Abstract
Objective
Nerve growth factor (NGF) and tropomyosin kinase receptors A (TrKA) exert a crucial effect on the regulation of autonomic nervous system which contributes to the progress of atrial fibrillation (AF). Valvular heart disease (VHD) patients are more easily to induce the AF. We investigated whether NGF/TrKA could impact the occurrence of AF in VHD patients.
Materials and methods
Atrial tissues were resected from 30 VHD patients with chronic AF (n = 15, AF >6 months) or sinus rhythm (SR, n = 15). The expression of NGF, TrKA, protein kinase B (PKB/Akt), beta-isoforms of glycogen synthase kinase-3 (GSK3β), Serine473 phosphorylation of Akt (p-Ser473 Akt), Serine9 phosphorylation of GSK-3β (p-Ser9 GSK3β) in right atrial tissues and peripheral blood lymphocyte were quantified by Western blot. The localization of those genes expression was measured by immunohistochemistry. Double sandwich enzyme-linked immunosorbent assay was used to observe the trace changes of NGF-β in peripheral plasma.
Results
Our results revealed that the NGF expression was markedly elevated in the tissue of right atrial appendage and peripheral blood lymphocytes from AF patients compared with the SR patients. But, the expression of TrKA, GSK3β, p-Akt and p-GSK3β were decreased. There was no difference about the expression of Akt from the AF patients and the SR patients. The NGF-β level in peripheral blood plasma of patients with AF and SR was not statistical difference.
Conclusion
Thus, we thought that NGF/TrKA signaling pathway may be involved in the AF in the patients with VHD, inactivation of GSK3β could increase the incidence of AF, but not relevant to phosphorylation.
Introduction
Atrial fibrillation (AF) is a common arrhythmia which increases with aging [Citation1,Citation2]. It leads to cardiac dysfunction and embolism, especially VHD patients. It is known that valvular AF is associated with atrial structural changes and electrophysiological abnormalities [Citation3]. However, the exact mechanism of the disease is still unclear. Growing studies reported that autonomic stimulation can induce AF and beta-blocker can reduce it. Therefore, we are interested in how the autonomic nervous system participates in AF.
NGF is a typical member of the neuro-trophic factor family, its high-affinity transmembrane receptor TrKA, and an endogenous survival factor of cardiac myocytes [Citation4]. NGF binding to TrkA, causing its dimerization and activation. TrkA is present on the plasma membrane and binds to dimerized NGF in the extracellular environment. Signaling then begins across the plasma membrane and into the intracellular cytoplasm, through major signaling cascades including phospholipase C-γ (PLC-γ), mitogen activated protein kinase (MAPK), and phosphatidylinositol 3-kinase (PI3K) pathways [Citation5]. Upon binding of neurotrophin, the cognate Trk is activated by tyrosine phosphorylation in the cytoplasmic domain and consequently recruits and activates downstream signaling molecules to trigger various physiological responses [Citation6]. The NGF expression in the innervation tissue is proportional to the innervation density [Citation7]. TrkA is a specific receptor of NGF. After NGF combines with TrKA, PI3K, the well-known protein in the proliferation and survival of cells, is activated [Citation5]. PI3K’s downstream molecule Akt plays an important role in myocardial protection [Citation8,Citation9]. Meanwhile, Akt as a potent upstream regulator of GSK3β. Activation of Akt (phosphorylation of Akt at Ser-473) can induce the phosphorylation of the Ser9 residue of GSK-3β [Citation10], which results in inactivation of GSK3β. Inactivation of GSK3β increases the accumulation of nuclear factor of activated T-cells (NFATs) in the nucleus through NFATs phosphorylation, which leads to myocardial hypertrophy [Citation11]. Previous studies have reported that calcineurin (CaN)-NFATs pathway participates in the pathogenesis of valvular AF [Citation12]. As NFATs are an effector of GSK3β, we hypothesized that NGF-TrkA-Akt-GSK3β pathway maybe involved in the occurrence of valvular AF.
In our study, we investigated clinical characteristics, immunohistochemical localization and gene expressions of NGF, TrkA, GSK3β and Akt of between the valvular AF and the SR patients.
Materials and methods
Ethical statement and clinical specimens
This investigation conformed to the principles presented in the Declaration of Helsinki and was approved by the Ethical Committee of the University. Written informed consent forms were obtained from all study subjects.
Human right atrial tissues and peripheral blood
Five milliliter of Peripheral venous blood was collected by venipuncture from each patient. The lymphocyte and upper plasma were separated immediately with the same amount of human peripheral blood lymphocyte separating fluid and then were stored at −80 °C for experiments. Right atrial tissues were collected from 30 patients with VHD. Patients were classified as two groups according to whether they had persistent AF or not: Chronic AF group and SR group. Demographic () and clinical data were collected (Table 2). Preoperative 2-dimensional color echocardiography was performed routinely. Patients who ever received Class I or III antiarrhythmic drugs and those with familial paroxysmal AF, pulmonary artery disease, sick sinus syndrome or cardiomyopathy were removed from this study.
Table 1. Clicinal characteristics of the patients.
Extraction of human peripheral blood lymphocytes and plasma
Five milliliter of fresh anticoagulant with saline at 1:1 was added carefully to the liquid surface of 5 ml Lymphocyte Separation Medium (Solarbio, Beijing, China). The mixture was centrifuged at 400 g for 20 min. Plasma and cells were collected. Lymphocytes were harvested after precipitation and washed twice.
Immunohistochemical localization of NGF, TrkA, GSK3β and AKT in right atrial appendage
Sections were incubated with 3% H2O2 for 15 min, then blocked with goat serum protein (Solarbio, Beijing, China) for 30 min at 37 °C. After pouring slowly, it was incubated with the corresponding primary antibody overnight at 4 °C. The primary antibody was presented as follows: Rabbit polyclonal to NGF (1:1500; ab6199, Abcam, Cambridge, MA), TrKA(1:1500; ab216626, Abcam), GSK3β (1:1500; ab131356, Abcam), Serine 9 phosphorylation of GSK-3β (p-Ser9 GSK3β) (1:1500; ab131097, Abcam), Akt (1:1500; ab18785, Abcam) and p-Ser473 Akt (1:800, Cell Signaling Technology, Shanghai, China). After incubation at 37 °C for 1 h and washing with 0.1 M PBS three times, secondary antibodies (1:3000; Abcam) were added to incubate for 20 min. After washing sections, it was visualized using DAB reagent. Nuclear counter stain hematoxylin dehydrated with ethanol and mounted with glycerol gelatin. Images were measured using Image J version 6.0 system (Wayne Rasband National Institutes of Health, USA)
Western blot analysis
Right atrial tissues and lymphocytes were extracted with a cold RIPA buffer (Solarbio, Beijing, China). The concentration of proteins was detected using a BCA assay kit (Jiancheng, Nanjing, China). Proteins (60 μg) were separated by 10% SDS-PAGE and transferred onto a PVDF membrane (Thermo Fisher Scientific, Inc., Waltham, MA). After washing with TBS containing 5% nonfat milk at 4 °C overnight, the blot was incubated with rabbit against NGF (1:1200; ab6199, Abcam, Cambridge, MA), TrKA (1:1200; ab216626, Abcam), GSK3β (1:1200; ab131356, Abcam), Serine9 phosphorylation of GSK-3β (p-Ser9 GSK3β) (1:1000; ab131097, Abcam), Akt (1:1000; ab18785, Abcam), and Serine473 phosphorylation of AKT (p-Ser473 Akt) (1:800, Cell Signaling Technology, Beijing, China). Washed again with TBS containing Tween-20 (TBST) for 10 min, the blot was added with HRP-conjugated goat anti-rabbit IgG (1:3000; ab6721, Abcam) for incubation at 37 °C for 30 min. After another washing with TBST for 10 min, the blot was detected by ECL reagent. Gray value of the blot was calculated by Image J software (Bethesda, MD).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was obtained from right atrial tissues using TRIzol Reagent (TaKaRa Bio, Tokyo, Japan), then reversed transcription into cDNA. The PCR assay was conducted using the SYBR Premix Ex Taq system under ABI 7900 Fast system. The primers were listed in Table 3. Relative expression was calculated by the 2−ΔΔCt method.
Enzyme-linked immunosorbent assay (ELISA)
NGF expression was detected using ELISA assay kit in accordance with the instruction manual (Abcam, Cambridge, MA). All the experiments were performed in duplicate.
Statistical analysis
Statistical analyses were conducted with SPSS version 19.0 (SPSS Inc., Chicago, IL). All data were presented as mean ± standard deviation (SD) or percentages. Differences in continuous and categorical data between AF and SR patients were compared using one-way analysis of variance (ANOVA) and Chi-square test, respectively. p<.05 indicates a significant difference.
Results
Baseline characteristics of patients
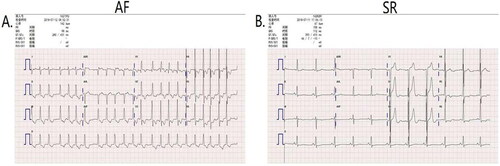
Baseline clinical and laboratory characteristics of the study patients were shown in and 2. The two groups (AF and SR) did not differ in gender, age, disease time and cardiac function. AF is defined as a sustained supraventricular tachyarrhythmia with disorganized atrial activation and ineffective contraction that has distinctive electrocardiogram characteristics including: fast atrial rate of ∼300 beats/min; absence of P waves; and irregular R-R intervals [Citation13,Citation14]. Preoperative 2-dimensional color echocardiography demonstrated that AF patients have no “P” wave () was consistent with it. With respect to clinical values, we could observe that the rest heart rates were markedly higher and diastolic blood pressure (BP; mmHg) was remarkedly decreased in AF patients compared with the SR patients (p<.01; ). With respect to laboratory values, we could observe that brain natriuretic peptide (BNP; pg/mL), direct bilirubin (DBIL; μmol/L), Urea nitrogen (BUN; mmol/L), phosphorus (P; mmol/L), osmotic pressure (OSM; mmol/L) and left atrial dimension (LAD; cm) in AF patients compared with the SR patients (p<.05; Table 2). There was no significant difference in the left ventricular ejection fraction (LVEF) between AF patients and SR individuals. These results demonstrated that our selection criteria for patients with AF and sinus rhythm, which is also consistent with previous reports [Citation15].
Localization and expression of NGF, TrKA, GSK3β, p-Ser 9 GSK3β, AKT and p-Ser 473 Akt in right atrial appendage
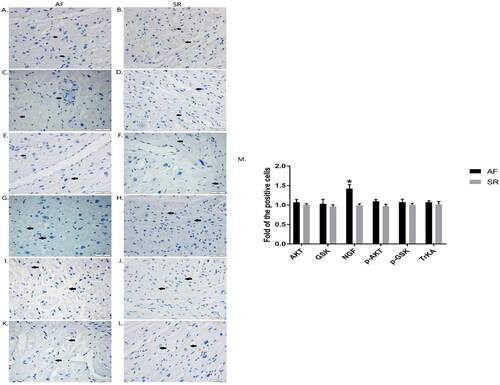
In the immunohistochemistry experiment, as shown in , The protein expression of AKT (), GSK3β (), NGF (), p-S473 AKT (), p-S9 GSK3β () and TrKA() were localized in the cytoplasm of the cardiac myocyte. Among them, NGF expression of AF patients was obviously increased than that of SR individuals (; p<.05).
Figure 2. Localizations of AKT, GSK3β, NGF, p-AKT, p-GSK3β and TrKA were detected in right atrial tissues by immunohistochemical assay. Expressions of AKT(A, B)、GSK3β (C, D), NGF(E, F), p-AKT (G, H), p-GSK3β (I, J) and TrKA (K, L) were demonstrated in right atrial tissues of AF patients and SR patients. The scale bar represents 50 μm. The arrows indicate the positive cells with brown granules. The graphs show the density of the target proteins in positive cells. Data were expressed as mean ± SD. *p<.05 vs. SR patients. AF: atrial fibrillation; SR: sinus rhythm; Akt: protein kinase B; GSK3β: glycogen synthase kinase 3β; NGF: nerve growth factor.
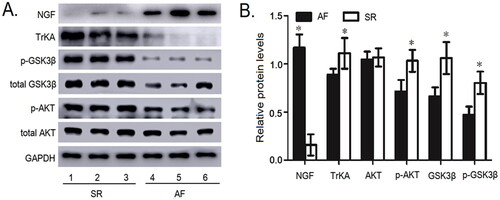
The protein expression of NGF, TrKA, GSK3β, p-Ser 9 GSK3β, AKT and p-Ser 473 Akt in the right atrial appendage
As illustrated in , the NGF protein expression in atrial appendage samples of the SR patients was lower than those with AF (p<.05). However, compared with the AF patients, the protein expression levels of TrKA (p<.05), GSK3β (p<.05), p-Ser 9 GSK3β (p<.05) and p-Ser 473 Akt (p<.05) were significantly increased in SR patients. There was no difference in the expression of AKT protein between the two groups (p>.05).
Figure 3. The protein expressions of NGF, TrKA, GSK3β, p-S9 GSK3β, AKT and p-S473 AKT were detected in the myocardial tissue of right atrium. (A) The protein expressions of NGF, TrKA, GSK3β, p-S9 GSK3β, AKT and p-S473 AKT in the myocardial tissue of right atrium by Western blot. (B) Quantitative assessment of the protein expressions of NGF, TrKA, GSK3β, p-S9 GSK3β, AKT and p-S473 AKT in the myocardial tissue of right atrium in AF patients compared to that in SR patients. *p< .05 vs. AF. AF, atrial fibrillation; SR, sinus rhythm; Akt, protein kinase B; GSK3β, Glycogen synthase kinase 3β; NGF: nerve growth factor.
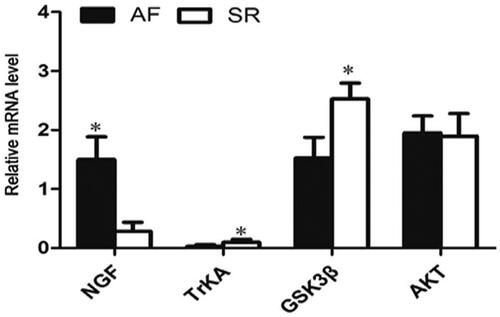
The mRNA expression of NGF, TrKA, GSK3β and AKT
As illustrated in , the mRNA expression of NGF was remarkably elevated in AF patients compared with the SR patients. Meanwhile, the mRNA expression of GSK3β (p<.05) and TrKA (p<.05) were significantly decreased. In addition, there was no statistical difference in the mRNA level of AKT between AF patients and SR patients (p>.05).
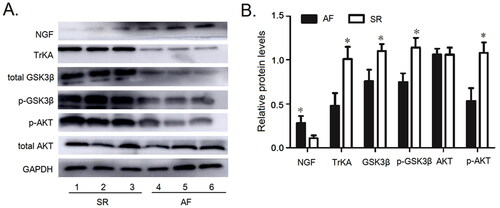
The Protein expression of NGF, TrKA, GSK3β, p-Ser 9 GSK3β, AKT and p-Ser 473 Akt in the peripheral blood lymphocytes
As illustrated
in . The profiles of NGF, TrKA, GSK3β, p-Ser 9 GSK3β, AKT and p-Ser 473 Akt in peripheral blood lymphocytes in AF patients and SR patients were consistent with those in right atrial appendage. The protein levels of NGF in peripheral blood lymphocyte from AF patients were higher than those with SR (; p<.05). There was no significant difference in the expression of AKT protein between the AF patients and SR patients (; p>.05), However, the expression of the other proteins in patients with AF was obviously decreased.
Figure 5. The protein expressions of NGF, TrKA, GSK3β, p-S9 GSK3β, AKT and p-S473 AKT were detected in the peripheral blood lymphocytes. (A) The protein expressions of NGF, TrKA, GSK3β, p-S9 GSK3β, AKT and p-S473 AKT in the peripheral blood lymphocytes. (B) Quantitative assessement of the protein expressions of NGF, TrKA, GSK3β, p-S9 GSK3β, AKT and p-S473 AKT in the Peripheral Blood Lymphocytes in AF patients compared to that in SR patients. *p<.05 vs. AF. AF: atrial fibrillation; SR: sinus rhythm; Akt: protein kinase B; GSK3β: glycogen synthase kinase 3β; NGF: nerve growth factor.
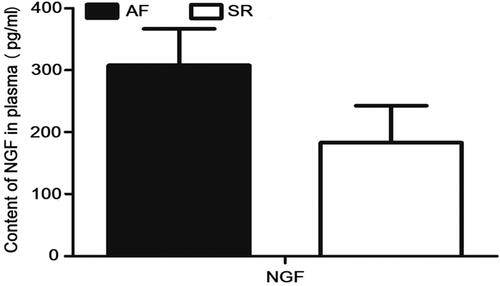
Detection of the microprotein-NGF in peripheral plasma
As shown in . There was no statistical significance in the expression of NGF in peripheral plasma between AF patients and SR patients (p>.05).
Discussion
In our study, we explored the role of NGF-TrKA-Akt-GSK3β pathway in mediating the occurrence of the VHD patients with AF. Our data revealed that the protein expression of NGF was obviously elevated in the right atrial tissue of AF patients, while TrKA, GSK3β, p-Ser 9 GSK3β and p-Ser 473 Akt were decreased. The gene expression and peripheral blood lymphocyte protein expression of NGF, TrKA, GSK3β, p-Ser 9 GSK3β, Akt and p-Ser 473 Akt had the same trend as the right atrial appendage. All of these indicated that NGF-TrKA is in association with AF. Inactivation of GSK3β could increase the incidence of AF, but not through phosphorylation. Akt activation has little effect on AF patients. Previous studies have reported that the activation of autonomic nervous system has significant changes in atrial structure [Citation16]. A mechanism for autonomic nervous system activation in adult heart was likely through paracrine secretion of NGF by parasympathetic neurons [Citation17]. NGF-TrKA can activate signal pathways through PI3K/Akt pathway in vivo and in vitro, and GSK3β is the downstream regulator of Akt. Therefore, these findings are consistent with previous studies.
NGF is a 118 amino acids glycoprotein composed of three subunits (α, β, and γ complex), The β-NGF is responsible for the biological activity. To some extent, NGF represents sympathetic nerve distribution and its increasing triggers nerve sprouting in non-infarcted ventricles and atria [Citation18]. Excessive sympathetic nerve sprouting may be an important element in the arrhythmogenicity of sympathetic remodeling [Citation19]. Elevated levels of NGF and excessive sympathetic innervation lead to arrhythmias, including AF [Citation20]. These were consistent with our results.
TrkA is a specific trk receptor that binds to NGF and is crucial for maintaining sympathetic neuron health and stimulating axon regeneration. Previous studies have demonstrated that NGF/TrkA signaling play an important role in enhancing normal cardiac calcium cycling and normal cardiovascular system function [Citation21,Citation22]. After the association between NGF and the TrkA receptor was demonstrated, it was revealed that this triggers TrkA receptor autophosphorylation. Therefore, the protein level of TrkA phosphorylation of Trk was investigated in this study. The TrkA protein level was decreased in AF patients. The results from this study also indicated that activation of the downstream Akt pathway was inhibited, which was detected by the phosphorylation of its mediators Akt. In addition, proper TrkA transport and sorting mediates the function of NGF. However, the concentration of TrKA did not increase with the increasing of NGF. Previous studies illustrated that stimulated cells with NGF and measured TrkA at different times, TrKA concentration decreased with time due to TrkA degradation [Citation23,Citation24]. This may explain the decreasion of TrkA concentration. Thus, as NGF/TrkA signaling was necessary for the function of neurotrophins in both neurons and heart, it was speculated that neurotrophic signaling may be a key factor of the AF patients.
The Akt/GSK-3β pathway is activated by signaling molecules, including NGF/TrKA [Citation5]. Activation of Akt inhibits GSK-3β expression by increasing its phosphorylation and leads to hypertrophy and/or fibrosis. In our study, we found that Akt/GSK3β phosphorylation was remarkably reduced in AF patients. But, in myofibrosis of the human AF, Akt activity enhanced in AF patients compared with the SR patients, suggesting that phosphorylation of Akt may be participate in the occurrence of myofibrosis in AF. These results were consistent with previous studies [Citation25]. The different considerations of these results maybe relate to the different basic diseases and early clinical medication of the patients. The patients we selected included not only those with rheumatic mitral valve disease, but also aortic valve disease. Wiersma et al. [Citation26] found that p-Ser 473 Akt expression decreased with the prolongation of pacing stimulation time in AF after 6 h. In animal experiment, the phosphorylation of Akt-GSK3β signaling pathway decreased in late stage [Citation27]. These results indicate that the phosphorylated Akt expression would reduce with the prolongation of AF. Meanwhile, exercise can improve cardiovascular health by increasing Akt activity [Citation28], which is consistent with the enhancement of Akt activity in SR patients in our study. Akt activation exerted little effects on reactive oxygen species (ROS) production and oxidative stress [Citation29], this is consistent with our findings that there is no significant difference in Akt expression of patients with AF and SR. P-Akt occurs at two sites: threonine 308 (Thr 308) and serine 473 (Ser 473), p-Thr308 increases the enzymatic activity of Akt by 10-fold higher than that by phosphorylation of Ser 473 [30]. Growing evidences demonstrated that these phosphorylation sites play different roles in Akt activation [Citation31]. Maybe, phosphorylation of Akt in Thr 308 exerts a crucial effect on the regulation of AF. In the future, we will pay attention to this signal protein.
GSK3β expression in patients with valvular AF was significantly reduced. Combined with the changes of phosphorylation level, we supposed that GSK3β was involved in the occurrence of valvular AF. Meanwhile, the inactivation of GSK3β in patients with valvular AF may not be through phosphorylation. GSK-3 is a serine/threonine kinase, regulates multiple cellular functions, consists of two similar isoforms (α, β). Previous study revealed that GSK-3β is an endogenous negative regulator of cardiac hypertrophy [Citation32]. GSK-3β activity is regulated by both phosphorylation-dependent and -independent mechanisms. Phosphorylation of GSK-3β occurs at two sites: serine 9 (Ser 9) and serine 389 (Ser 389). Akt, an upstream kinase, can induce the phosphorylation of the Ser 9 residue of GSK-3β, which blocks the entry of substrates to the GSK-3β catalytic domain. GSK-3β could be suppressed through Ser 389 phosphorylation by p38-MAPK [Citation33]. It has also been proved that p-Ser 21/Ser 9 in GSK-3β may not be necessary for cardiac hypertrophy [Citation34]. Therefore, the reduction of phosphorylated AKT and GSK3β does not negate the role of GSK3β in patients with valvular AF.
Clinical data from the patients revealed that BNP in patients with valvular heart disease and AF was higher than that in those with SR, but there was no significant difference in the classification of NYHA and LVEF. BNP, as a quantitative marker of heart failure, not only reflects left ventricular systolic dysfunction, but also reflects left ventricular diastolic dysfunction, valvular dysfunction and right ventricular dysfunction. Although the morphological and clinical manifestations are not present, the increasion of BNP suggests the impairment of cardiac function. Considering the expression of NGF was increased in patients with cardiac insufficiency or AF, previous studies have proved this conclusion [Citation35].
It is a limitation of this study that direct evidence was not provided to verify the functions of NGF/TrKA pathways in AF patients and SR patients. To provide the direct evidence supporting NGF/TrkA signaling pathways in AF patients, we plan to establish an animal model of AF to study this pathway in future studies, such as by adding NGF/TrKA pathway inhibitors, the protein expression of AF-related indicators is then detected, including the determination of some clinical basic indicators.
To the best of our knowledge, this study is the first to demonstrate that that the signaling pathway of NGF/TrkA is associated with the AF patients, which raises the possibility for the involvement of neurotrophic signaling in AF patients. Taken together, the results from this study lead to an improved understanding of the role of neurotrophic signaling in AF patients and provided evidence for new strategies to prevent heart disease.
In conclusion, our results revealed that NGF/TrKA signaling pathway may be associated with AF, inactivation of GSK3β could increase the incidence of AF, but not through phosphorylation. Akt activation (ser473) has little effect on AF in patients with VHD.
Author contributions
Q.W., Y.Z. and J.W. conceived and designed the project, Q.W., X.D., C.L., L.Z. and J.W. acquired the data, C.Z., X.L., N.Z., J.L. and Y.S. analyzed and interpreted the data, Q.W. and J.W. wrote the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–2751. doi:https://doi.org/10.1093/eurheartj/eht280.
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–847. doi:https://doi.org/10.1161/CIRCULATIONAHA.113.005119.
- January CT, Wann LS, Alpert JS, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2014;64(21):e1–e76. doi:https://doi.org/10.1016/j.jacc.2014.03.022.
- Ieda M, Fukuda K, Hisaka Y, et al. Endothelin-1 regulates cardiac sympathetic innervation in the rodent heart by controlling nerve growth factor expression. J Clin Invest. 2004;113(6):876–884. doi:https://doi.org/10.1172/JCI200419480.
- Marlin MC, Li G. Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol. 2015;314:239–257. doi:https://doi.org/10.1016/bs.ircmb.2014.10.002.
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. doi:https://doi.org/10.1038/nrn1078.
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77(5):627–638. doi:https://doi.org/10.1016/0092-8674(94)90048-5.
- Bernardo BC, Weeks KL, Pretorius L, et al. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128(1):191–227. doi:https://doi.org/10.1016/j.pharmthera.2010.04.005.
- Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38(1):63–71. doi:https://doi.org/10.1016/j.yjmcc.2004.11.005.
- Tsujita Y, Muraski J, Shiraishi I, et al. Nuclear targeting of Akt antagonizes aspects of cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2006;103(32):11946–11951. doi:https://doi.org/10.1073/pnas.0510138103.
- Kim MS, Shutov LP, Gnanasekaran A, et al. Nerve growth factor (NGF) regulates activity of nuclear factor of activated T-cells (NFAT) in neurons via the phosphatidylinositol 3-kinase (PI3K)-Akt-glycogen synthase kinase 3β (GSK3β) pathway. J Biol Chem. 2014;289(45):31349–31360. doi:https://doi.org/10.1074/jbc.M114.587188.
- Zhao Y, Cui GM, Zhou NN, et al. Calpain-calcineurin-nuclear factor signaling and the development of atrial fibrillation in patients with valvular heart disease and diabetes. J Diabetes Res. 2016;2016:4639654. doi:https://doi.org/10.1155/2016/4639654.
- January CT, Wann LS, Alpert JS, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130(23):2071–2104. doi:https://doi.org/10.1161/CIR.0000000000000040.
- Fye WB. Tracing atrial fibrillation-100 years. N Engl J Med. 2006;355(14):1412–1414. doi:https://doi.org/10.1056/NEJMp068059.
- Chishaki A, Chishaki H. To know the exact prevalence and prognosis of atrial fibrillation from a clinical survey-comments on the “The Fushimi AF Registry”. J Cardiol. 2013;61(4):304–306. doi:https://doi.org/10.1016/j.jjcc.2013.02.005.
- Chen PS, Chen LS, Fishbein MC, et al. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114(9):1500–1515. doi:https://doi.org/10.1161/CIRCRESAHA.114.303772.
- Hasan W, Smith PG. Modulation of rat parasympathetic cardiac ganglion phenotype and NGF synthesis by adrenergic nerves. Auton Neurosci. 2009;145(1–2):17–26. doi:https://doi.org/10.1016/j.autneu.2008.10.012.
- Miyauchi Y, Zhou S, Miyauchi M, et al. Altered atrial electrical restitution and heterogeneous sympathetic hyperinnervation in hearts with chronic left ventricular myocardial infarction: implications for atrial fibrillation. Circulation. 2003;108(3):360–366. doi:https://doi.org/10.1161/01.CIR.0000080327.32573.7C.
- Li CY, Li YG. Cardiac sympathetic nerve sprouting and susceptibility to ventricular arrhythmias after myocardial infarction. Cardiol Res Pract. 2015;2015:698368. doi:https://doi.org/10.1155/2015/698368.
- Cao JM, Chen LS, KenKnight BH, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86(7):816–821. doi:https://doi.org/10.1161/01.RES.86.7.816.
- Feng N, Huke S, Zhu G, et al. Constitutive BDNF/TrkB signaling is required for normal cardiac contraction and relaxation. Proc Natl Acad Sci USA. 2015;112(6):1880–1885. doi:https://doi.org/10.1073/pnas.1417949112.
- Hou Y, Jia L, Zhang Y, et al. Activation of the NGF/TrkA signaling pathway attenuates diabetic erectile dysfunction. Oncotarget. 2017;8(62):105692–105702. doi:https://doi.org/10.18632/oncotarget.22389.
- Anta B, Martin-Rodriguez C, Gomis-Perez C, et al. Ubiquitin-specific protease 36 (USP36) controls neuronal precursor cell-expressed developmentally down-regulated 4-2 (Nedd4-2) actions over the neurotrophin receptor TrkA and potassium voltage-gated channels 7.2/3 (Kv7.2/3). J Biol Chem. 2016;291(36):19132–19145. doi:https://doi.org/10.1074/jbc.M116.722637.
- Yu T, Calvo L, Anta B, et al. Regulation of trafficking of activated TrkA is critical for NGF-mediated functions. Traffic. 2011;12(4):521–534. doi:https://doi.org/10.1111/j.1600-0854.2010.01156.x.
- Zhang P, Wang W, Wang X, et al. Focal adhesion kinase mediates atrial fibrosis via the AKT/S6K signaling pathway in chronic atrial fibrillation patients with rheumatic mitral valve disease. Int J Cardiol. 2013;168(4):3200–3207. doi:https://doi.org/10.1016/j.ijcard.2013.04.113.
- Wiersma M, Meijering RAM, Qi XY, et al. Endoplasmic reticulum stress is associated with autophagy and cardiomyocyte remodeling in experimental and human atrial fibrillation. J Am Heart Assoc. 2017;6(10):e006458.
- Prévilon M, Pezet M, Dachez C, et al. Sequential alterations in Akt, GSK3β, and calcineurin signalling in the mouse left ventricle after thoracic aortic constriction . Can J Physiol Pharmacol. 2010;88(11):1093–1101. doi:https://doi.org/10.1139/y10-087.
- Cheng SM, Ho TJ, Yang AL, et al. Exercise training enhances cardiac IGFI-R/PI3K/Akt and Bcl-2 family associated pro-survival pathways in streptozotocin-induced diabetic rats. Int J Cardiol. 2013;167(2):478–485. doi:https://doi.org/10.1016/j.ijcard.2012.01.031.
- Dong M, Hu N, Hua Y, et al. Chronic Akt activation attenuated lipopolysaccharide-induced cardiac dysfunction via Akt/GSK3β-dependent inhibition of apoptosis and ER stress. Biochim Biophys Acta. 2013;1832(6):848–863. doi:https://doi.org/10.1016/j.bbadis.2013.02.023.
- Alessi DR. Discovery of PDK1, one of the missing links in insulin signal transduction. Biochem Soc Trans. 2001;29(Pt 2):1–14. doi:https://doi.org/10.1042/bst0290001.
- Vincent EE, Elder DJ, Thomas EC, et al. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br J Cancer. 2011;104(11):1755–1761. doi:https://doi.org/10.1038/bjc.2011.132.
- Hardt SE, Sadoshima J. Negative regulators of cardiac hypertrophy. Cardiovasc Res. 2004;63(3):500–909. doi:https://doi.org/10.1016/j.cardiores.2004.03.015.
- Thornton TM, Pedraza-Alva G, Deng B, et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320(5876):667–670. doi:https://doi.org/10.1126/science.1156037.
- Matsuda T, Zhai P, Maejima Y, et al. Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc Natl Acad Sci USA. 2008;105(52):20900–20905. doi:https://doi.org/10.1073/pnas.0808315106.
- Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol. 2014;63(22):2335–2345. doi:https://doi.org/10.1016/j.jacc.2014.02.555.