Abstract
Purpose
Topical hemostatic agents can be classified as active or passive. This study compared the hemostatic efficacy of an active agent, recombinant thrombin (RECOTHROM® [rT]) plus gelatin sponge carrier versus a passive agent, oxidized regenerated cellulose (TABOTAMP®/SURGICEL® [ORC]), in a porcine liver abrasion model.
Materials and Methods
Eight pigs were used, four of them were heparinized. A total of 80 liver lesions were created, 40 of them in heparinized pigs. Lesions were treated with rT plus gelatin sponge or ORC. Bleeding rate was quantified before treatment by applying pre-weighed gauze. Time to hemostasis was assessed visually for 10 minutes.
Results
Seven of the 80 lesions were excluded for having initial bleeding rates exceeding the target of 10 g/min. Sixteen and 20 lesions were treated with rT plus gelatin sponge and 19 and 18 lesions were treated with ORC, in non-heparinized and heparinized animals, respectively. Time to hemostasis (median [IQR]) was significantly shorter with rT plus gelatin sponge (30 [30,30] seconds) in heparinized and non-heparinized animals versus ORC in non-heparinized (180 [120,210] seconds) and heparinized animals (215 [135,345] seconds); P < 0.0001 for both comparisons. In heparinized animals, ORC took longer to achieve hemostasis, with treatment failure in 2/18 lesions. Time to hemostasis with ORC was longer for lesions in heparinized animals with initial bleeding rates of >5–10 g/min (285 [225,394] seconds) versus ≤5 g/min (175 [108,290] seconds).
Conclusions
In this model, rT plus gelatin sponge carrier (active) was a more effective hemostat than ORC (passive) in both heparinized and non-heparinized animals.
Introduction
Management of bleeding during surgery is important for reducing the risk of complications which can lead to increased morbidity and mortality.Citation1 Major bleeding often necessitates blood product transfusion which is associated with potential adverse events and significant costs.Citation2–4 Incidence and severity of surgical bleeding can be minimized to some degree by optimizing the surgical technique used.Citation5,Citation6 However, patients with coagulopathy (either prior to, or developed during surgery) are at much greater risk of experiencing bleeding complications, regardless of surgical technique.Citation7,Citation8
Topical hemostatic agents can be used to supplement coagulation and prevent or control bleeding. Time to hemostasis is an important efficacy measure for hemostatic agents, as shorter times not only result in better clinical outcomesCitation4,Citation9 but also save hospital resources in terms of reduced blood product use,Citation4 reduced operating times,Citation10 shorter duration of hospital stayCitation9 and reduced likelihood of needing to apply additional hemostatic agents.Citation4 A variety of hemostatic agents are available, with oxidized regenerated cellulose (ORC) being one of the most widely used.Citation11 However, the efficacy of ORC is reduced in patients with coagulopathy and may therefore be an inappropriate choice of hemostat when treating these patients.Citation12
Hemostatic agents can be broadly characterized as active or passive based on their mechanism of action (MoA). Active hemostatic agents, such as thrombin (stand-alone or combined with gelatin), fibrin sealants and advanced patches act biologically at the end of the coagulation cascade, accelerating the natural clotting process, and are effective regardless of whether patients have received anticoagulants or antiplatelet therapies, or not.Citation13–15 This is due to their MoA, which is effective independently of the state of the coagulation cascade. Active hemostatic agents are also effective over a broader range of bleeding rates than passive agents.Citation14 As a result of this broad range of efficacy, active hemostatic agents can be a more cost-effective option in some patients by lowering risk of requiring additional treatment due to hemostatic failure and shorter operative and recovery times.Citation4,Citation14 Passive hemostatic agents, such as collagens, cellulose, gelatins and polysaccharide sphere powder work via contact activation and promote platelet aggregation by providing a structure to assist platelet aggregation and clot formation.Citation13–16 As their MoA is dependent on an intact or functioning coagulation cascade, the efficacy of passive agents is reduced in patients treated with anticoagulant or antiplatelet medications, or in those with other coagulation disorders.
Comparison of the efficacy of hemostatic agents used in routine surgery is important for providing evidence to guide clinical practice. The Validated Intraoperative Bleeding Scale (VIBe SCALE)Citation17 has been developed for studies of hemostatic agents, and can also be used to assess the severity of bleeding during surgery and guide the choice of hemostatic technique or agent. This scale classifies bleeding into 5 grades (ranging from 0: No bleed, to 4: Life threatening) and aligns the rate of blood flow with the appearance of the bleed, as shown in . Active hemostatic agents are effective in a wider range of bleeding grades than passive agents, with passive agents being limited to mild or moderate bleeding rates.Citation13,Citation14 The aim of this study was to compare an active hemostatic agent, recombinant thrombin (rT; RECOTHROM®), with a passive agent, oxidized regenerated cellulose (ORC; TABOTAMP®/SURGICEL®) for VIBe SCALE grade 1–2 bleeds using a porcine liver abrasion model.
Table 1. The validated intraoperative bleeding scale (VIBe SCALE).
Materials and methods
The porcine liver abrasion model was chosen as it is an established method for assessing the efficacy of hemostats on mild to moderate (VIBe SCALE grade 1–2) bleeds.Citation18,Citation19 An animal experiment permit was issued by the municipal government of Vienna and all experimental methods are consistent with the Guide for the Care and Use of Laboratory Animals of the National Institute of Health.Citation20
Two treatment arms were investigated. The rT (active treatment) used was RECOTHROM®; Baxter Healthcare Corporation, Deerfield, IL, USA, with a gelatin sponge carrier (SPONGOSTAN™; Ethicon, Norderstedt, Germany). The ORC (passive treatment) used was TABOTAMP® Original/SURGICEL® Original; Ethicon, Norderstedt, Germany. Treatments were tested in 8 male pigs weighing approximately 35 kg, with a target of 15 applications per animal. Premedication and anesthesia were performed as previously described.Citation21 Briefly, animals were premedicated with a combination of tiletamine and zolazepam intramuscularly, followed by inhalation anesthesia with isoflurane. Upon completion of the experiment, animals were humanely euthanized under deep anesthesia with a lethal intravenous dose of thiopental sodium and embutramide/mebezonium iodine/tetracaine hydrochloride.
Surgical procedure
Animals were placed in dorsal recumbency. After surgical preparation of the carotid artery and jugular vein, these vessels were cannulated for blood pressure monitoring and intravenous access. A urinary catheter was inserted into the bladder after preparation. A median laparotomy was performed and the liver was exposed. Half of the animals (N = 4) were heparinized with 1000 IU/mL sodium heparin (Gilvasan Pharma, Vienna, Austria) to obtain an activated clotting time 1.5–2.5 fold of the initial measurement. These animals were heparinized for the duration of the investigation and activated clotting time was measured approximately every 60 minutes, with heparin being re-administered as needed.
Circular lesions of about 1.8 cm in diameter and 2 mm in depth were created on the liver surface using a grinding disc. Initial blood loss was quantified as described below, and lesions were excluded from the study if the bleeding rate was >10 mL/min. Treatments were allocated to lesions in an alternating pattern in each animal (ABAB, etc.), with 20 applications in heparinized plus 20 applications in non-heparinized animals per treatment. The rT plus gelatin sponge carrier and ORC treatments were prepared and applied according to the Prescribing InformationCitation22 or Instructions for Use.Citation23,Citation24 Each sheet of gelatin sponge carrier (supplied as 7 × 5 cm) was cut to approximately 3.5 × 3.5 cm before treating with rT solution. Each sheet of ORC (supplied as 7.5 × 5 cm) was cut to approximately 4 × 4 cm and an additional layer of ORC was added after every evaluation of hemostatic effect, up to a maximum of 3 layers or until hemostasis was achieved.
Assessment of bleeding and quantification of blood loss
Initial bleeding rates were assessed by measuring the amount of blood absorbed by a pre-weighed gauze (measuring 5 × 5 cm) in 6 seconds, and the bleeding rate in grams of blood per minute was calculated. Initial bleeding grades were also visually assessed using the VIBe SCALE (). All lesions with initial bleeding rates greater than the target of 10 g/min (i.e., > grade 2 VIBe SCALE) were excluded from the study.
Once the hemostatic agent had been applied to the lesion, residual bleeding rates and time to hemostasis were assessed by application of fresh dry gauze over the hemostatic agent for 30 seconds followed by careful removal of the gauze and visual assessment of bleeding through and around the hemostatic agent for up to 1 minute. Hemostasis was defined as the absence of observable active bleeding or the absence of sustained soaking of blood into the hemostatic material within the observation period of 1 minute. In the case of bleeding during that period, fresh gauze was re-applied immediately for 30 seconds followed by another minute of observation. This was repeated for up to 10 minutes, or until hemostasis was achieved, whichever came first. A treatment was regarded as a failure if hemostasis was not achieved within 10 minutes.
Histology
Samples were prepared and stained as described previously.Citation21 Briefly, tissue samples were harvested postmortem, fixed in formalin and stained with Martius, Scarlet and Blue (MSB) for microscopic assessment.
Statistics
Bleeding rates were reported as median values with interquartile range (IQR) and mean values with standard deviation (SD). For consistency with the VIBe SCALE, all bleeding rates were reported in g/min which corresponds to mL/min due to the close similarity in the density of blood and water.Citation25 All statistical comparisons were performed using the Mann-Whitney test. GraphPad Prism (version 8.2.0 for Windows, GraphPad Software, San Diego, CA, USA) was used to conduct the statistical analyses.
Results
Initial bleeding rates
The mean initial bleeding rate was 3.485 ± 2.388 g/min in the non-heparinized animals and 3.975 ± 1.865 g/min in the heparinized animals. Initial bleeding rates by treatment group and heparin status are shown in . There was no significant difference in initial bleeding rates between treatment groups or heparinization status. A total of 7 out of 80 lesions were excluded as they had an initial bleeding rate >10 g/min (i.e., > grade 2 VIBe SCALE).
Table 2. Initial bleeding rates in non-heparinized and heparinized animals.
Time to hemostasis
All lesions with initial bleeding rates within intended range (≤10 g/min)
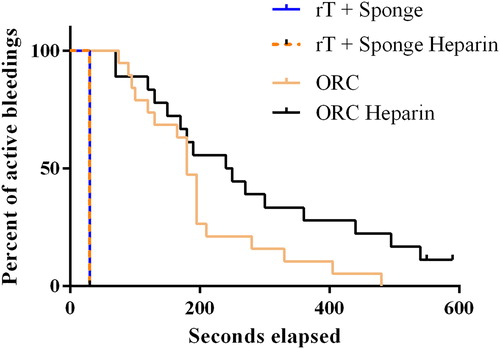
Time to hemostasis for all included lesions is shown in and . In lesions treated with rT and gelatin sponge carrier, all bleeding stopped within the first 30 seconds in both non-heparinized and heparinized animals (16 and 20 applications, respectively), with no treatment failures. With ORC, hemostasis was achieved in all non-heparinized lesions but there were 2 failures out of 18 ORC-treated lesions (11.1%) in heparinized animals (). The 2 failed applications were not accounted for in the time to hemostasis for ORC in heparinized animals.
Figure 1. Comparison of time to hemostasis – all initial bleeding rates within intended range (≤10 g/min).
ORC, oxidized regenerated cellulose; rT, recombinant thrombin. Horizontal line and box represent the median and interquartile ranges; error bars represent maximum/minimum values.
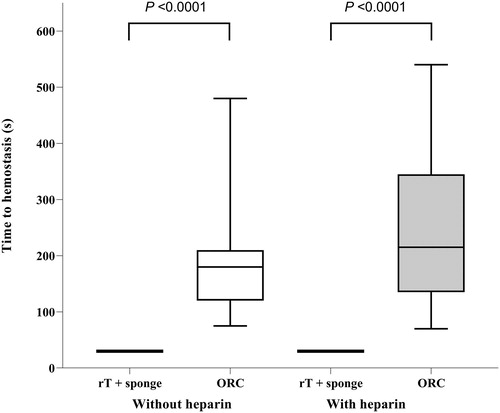
Table 3. Time to hemostasis and number of applications by treatment in non-heparinized and heparinized animals.
The mean time to hemostasis in the ORC treatment group was 200 ± 107 seconds (median 180 [120, 210] seconds) in non-heparinized animals (19 applications) and mean 248 ± 145 (median 215 [135, 345] seconds) in heparinized animals (16 applications). Time to hemostasis was significantly shorter with rT plus gelatin sponge carrier (median 30 [30, 30] seconds) versus the ORC group, both in heparinized and non-heparinized animals (P < 0.0001 for both comparisons). For lesions where hemostasis was achieved with ORC, an average of 3 layers was required. Heparinization did not significantly affect time to hemostasis in either treatment group versus non-heparinized lesions in successful applications of ORC. However, as mentioned above, the 2 failed applications with ORC in heparinized animals were not included when calculating time to hemostasis.
The proportion of active bleeding events over time for each treatment group is shown in . The plots for rT plus gelatin sponge carrier are identical regardless of whether the applications were in heparinized on non-heparinized animals. Conversely in the ORC group, there is a clear reduction in efficacy in heparinized animals, where more than 50% of lesions still had active bleeding at 200 seconds compared with approximately 25% of lesions in non-heparinized animals.
Lesions with initial bleeding rates ≤5 g/min (VIBe SCALE grade 1) versus >5–10 g/min (VIBe SCALE grade 2)
A sub-analysis distinguished between an initial bleeding rate ≤5 g/min (VIBe SCALE grade 1) and an initial bleeding rate >5–10 g/min (VIBe SCALE grade 2). Hemostasis was achieved within 30 seconds regardless of initial bleeding rate or presence or absence of heparin for all lesions treated with rT and gelatin sponge carrier (). In the ORC group, time to hemostasis in non-heparinized animals with initial bleeding rates ≤5 g/min (VIBe SCALE grade 1; 16 applications) was comparable with non-heparinized animals with initial bleeding rates >5–10 g/min (VIBe SCALE grade 2; 3 applications); durations of 203 ± 117 and 185 ± 9 seconds, respectively; P = 0.9742. In heparinized animals, however, mean time to hemostasis with ORC was numerically longer for lesions with initial bleeding rates of >5–10 g/min (304 ± 116 seconds; 6 applications) versus ≤5 g/min (215 ± 156 seconds; 10 applications), although this difference did not reach statistical significance (P = 0.1130). The mean number of layers of ORC required to achieve hemostasis was increased non-significantly from 2.6 to 3 in lesions with initial bleeding rates ≤5 g/min versus >5–10 g/min.
Figure 3. Comparison of time to hemostasis – A. Initial bleeding rates ≤5 g/min (VIBe SCALE grade 1), and B. Initial bleeding rates >5–10 g/min (VIBe SCALE grade 2). A. B. ORC, oxidized regenerated cellulose; rT, recombinant thrombin. Horizontal line and box represent the median and interquartile ranges; error bars represent maximum/minimum values.
Visual and histologic observations
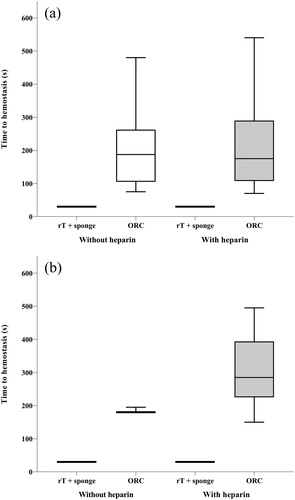
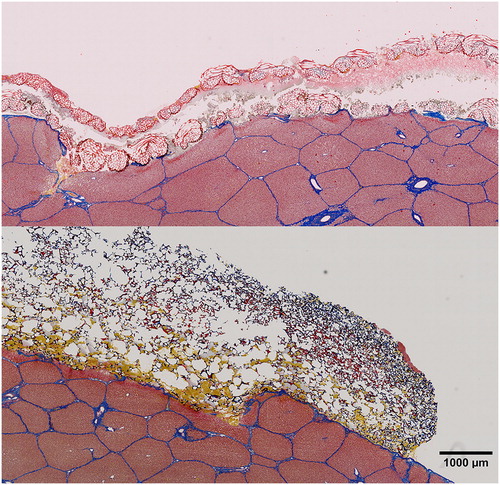
A representative photograph of each treatment post-application is shown in . Representative samples of rT plus gelatin sponge carrier and ORC stained for fibrin, erythrocytes, and connective tissue are shown in .
Figure 4. Test samples post application.
ORC, oxidized regenerated cellulose; rT, recombinant thrombin plus gelatin sponge on the left, ORC on the right, after 3 layers have been applied.
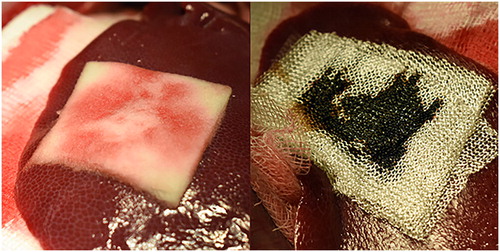
Figure 5. Martius, Scarlet and Blue staining of ORC (top) and rT plus gelatin sponge carrier (bottom).
ORC, oxidized regenerated cellulose; rT, recombinant thrombin.
Orange-yellow coloring represents fresh fibrin, red represents mature fibrin, erythrocytes are stained yellow, and connective tissue is shown in blue.
Liver tissue can be seen in the lower part of each picture with the patch material covering the superficial organ defect on top. Note the spongious structure of the gelatin sponge carrier that has been partially penetrated with blood in the lower regions, whereas ORC shows significant amounts of coagulated blood between the individual layers and blood penetration throughout the top layer.
Discussion
In this bleeding model study, hemostasis was achieved fastest in the rT plus gelatin sponge carrier (active) group, occurring consistently within 30 seconds regardless of heparinization status or initial bleeding intensity tested (VIBe SCALE grades 1 and 2). There were no treatment failures in the rT plus gelatin sponge carrier group. It is important to note that due to the experimental design, the minimum time that could be recorded for hemostasis was 30 seconds, so hemostasis may have been achieved in <30 seconds. In the ORC group, hemostasis was achieved in all applications in non-heparinized animals, but there were a clinically relevant number of failures under heparinized conditions (2/18). When hemostasis was achieved with ORC, it was at a significantly later time (an average of 200 seconds in non-heparinized animals and 248 seconds in heparinized animals) than with rT plus gelatin sponge carrier. It is also important to note that the lesions that failed to reach hemostasis in the ORC group are not included in these values.
Initial bleeding rates in this study for the rT plus gelatin sponge carrier treatment group in non-heparinized animals (93.75% mild; 6.25% moderate) were similar to those reported in a previous porcine liver abrasion study (95% of lesions had mild bleeding).Citation19 The performance of rT plus gelatin sponge carrier is in agreement with a phase 2 study that suggested that rT plus gelatin sponge had greater clinically meaningful efficacy versus a control gelatin sponge in a variety of surgical indications.Citation26 Furthermore, the presence of heparin did not reduce the hemostatic efficacy of rT plus gelatin sponge carrier, in line with previous findings in a rabbit model of vascular bleeding.Citation27 Treatment with ORC was generally effective in non-heparinized animals, although 3 layers of ORC (cut from separate sheets) were required to achieve hemostasis in the majority of cases (potentially conflicting with recommendations to use the minimum amount of material)Citation13,Citation23 and hemostasis was significantly slower than with rT plus gelatin sponge carrier. Although there was no significant increase in time to hemostasis with ORC in heparinized versus non-heparinized conditions in successful applications, the failure of 2 out of 18 ORC applications in heparinized conditions (which is not reflected in the time to hemostasis) is clinically relevant and represents a key finding of this study. Hemostatic agent failure is associated with increased blood loss and the corresponding greater likelihood of blood product transfusion, longer operative times, and greater use of surgical consumables, which result in poorer patient outcomes and higher economic costs.Citation4,Citation9,Citation10,Citation12 When the time to hemostasis of all ORC applications was examined, there was an apparent reduction in efficacy in heparinized animals. These results demonstrate the importance of using an appropriate hemostat for the individual patient, with active hemostatic agents (in this case rT plus gelatin sponge carrier) performing reliably in both intact and impaired coagulation settings due to their biological action that is independent of the coagulation cascade.Citation13,Citation15 Passive agents (in this case ORC) on the other hand, rely on contact activation and are only suitable for patients with a fully functioning coagulation cascade,Citation13 a factor which may not be immediately apparent in a traumatic clinical situation. This study adds to previous evidenceCitation15,Citation28,Citation29 that active agents such as rT plus gelatin sponge carrier have higher hemostatic success rates than passive agents such as ORC across a range of bleeding rates and patient coagulation profiles. Additionally, as an active hemostatic agent, rT plus gelatin sponge carrier meets more of the criteria for an ‘ideal’ hemostatic agent (which include rapid, effective hemostasis and reliability for different types of bleeding) than ORC, a passive agent.Citation13 Therefore rT plus gelatin sponge carrier is appropriate for treating a wider range of patients and lesion types than ORC.
In heparinized test conditions, ORC showed a non-significant trend for better results with VIBe SCALE grade 1 bleeding events compared with grade 2 bleeding events, but comparable efficacy under non-heparinized test conditions for grade 1 and 2 bleeding. This investigation was not powered to identify a difference between bleeding grades and therefore the low number of lesions with an initial bleeding grade of 2 (≤7 per treatment/heparinized group) may explain the trend of poorer performance of ORC in heparinized conditions not reaching statistical significance. However, a previous study has shown greater hemostatic efficacy with active agents versus passive agents in moderate-to-severe bleeds.Citation13 Therefore passive hemostatic agents such as ORC may be inappropriate for use in patients with grade 2 bleeds and impaired coagulation, and active agents such as rT may be a preferred choice. The instructions for use for ORC indicate that although ORC is absorbable, care should be taken to avoid excess material when used for wounds which are surgically sealed, to minimize risk of foreign body reactions, and that all ORC material should be removed once hemostasis is achieved when applied to open wounds, the spinal cord, the optic nerve and chiasm, or in proximity of bone.Citation23 Furthermore, it has been recommended to remove ORC after hemostasis due to serious adverse events when left in place.Citation13 Adverse events resulting from ORC may be due to incomplete absorption,Citation13 or swelling of the material.Citation23 Foreign-body reactions have been documented with ORC, where it has led to a granuloma-like presentation in several patients.Citation30–33 Swelling of ORC due to its hygroscopic nature has been attributed to compression of luminal tissue when used in cardiac surgeryCitation34 and compression of nerves after use in spinal surgery.Citation35,Citation36 Granuloma has been reported with gelatin sponge when used in neurosurgery.Citation37 The Instructions for Use for gelatin sponge also recommend removal once hemostasis is achieved to avoid possible dislodgment or compression of surrounding tissues.Citation24 Gelatin sponge has led to spinal cord compression due to swelling of the materialCitation38,Citation39 and the Instructions for Use therefore specify that it should be removed after use on or near the spine, bone or optic nerve.Citation24 Swelling of hemostatic material has also been observed with gelatin-thrombin hemostatic matricies,Citation40,Citation41 but to a lesser degree than ORC or gelatin sponge. Removal of hemostatic material may lead to rebleeding, which requires additional treatment, longer operative times and/or hospital stays, and consumption of expensive blood products in severe cases.Citation4,Citation14 Adverse events reported with rT in clinical trials include thromboembolic adverse reactions (6% of patients) and antibody formation to rT (<1% of patients).Citation22
A limitation of this study was the low number of lesions with initial bleed rates of VIBe SCALE grade 2, making it difficult to compare efficacy in lesions with less severe initial bleeds. Furthermore, the experimental design limited the minimum possible time to observation of hemostasis to 30 seconds, so hemostasis that occurred in <30 seconds could not be more precisely assessed.
In conclusion, rT plus gelatin sponge carrier demonstrated superior hemostatic properties compared with ORC, regardless of coagulation status or initial bleeding rate. When the consistent efficacy of rT plus gelatin sponge carrier resulting from its active hemostasis properties is considered, together with the compromised efficacy of ORC in heparinized conditions, multiple layers of ORC required in every application, and the risk of adverse events when left in place, this study suggests that an active hemostatic agent such as rT (plus gelatin sponge carrier) may be a preferable first choice of hemostatic agent versus a passive agent such as ORC for all mild and moderate bleeds.
Disclosure statement
The authors report conflicts of interest. PS is CEO of Trauma Care Consult GmbH, the organization that received the funding to conduct the study. DL and HG are full-time employees of Baxter Medical Products GmbH. The studies reported here were designed and performed using established scientific methods with impartial data collection and analysis. The authors are responsible for the content of the article. Meridian Health Comms Ltd provided writing assistance, which was paid for by Baxter Healthcare Corporation.
Additional information
Funding
References
- Ghadimi K, Levy JH, Welsby IJ. Perioperative management of the bleeding patient. Br J Anaesth. 2016;117(suppl 3):iii18–iii30. doi:10.1093/bja/aew358.
- Tackett SM, Sugarman R, Kreuwel HT, et al. Hospital economic impact from hemostatic matrix usage in cardiac surgery. J Med Econ. 2014;17(9):670–676. doi:10.3111/13696998.2014.928638.
- Carson JL, Stanworth SJ, Roubinian N, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;10:CD002042. doi:10.1002/14651858.CD002042.pub4.
- Ramirez Manuel G, Castillo George F, Ramirez Manuel A. The economic burden of bleeds and transfusions in selected surgeries: A retrospective multi center analysis from the US perspective. Am J Biomed Sci Res. 2019;2(5):211–216. doi:10.34297/AJBSR.2019.02.000610.
- Pedziwiatr M, Malczak P, Pisarska M, et al. Minimally invasive versus open pancreatoduodenectomy-systematic review and meta-analysis. Langenbecks Arch Surg. 2017;402(5):841–851. doi:10.1007/s00423-017-1583-8.
- Hughes MJ, Ventham NT, Harrison EM, et al. Central venous pressure and liver resection: a systematic review and meta-analysis. HPB (Oxford). 2015;17(10):863–871. doi:10.1111/hpb.12462.
- Staerkle RF, Hoffmann H, Kockerling F, et al. Does coagulopathy, anticoagulant or antithrombotic therapy matter in incisional hernia repair? Data from the Herniamed Registry. Surg Endosc. 2018;32(9):3881–3889. doi:10.1007/s00464-018-6127-y.
- Grottke O, Fries D, Nascimento B. Perioperatively acquired disorders of coagulation. Curr Opin Anaesthesiol. 2015;28(2):113–122. doi:10.1097/ACO.0000000000000176.
- Bennett KM, Kent KC, Schumacher J, et al. Targeting the most important complications in vascular surgery. J Vasc Surg. 2017;65(3):793–803. doi:10.1016/j.jvs.2016.08.107.
- Vyas KS, Saha SP. Comparison of hemostatic agents used in vascular surgery. Expert Opin Biol Ther. 2013;13(12):1663–1672. doi:10.1517/14712598.2013.848193.
- Lewis KM, Spazierer D, Urban MD, et al. Comparison of regenerated and non-regenerated oxidized cellulose hemostatic agents. Eur Surg. 2013;45(4):213–220. doi:10.1007/s10353-013-0222-z.
- Chiara O, Cimbanassi S, Bellanova G, et al. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 2018;18(1):68. doi:10.1186/s12893-018-0398-z.
- Samudrala S. Topical hemostatic agents in surgery: a surgeon's perspective. AORN J. 2008;88(3):S2–S11. doi:10.1016/S0001-2092(08)00586-3.
- Price JS, Tackett S, Patel V. Observational evaluation of outcomes and resource utilization from hemostatic matrices in spine surgery. J Med Econ. 2015;18(10):777–786. doi:10.3111/13696998.2015.1046879.
- Bracey A, Shander A, Aronson S, et al. The use of topical hemostatic agents in cardiothoracic surgery. Ann Thorac Surg. 2017;104(1):353–360. doi:10.1016/j.athoracsur.2017.01.096.
- Moss R. Management of surgical hemostasis: an independent study guide. 2013. https://www.aorn.org/-/media/aorn/guidelines/tool-kits/medication-safety/management-of-surgical-hemostasis-independent-study-guide.pdf?la=en&hash=9FED3DF8BFDEF8B1C8D1899F8FC7BE79. Accessed July 2019.
- Lewis KM, Li Q, Jones DS, et al. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. 2017;161(3):771–781. doi:10.1016/j.surg.2016.09.022.
- Lewis KM, Atlee HD, Mannone AJ, et al. Comparison of two gelatin and thrombin combination hemostats in a porcine liver abrasion model. J Invest Surg. 2013;26(3):141–148. doi:10.3109/08941939.2012.724519.
- MacDonald MH, Wang AY, Clymer JW, et al. An in vivo comparison of the efficacy of hemostatic powders, using two porcine bleeding models. Med Devices (Auckl). 2017;10:273–279. doi:10.2147/MDER.S140663.
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. https://grantsnihgov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animalspdf. 2011. Accessed July 2019.
- Slezak P, Keibl C, Redl H, et al. An efficacy comparison of two hemostatic agents in a porcine liver bleeding model: Gelatin/thrombin flowable matrix versus collagen/thrombin powder. J Invest Surg. 2019. [Epub ahead of print]. doi:10.1080/08941939.2019.1571130.
- Baxter Healthcare Corporation. RECOTHROM Thrombin topical (Recombinant) Prescribing Information. https://advancedsurgery.baxter.com/sites/g/files/ebysai1521/files/2019-02/Recothrom%20PI_Oct%202018.pdf. Published 2018. Accessed September 2019.
- Ethicon SARL. SURGICEL® Original Absorbable Hemostat Instructions for Use. https://hostedvl106.quosavl.com/qb/doc/7tet975fdm64l9f9qdpo6470us. Published 2016. Accessed July 2019.
- Ethicon Inc. SPONGOSTAN™ Absorbable Hemostatic Gelatin Sponge Instructions for Use. https://webroot146.s3.amazonaws.com/img/catalogues/ethicon-spongostan-sponge-IFU.pdf. Published 2010. Accessed September 2019.
- Vitello DJ, Ripper RM, Fettiplace MR, et al. Blood density is nearly equal to water density: A validation study of the gravimetric method of measuring intraoperative blood loss. J Vet Med. 2015;2015:1–4. doi:10.1155/2015/152730.
- Chapman WC, Lockstadt H, Singla N, et al. Phase 2, randomized, double-blind, placebo-controlled, multicenter clinical evaluation of recombinant human thrombin in multiple surgical indications. J Thromb Haemost. 2006;4(9):2083–2085. doi:10.1111/j.1538-7836.2006.02067.x.
- Hughes SD, Bishop PD, Garcia R, et al. Topical recombinant thrombin at a concentration of 1000 IU/mL reliably shortens in vivo TTH and delivers durable hemostasis in the presence of heparin anticoagulation and clopidogrel platelet inhibition in a rabbit model of vascular bleeding. Ann Surg Innov Res. 2009;3(1):14. doi:10.1186/1750-1164-3-14.
- Testini M, Marzaioli R, Lissidini G, et al. The effectiveness of FloSeal matrix hemostatic agent in thyroid surgery: a prospective, randomized, control study. Langenbecks Arch Surg. 2009;394(5):837–842. doi:10.1007/s00423-009-0497-5.
- Kakaei F, Seyyed Sadeghi MS, Sanei B, et al. A randomized clinical trial comparing the effect of different haemostatic agents for haemostasis of the liver after hepatic resection. HPB Surg. 2013;2013:1–5. doi:10.1155/2013/587608.
- Badenes D, Pijuan L, Curull V, et al. A foreign body reaction to Surgicel((R)) in a lymph node diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Ann Thorac Med. 2017;12(1):55–56. doi:10.4103/1817-1737.197780.
- Gao HW, Lin CK, Yu CP, et al. Oxidized cellulose (Surgicel) granuloma mimicking a primary ovarian tumor. Int J Gynecol Pathol. 2002;21(4):422–423. doi:10.1097/00004347-200210000-00015.
- Sandhu GS, Elexpuru-Camiruaga JA, Buckley S. Oxidized cellulose (Surgicel) granulomata mimicking tumour recurrence. Br J Neurosurg. 1996;10(6):617–619. doi:10.1080/02688699646989.
- Wang H, Chen P. Surgicel((R)) (oxidized regenerated cellulose) granuloma mimicking local recurrent gastrointestinal stromal tumor: A case report. Oncol Lett. 2013;5(5):1497–1500. doi:10.3892/ol.2013.1218.
- Arora ND, Varghese R, Pavithran S, et al. The pressures of surgicel((R)) in cardiac surgery. Ann Pediatr Card. 2015;8(2):167–169. doi:10.4103/0974-2069.157040.
- Banerjee T, Goldschmidt K. Surgiceloma' manifested as cauda equina syndrome. South Med J. 1998;91(5):481–483. doi:10.1097/00007611-199805000-00016.
- Rustagi T, Patel K, Kadrekar S, et al. Oxidized cellulose (surgicel) causing Postoperative Cauda Equine Syndrome. Cureus. 2017;9(7):e1500. doi:10.7759/cureus.1500.
- Knowlson GT. Gel-foam granuloma in the brain. J Neurol Neurosurg Psychiatry. 1974;37(8):971–973. doi:10.1136/jnnp.37.8.971.
- Epstein NE, Silvergleid RS, Hollingsworth R. Increased postoperative cervical myelopathy and cord compression resulting from the use of Gelfoam. Spine J. 2009;9(2):e19–21. doi:10.1016/j.spinee.2008.03.009.
- Herndon JH, Grillo HC, Riseborough EJ, et al. Compression of the brain and spinal cord following use of gelfoam. Arch Surg. 1972;104(1):107. doi:10.1001/archsurg.1972.04180010101027.
- Gazzeri R, Galarza M, Neroni M, et al. Hemostatic matrix sealant in neurosurgery: a clinical and imaging study. Acta Neurochir. 2011;153(1):148–155. discussion 155. doi:10.1007/s00701-010-0762-y.
- Gazzeri R, De Bonis C, Galarza M. Use of a thrombin-gelatin hemostatic matrix (Surgiflo) in spinal surgery. Surg Technol Int. 2014;25:280–285.