moodley, C. Diagne, A. J. Turbelin, D. Renault, T. Dalu, and F. Courchamp. 2022. Massive economic costs of biological invasions despite widespread knowledge gaps: A dual setback for India. Biological Invasions 24 (7):2017–39. doi: 10.1007/s10530-022-02780-z.
- Barahukwa, A., C. A. Chapman, M. Namaganda, G. Eilu, P. A. Omeja, and M. J. Lawes. 2023. The effects of the invasive species, Lantana camara, on regeneration of an African rainforest. African Journal of Ecology 61 (2):451–60. doi: 10.1111/aje.13133.
- Choksi, P., M. Kotian, Z. Burivalova, and R. DeFries. 2023. Social and ecological outcomes of tropical dry forest restoration through invasive species removal in central India. Ecological Indicators 155:111054. doi: 10.1016/j.ecolind.2023.111054.
- Day, M. D., and S. Neser. 2000. Factors influencing the biological control of Lantana camara in Australia and South Africa. In Proceedings of the X International Symposium on Biological Control of Weeds, 4–14 July 2000, ed. N. R. Spencer, 897–908. Bozeman, MT: Montana State University.
- Dutta, H. 2018. Insights into the phenomenon of alien plant invasion and its synergistic interlinkage with three current ecological issues. Journal of Asia-Pacific Biodiversity 11 (2):188–98. doi: 10.1016/j.japb.2018.03.002.
- Eschen, R., T. Beale, J. M. Bonnin, K. L. Constantine, S. Duah, E. A. Finch, F. Makale, W. Nunda, A. Ogunmodede, C. F. Pratt, et al. 2021. Towards estimating the economic cost of invasive alien species to African crop and livestock production. CABI Agriculture and Bioscience 2 (1):18. doi: 10.1186/s43170-021-00052-9.
- Fantle-Lepczyk, J. E., P. J. Haubrock, A. M. Kramer, R. N. Cuthbert, A. J. Turbelin, R. Crystal-Ornelas, C. Diagne, and F. Courchamp. 2022. Economic costs of biological invasions in the United States. The Science of the Total Environment 806 (Pt 3):151318. doi: 10.1016/j.scitotenv.2021.151318.
- Faulkner, K. T., A. Burness, M. J. Byrne, S. Kumschick, K. Peters, M. P. Robertson, D. L. Saccaggi, O. L. F. Weyl, and V. L. Williams. 2020. South Africa’s pathways of introduction and dispersal and how they have changed over time. In Biological invasions in South Africa, ed. B. W. van Wilgen, J. Measey, D. M. Richardson, J. R. Wilson, and T. A. Zengeya, 313–54. Berlin: Springer.
- Haubrock, P. J., A. J. Turbelin, R. N. Cuthbert, A. Novoa, N. G. Taylor, E. Angulo, L. Ballesteros-Mejia, T. W. Bodey, C. Capinha, C. Diagne, et al. 2021. Economic costs of invasive alien species across Europe. NeoBiota 67:153–90. doi: 10.3897/neobiota.67.58196.
- Hirsch, H., M. H. Allsopp, S. Canavan, M. Cheek, S. Geerts, C. J. Geldenhuys, G. Harding, B. P. Hurley, W. Jones, J.-H. Keet, et al. 2020. Eucalyptus camaldulensis in South Africa – Past, present, future. Transactions of the Royal Society of South Africa 75 (1):1–22. doi: 10.1080/0035919X.2019.1669732.
- Jevon, T., and C. M. Shackleton. 2015. Integrating local knowledge and forest surveys to assess Lantana camara impacts on indigenous species recruitment in Mazeppa Bay, South Africa. Human Ecology 43 (2):247–54. doi: 10.1007/s10745-015-9748-y.
- Kannan, R., C. M. Shackleton, and R. U. Shaanker. 2014. Invasive alien species as drivers in socio-ecological systems: Local adaptions towards use of Lantana in Southern India. Environment, Development and Sustainability 16 (3):649–69. doi: 10.1007/s10668-013-9500-y.
- Kato-Noguchi, H., and D. Kurniadie. 2021. Allelopathy of Lantana camara as an invasive plant. Plants 10 (5):1028. doi: 10.3390/plants10051028.
- Le Maitre, D. C., M. Gaertner, E. Marchante, E. Ens, P. M. Holmes, A. Pauchard, P. J. O’Farrell, A. M. Rogers, R. Blanchard, J. Blignaut, et al. 2011. Impacts of invasive Australian acacias: Implications for management and restoration. Diversity and Distributions 17 (5):1015–29. doi: 10.1111/j.1472-4642.2011.00816.x.
- Leishman, M. R., and R. V. Gallagher. 2016. Will alien plant invaders be advantaged under future climates? In Biological invasions in changing ecosystems, ed. J. Canning-Clode, 368–88. Warsaw; Berlin: De Gruyter Open Ltd.
- Machado, M., L. G. S. Oliveira, C. O. Schild, F. Boabaid, M. Lucas, F. Buroni, M. B. Castro, and F. Riet-Correa. 2023. Lantana camara poisoning in cattle that took refuge during a storm in a forest invaded by this plant. Toxicon 229:107124. doi: 10.1016/j.toxicon.2023.107124.
- Mhlongo, E. S. 2021. Perceptions of the role of Lantana camara on human well-being and rural livelihoods in Vhembe Biosphere Reserve, South Africa. MSc thesis, Rhodes University.
- Mucina, L., and M. C. Rutherford. 2006. The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19. Pretoria: South African National Biodiversity Institute.
- Negi, G. C., S. Sharma, S. C. Vishvakarma, S. S. Samant, R. K. Maikhuri, R. C. Prasad, and L. M. Palni. 2019. Ecology and use of Lantana camara in India. The Botanical Review 85 (2):109–30. doi: 10.1007/s12229-019-09209-8.
- Ngorima, A., and C. M. Shackleton. 2019. Livelihood benefits and costs from an invasive alien tree (Acacia dealbata) to rural communities in the Eastern Cape, South Africa. Journal of Environmental Management 229:158–65. doi: 10.1016/j.jenvman.2018.05.077.
- Rai, P. K., and J. S. Singh. 2020. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecological Indicators 111:106020. doi: 10.1016/j.ecolind.2019.106020.
- Rai, R. K., H. Scarborough, N. Subedi, and B. Lamichhane. 2012. Invasive plants – Do they devastate or diversify rural livelihoods? Rural farmers’ perception of three invasive plants in Nepal. Journal for Nature Conservation 20 (3):170–6. doi: 10.1016/j.jnc.2012.01.003.
- Reynolds, C., N. Venter, B. W. Cowie, D. Marlin, S. Mayonde, C. Tocco, and M. J. Byrne. 2020. Mapping the socio–ecological impacts of invasive plants in South Africa: Are poorer households with high ecosystem service use most at risk? Ecosystem Services 42:101075. doi: 10.1016/j.ecoser.2020.101075.
- Richardson, D. M., F. C. Foxcroft, G. Latombe, D. C. Le Maitre, M. Rouget, and J. R. U. Wilson. 2020. The biogeography of South African terrestrial plant invasions. In Biological invasions in South Africa, ed. B. W. van Wilgen, J. Measey, D. M. Richardson, J. R. Wilson, and T. A. Zengeya, 67–96. Berlin: Springer.
- Ruwanza, S., and C. M. Shackleton. 2016. Effects of the invasive shrub, Lantana camara, on soil properties in the Eastern Cape, South Africa. Weed Biology and Management 16 (2):67–79. doi: 10.1111/wbm.12094.
- Ruwanza, S., and G. Thondhlana. 2022. People’s perceptions and uses of invasive plant Psidium guajava in Vhembe Biosphere Reserve, Limpopo Province of South Africa. Ecosystems and People 18 (1):64–75. doi: 10.1080/26395916.2021.2019834.
- Semenya, S., and A. Maroyi. 2020. Assessment of useful alien plant species cultivated and managed in rural home gardens of Limpopo Province, South Africa. Scientifica 2020:3561306. doi: 10.1155/2020/3561306.
- Shackleton, C. M., D. McGarry, S. Fourie, J. Gambiza, S. E. Shackleton, and C. Fabricius. 2007. Assessing the effects of invasive alien species on rural livelihoods: Case examples and a framework from South Africa. Human Ecology 35 (1):113–27. doi: 10.1007/s10745-006-9095-0.
- Shackleton, R. T., A. B. R. Witt, F. M. Piroris, and B. W. van Wilgen. 2017b. Distribution and socio-ecological impacts of the invasive alien cactus Opuntia stricta in eastern Africa. Biological Invasions 19 (8):2427–41. doi: 10.1007/s10530-017-1453-x.
- Shackleton, R. T., A. B. R. Witt, W. Aool, and C. F. Pratt. 2017a. Distribution of the invasive alien weed, Lantana camara, and its ecological and livelihood impacts in eastern Africa. African Journal of Range & Forage Science 34 (1):1–11. doi: 10.2989/10220119.2017.1301551.
- Shackleton, R. T., A. Novoa, C. M. Shackleton, and C. A. Kull. 2020. The social dimensions of biological invasions in South Africa. In Biological invasions in South Africa, ed. B. W. van Wilgen, J. Measey, D. M. Richardson, J. R. Wilson, and T. A. Zengeya, 697–726. Berlin: Springer.
- Shackleton, R. T., B. M. H. Larson, A. Novoa, D. M. Richardson, and C. A. Kull. 2019b. The human and social dimensions of invasion science and management. Journal of Environmental Management 229:1–9. doi: 10.1016/j.jenvman.2018.08.041.
- Shackleton, R. T., D. C. Le Maitre, and D. M. Richardson. 2015. Stakeholder perceptions and practices regarding Prosopis (Mesquite) invasions and management in South Africa. Ambio 44 (6):569–81. doi: 10.1007/s13280-014-0597-5.
- Shackleton, R. T., D. M. Richardson, C. M. Shackleton, B. Bennett, S. L. Crowley, K. Dehnen-Schmutz, R. A. Estévez, A. Fischer, C. Kueffer, C. A. Kull, et al. 2019a. Explaining people’s perceptions of invasive alien species: A conceptual framework. Journal of Environmental Management 229:10–26. doi: 10.1016/j.jenvman.2018.04.045.
- Shackleton, S. E., and R. T. Shackleton. 2018. Local knowledge regarding ecosystem services and disservices from invasive alien plants in the arid Kalahari, South Africa. Journal of Arid Environments 159:22–33. doi: 10.1016/j.jaridenv.2017.07.001.
- Shackleton, S. E., D. Kirby, and J. Gambiza. 2011. Invasive plants: Friends or foes? Contribution of prickly pear (Opuntia ficus-indica) to livelihoods in Makana municipality, Eastern Cape. South Africa. Development Southern Africa 28 (2):177–93. doi: 10.1080/0376835X.2011.570065.
- Sharma, G. P., A. S. Raghubanshi, and J. S. Singh. 2005. Lantana invasion: An overview. Weed Biology and Management 5 (4):157–65. doi: 10.1111/j.1445-6664.2005.00178.x.
- Simberloff, D., J.-L. Martin, P. Genovesi, V. Maris, D. A. Wardle, J. Aronson, F. Courchamp, B. Galil, E. García-Berthou, M. Pascal, et al. 2013. Impacts of biological invasions: What’s what and the way forward. Trends in Ecology & Evolution 28 (1):58–66. doi: 10.1016/j.tree.2012.07.013.
- Simelane, D. O., N. Katembo, and K. V. Mawela. 2021. Current status of biological control of Lantana camara L. (Sensu lato) in South Africa. African Entomology 29 (3):775–83. doi: 10.4001/003.029.0775.
- Stats SA (Statistics South Africa). 2011. Census 2011 Statistical Release–P0301.4. Accessed June 21, 2019.
- Subhashni, T., and K. Lalit. 2014. Impacts of climate change on invasive Lantana camara L. distribution in South Africa. African Journal of Environmental Science and Technology 8 (6):391–400. doi: 10.5897/AJEST2014.1705.
- Sundaram, B., S. Krishnan, A. J. Hiremath, and G. Joseph. 2012. Ecology and impacts of the invasive species, Lantana camara, in a social-ecological system in South India: Perspectives from local knowledge. Human Ecology 40 (6):931–42. doi: 10.1007/s10745-012-9532-1.
- Taylor, S., and L. Kumar. 2013. Potential distribution of an invasive species under climate change scenarios using CLIMEX and soil drainage: A case study of Lantana camara L. in Queensland, Australia. Journal of Environmental Management 114:414–22. doi: 10.1016/j.jenvman.2012.10.039.
- Thondhlana, G., and S. Ruwanza. 2020. Homestead tree holdings: Composition, uses and challenges in Checheche Growth Point, South East Lowveld, Zimbabwe. African Journal of Ecology 58 (2):260–71. doi: 10.1111/aje.12691.
- TIBCO Software Inc. 2019. Statistica (data analysis software system), version 14. Accessed June 19, 2022. http://tibco.com.
- Turner, P. J., and P. O. Downey. 2010. Ensuring invasive alien plant management delivers biodiversity conservation: insights from an assessment of Lantana camara in Australia. Plant Protection Quarterly 25:102–10.
- van Wilgen, B. W., and A. Wannenburgh. 2016. Co-facilitating invasive species control, water conservation and poverty relief: Achievements and challenges in South Africa’s working for water programme. Current Opinion in Environmental Sustainability 19:7–17. doi: 10.1016/j.cosust.2015.08.012.
- van Wilgen, B. W., G. C. Forsyth, D. C. Le Maitre, A. Wannenburgh, J. D. F. Kotzé, E. van den Berg, and L. Henderson. 2012. An assessment of the effectiveness of a large, national-scale invasive alien plant control strategy in South Africa. Biological Conservation 148 (1):28–38. doi: 10.1016/j.biocon.2011.12.035.
- van Wilgen, B. W., J. Measey, D. M. Richardson, R. U. Wilson, and T. A. Zengeya. 2020a. Biological invasions in South Africa: An overview. In Biological invasions in South Africa, ed. B. W. van Wilgen, J. Measey, D. M. Richardson, J. R. Wilson, and T. A. Zengeya, 3–32. Berlin: Springer.
- van Wilgen, B. W., J. R. Wilson, A. Wannenburgh, and L. C. Foxcroft. 2020b. The extent and effectiveness of alien plant control projects in South Africa. In Biological invasions in South Africa, ed. B. W. van Wilgen, J. Measey, D. M. Richardson, J. R. Wilson, and T. A. Zengeya, 597–628. Berlin: Springer.
- Vardien, W., D. M. Richardson, L. C. Foxcroft, G. D. Thompson, J. R. Wilson, and J. J. Le Roux. 2012. Invasion dynamics of Lantana camara L. (Sensu lato) in South Africa. South African Journal of Botany 81:81–94. doi: 10.1016/j.sajb.2012.06.002.
- Zengeya, T., P. Ivey, D. J. Woodford, O. Weyl, A. Novoa, R. Shackleton, D. Richardson, and B. Van Wilgen. 2017. Managing conflict-generating invasive species in South Africa: Challenges and trade-offs. Bothalia 47 (2):1–11. doi: 10.4102/abc.v47i2.2160.