Abstract
Purpose: Management of bleeding during surgery can be aided by the application of topical hemostatic agents. This study compared the hemostatic efficacy of a new powder agent containing collagen, chondroitin sulfate, and thrombin (PCCT) with a flowable gelatin-thrombin matrix with smooth particles (SmGM) in a porcine liver bleeding model. Materials and Methods: Lesions 4–6 mm deep and ∼10 mm in diameter were created in porcine livers and treated with either SmGM or PCCT. Bleeding rate and grade were quantified before and 3, 7, and 11 minutes after treatment. Results: Thirty-two lesions each were treated with SmGM or PCCT; the median (Q1, Q3) initial bleeding rate was comparable between the two groups (8.43 [6.18, 10.68] g/min and 7.15 [5.16, 9.63] g/min, respectively). The residual bleeding rate was significantly lower at all time-points post treatment for SmGM compared with PCCT (3 minutes: 0.14 [0.07, 0.21] versus 0.46 [0.20, 1.20] g/min, p < 0.0001; 7 minutes: 0.07 [0.04, 0.11] versus 0.12 [0.08, 0.39] g/min, p = 0.001; 11 minutes: 0.05 [0.03, 0.08] versus 0.07 [0.05, 0.12] g/min, p = 0.043). Bleeding grade at 3 minutes was also significantly lower for SmGM compared with PCCT (median [Q1, Q3] 0.0 [0.0, 0.0] versus 1.0 [1.0, 2.0], p < 0.0001). PCCT required reapplication in approximately one-third of applications due to insufficient hemostasis 4 minutes after initial application and showed a tendency to stick to the wet gauze during approximation. Conclusions: In this bleeding model, treatment with SmGM resulted in reduced blood loss, no need for reapplication and was easier to apply compared with PCCT.
INTRODUCTION
Management of bleeding during surgery or trauma is vital to optimize patient outcomes such as morbidity and mortality. The consequences of major bleeding are frequently treated with allogeneic blood product transfusion, with over 11 million units of red blood cells transfused in 2015 in the USA alone [Citation1]. However, transfusion of blood products is associated with significant costs as well as a range of adverse events, [Citation2–5] highlighting the importance of minimizing bleeding intraoperatively, thereby reducing transfusion requirements. Topical hemostatic agents can be used to supplement coagulation and help control bleeding following surgery or trauma either in conjunction with conventional procedures, or when conventional methods are inefficient or impractical [Citation6].
Powdered hemostatic agents have been developed for use in surgical procedures. These agents are typically mechanical in nature, with examples including polysaccharide-, mineral-, and gelatin-based powders which can create a barrier layer at the bleeding site [Citation7–9]. In comparison with flowable agents, powdered hemostatic agents can be directly applied without reconstitution and can cover large surface areas when the exact site of bleeding is not known; however, their efficacy has been limited to low-level bleeds. An active powdered hemostatic agent consisting of porcine collagen, bovine chondroitin sulfate, and plasma-derived human thrombin (PCCT) has recently been developed for direct application to the bleeding site [Citation10]. This is the first hemostatic agent to combine collagen powder with thrombin, and was launched in the EU and USA in 2018. Once applied, the collagen absorbs blood and concentrates coagulation factors and platelets, while the thrombin facilitates conversion of fibrinogen to fibrin for clot formation. PCCT was approved by the FDA in December 2017 for use in surgical procedures as an adjunct to hemostasis when control of minimal, mild, and moderate bleeding by conventional procedures is ineffective or impractical, except in neurosurgical, ophthalmic, and urological procedures [Citation10, Citation11]. The FDA approval was based on the results of the pivotal clinical study in patients undergoing either cardiothoracic, abdominal, or orthopedic lower extremity surgery, which demonstrated non-inferiority of PCCT relative to treatment with an absorbable gelatin sponge, USP, and recombinant thrombin [Citation10].
Flowable hemostatic agents comprised of a gelatin-thrombin matrix have also been successfully used to achieve hemostasis and reduce blood loss in a variety of surgical settings [Citation12–16]. These can typically be applied as a liquid or paste via a syringe, allowing precise application and conformation to wound contours [Citation6, Citation13]. One such agent is comprised of bovine gelatin combined with plasma-derived human thrombin to form a gelatin-thrombin matrix with smooth particles (SmGM). SmGM is indicated for use in surgical procedures (other than ophthalmic) as an adjunct to hemostasis when control of bleeding, by ligature or conventional procedures, is ineffective or impractical [Citation12, Citation17]. This agent can be rapidly prepared from a kit within 2–3 minutes, before application to the bleeding site with a syringe. Clinical trials in cardiac surgery demonstrated superior hemostatic efficacy with SmGM compared to alternative hemostatic agents [Citation14, Citation18]. Additionally, a systematic review evaluated SmGM use across a variety of surgical settings and concluded that SmGM is efficacious in terms of achieving complete and stable hemostasis, and resulted in reduced consumption of health resources compared with other hemostatic agents or conventional methods of hemostatic control [Citation12].
As yet, no published studies have compared the performance of PCCT with current standards of care, although a comparison with gelatin sponge plus thrombin in cardiothoracic surgery was recently completed (NCT02780869). Although there may be benefits relating to the ease of application and rapid preparation times with PCCT, it is vital to also determine the in vivo efficacy of this novel agent. We have therefore compared the hemostatic efficacy of SmGM and PCCT in a porcine liver punch biopsy model.
MATERIALS AND METHODS
The porcine liver punch biopsy model was selected as it represents a standard model of bleeding in pigs [Citation9, Citation19]. An animal experiment permit was issued by the municipal government of Vienna, and all experimental methods were consistent with the Guide for the Care and Use of Laboratory Animals of the US National Research Council [Citation20]. The bleeding model used was aimed to reflect a bleeding intensity below 25.3 mL/min/cm2, which is consistent with PCCT product labeling [Citation10, Citation21]. This translates into <20 mL/min for the wound size of 0.785cm2 used in this study. The study consisted of two treatment arms in three male pigs, each weighing approximately 35 kg. A total of 32 valid applications per test item were performed.
Animals were premedicated with Tiletamine ∼3.5 mg/kg and Zolazepam ∼3.5 mg/kg (Zoletil; Virbac, Bury St Edmunds, Suffolk, UK), Xylazin ∼1.6 mg/kg (Rompun, Bayer Austria GmbH, Vienna, Austria) and Butorphanol ∼0.33 mg/kg (Butomidor, Richter Pharma AG, Wels, Austria) intramuscular. Inhalation anesthesia consisting of isoflurane and oxygen was then administered via intubation. To ensure analgesia and maintain anesthesia, acetate-buffered crystalloid solution (Elomel; Fresenius Kabi, Graz, Austria; 10 mL/kg/h), sufentanil 0.008 mg/kg/h and rocuronium bromide 5 mg/kg/h were administered in a standardized fashion. No systemic heparinization was applied. A mean arterial blood pressure of 70–110 mmHg was aimed for, with 80 mmHg being an actionable level; hydroxyethyl starch (Voluven; Fresenius Kabi, Graz, Austria; 10 mL/kg/h) and Norepinephrine (Arterenol; Sanofi Aventis, Frankfurt, Germany; 5 μg/kg/h) administered in case of major blood loss or major drop in blood pressure, respectively.
Once the experiment was complete, animals were humanely euthanized under deep anesthesia with a lethal dose of thiopental sodium and embutramide/mebezonium iodine/tetracaine hydrochloride (T61; Hoechst Marion Roussel, Brussels, Belgium).
Surgical Procedure
Animals were placed in dorsal recumbency and, following insertion of jugular, carotid, and urinary catheters, a median laparotomy was performed and the liver was exposed. A baseline measurement of activated clotting time (ACT) was performed. Following preparation, a 10-mm diameter biopsy punch was used to create lesions ∼4–6 mm deep in the liver. Initial blood loss was quantified as described below, and lesions were excluded from the study if the bleeding rate was ≥20 mL/min. Lesions with bleeding rates below 20 mL/min were treated topically following an alternating pattern (ABAB) scheme with either SmGM (Floseal; Baxter Healthcare SA, Zurich, Switzerland) or PCCT (Hemoblast; Biom’up, Saint-Priest, France).
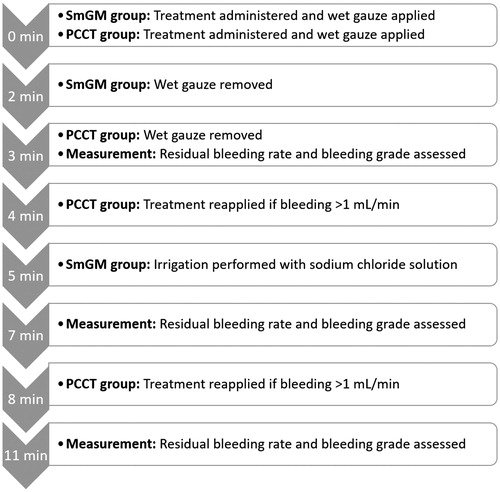
SmGM (lot number Y2T007-AA, expiry date October 2018) and PCCT (lot number BQF17018.134039, expiry date December 2018) were prepared and applied according to the Instructions for Use [Citation10, Citation17]. Following application of as much agent as was required to fill/cover the tissue lesions, a wet gauze was manually applied for 2 minutes (SmGM) or 3 minutes (PCCT) for approximation. In the case of sustained bleeding >1 mL/min (≥Grade 1 per the VIBe SCALE22), reapplication of PCCT was performed after 4 or 8 minutes; no reapplication was performed for SmGM. At the 5-minute time-point, irrigation with sodium chloride solution was used to remove excess material from the lesion for SmGM; no irrigation was performed for PCCT. A timeline for treatment application is given in .
Assessment of Bleeding and Quantification of Blood Loss
An assessment of bleeding severity was performed before hemostatic agent application, and at 3, 7, and 11 minutes after application. A validated intraoperative bleeding scale (VIBe SCALE) was used to assess bleeding: Grade 0, no bleeding (visually estimated rate of blood loss: ≤1.0 mL/min); Grade 1, ooze or intermittent flow (>1.0–5.0 mL/min); Grade 2, continuous flow (>5.0–10.0 mL/min); Grade 3, controllable spurting and/or overwhelming flow (>10.0–50.0 mL/min); Grade 4, unidentified or inaccessible spurting or gush (>50.0 mL/min)[Citation22]. The amount of blood loss was also quantified at the same time points from the amount of blood absorbed by a pre-weighed gauze over 30 seconds (6 seconds for the initial bleed).
Histology
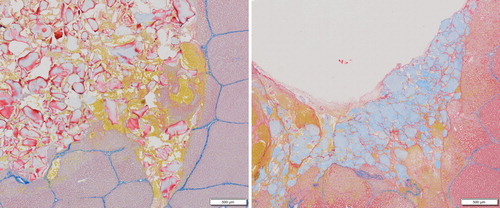
Four tissue samples were harvested postmortem for each hemostatic agent to obtain a representative histologic section. These were fixed in formalin for 24 hours and rinsed in tap water for 1 hour, before being immersed in 50% alcohol for 1 hour and storage in 70% alcohol. Microscopic assessment was then performed via staining with Martius, Scarlet and Blue (MSB), to detect fibrin formation, collagen content, and blood clot formation.
Statistics
Bleeding rates are reported as median values with 25% and 75% quartiles (Q1 and Q3) and as mean values with standard deviation (SD). All bleeding rates are reported in g/min, corresponding to mL/min due to the similar density of blood and water (1,060 kg/m3 vs. 1,000 kg/m3). Bleeding grades are reported as median values with 25% and 75% quartiles (Q1 and Q3). A D’Agostino–Pearson normality test revealed that the data sets were not normally distributed; therefore, a non-parametric Mann–Whitney test was used to determine the statistical significance of differences between groups. p-values ≤0.050 were considered statistically significant. GraphPad Prism (version 6.01 for Windows, GraphPad Software, La Jolla, California, USA) was used to conduct the statistical analyses.
RESULTS
Initial Bleeding Rates and Activated Clotting Time (ACT)
The ACT at the start of surgery was 77, 86, and 82 seconds, respectively, in the three animals used for this study. Mean initial bleeding rates were comparable between the two test groups (SmGM, mean ± SD 8.84 ± 3.96 g/min, median [Q1, Q3] 8.43 [6.18, 10.68] g/min; PCCT, 7.45 ± 3.03 g/min, 7.15 [5.16, 9.63] g/min; all lesions, 8.14 ± 3.57 g/min, 7.71 [5.38, 10.13] g/min).
Residual Bleeding Rates and Bleeding Grades
At 3 minutes after application of the hemostatic agent, bleeding rates were significantly lower with SmGM than PCCT (mean ± SD, 0.18 ± 0.18 g/min, median [Q1, Q3], 0.14 [0.07, 0.21] g/min versus 1.26 ± 1.97 g/min, 0.46 [0.20, 1.20] g/min; p < 0.0001) (). Bleeding rates were also significantly lower with SmGM than PCCT at the 7- and 11-minute time-points (7 minutes: 0.09 ± 0.05 g/min, 0.07 [0.04, 0.11] g/min versus 0.57 ± 1.36 g/min, 0.12 [0.08, 0.39] g/min, p = 0.001; 11 minutes: 0.07 ± 0.08 g/min, 0.05 [0.03, 0.08] g/min versus 0.11 ± 0.10 g/min, 0.07 [0.05, 0.12] g/min, p = 0.043).
FIGURE 2. Comparison of bleeding rates and grade for all lesions at 3, 7, and 11 minutes after application of the hemostatic agent. Horizontal line and box represent the median and interquartile ranges; error bars represent maximum/minimum values. Left side: residual bleeding rate for PCCT and SmGM. Right side: bleeding grades for PCCT and SmGM.
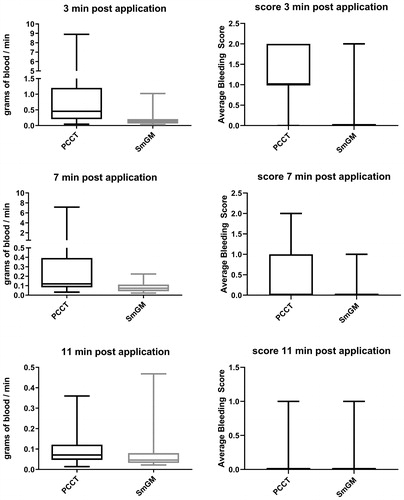
When considering bleeding grade, SmGM was significantly more efficient than PCCT at the 3-minute time-point (0.0 [0.0, 0.0] versus 1.0 [1.0, 2.0], p < 0.0001) (). At 7 and 11 minutes, the observed bleeding grade was lower for SmGM, although this was not significant (7 minutes: 0.0 [0.0, 0.0] versus 0.0 [0.0, 1.0], p = 0.064; 11 minutes: 0.0 [0.0, 0.0] versus 0.0 [0.0, 0.0], p = 0.426).
Residual bleeding rates and bleeding grades where initial bleeding was <8.1 g/min
To evaluate whether there were differences in efficacy between lesions with mild or moderate initial bleeding rates [Citation21], a further analysis was conducted, focusing on the results for lesions where the initial bleeding rate was lower than the average (8.1 g/min, ). This analysis included 15 lesions for SmGM and 19 for PCCT. At both the 3- and 7-minute time-points, the bleeding rate for SmGM was significantly lower than for PCCT (3 minutes: mean ± SD, 0.10 ± 0.07 g/min, median [Q1, Q3], 0.07 [0.06, 0.13] g/min versus 0.61 ± 0.75 g/min, 0.37 [0.18, 0.70] g/min; p = 0.0004; 7 minutes: 0.08 ± 0.05 g/min, 0.07 [0.04, 0.12] g/min versus 0.22 ± 0.25 g/min, 0.12 [0.08, 0.17] g/min; p = 0.013). By 11 minutes, there was no significant difference in bleeding rates (SmGM 0.08 ± 0.11 g/min, 0.05 [0.03, 0.06] g/min versus 0.11 ± 0.11 g/min, 0.06 [0.04, 0.18] g/min; p = 0.282).
FIGURE 3. Comparison of bleeding rates and grade for lesions with an initial bleeding rate lower than 8.1 mL/min at 3, 7, and 11 minutes after application of the hemostatic agent. Horizontal line and box represent the median and interquartile ranges; error bars represent maximum/minimum values. Left side: residual bleeding rate for PCCT and SmGM. Right side: mean bleeding grades for PCCT and SmGM.
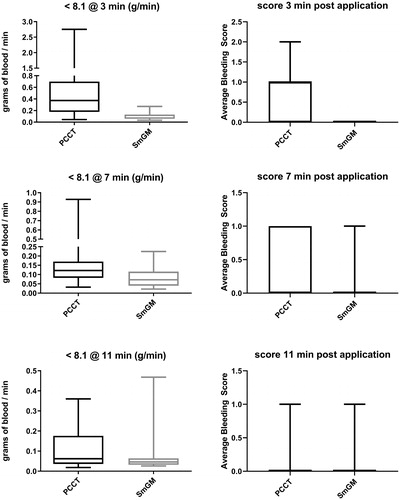
At the 3-minute time-point, the bleeding grade was significantly lower for SmGM than for PCCT (0.0 [0.0, 0.0] versus 1.0 [0.0, 1.0], p ≤ 0.0001). For both the 7- and 11-minute time-points, the bleeding grade was lower for SmGM but was not significant (7 minutes: 0.0 [0.0, 0.0] versus 0.0 [0.0, 1.0], p = 0.196; 11 minutes: 0.0 [0.0, 0.0] versus 0.0 [0.0, 0.0], p = 0.613).
Residual bleeding rates and bleeding grades where initial bleeding was >8.1 g/min
Lesions where the initial bleeding rate was higher than the average were also considered, with 17 lesions with SmGM (16 lesions for the 3-minute time-point) and 13 lesions with PCCT (). Bleeding rates were significantly lower with SmGM than PCCT at all time-points (3 minutes: mean ± SD, 0.25 ± 0.22 g/min, median [Q1, Q3], 0.20 [0.14, 0.28] g/min versus 2.16 ± 2.72 g/min, 0.89 [0.41, 3.27] g/min; p = 0.0001; 7 minutes: 0.09 ± 0.05 g/min, 0.09 [0.04, 0.12] g/min versus 1.07 ± 2.05 g/min, 0.11 [0.09, 1.44] g/min, p = 0.034; 11 minutes: 0.06 ± 0.03 g/min, 0.06 [0.03, 0.08] g/min versus 0.10 ± 0.08 g/min, 0.08 [0.06, 0.12] g/min; p = 0.042).
FIGURE 4. Comparison of bleeding rates and grade for lesions with an initial bleeding rate higher than 8.1 mL/min at 3, 7, and 11 minutes after application of the hemostatic agent. Horizontal line and box represent the median and interquartile ranges; error bars represent maximum/minimum values. Left side: residual bleeding rate for PCCT and SmGM. Right side: mean bleeding grades for PCCT and SmGM.
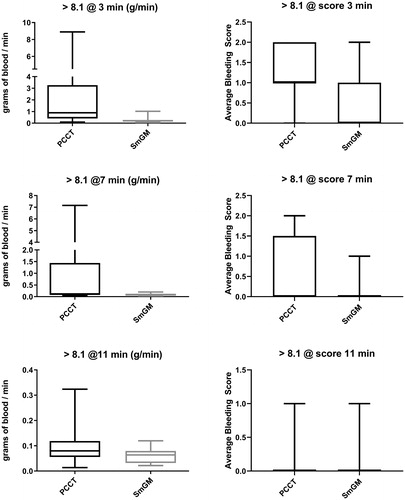
A significantly lower bleeding grade was observed at the 3-minute time-point with SmGM compared with PCCT (0.0 [0.0, 1.0] versus 1.0 [1.0, 2.0], p = 0.001). Bleeding grades were also lower with SmGM at the 7- and 11-minute time-points (7 minutes: 0.0 [0.0, 0.0] versus 0.0 [0.0, 1.5], p = 0.119; 11 minutes: 0.0 [0.0, 0.0] versus 0.0 [0.0, 0.0], p = 0.565).
Histologic Observations
Representative samples stained for fibrin, erythrocytes, connective tissue, and collagen are shown in . When treating with PCCT a layer of fibrin formed on the surface of the material (shown in the center of ).
Additional Observations
Reapplications
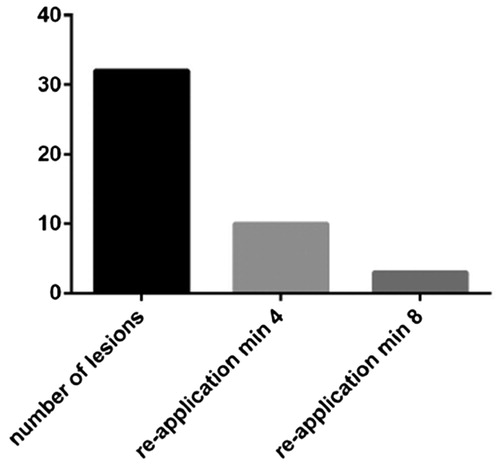
Reapplication of PCCT was required in 10/32 lesions at 4 minutes after initial application, and a second reapplication was required in 3/32 lesions at 8 minutes (). Reapplication of SmGM was not necessary based on earlier experience with this product; only one lesion was observed with a residual bleeding rate just above 1 mL/min at 3 minutes (measured at 1.02 mL/min).
Visual observations
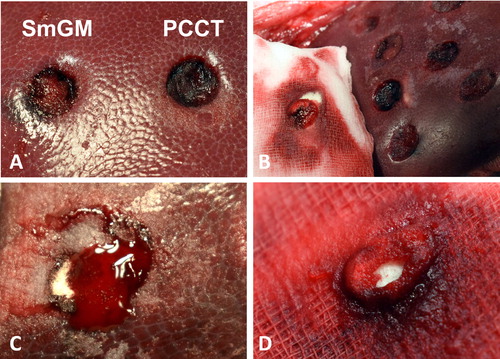
A representative photograph of both products post-application is shown in . Little material was left after irrigation for SmGM or gauze removal for PCCT. SmGM application was easily performed and convenient in all tested lesions. PCCT application was slightly more challenging in lesions with higher bleeding rates, as the power material tended to be washed away by the blood stream. This required a quick application of surplus amounts of PCCT followed by immediate approximation. PCCT also showed a tendency to stick to the wet gauze used for approximation, and considerable portions of the applied material were occasionally removed from the wound site when removing the gauze (). Additionally, PCCT was less capable than SmGM in absorbing blood ().
FIGURE 7. (A) Left: liver punch biopsy lesion treated with SmGM. Right: Liver punch biopsy lesion treated with PCCT. (B) PCCT material residues sticking to the wet gauze used for approximation. (C) Residual bleeding after application of PCCT. The lesion is filled with partially white, non-saturated powder, demonstrating a limited absorption capacity for blood. (D) PCCT residues sticking to the gauze after approximation. Note the blood-soaked gauze and the dry, non-soaked core of the powder.
DISCUSSION
In this model, both SmGM and PCCT were effective at inducing hemostasis for minimal to moderate bleeding. However, the residual bleeding rates observed with SmGM were significantly lower at all time-points, indicating greater hemostatic efficacy for SmGM than PCCT. This was consistent with bleeding grades, which were significantly lower with SmGM than PCCT at the 3-minute time-point and showed a trend toward lower bleeding grades at later time-points.
To obtain further detail regarding the clinical applicability and efficacy of these results for minimal or moderate bleeding wounds, the data were split to two sub-sets to consider lesions with bleeding rates either above or below the overall average rate of 8.14 g/min. These results confirmed the superior hemostatic efficacy of SmGM, with both sub-sets showing significantly lower bleeding rates and grade with SmGM at 3 minutes. Additionally, significantly lower bleeding rates were observed with SmGM at 7 minutes for the low-intensity sub-set and at 7 and 11 minutes for the high-intensity sub-set. The efficacy of SmGM is in line with results from a previous study in which SmGM was able to induce clotting in all wounds in a porcine model with liver stab wounds under both normal physiological conditions and under conditions of hypotension, hypothermia, and hemodilution [Citation23]. Similarly, in a porcine liver abrasion model, SmGM displayed superior hemostatic success when compared with a stellate gelatin hemostatic agent, with reduced bleeding and blood loss [Citation24]. During the development of PCCT, a comparison of SmGM and PCCT was conducted in an open acute liver surgery model in pigs under aspirin treatment [Citation25]. Hemostatic success was achieved in 100% of wounds treated with SmGM at all time-points, but only in 50%, 85%, and 98% of wounds treated with PCCT at the 3-, 6-, and 10-minute time-points, respectively [Citation25]. SmGM also showed greater hemostatic efficacy in a second porcine open hepatic surgery model, with hemostatic success achieved in 50%, 100%, and 100% of wounds at 3, 6, and 10 minutes, respectively, compared with 0%, 67%, and 83% with PCCT linked to a long nozzle and 17%, 67%, and 100% for PCCT linked to a short nozzle.
The porcine liver punch biopsy bleeding model was selected to reflect the indication for PCCT, which is limited to minimal to moderate bleeding corresponding to its approved indication [Citation10, Citation21]. These bleeding rates are also well within the indication for SmGM for treatment of oozing to spurting bleeding. For all lesions treated, the initial bleeding rates were below 20 mL/min with a mean rate of 8.14 g/min, demonstrating that they were within the indication for both products and so formed a relevant model. By conducting the study within one organ with multiple standardized lesions per animal, it was possible to ensure high consistency and sensitivity for comparing the two hemostatic agents, and to accurately assess the relevant levels of bleeding. This level of consistency would not be achievable in a clinical setting.
Approximately 1 in 3 applications of PCCT required reapplication after 3 minutes due to residual bleeding rate above 1 g/min, corresponding to grade 1 of the VIBe SCALE [Citation22]. This is accounted for within the product Instructions for use, which note that bleeding should be assessed after 3 minutes, and PCCT should be reapplied if hemostasis has not been achieved [Citation10]. The reapplication time point is given as 4 minutes, to allow 3 minutes of initial approximation, followed by 1 minute for assessment and bleeding rate measurement. Based on our observations, we speculate that this may be due to blood not properly penetrating the PCCT matrix, as shown by dry residue visible on the blood-soaked gauze removed from the lesion after the period of approximation. This may contribute to the greater hemostatic efficacy observed at the 3-minute time point with SmGM. A hemostatic agent that can readily take up blood, swell, and produce a tamponade effect would be of advantage at the early time points, when thrombin-inducted coagulation is not yet able to provide a tight and mechanically stable clot within the lesion. The fine powder structure of PCCT was also challenging when dealing with stronger bleeds, as the powder was quickly dislodged from the lesion by blood flow, requiring the application of excess or surplus amounts of material followed by immediate approximation. Additionally, PCCT showed a tendency to stick to the gauze upon removal in varying amounts, sometimes resulting in residual bleeding at the wound edges, where less material remained. In very few cases, this resulted in residual bleeding like the initial bleeding intensity. A powdered hemostatic agent can offer some advantages compared to a flowable paste, such as reduced preparation time and ease of application. However, the disadvantages we have observed, such as the need for reapplication or the adhesion to gauze, indicate that alternative agents may be required. The superior efficacy of SmGM, regardless of initial bleeding rate, coupled with the occasional dislocation of PCCT upon application by moderate bleeds, indicates that SmGM could be preferred in the clinical setting as the full intensity of bleeding may not be apparent when the decision is taken to apply a hemostatic agent. This is in line with a study which demonstrated superior hemostatic success, greater control of bleeding, and reduced blood loss when comparing SmGM with a powdered hemostatic agent (microporous polysaccharide hemospheres, MPH) in a heparinized porcine abrasion model [Citation26]. It is worth noting that the difference between the two products might be less pronounced in an oozing bleeding model, e.g. a liver abrasion, as opposed to the liver biopsy model used in this study.
In conclusion, SmGM was easier to apply and showed greater hemostatic efficacy than PCCT, with lower residual bleeding rates and grades observed at all time points in a model of minimal to moderate bleeding at clinically relevant bleeding rates. Both products were able to induce hemostasis in this model, with no statistical difference between the two treatment groups by the end of the 11-minute observation period. The superior efficacy of SmGM was not affected by the initial bleeding rate, indicating consistent performance of SmGM across bleeding intensities.
Declaration of interest
The authors report conflicts of interest. HR and PS are CEOs of Trauma Care Consult GmbH, the organization that received the funding to conduct the study. DL and HG are full-time employees of Baxter Medical Products GmbH. The studies reported here were designed and performed using established scientific methods with impartial data collection and analysis. The authors are responsible for the content of the article. Meridian HealthComms Ltd provided writing assistance, which was paid for by Baxter Healthcare Corporation.
Acknowledgments
We would like to thank Kevin M. Lewis DVM and Mary Anne Sanford RN, BSN, CNOR for reviewing the manuscript, Dr. Sylvia Nürnberger for her support with histology, and Karaleen Anderson and Karl Kropik for technical support.
Additional information
Funding
References
- Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States-2015. Transfusion. 2017;57(2):1588–1598.
- Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552.
- Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–765.
- Shander A, Ozawa S, Hofmann A. Activity-based costs of plasma transfusions in medical and surgical inpatients at a US hospital. Vox Sang. 2016;111(1):55–61.
- Velasquez CA, Singh M, Bin Mahmood SU, et al. The effect of blood transfusion on outcomes in aortic surgery. Int J Angiol. 2017;26(3):135–142.
- Bracey A, Shander A, Aronson S, et al. The use of topical hemostatic agents in cardiothoracic surgery. Ann Thorac Surg. 2017;104(1):353–360.
- Singh RK, Baumgartner B, Mantei JR, et al. Hemostatic comparison of a polysaccharide powder and a gelatin powder. J Invest Surg. 2018;1–9.
- Mourad FH, Leong RW. Role of hemostatic powders in the management of lower gastrointestinal bleeding: A review. J Gastroenterol Hepatol. 2018;33(8):1445–1453.
- MacDonald MH, Wang AY, Clymer JW, Hutchinson RW, Kocharian R. An in vivo comparison of the efficacy of hemostatic powders, using two porcine bleeding models. Med Devices (Auckl). 2017;10:273–279.
- Biom'Up SA. HEMOBLAST Bellows hemostatic agent. Published 2018 Jan [cited 2018, Nov 15]. Available from: https://www.hemoblast.com/content/uploads/2018/02/ifu-us-2018.pdf.
- Business W. Biom’Up receives FDA marketing approval for HEMOBLAST Bellows, its flagship product. Published 2017 Dec 18 [cited 2018 Nov 15]. https://www.businesswire.com/news/home/20171217005101/en/Biom%E2%80%99Up-Receives-FDA-Marketing-Approval-HEMOBLAST%E2%84%A2-Bellows. Available from: https://www.biomup.com/file/0654/pr-18-12-17.PDF.
- Echave M, Oyaguez I, Casado MA. Use of Floseal(R), a human gelatine-thrombin matrix sealant, in surgery: a systematic review. BMC Surg. 2014;14:111.
- Tackett SM, Calcaterra D, Magee G, Lattouf OM. Real-world outcomes of hemostatic matrices in cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28(6):1558–1565.
- Oz MC, Cosgrove DM, Badduke BR, et al. Controlled clinical trial of a novel hemostatic agent in cardiac surgery. Ann Thorac Surg. 2000;69(5):1376–1382.
- Watrowski R, Jager C, Forster J. Improvement of perioperative outcomes in major gynecological and gynecologic-oncological surgery with hemostatic gelatin-thrombin matrix. IV. 2017;31(2):251–258.
- Weaver FA, Hood DB, Zatina M, Messina L, Badduke B. Gelatin-thrombin-based hemostatic sealant for intraoperative bleeding in vascular surgery. Ann Vasc Surg. 2002;16(3):286–293.
- Baxter Healthcare C. Baxter Healthcare SA. FLOSEAL Hemostatic Matrix, instructions for use. Published 2015 Apr 04 [cited 2018 Nov 15]. Available from: http://www.floseal.com/int/instructions-for-use.html.
- Nasso G, Piancone F, Bonifazi R, et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann Thorac Surg. 2009;88(5):1520–1526.
- Ereth MH, Schrader LM, Dong Y, et al. Efficacy of microporous polysaccharide hemospheres on liver punch biopsies porcine model. Anesthesiology Annual Meeting. 2003. A153. San Francisco, CA: American Society of Anesthesiologists. CrossRef]
- US National Research Council. Guide for the care and use of laboratory animals. 8th edition. Washington (DC): National Academies Press (US); 2011.
- Spotnitz WD, Zielske D, Centis V, et al. The SPOT GRADE: a new method for reproducibly quantifying surgical wound bleeding. Spine (Phila Pa 1976). 2018;43(11):E664–E671.
- Lewis KM, Li Q, Jones DS, et al. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. 2017;161(3):771–781.
- Coenye KE, Bourgain C, Keibl C, Nurnberger S, van Griensven M. A qualitative morphological comparison of two haemostatic agents in a porcine liver trauma model. SS. 2013;4(8):359–364.
- Lewis KM, Atlee HD, Mannone AJ, et al. Comparison of two gelatin and thrombin combination hemostats in a porcine liver abrasion model. J Invest Surg. 2013;26(3):141–148.
- Agency EM. Committee for medicinal products for human use. Consultation Procedure Public Assessment Report (CPAR): Consultation on an ancillary medicinal substance incorporated in a medical device. EMA/66720/2015. Published 2014 Sep 25 [cited 2018 Nov 15]. Available from: https://www.ema.europa.eu/documents/other/consultation-ancillary-medicinal-substance-incorporated-medical-device-hemoblast_en.pdf.
- Lewis KM, Atlee H, Mannone A, Lin L, Goppelt A. Efficacy of hemostatic matrix and microporous polysaccharide hemospheres. J Surg Res. 2015;193(2):825–830.