Abstract
Background
Transcatheter aortic valve replacement (TAVR) is an effective therapy in treating high-risk patients suffering from aortic stenosis. Animal models used to evaluate safety and efficacy of TAVR devices prior to clinical use lack a stenotic aortic annulus, a critical impediment to long-term TAVR device evaluation. We sought to create a reproducible model of aortic stenosis using a modified aortic annuloplasty (MAA) procedure in sheep, followed by deployment and long-term evaluation of TAVR devices using this model.
Methods
Twelve sheep underwent the MAA procedure and were recovered. Transthoracic echocardiography (TTE) was used to monitor changes in the aortic annulus in the postoperative period. At 60 days post-MAA, Test group animals were anesthetized for TAVR insertion and Control animals underwent a necropsy. Test animals were recovered following TAVR insertion and observed for a postoperative period of 140 days.
Results
Twelve sheep survived the annuloplasty procedure and the 60-day recovery period. Gross examination of seven Control group animals revealed the implanted annuloplasty ring segments formed hard protrusions into the aortic annulus. Five sheep in the Test group underwent successful deployment of Abbott’s experimental TAVR device without evidence of migration. Examination at 140 days post-TAVR insertion showed all devices tightly anchored within the modified aortic annulus.
Conclusions
The MAA procedure creates stenotic segments in the aortic annulus with adequate rigidity for anchorage and long-term evaluation of TAVR devices. This represents the first model that successfully mimics human aortic stenosis and provides a clinically relevant TAVR deployment platform for long-term evaluation in sheep.
Introduction
Aortic stenosis is the most common cardiac valve lesion in the United States [Citation1]. Patients exhibiting clinical symptoms of AS have a high risk of death [Citation1, Citation2]. AS is characterized by progressive calcification and narrowing of the aortic valve, resulting in an obstruction forcing the left ventricle to generate a continuously increasing pressure. Over time, aortic stenosis causes damage to the left ventricle, alters coronary flow, and ultimately causes systolic and diastolic left ventricular dysfunction.
Historically, the standard of care for patients with symptomatic AS has been surgical aortic valve replacement using cardiopulmonary bypass [Citation3, Citation4]. However, surgical replacement can be extremely risky for some critically ill patients. Thus, over the last 20 years, less invasive catheter-based devices have been developed as an alternative to treat high risk patients [Citation5–8]. At present, an estimated 200,000 patients have been treated at 750 global centers using transcatheter aortic valve replacement (TAVR) devices for the treatment of aortic valve stenosis, establishing the use of this treatment modality as a clinically viable and effective therapy [Citation9]. Moreover, regulatory approval of TAVR devices will undoubtedly stimulate the creation and development of novel transcatheter-based devices for the treatment of AS [Citation10–12].
Prior to human use, the safety and performance of new TAVR devices must be evaluated in a large animal model [Citation12]. However, healthy animals lack a calcified and stenotic aortic annulus, critical to the securement of a TAVR stent, and a key characteristic of AS. This presents a critical impediment to long-term TAVR device evaluation. While a number of surgical techniques have been developed to circumvent this problem, including surgical implant, heterotopic delivery, aortic banding and valve-in-valve deployment, none effectively model the cardiac disease state of AS for which patients would receive a TAVR device.
Therefore, the aim of this study was to 1) model AS in sheep by performing a modified aortic annuloplasty (MAA) procedure and 2) implant a clinically relevant TAVR device in the model for long-term evaluation.
Materials and methods
Approval was obtained by the Institutional Animal Care and Use Committee prior to the start of the study.
Experimental design
Experimental design is provided in . All animals underwent a modified aortic annuloplasty (MAA) procedure using cardiopulmonary bypass (CPB) and were recovered. At 14 and 60 days post-MAA, all animals were monitored with transthoracic echo (TTE). At 60 days post-MAA, animals were divided into Test and Control groups. Animals in the Control group (n = 7) were euthanized. Animals in the Test group (n = 5) underwent TAVR insertion into the modified aortic annulus and were recovered. At 140 days post-TAVR insertion animals were humanely euthanized. All animals underwent a comprehensive gross necropsy at the end of the study term.
Table 1. Experimental design and data collection events.
Annuloplasty ring design
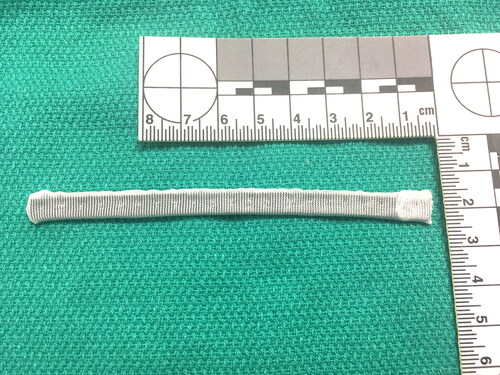
The MAA procedure is facilitated by an annuloplasty ring designed by our laboratory, consisting of a silicone core covered in Dacron fabric, measuring 110 mm × 7 mm × 1 mm in dimension. The silicone core is seeded with Tantalum powder prior to manufacture to make the ring radiolucent. The ring can easily be cut with a scissors for customization of the segments specific to each animal’s aortic anatomy. The ring was produced using standard manufacturing procedures and was sterilized appropriately prior to implantation. A photo of the annuloplasty ring is presented in .
Animals
Twelve domestic sheep were selected for this study, consisting of 10 females and 2 castrated males, 15.4 ± 3.8 months old (mean ± SD); weighing 75.6 ± 7.2 kg. More females were available from the farmer than castrated males at time of purchase. Upon arrival to the laboratory, a veterinarian performed a physical examination for heath assessment, including heart rate, body temperature, respiratory rate and capillary refill time, and were vaccinated and dewormed as appropriate. Animals were housed in AAALAC accredited pens in large animal housing.
Fasting/preoperative preparation
Animals were fasted for 12–24 h prior to anesthetic events with water provided ad libitum. Sustained release (SR) Buprenorphine (SQ) was used for pre- and post- operative analgesia at a dose of 0.12–0.27 mg/kg, given in the 24 h period prior to surgical induction.
Modified aortic annuloplasty (MAA)
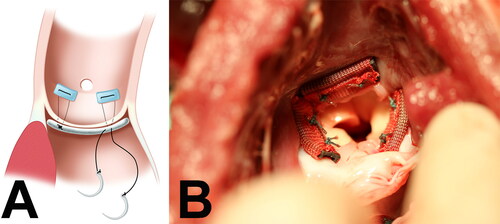
Animals were sedated with 0.04 mg/kg atropine IM, 10 mg/kg Ketamine IM and 2–6 mg/kg propofol IV. Animals were intubated, maintained on isoflurane at 2–4% for the duration of surgery and monitored for heart rate, mean blood pressure, fixed pupil location, corneal reflex absence, and oxygen saturation to ensure proper anesthesia. Surgery was performed in the right decubitus position with left 3rd intercostal space thoracotomy to expose the heart and the aorta. The animals were anticoagulated and placed on CPB using standard techniques [Citation13–16]. The animal was cooled to 28˚C, and the aorta was cross clamped proximal to the junction of the brachiocephalic trunk. A partial transverse aortotomy was made. The native aortic annulus was measured and the modified annuloplasty material was cut into three appropriately sized sections. These sections were then implanted directly below each of the three native aortic valve leaflets, using interrupted 3-0 braided polyester mattress stitches, as illustrated in . A photograph of the annuloplasty segments sewn to the aortic annulus is provided in . The aortotomy was closed and the animal was warmed and bypass cannulas removed. Postoperatively, the animals recovered under the care of a veterinarian and received Ketoprofen 1–2 mg/kg IM or Carprofen 2–4 mg/kg IM, as needed for pain management.
TAVR insertion
Test group animals underwent TAVR insertion 60 days following the MAA procedure. Animals were sedated as described above. A right neck incision was made and the carotid artery was exposed and cannulated with a 20 Fr. introducer using the Seldinger technique. The TAVR valve deployment catheter was introduced into the carotid artery. The TAVR valve was deployed in the modified aortic annulus, under fluoroscopy and intracardiac echocardiography (ICE) visualization. The carotid artery was repaired and the animal was recovered from anesthesia. Postoperatively, the animals recovered under the care of a veterinarian and received analgesia as previously described.
Postoperative care
Animals were observed post operatively for normal recovery from surgery, appetite, fluid intake, voiding, ambulation, respiratory rate, respiratory effort, heart rate and rhythm, willingness to stand when approached, willingness to ambulate, development of ascites, and survival. Independent veterinary assessment was performed in the presence of atypical clinical appearance. After discharge from post-operative care, animals were housed long-term in a natural environment with pasture and appropriate shelter with on-site veterinary technical support.
Echocardiography
Transthoracic Echocardiography (TTE) exams were performed at 14 and 60 days after MAA implant, and monthly for Test group animals following TAVR insertion. A comprehensive exam was performed following established guidelines [Citation17].
Endpoint data collection
Prior to scheduled sacrifice, animals in both the Control and Test groups underwent TTEs and were humanely euthanized with administration of Beuthanasia-D IV at 87 mg/kg. A comprehensive gross necropsy was completed following euthanasia.
Statistics
All data were collected using Microsoft Excel 2016 and a paired t-test was used to analyze the data. Statistical significance was defined as p ≤ 0.05.
Results
All 12 Test and Control group sheep survived the modified aortic annuloplasty procedure. Cardiac function was monitored in the postoperative period with transthoracic echo (TTE). Data is presented in . At 14 and 60 day post-MAA time points, there were no statistically significant differences in the maximum and mean systolic left ventricle to aorta pressure gradients between the animals selected to be part of the Control group (n = 7) and the Test group (n = 5). At the 60 day TTE, mild to moderate aortic insufficiency was appreciable in 4 of 7 Control group animals and 5 of 5 Test group animals. This finding in the Test group was supported by angiograms captured at the time of TAVR insertion.
Table 2. Transthoracic echo data at 14 and 60 days post-MAA collected on Test and Control group animals.
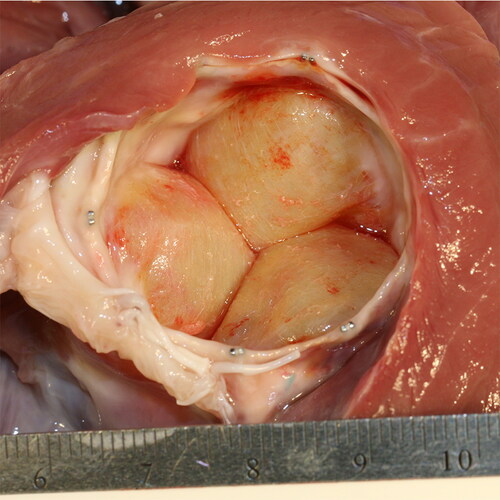
Comprehensive gross necropsy of Control group animals at 60 days post-MAA revealed that the implanted annuloplasty ring segments formed hard, stenotic, fibrous protrusions into the left ventricle outflow tract, appreciable in .
Figure 3. Photograph of annuloplasty ring segments healed into the aortic annulus in a Control group animal at 60-day gross exam. Note the fibrous appearance of the ring segments and the circumferential scarring of the left ventricle outflow tract and aortic annulus.
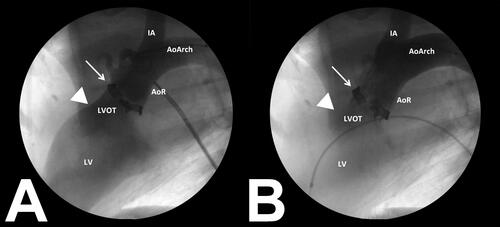
At 60 days post-MAA, Test group animals underwent TAVR insertion within the modified aortic annulus, and were implanted with an experimental Abbott next generation TAVR device. shows the fluoroscopy view of the aortic annulus with implanted material before TAVR deployment; note the presence of aortic insufficiency as contrast leaked back into the left ventricle. shows the TAVR device was positioned within the aortic annulus immediately following insertion; note that there was no aortic insufficiency after insertion of the TAVR valve.
Figure 4. Fluoroscopic images obtained during TAVR device deployment. presents an angiogram collected prior to TAVR insertion. Note the modified aortic annulus (white arrow) with aortic insufficiency present (arrow head). presents a post-insertion angiogram with the TAVR device seated within the modified aortic annulus (white arrow). Note that aortic insufficiency is no longer present (arrow head) following TAVR insertion.
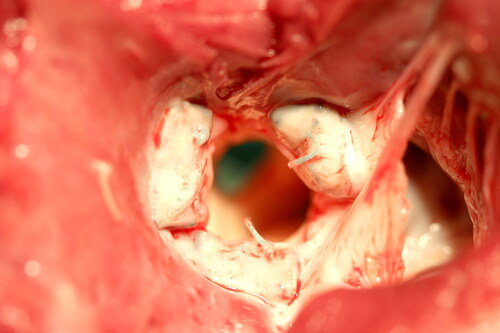
Five of five animals survived the TAVR procedure and recovered normally. Animals were monitored with TTE in the postoperative period. Three of five Test animals survived to the scheduled study term of 140 postoperative days. Two sheep died on post-TAVR days 6 and 19 respectively, of causes determined to be unrelated to the TAVR valve. Gross examination of the Test group animals at necropsy revealed well-positioned, tightly anchored TAVR device within stenotic aortic annuli. A photo of the TAVR device healed into the modified aortic annulus is presented in . There was no TAVR device migration in any of the Test group animals examined.
Discussion
In this study, we present the first animal model of aortic stenosis, structurally and functionally mimicking human pathology, valvular stenosis and calcification at the level of the native aortic annulus. The MAA model is not intended to create a calcified aortic annulus as observed in the clinical setting, rather its purpose is to create fibrotic scarring at the level of the annuloplasty ring segments, resulting in a mechanical stenosis, where the aortic annulus is narrowed by fibrous, bulbous protrusions into the aortic annulus, functionally similar to calcific aortic stenosis in human patients.
Despite AS being the most common valvular disease in the US, with a high surgical risk for many patients, an adequate large animal model is not currently available for long-term testing of TAVR devices. Creating a large animal model has been challenging, as in a normal healthy animal with an elastic non-calcified annulus, the radial expansion force of the stent prevents adequate anchoring of the TAVR device. This consequently results in the common occurrence of device migration and regurgitant blood flow between the stent and annulus during diastole leading to paravalvular leaks [Citation5,Citation18,Citation19]. In contrast, human AS patients have a narrowed and calcified aortic annulus, and the resultant stiffness secures the TAVR in place by providing resistance against the radial expansion force of the stent.
A number of techniques have been developed to circumvent this problem, including surgical implantation under CPB, heterotopic delivery into the main pulmonary artery or the Hufnagel position, valve-in-valve deployment, aortic banding, and interventional insertion into a healthy annulus [Citation5,Citation18,Citation19]. Each model presents its own set of challenges or imperfections not observed in the clinical setting of AS. Surgical implant of TAVR devices under CPB can be technically challenging due to the dimensions of the TAVR stent, particularly TAVRs with a tall height profile, as the distance between the aortic annulus and brachiocephalic arch is short in sheep and complicates closure of the aorta. Heterotopic delivery of TAVR devices is clinically irrelevant, as devices are deployed either into the main pulmonary artery or the descending thoracic aorta (Hufnagel position), neither of which is the anatomic position for which the devices will be used in humans. Moreover, in the pulmonary arterial position, TAVR devices are subject to less hemodynamic stress than within the aortic annulus. Valve-in-valve TAVR is used clinically but problematic to model in sheep due to expense and restriction to the implantation of 19—21 mm devices. The aortic banding model requires a TAVR stent with a tall profile, such as the Abbott SJM Portico and Medtronic CoreValve, to reach the band situated distal to the Sino tubular junction region of the aorta and is not appropriate for short profile TAVR stents, like the Edwards Sapien. Moreover, the banding model requires a thoracotomy and band placement before TAVR deployment, which makes a more complicated surgical procedure and recovery on the day of TAVR deployment. There have also been attempts to interventionally insert oversized valves into the healthy aortic valve to overcome the lack of stiff calcified anchoring points, but even this approach is subject to valve migration, paravalvular leaks, and aortic insufficiency [Citation18–25].
We designed our novel annuloplasty ring and the MAA procedure based on our previously published work performing aortic annuloplasty and aortic valve replacement in sheep [Citation13]. Since there is limited access to the aortic annulus, we designed a ring that was flexible and low profile to facilitate visualization and insertion. Therefore, we elected to implant three sections of the material. We selected material that, given enough time after implantation, would develop fibrotic scarring and provide a stiff landing and anchoring platform for TAVR device insertion, similar to how aortic valve calcifications anchor valves in human patients.
In the first phase of our study, the MAA procedure was successfully performed in 12 animals. Follow up echocardiography data demonstrated that the MAA procedure does not create a functional aortic stenosis or left ventricular outflow obstruction. The maximum and mean pressure gradients across the aortic valve were within normal range and similar at 14 days and 60 days post-operatively, the latter being the estimated time point of satisfactory material incorporation and fibrotic scar formation as expected per wound healing literature [Citation26]. Interestingly, mild to moderate aortic insufficiency (AI) was noted post-operatively in most of the animals, suggesting that the implanted material either alters the shape of the aortic annulus or in some way interferes with the valve leaflets themselves. However, the AI did not negatively impact hemodynamics or have a notable clinical impact on the animal. Gross examination of Control group animals at necropsy demonstrated adequate fibrotic scar formation and good incorporation of the material into the surrounding tissue into the aortic annulus at 4-6 weeks after implantation, establishing a firm anchoring platform for TAVR devices.
In the second phase of our study, experimental Abbott next generation TAVR devices were successfully deployed into five Test group sheep having previously undergone the MAA procedure. Importantly, these TAVR devices had not been altered in any way to facilitate animal implant. The animals thrived clinically and no longer showed evidence of AI on post-TAVR insertion TTE. No device migration, paravalvular leaks, or any other complications secondary to the model or device deployment were observed.
Our animal model has many advantages over the currently utilized models for AS, a major one being that large animals can be prepared in advance of a TAVR device study. This is ideal for the medical device industry, where high volumes of devices need to be tested, analyzed, improved and approved for clinical use as efficiently and quickly as possible in a clinically relevant setting.
Allowing for the 4–6 weeks of healing and adequate scar formation at the aortic annulus, a large number of TAVR devices can be deployed and tested at one time. This is in contrast to the limited number of devices that can be tested in other models, such as the ascending aortic band approach, which require a thoracotomy at the time of the device deployment.
Another advantage of our aortic stenosis model is that follow-up can be easily accomplished with TTE because TAVR devices are deployed in the orthotopic aortic valve position, In contrast, heterotopic insertion of a TAVR into the pulmonary artery or the descending aorta (Hufnagel) positions are challenging, if not impossible, to visualize and attain adequate windows for accurate measurements via TTE, since the TAVR device is deeper in the mediastinum or tucked behind a lobe of the left lung. Alternative follow up modalities such as angiography, transesophageal echocardiography or intra-cardiac echo can be used to visualize the above device positions; however, all of those modalities require animal sedation as well as an invasive procedure, which necessitates added expertise and cost to the testing process.
A limitation to our model is that although conceptually very simple, the MAA procedure can be challenging and requires precise surgical skill. Extreme care and methodical placement of each anchoring suture of the annuloplasty ring segments to the nadirs of the valve cusps has to be taken to avoid surgical complications such as damage or entrapment of the native aortic valve leaflets leading to severe perioperative AI, or trauma to the atrioventricular node or mitral valve leaflets when placing the anchoring sutures. Any of these could result in acute heart failure and inability to wean the animal from CPB. Furthermore, the amount of implanted annuloplasty material had to be adjusted for each annulus based on shape, size and degree of visualization.
Another limitation to the model is that while the MAA procedure creates a functional stenosis at the level of the aortic annulus, the model does not create idiopathic calcification of the aortic leaflets and root as commonly observed in aortic stenosis in the clinical setting. In human patients, calcification of the native aortic leaflets and root present the potential for calcific nodules to embolize during positioning and insertion of the TAVR device within the stenotic aortic annulus. In our animal model, the fibrous protrusions created by annuloplasty ring segments were secured to the aorta by suture, and observed circumferential scarring was confined to the level of the aortic annulus, leaving the native leaflets and aortic root unaffected. This presents a limitation to the model in evaluating the potential of a TAVR device to embolize calcific nodules during insertion into a human patient. The stenotic nodules created by the MAA in the model are unlikely to behave in the manner calcific nodules in a human would during TAVR insertion. This is a limitation shared by the previously described large animal models developed for TAVR insertion.
Conclusion
Our aortic stenosis model, using modified aortic annuloplasty, creates stenotic segments in the aortic annulus with adequate rigidity for deployment, anchoring and long-term evaluation of TAVR devices. This procedure represents the first animal model that successfully mimics human aortic stenosis and provides a clinically relevant TAVR insertion model for long-term evaluation of TAVR devices.
Acknowledgements
We thank UMN Experimental Surgical Services Laboratory staff and students with their help in conducting the animal experiments; the UMN Research Animal Resources staff for their care and support of the research animals used in this study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding
This research was supported by an Innovation Research Grant from the University of Minnesota Medical School.
Data availability statement
The data that support the findings of this study are available from the corresponding author, JPC, upon reasonable request.
References
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update. Circulation. 2016;133(4):e38–e360. http://circ.ahajournals.org/content/133/4/e38.short. Accessed July 10, 2017.
- Paradis JM, Fried J, Nazif T, et al . Aortic stenosis and coronary artery disease: What do we know? What don't we know? A comprehensive review of the literature with proposed treatment algorithms. Eur Heart J. 2014;35(31):2069–2082. doi:https://doi.org/10.1093/eurheartj/ehu247.
- Bourantas CV, Serruys PW. Evolution of transcatheter aortic valve replacement. Circ Res. 2014;114(6):1037–1051. doi:https://doi.org/10.1161/CIRCRESAHA.114.302292.
- Bajona P, Suri RM, Greason KL, Schaff HV. Outcomes of surgical aortic valve replacement: the benchmark for percutaneous therapies. Prog Cardiovasc Dis. 2014;56(6):619–624. doi:https://doi.org/10.1016/j.pcad.2014.02.004.
- Leon MB, Kodali S, Williams M, et al. Transcatheter aortic valve replacement in patients with critical aortic stenosis: rationale, device descriptions, early clinical experiences, and perspectives. Semin Thorac Cardiovasc Surg. 2006;18(2):165–174. doi:https://doi.org/10.1053/j.semtcvs.2006.08.002.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi:https://doi.org/10.1056/NEJMoa1103510.
- Webb JG, Doshi D, Mack MJ, et al. A randomized evaluation of the SAPIEN XT transcatheter heart valve system in patients with aortic stenosis who are not candidates for surgery. JACC Cardiovasc Interv. 2015;8(14):1797–1806. doi:https://doi.org/10.1016/j.jcin.2015.08.017.
- Zeeshan A, Tuzcu EM, Krishnaswamy A, Kapadia S, Mick S. Transcatheter aortic valve replacement: history and current indications. Cleve Clin J Med. 2015;82(12 Suppl 2):S6–S10. doi:https://doi.org/10.3949/ccjm.82.s2.02.
- Waksman R, Pichard AD. Will TAVR become the default treatment for patients with severe aortic stenosis? J Am Coll Cardiol. 2015;66(2):122–124. doi:https://doi.org/10.1016/j.jacc.2015.05.016.
- Cribier A. Development of transcatheter aortic valve implantation (TAVI): a 20-year odyssey. Arch Cardiovasc Dis. 2012;105(3):146–152. doi:https://doi.org/10.1016/j.acvd.2012.01.005.
- Cribier A. Development of transcatheter aortic valve implantation (TAVI): a heart-warming adventure. Eur Geriatr Med. 2013;4(6):401–406. doi:https://doi.org/10.1016/j.eurger.2013.09.010.
- Webb JG, Binder RK. Transcatheter aortic valve implantation: the evolution of prostheses, delivery systems and approaches. Arch Cardiovasc Dis. 2012;105(3):153–159. doi:https://doi.org/10.1016/j.acvd.2012.02.001.
- Schomburg JL, Lahti MT, Ruth GR, Bianco RW. Internal aortic annuloplasty: a novel technique. J Invest Surg. 2011;24(5):222–226. doi:https://doi.org/10.3109/08941939.2011.575916.
- Harvey L, Bianco R, Lahti M, Carney J, Zhang L, Robinson N. Carpentier-Edwards aortic pericardial bioprosthetic valve as a valid control in preclinical in vivo ovine studies. Eur J Pharmacol. 2015;759:192–199. doi:https://doi.org/10.1016/j.ejphar.2015.03.033.
- Azakie A, Carney JP, Lahti MT, Moklyak Y, Bianco RW. Anisotropic polytetrafluoroethylene cardiovascular conduits spontaneously expand in a growing lamb model. J Invest Surg. 2020. doi:https://doi.org/10.1080/08941939.2020.1805056.
- Carney JP, Zhang LM, Larson JJ, et al. The Hancock® valved conduit for right ventricular outflow tract reconstruction in sheep for assessing new devices. J Heart Valve Dis. 2017;26(4):472–480.
- Zoghbi WA, Chambers JB, Dumesnil JG, et al . Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22(9):975–1014. doi:https://doi.org/10.1016/j.echo.2009.07.013.
- Dewey TM, Walther T, Doss M, et al. Transapical aortic valve implantation: an animal feasibility study. Ann Thorac Surg. 2006;82(1):110–116. doi:https://doi.org/10.1016/j.athoracsur.2006.02.035.
- Wendt D, Pasa S, Kahlert P, et al. A new self-expandable transcatheter aortic valve for transapical implantation: feasibility in acute and chronic animal experiments. Scand Cardiovasc J. 2013;47(3):145–153. doi:https://doi.org/10.3109/14017431.2012.743675.
- Cai J, Sheng Y, Zhang S, et al. Preliminary feasibility and hemodynamic performance of a newly-developed self-expanding bioprosthesis and 16-F delivery system in transcatheter aortic valve implantation in sheep. J Biomed Res. 2012;26(3):211–218. doi:https://doi.org/10.7555/JBR.26.20120011.
- Emmert MY, Weber B, Behr L, et al. Transcatheter aortic valve implantation using anatomically oriented, marrow stromal cell-based, stented, tissue-engineered heart valves: technical considerations and implications for translational cell-based heart valve concepts. Eur J Cardiothorac Surg. 2014;45(1):61–68. doi:https://doi.org/10.1093/ejcts/ezt243.
- Horvath KA, Mazilu D, Cai J, Kindzelski B, Li M. Transapical sutureless aortic valve implantation under magnetic resonance imaging guidance: Acute and short-term results. J Thorac Cardiovasc Surg. 2015;149(4):1067–1072. doi:https://doi.org/10.1016/j.jtcvs.2014.10.101.
- Mazilu D, Li M, Kocaturk O, Horvath KA. Self-expanding stent and delivery system for aortic valve replacement. J Med Device. 2012;6(4):410061–410069. doi:https://doi.org/10.1115/1.4007750.
- Miller JG, Li M, Mazilu D, Hunt T, Horvath KA. Robot-assisted real-time magnetic resonance image-guided transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2016;151(5):1407–1412. doi:https://doi.org/10.1016/j.jtcvs.2015.11.047.
- Nakatsuma K, Saito N, Watanabe H, et al. Antegrade transcatheter aortic valve implantation using the looped Inoue balloon technique: a pilot study in a swine model. J Cardiol. 2017;69(1):260–263. doi:https://doi.org/10.1016/j.jjcc.2016.04.008.
- Witte M, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77(3):509–528. doi:https://doi.org/10.1016/S0039-6109(05)70566-1.