Abstract
Background
Insertion of conduits from the right ventricle (RV) to the pulmonary artery (PA) is a commonly used technique for repair of congenital heart defects. The vast majority of infants and children will require reoperation and/or re-intervention to replace the conduit. Some children may require multiple reoperations, with the risk of death and morbidity increasing significantly with each subsequent operation. We evaluated the feasibility and performance of a relatively novel anisotropic conduit for cardiovascular repair in the growing lamb model.
Materials and Methods
Lambs were allocated into a control (n = 3) or test (n = 4, anisotropic) conduit group. Control conventional polytetrafluoroethylene (PTFE) conduits or test anisotropic expanded PTFE (ePTFE) based test conduits measuring 10–11 mm in diameter were sewn as interpositional grafts in the main pulmonary artery (MPA) and followed up to 6 months. Clinical and echocardiographic evaluations were performed monthly with hemodynamic and angiographic assessment at 3 and 6 months.
Results
Control conduits did not expand, all 3 animals developed one or more adverse events including tachypnea, ascites, inappetence, lethargy, and mortality due to severe right heart failure and significantly higher peak trans-conduit gradients (48.5 ± 5.1 p = 0.02). The test conduits spontaneously expanded up to 14.8 ± 0.8 mm in diameter, no adverse events were observed in any animals and trans-conduit gradients were significantly lower (27.0 ± 8.3, p = 0.02).
Conclusions
Anisotropic ePTFE conduits can be safely implanted in growing lambs with stable hemodynamics. This spontaneously expanding anisotropic conduit may represent a novel approach to congenital heart repairs that would avoid the need for reoperation or multiple operations.
Introduction
Congenital heart disease is the leading cause of birth defects and leading cause of infant mortality in the US, occurring in approximately 1/100 live-births [Citation1,Citation2]. There are 30–40 different types of operations performed to repair a wide variety of heart defects. Conduit insertion is one of the most common procedures performed by heart surgeons to address many of these defects, including tetralogy of Fallot, pulmonary atresia/ventricular septal defect, truncus arteriosus, certain transposition complexes and some variants of double outlet right ventricle.
Current conduits inserted from the RV to the PA have no growth potential, are subject to deterioration, calcification, infection and not always easily amenable to catheter-based interventions [Citation3–17]. The vast majority of infants and children will require reoperation and/or re-intervention to replace the conduit. Some children may require multiple reoperations, with the risk of death and morbidity increasing significantly with each subsequent operation [Citation14].
Current options for conduit insertion in children include aortic and pulmonary homografts, venous homografts, bovine jugular vein grafts, or PTFE conduits [Citation3–7,Citation12–16,Citation18–23]. Homografts are the conduits of choice for most surgeons and the standard by which any new conduit is compared. They handle easily and may be resistant to infection but suffer from deterioration, calcification, limited durability and need for reoperation. Homografts are in limited supply, especially in the smaller sizes.
Bovine jugular vein grafts are currently approved in the US under humanitarian device exemption [Citation14–16]. They are widely available, but may have a higher incidence of obstruction and infection. There is an 83.3% stenosis rate for the 12-mm-size conduit (used for neonates and infants). The risk of endocarditis in bovine jugular vein grafts is significantly higher compared to other conduits, with a 9-fold hazard increase and 83% freedom from endocarditis at 10 years after implantation, versus 95% to 100% freedom for other conduit choices. Several reports have highlighted the higher occurrence of endocarditis for jugular vein grafts. Bovine jugular vein grafts are short-lived in smaller sizes, and their handling characteristics result in variable outcomes that are surgeon or institution specific [Citation15,Citation16]. In order to address the problem with cardiovascular conduit insertion in infants and children we manipulated an ePTFE-polymer sheet with directionally dependent expansion properties. The sheet is circularized in such a way that the resultant tube has expansion potential in a radial direction, requires low pressure to expand its circumference and is easily amenable to having a valve mechanism inserted.
The resulting anisotropic conduits have spontaneous radial expansion potential to accommodate natural growth, potentially obviating the need for multiple surgeries or make future catheter-based interventions highly successful. In the current study, we report the feasibility and performance of anisotropic conduits for cardiovascular repair in a growing lamb model.
The aim of this study is to determine whether anisotropic conduits can be a) safely implanted in growing lambs, and b) have favorable hemodynamics, and show radial expansion properties in vivo capable of accommodating growth.
Materials and methods
Approval was obtained from the laboratory’s Institutional Animal Care and Use Committee (IACUC).
Test conduit design
A 20 or 22 mm Stretch Gore-Tex™ graft was longitudinally cut and re-circularized using suture in a perpendicular direction such that the resultant diameter was 10–11 mm. The length of the test conduit was 2 cm for interposition (IP) implants. Alternately, the test conduit design can incorporate a 2 cm beveled cobra hood extension allowing for right ventricular outflow tract (RVOT) anastomosis. A 22 mm Braun Z-Med II™ valvuloplasty balloon catheter was used to verify the expansion capacity of the conduit design prior to implant.
Animals
Polypay lambs aged 8–12 weeks, weighing 15.3–21.1 kg were used for the study. Animals were fed Lamb Creep B90 (a ruminant diet), mixed with a sweet feed (may be medicated with lasalocid sodium 11.6 mg/lb), timothy hay cubes and loose hay. They are fed once daily and allowed to consume their feed throughout the day. All animals were given water ad libitum throughout the study.
Fasting/preoperative preparation
Animals were fasted for 12–24 h prior to anesthetic events with water provided ad lib. Sustained release (SR) Buprenorphine (SQ) was used for pre- and post- operative analgesia at a dose of 0.12–0.27 mg/kg, given in the 24 h period prior to surgical induction.
Induction
Animals were sedated with 0.04 mg/kg atropine IM and 10 mg/kg ketamine IM. Supportive fluids, 0.9% normal saline (NaCl), were administered IV. Anesthesia was induced using 2–6 mg/kg Propofol IV. Animals were endotracheally intubated for mechanical ventilation at 10–15 breaths per minute, 4 LO2/min, and 1–4% isoflurane. A stomach tube was inserted and approximately 1 pint of antacid was administered via the tube to prevent bloat. An antibiotic, 3 mg/kg ceftiofur IM, and corticosteroid, 250 mg methylprednisolone (or equivalent) IV, were administered prior to incision. The animal was positioned in the right decubitus position, aseptically prepped, and draped. Heart rate, respiratory rate, oxygen saturation, body temperature and intravenous fluid infusion rate were monitored.
Conduit implant
When the animal reached a deep plane of anesthesia 0.4–0.8 mg/kg succinylcholine chloride was administered IV. A left thoracotomy was performed through the 4th intercostal space, the animal was heparinized (250 units/kg) and placed on single stage cardiopulmonary bypass. Conduit implant was performed on a beating heart during hypothermia. In both groups the main pulmonary artery was isolated, and cross clamped proximal to the pulmonary artery bifurcation, as previously described in the literature [Citation24].
A section of the MPA was excised to allow space for the conduit. The conduit was sewn interpositionally in place with a running stitch using 5-0 or 6-0 absorbable suture on each side of the conduit. For interpositional implantation of the anisotropic conduit directly to the RVOT, the MPA was transected 5–15 mm distal to the pulmonary valve. A longitudinal arteriotomy was subsequently made from the transected edge of the main PA, extending across the pulmonary annulus and into the RVOT (ventriculotomy) so that a 1.5–2 cm length of beveled conduit could be used for the proximal anastomosis. Once the conduit was implanted, mechanical ventilation recommenced and the animal was warmed to normothermia and weaned off cardiopulmonary bypass. When bypass cannulas were removed, heparin was reversed (Protamine, IV, approximate ratio of 10 mg protamine:1000IU of heparin). The thoracotomy was closed and animals were recovered from anesthesia.
Hemodynamic and angiographic assessment
Animals were anesthetized for hemodynamic and angiographic assessments at three and six months post-conduit implant. The heart was catheterized for measurement of intracardiac pressure data, dimensional measurements, and fluoroscopic angiogram. Anesthesia was the same as previously described, the left neck was shaved and aseptically prepped for vascular access using a modified Seldinger technique to insert a 7 Fr. introducer into the jugular vein. Intracardiac catheters (7 Fr. pigtail, 7 Fr. wedge pressure and 7 Fr. Swan-Ganz catheters) were inserted into the jugular vein introducer and advanced into the heart under fluoroscopic guidance. At the completion of the procedure, the introducer was removed and the vein repaired with 7-0 monofilament suture and the skin incision was closed in a normal fashion with 2-0 and 3-0 absorbable suture and animals were recovered from anesthesia. Buprenorphine (SQ) was used for pre- and post- operative analgesia at a dose of 0.12–0.27 mg/kg, given in the 24 h period prior to surgical induction.
Postoperative care
Animals were observed post operatively for normal recovery from surgery, appetite, fluid intake, voiding, ambulation, respiratory rate, respiratory effort, heart rate and rhythm, willingness to stand when approached, willingness to ambulate, development of ascites, and survival. Independent veterinary assessment was performed in the presence of atypical clinical appearance. After discharge from post-operative care, animals were housed long-term in a natural environment with pasture and appropriate shelter with on-site veterinary technical support. Tachypnea or ascites symptomatic animals were treated with furosemide 2–4 mg/kg at the onset of symptoms.
Echocardiography
Transthoracic Echocardiography (TTE) exams were performed at 14 days after graft implant, and monthly throughout each animal’s postoperative period. A comprehensive exam was performed following established guidelines [Citation25].
Statistics
Data is presented (mean ± SD) and analyzed using Microsoft Excel 2016. Paired t-tests were used to compare groups, with statistical significance defined as p < 0.05.
Results
Anisotropic (test) conduits were engineered to a diameter of 10–11 mm, and 2 cm length. Prior to implant the expansion capacity of the engineered conduit was evaluated in vitro using a 22 mm balloon at varying pressures presented in . The results are summarized in . At baseline, conduits have an internal diameter of 10-11mm, which corresponds to a desired size in neonatal or infant insertion of RV-PA conduit in biventricular repair. Progressive increases in balloon inflation pressure from 0.5 atm to 3.5 atm resulted in conduit radial expansion to 21.5 mm diameter (adequate for adult RVOT). When a 25 mm balloon was used, the conduits expanded up to 22–24 mm in diameter and began to tear at the suture line at an insufflation pressure greater than 4 atm with the oversized balloon. Wall tension was not calculated as a design specification.
Figure 1. In vitro benchtop balloon dilation of the anisotropic (test) conduit. A 10 mm test conduit is dilated with a 20 mm catheter based balloon to its final diameter of 21.5 mm. Wall tension was not calculated during this test. The black thumb drive is in the image for perspective, measuring 4.5 cm x 1.5 cm.

Table 1. Verification of Test conduit expansile properties with a pressurized balloon catheter with reference to corresponding patient size.
The in vivo evaluation of anisotropic conduit performance was performed in a well-established growing lamb model and there was typical weight gain and a normal growth curve () [Citation26–28]. All three control animals developed adverse events including progressive tachypnea (3/3), ascites (3/3), inappetence(1/3), lethargy (3/3), and death (1/3) due to severe right heart failure and showed significant peak trans-conduit gradients that ranged from 46–110 mmHg by echocardiography (). The RV was subjectively dilated and dysfunctional, with moderate tricuspid valve regurgitation (TR) [Citation25]. Test animals (n = 4) were clinically asymptomatic, active and there were no adverse events observed. Compared to controls, the test conduits demonstrated spontaneous expansion during growth, with a significant (p < 0.002 at 3 months and p < 0.01 at 6 months) increase in diameter and, significantly lower peak and mean echo gradients (p < 0.03) across the conduits at both 3 and 6 months ().
Table 2. Animal weight.
Table 3. Assessment of cardiac function using transthoracic echocardiography at 3 and 6 months after implant of the Test and Control conduits.
At 3 months, cardiac catheterization of control animals showed a mean trans-conduit peak gradient of 35.6 ± 5.8 mmHg. By 6 months the mean trans-conduit peak gradient in controls increased to 48.5 ± 5.1 mmHg. Conversely, in test animals (n = 4) there was reduced obstruction and lower mean trans-conduit peak gradients at 3 months (12.2 ± 6.7 mmHg vs 35.6 ± 5.8 mmHg, p < 0.05), and 6 months (test gradient of 27.0 ± 8.3 mmHg vs control to 48.5 ± 5.1 mmHg, p < 0.05) ().
Representative angiograms in growing lambs 3 months after control conduit insertion and of the test anisotropic conduit at 3 months post-implantation are shown in and , respectively. Application of the test conduit as an interposition graft in the MPA and a test conduit sewn from a right ventriculotomy to the PA are shown in , respectively. At both the 3 and 6 months timepoint after implant the diameter of the test grafts were greater than controls, 15.2 ± 0.8 mm vs 9.7 ± 0.7 mm at 3 months, and 14.8 ± 0.8 mm vs 9.6 ± 0.6 mm at 6 months, and this difference was significant p < 0.05, ().
Figure 2. Angiograms of a control conduit at 3 months post-implant. There is severe obstruction of the pulmonary outflow at the interposition graft insertion site with the conduit diameter measuring approximately 9 mm.
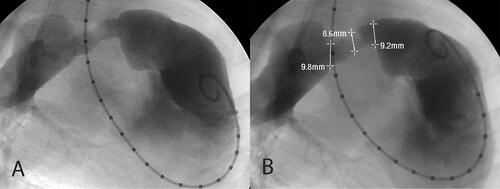
Figure 3. Angiograms of Test conduits at 3 months post-implant. shows the test conduit as an interposition graft in the main PA. The conduit measures approximately 16 mm along its entire length from proximal to distal anastomosis, spontaneously expanding from its baseline insertion diameter of 10–11 mm. shows a representative angiogram of a test conduit sewn from a right ventriculotomy to the PA. There is no contour deformity, nor obstruction of the RVOT. Angiographic images were collected with an OEC 9900 Elite C-Arm positioned at AP 0° with the animal in the right decubitus position.
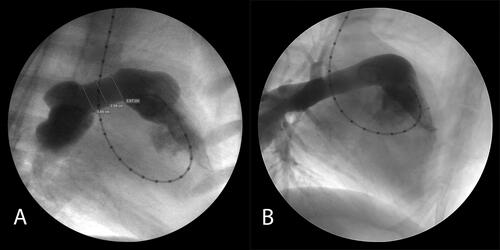
Table 4. Assessment of cardiac function using intracardiac catheterization at 3 and 6 months after implant of the Test and Control conduits.
Discussion
A new technology or option that has “growth” potential or radial expansion properties for use in pediatric cardiovascular surgery would have immediate and direct clinical/translational impact on patient care. We have designed a unique cardiovascular conduit construct with an anisotropic capacity for stretching. Anisotropic conduits have impressive expansion capacity, evident in balloon dilation measures evaluating conduit design prior to implant on the benchtop. Anisotropic conduit performance in the growing lamb model demonstrated the spontaneous expansion from a starting diameter of 10–11 mm to a diameter of 15–16 mm driven by RV pressure and improved hemodynamics compared with controls. We postulate that the conduit would also support balloon expansion and stenting in vivo. Anisotropic conduits can be deployed in vascular systems to provide a pathway of care, as they can be expanded circumferentially to provide a cross-section for blood flow that is appropriate for the size and growth needs of the patient. These ePTFE-based conduits are biocompatible, easily available off-the-shelf, and resist calcification and degeneration.
Our novel approach generated a conduit that is anisotropic by stretching more circumferentially than longitudinally and has some hysteresis or malleability. The conduit will remain expanded at least some portion of the amount it was stretched. Its malleability permits for circumferential expansion as a result of pressure within the plenum. Based on material properties, it seems logical to conclude that the conduit could additionally or alternatively be manipulated to expand over time by balloon expansion and/or stenting.
Depending on the age and size of the patient, an appropriately sized conduit connecting the right ventricle and the pulmonary artery can be anywhere from less than 10 mm for an infant to 2 cm or more for an adult. The conduit thereby provides a replacement for defective or missing components of the vasculature of a growing child without requiring reoperation as frequently, because the conduit is capable of expanding to match a desired size for the child. Based on the pre-implant balloon experiments evaluation of expansion capacity, it is clear that relatively low pressures are required to dilate the conduit to an increased diameter. The expansion in the growing lamb demonstrates that the conduit naturally expands radially in response to the pressure generated from the RV. In control lambs, the 10 mm conduit becomes inadequate by 3 months with the mean peak RV pressures rising to 51 mmHg, and the mean trans-conduit peak gradient reaching 35 mmHg. Long-term, 6 months after implantation, with additional growth and increasing metabolic demands, the mean peak RV pressure increases to greater than 60 mmHg and the mean trans-conduit peak gradient reaches 48 mmHg. While lambs in the control group experienced symptoms and complications of right heart failure, there was no evidence of heart failure in the test group and animals demonstrated superior graft hemodynamics in comparison with controls through the duration of follow-up. In this study, the test graft spontaneously increased in diameter by more than 50%, with normal RV pressures of about 25 mmHg at 3 months and very mild to minimal trans-conduit gradient. By 6 months mild increases in RV pressure and trans-conduit gradient on cardiac catheterization were observed in test lambs that were not present on echo analysis. Echocardiography revealed test animals had stable cardiac output at 3 and 6 months after implantation and low mean gradients across the test conduit. The discrepancy between echo and catheterization data at 6 months may be a reflection of different testing conditions and technique. In general, test animals with anisotropic conduits had near normal RV pressures, low trans-conduit gradients, and significant increases in the diameter of the RV-PA connection versus controls that developed heart failure during follow-up.
This exploratory study shows anisotropic conduits can be safely implanted, as evidenced by: (1) the absence of adverse events during follow-up, (2) spontaneous expansion of the graft confirmed by angiography, (3) and stable hemodynamics over the course of normal lamb growth during the study period. After establishing favorable outcomes for interposition grafting with anisotropic conduits compared to controls we sought to determine whether direct anastomosis to a right ventriculotomy would affect the radial expansile properties of the conduit. Our results showed that the conduits radial expansion is not limited by anastomosis to the RV, a highly clinically relevant finding.
Another clinical example where this approach can be used includes the extra-cardiac conduit (ECC) Fontan operation for final stage palliation of children with single ventricles. Approximately 1000 Fontan operations are done each year in the US. The ECC Fontan is usually delayed until the child is at least four or five years of age, or at least 15 kg, so that an adult sized conduit (about 20 mm) can be implanted [Citation29–33]. Using an anisotropic conduit, the ECC Fontan can be performed at 2 or 3 years of age, with a 14 mm conduit that we postulate can be expanded to 20–22 mm over time accommodating patient growth, for example. Hence, an earlier Fontan operation can be performed, allowing the child to avoid the prolonged effects of cyanosis, and reduce the risk of stroke.
There is tremendous potential for further engineering of this conduit, for example, valve cusps could be added to the conduit or small intestinal submucosal (SIS) patches, thin-walled (0.1 mm–0.4 mm) PTFE, femoral venous valves or other polymer can be intussuscepted to variable heights to create a bicuspid or tricuspid valve mechanism [Citation21,Citation22]. Valveless RVOT connections have been used for the repair of several lesions, such as truncus arteriosus, pulmonary atresia with ventricular septal defect, tetralogy of Fallot with absent pulmonary valve, and D-Transposition of the great arteries with ventricular septal defect and severe pulmonary stenosis. Valve-less repair for reconstruction of the RVOT in infants has been shown to be associated with lower risk of reoperation. However, many surgeons have not adopted this approach because of concern with pulmonary insufficiency, which may make the early post-operative course more challenging. Dr. Alsoufi et al expressed that given the current advances in perioperative care, valve-less repair strategies might need to be reconsidered, especially because many of the conduits in small neonates and infants show early incompetence [Citation34]. The detrimental effects of chronic pulmonary regurgitation on the late RV function and size can be addressed with transcatheter implantation of a stented-valve, which for anisotropic conduits, would allow for radial expansion to “adult” diameters while establishing pulmonary valve competence [Citation35].
The anisotropic conduits do not stenose, are easy to handle, are more readily available than donor material, and are hemostatic. Conduits that are amenable to catheter-based manipulation with reduced risk of rupture minimize the number of relatively riskier and more costly surgical replacement procedures. Future studies will examine the feasibility, safety and efficacy of anisotropic conduit dilation and/or stenting. This approach is advantageous in that it creates a “pathway” of treatment, wherein the conduit can expand spontaneously, or alternately via angioplasty if future studies validate this material property, to accommodate patient growth.
Conclusions
This novel ePTFE anisotropic conduit may be a safe and efficacious approach for repairing congenital heart defects in a growing lamb model with midterm favorable hemodynamics. Anisotropic conduits spontaneously expand in vivo and may represent a novel approach to congenital heart repairs that would avoid the need for reoperation or multiple operations.
Acknowledgment
We thank the UMN Experimental Surgical Services Laboratory staff and students with their help in conducting the animal experiments; the UMN Research Animal Resources staff for their care and support of the research animals used in this study; Melanie Graham for her help with the editing process; Gurumurthy Hiremath for interventional cardiology expertise and helpful discussions.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
The data that support the findings of this study are available from the corresponding author, JPC, upon reasonable request.
Additional information
Funding
References
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi:https://doi.org/10.1161/CIR.0b013e31828124ad.
- Triedman JK, Newburger JW. Trends in congenital heart disease: the next decade. Circulation. 2016;133(25):2716–2733. doi:https://doi.org/10.1161/CIRCULATIONAHA.116.023544.
- Arsdell GS, Azakie A. Surgery for common arterial trunk. Progr Pediatr Cardiol. 2002;15(1):53–58. doi:https://doi.org/10.1016/S1058-9813(02)00008-5.
- Stark J. The use of valved conduits in pediatric cardiac surgery. Pediatr Cardiol. 1998;19(4):282–288. doi:https://doi.org/10.1007/s002469900311.
- Albert JD, Bishop DA, Fullerton DA, Campbell DN, Clarke DR. Conduit reconstruction of the right ventricular outflow tract. J Thorac Cardiovasc Surg. 1993;106(2):228–236.
- Bando K, Danielson GK, Schaff HV, Mair DD, Julsrud PR, Puga FJ. Outcome of pulmonary and aortic homografts for right ventricular outflow tract reconstruction. J Thorac Cardiovasc Surg. 1995;109(3):509–518. doi:https://doi.org/10.1016/S0022-5223(95)70282-2.
- Baskett RJ, Ross DB, Nanton MA, Murphy DA. Factors in the early failure of cryopreserved homograft pulmonary valves in children: preserved immunogenicity? J Thorac Cardiovasc Surg. 1996;112(5):1170–1179. doi:https://doi.org/10.1016/S0022-5223(96)70130-7.
- Dobell ARC, Jain AK. Catastrophic hemorrhage during redo sternotomy. Ann Thorac Surg. 1984;37(4):273–278. doi:https://doi.org/10.1016/S0003-4975(10)60728-X.
- Hawkins JA, Bailey WW, Dillon T, Schwartz DC. Midterm results with cryopreserved allograft valved conduits from the right ventricle to the pulmonary arteries. J Thorac Cardiovasc Surg. 1992;104(4):910–916. doi:https://doi.org/10.1016/S0022-5223(19)34671-9.
- Jonas RA, Freed MD, Mayer JE, Castaneda AR. Long-term follow-up of patients with synthetic right heart conduits. Circulation. 1985;72:77–83.
- Lacour-Gayet F, Serraf A, Komiya T, et al. Truncus arteriosus repair: influence of techniques of right ventricular outflow tract reconstruction. J Thorac Cardiovasc Surg. 1996;111(4):849–856. doi:https://doi.org/10.1016/s0022-5223(96)70346-x.
- Razzouk AJ, Williams WG, Cleveland DC, et al. Surgical connections from ventricle to pulmonary artery. Comparison of four types of valved implants. Circulation. 1982;86(II):154–158.
- Iyer KS. The Contegra bovine jugular valved conduit: living up to expectations? Ann Pediatr Cardiol. 2012;5(1):34–35.
- Holmes AA, Co S, Human DG, Leblanc JG, Campbell AIM. The Contegra conduit: late outcomes in right ventricular outflow tract reconstruction. Ann Pediatr Card. 2012;5(1):27–33. doi:https://doi.org/10.4103/0974-2069.93706.
- Protopapas AD, Athanasiou T. Contegra conduit for reconstruction of the right ventricular outflow tract: a review of published early and mid-time results. J Cardiothorac Surg. 2008;3:62doi:https://doi.org/10.1186/1749-8090-3-62.
- Breymann T, Blanz U, Wojtalik MA, et al. European Contegra multicentre study: 7-year results after 165 valved bovine jugular vein graft implantations. Thorac Cardiovasc Surg. 2009;57(5):257–269. doi:https://doi.org/10.1055/s-0029-1185513.
- Penford G, Quandt D, Mehta C, et al. Stenting and overdilating small Gore-Tex vascular grafts in complex congenital heart disease. Catheter Cardiovasc Interv. 2018;91(1):71–80. doi:https://doi.org/10.1002/ccd.27310.
- Manavitehrani I, Ebrahimi P, Yang I, et al. Current challenges and emergent technologies for manufacturing artificial right ventricle to pulmonary artery (RV-PA) cardiac conduits. Cardiovasc Eng Technol. 2019;10(2):205–215. doi:https://doi.org/10.1007/s13239-019-00406-5.
- Dearani JA, Connolly HM, Martinez R, Fontanet H, Webb GD. Caring for adults with congenital cardiac disease: successes and challenges for 2007 and beyond. Cardiol Young. 2007;17(S4):87–96. doi:https://doi.org/10.1017/S1047951107001199.
- Jacobs JP, Mavroudis C, Quintessenza JA, et al. Reoperations for pediatric and congenital heart disease: an analysis of the Society of Thoracic Surgeons (STS) congenital heart surgery database. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2014;17(1):2–8. doi:https://doi.org/10.1053/j.pcsu.2014.01.006.
- Schiller O, Sinha P, Zurakowski D, Jonas RA. Reconstruction of right ventricular outflow tract in neonates and infants using valved cryopreserved femoral vein homografts. J Thorac Cardiovasc Surg. 2014;147(3):874–879. doi:https://doi.org/10.1016/j.jtcvs.2013.11.006.
- Yoshida M, Wearden PD, Dur O, Pekkan K, Morell VO. Right ventricular outflow tract reconstruction with bicuspid valved polytetrafluoroethylene conduit. Ann Thorac Surg. 2011;91(4):1235–1239. doi:https://doi.org/10.1016/j.athoracsur.2010.11.010.
- Sharifulin R, Bogachev-Prokophiev A, Demin I, et al. Right ventricular outflow tract reconstruction using a polytetrafluoroethylene conduit in Ross patients. Eur J Cardiothorac Surg. 2018;54(3):427–433. doi:https://doi.org/10.1093/ejcts/ezy128.
- Carney JP, Zhang LM, Larson JJ, et al. The Hancock® valved conduit for right ventricular outflow tract reconstruction in sheep for assessing new devices. J Heart Valve Dis. 2017;26(4):472–480.
- Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for Evaluation of Prosthetic Valves With Echocardiography and Doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22(9):975–1014. doi:https://doi.org/10.1016/j.echo.2009.07.013.
- Boudjemline Y, Laborde F, Pineau E, et al. Expandable right ventricular-to-pulmonary artery conduit: an animal study. Pediatr Res. 2006;59(6):773–777. doi:https://doi.org/10.1203/01.pdr.0000219396.34610.4a.
- Hilbert SL, Yanagida R, Souza J, et al. Prototype anionic detergent technique used to decellularize allograft valve conduits evaluated in the right ventricular outflow tract in sheep. J Heart Valve Dis. 2004;13(5):831–840.
- Reimer J, Syedain Z, Haynie B, Lahti M, Berry J, Tranquillo R. Implantation of a tissue-engineered tubular heart valve in growing lambs. Ann Biomed Eng. 2017;45(2):439–451. doi:https://doi.org/10.1007/s10439-016-1605-7.
- Petrossian E, Reddy VM, Mcelhinney DB, et al. Early results of the extracardiac conduit Fontan operation. J Thorac Cardiovac Surg. 1999;117(4):688–696. doi:https://doi.org/10.1016/S0022-5223(99)70288-6.
- Petrossian E, Reddy VM, Collins KK, et al. The extracardiac conduit Fontan operation using minimal approach extracorporeal circulation: Early and midterm outcomes. J Thorac Cardiovasc Surg. 2006;132(5):1054–1063. doi:https://doi.org/10.1016/j.jtcvs.2006.05.066.
- Azakie A, Mccrindle BW, Arsdell GV, et al. Extracardiac conduit versus lateral tunnel cavopulmonary connections at a single institution: Impact on outcomes. J Thorac Cardiovasc Surg. 2001;122(6):1219–1228. doi:https://doi.org/10.1067/mtc.2001.116947.
- Shinkawa T, Anagnostopoulos PV, Johnson NC, et al. Early results of the “clamp and sew” Fontan procedure without the use of circulatory support. Ann Thorac Surg. 2011;91(5):1453–1459. doi:https://doi.org/10.1016/j.athoracsur.2010.12.030.
- The Society of Thoracic Surgeons. The STS Congenital Heart Surgery Database. https://[www.sts.org/registries-research-center/sts-national-database/congenital-heart-surgery-database/data-collection. Published April 2020. Accessed April 3, 2020.
- Alsoufi B. Right ventricle to pulmonary artery conduits: do we really have an option? J Thorac Cardiovasc Surg. 2016;151(2):442–443. doi:https://doi.org/10.1016/j.jtcvs.2015.10.100.
- Hofferberth SC, Saeed MY, Tomholt L, et al. A geometrically adaptable heart valve replacement. Sci Transl Med. 2020;12(531):eaay4006. https://[stm.sciencemag.org/content/12/531/eaay4006/tab-pdf]. Published Feb 2020. Accessed May 20, 2020. doi:https://doi.org/10.1126/scitranslmed.aay4006.
