Abstract
Surgical procedures that maintain continence with minimal complication following resection of trigono-urethral urothelial carcinoma (UC) are limited in canines; therefore, palliative options are often pursued. A feasible tumor resection option may improve disease control and survival. The study’s objective was to evaluate a continent urine reservoir created from the urinary bladder body and vascularized solely by omentum. We hypothesized that a viable urine reservoir could be created, and staged omentalization would provide improved vascularity. Nine normal female Beagles were randomized to one of three groups. Group A urinary bladders were transected cranial to the ureteral papillae to create a closed bladder vesicle which was concomitantly omentalized. Group B underwent omentalization two weeks prior to vesicle creation. Based on Group A and B results, Group C underwent neoureterocystostomy and omentalization followed by neoreservoir formation and tube cystostomy 2 weeks later. Serial ultrasounds and histopathology confirmed adequate omental neovascularization in Groups B and C with continent Group C neoreservoirs maintained for 2 months. Some pylectasia and ureteral dilation was documented in all Group C dogs at variable timepoints. Progressive hydroureteronephrosis developed in 2/6 kidneys. Transient azotemia was noted in only 1 Group C dog, although all developed treatable urinary tract infections. The sample size is limited, and the efficacy of this technique in providing disease control for UC is unknown. However, this novel option could allow for primary UC resection while providing continence and limiting complications. Postoperative local or systemic adjuvant therapy, ultrasonographic neoreservoir monitoring, and BRAF analysis would be indicated.
Introduction
Urothelial (transitional cell) carcinoma (UC) is the most commonly reported lower urinary tract tumor in dogs [Citation1–3]. UC is most frequently located at the trigonal region of the bladder but 56% of affected dogs may also have urethral involvement, predisposing them to urethral obstruction [Citation1, Citation3, Citation4]. In some cases, the ureteral papilla may be involved, resulting in ureteral obstruction [Citation5–7]. Dogs with UC are considered to have a poor prognosis with median survival times (MST) ranging from 78 to 450 days depending on the treatment modality(s) instituted [Citation1, Citation4, Citation8–10]. Both curative- and palliative-intent surgical procedures have been implemented to address UC in canine patients. While radical surgeries aimed at removal of all gross disease have been shown to improve MST, this is often not attempted due to the trigonal/urethral location of the tumor [Citation4, Citation9, Citation11]. Radical/total cystectomy with urinary diversion procedures such as ureterocolonic anastomosis [Citation12], uretero-urethral/vaginal/preputial anastomosis [Citation6, Citation13], enterocystoplasty [Citation14], bladder neck excision with vesicourethral anastomosis and neoureterocystostomy [Citation7], and various forms of neobladders including gastrointestinal reservoirs [Citation15, Citation16] have been described in dogs but are often associated with complications affecting their welfare and quality of life. Gastrointestinal reservoirs, which lack a urothelial lining, result in metabolic derangements and neurologic complications in dogs [Citation12, Citation15]. Other complications seen with certain surgical techniques include ascending urinary tract infections, incontinence, and/or pollakiuria [Citation8, Citation12–14]. Because of these complications and the lack of substantial long-term benefit [Citation17], palliative procedures such as urethral stenting and tube cystostomy have been employed to manage urinary obstruction [Citation6, Citation8, Citation18–20]. However the MST for these palliative procedures is low, 78 and 106 days reported respectively [Citation18, Citation20]. Regardless of treatment modality, without resection of the primary tumor, patients with trigonal UC often experience continued stranguria and dysuria due to partial obstruction and/or tumor-associated inflammation [Citation18, Citation20, Citation21]. Overall systemic treatment is typically recommended with chemotherapeutics such as mitoxantrone and cyclooxygenase (COX) inhibitors as the mainstays of medical therapy [Citation9, Citation17, Citation21, Citation22].
In humans, radical cystectomy is the treatment of choice for invasive UC along with adjunctive chemotherapy [Citation23]. As in dogs, UC is the most commonly described primary neoplasm of the human urinary bladder. Both non-muscle invasive “superficial” (low-grade) forms and invasive (high-grade) forms are described [Citation23]. It is estimated that 25% of affected people present with muscle invasive tumors (muscularis involvement), while the remaining tumors are superficial (mucosa/submucosa involvement) [Citation24]. The most common location of occurrence for this tumor in humans is the lateral wall (37%), followed by the posterior wall (18%) [Citation25]. In comparison, 78% of canine UCs are invasive, with involvement of the muscularis propria [Citation4, Citation17], and a trigonal or urethral origin is most common. In humans, cystoscopic transurethral resection of bladder tumors (TURBT) is recommended for non-muscle-invasive bladder tumors [Citation26], and radical cystectomy in conjunction with various urinary diversion procedures is recommended for more invasive cases [Citation24, Citation27]. Approximately 20% of human patients with invasive urothelial carcinoma have metastases at the time of presentation, which is similar to the 20% of dogs found to have metastases at the time of diagnosis [Citation17, Citation28]. Radical cystectomy is considered advantageous, removing the primary source of possible dissemination, and is more feasible in humans when compared to canine patients, given the low rate (2%) of urethral and ureteral involvement in humans [Citation27]. For those human patients who do require radical cystectomy, reconstructive surgery afterward has involved both continent and incontinent diversion techniques as well as the formation of neobladders that reattach to the urethra [Citation29]. Overall urinary diversion procedures seem to be tolerated better in humans. Gastrointestinal reservoirs can provide a level of continence without causing significant electrolyte imbalances and neurologic signs as reported in canine patients [Citation12, Citation29]. Due to the trigonal/urethral location of a majority of canine tumors, partial cystectomy is not a viable option because it disrupts the supporting neurovascular structures. Alternative surgical procedures for canine patients with urethral/trigonal UC are therefore needed to avoid systemic complications while also providing better control of the disease.
We postulate that the grossly normal portion of urinary bladder could serve as a continent reservoir in dogs after removal of the affected trigone and urethra, eliminating the side effects seen with gastrointestinal pouches. As previously described, preservation of the neurovascular structures in this region can be challenging [Citation7]. The omentum has many different functions, one of which is providing vascular support to surrounding organs in contact with it by inducing angiogenesis and neovascularization [Citation30–33]. Previous studies have shown that for tissues lacking an intrinsic blood supply, such as isolated segments of intestine, viability can be maintained via the omentum, especially after a period of ingrowth [Citation31, Citation34, Citation35]. As such the omentum may be used to provide neovascularization and ensure viability of an isolated bladder reservoir.
The objective of this pilot study was to evaluate whether the body of the urinary bladder could be utilized as a continent reservoir if detached from intrinsic vasculature and supported solely by the omentum. If successful, this type of procedure could be implemented to allow for resection of trigono-urethral UC. We hypothesized that 1) a continent reservoir could be formed from the body of the urinary bladder and 2) the omentum would provide appropriate vascularity to the reservoir although a longer period of ingrowth would improve viability.
Materials and methods
Research animals
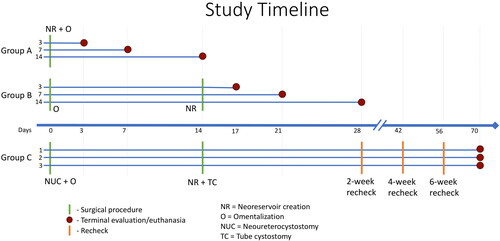
A prospective terminal study was carried out with 9 healthy adult intact female beagles. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC), and all animals were cared for following guidelines for humane care and use of laboratory animals. Taking into account the possible loss of one dog in any group, three dogs per group was considered the minimum number required in this pilot study to be able to draw conclusions regarding the feasibility of this technique. Dogs were evenly distributed and randomly assigned to one of three treatment groups by drawing out of a sealed envelope. As experimental design for Group C was contingent upon results from Group A and B, Group C was not pursued until completion and evaluation of results from Groups A and B. All dogs were quarantined for 2 weeks prior to the start of the project. Dogs were housed in individualized kennels for the duration of the study. Terminal dates were randomly assigned with dates dictated by the Group specification. As depicted in the timeline in , dogs in Group A were followed for 3, 7, or 14 days following a single surgery prior to termination and were referred to as A3, A7, and A14 in a manner corresponding to their termination date. In Group B, two surgical procedures were staged two weeks apart. Dogs in Group B were followed for 3, 7, or 14 days after completing the second surgery, prior to termination, and referred to B3, B7, and B14, respectively. Dogs in Group C were followed for a total of 10 weeks, with termination 2 months after the completion of the second surgery; designations of C1, C2, and C3 were given. All dogs were euthanized under general anesthesia at their pre-determined timepoints.
Figure 1. Research timelines (x-axis) for Groups A, B, and C (y-axis). Dogs in Groups A and B were designated by their group and pre-determined study endpoint of 3, 7, or 14 days after undergoing a single or staged surgery. Timepoints for surgical procedures are marked by green lines. Red dots indicate the study endpoint for that dog. Group C was carried out after completion of Groups A and B. Dogs were randomly designated C1-C3. As with Group B, 2 different surgical procedures were carried out 2 weeks apart. Follow-up recheck evaluations were performed every 2 weeks as marked by the orange lines until terminal endpoint 2 months after the second surgery. Laboratory analyses and ultrasounds were performed concomitant with each surgical procedure, recheck if applicable, and terminal time-point.
Laboratory analyses
Complete blood counts and chemistry panels were evaluated for all dogs prior to all surgical procedures, including the terminal surgical procedure. For Group C dogs, a complete blood count, chemistry panel, urinalysis, and urine culture were evaluated prior to each surgery, at two-week intervals during the 2-month follow-up period, and at terminal evaluation. Urine in this group was collected intraoperatively when relevant and then subsequently via a cystostomy tube. Additional testing was performed if indicated based on clinical status. Terminal urine cultures in Group C were taken from the neoreservoir and both renal pelves via aspiration using 22ga needles under ultrasound guidance.
Anesthesia
Dogs were premedicated with acepromazine at 0.03-0.05 mg/kg IV and induced with propofol to effect with doses ranging between 3-8mg/kg IV. All dogs undergoing surgery received a lumbar epidural with buprenorphine (0.01 mg/kg) and 0.5% bupivacaine (0.5 mg/kg). Anesthesia was maintained with 1-4% isoflurane and oxygen. An intra-incisional local block was performed prior to closure using 0.5% bupivacaine (2 mg/kg). The same protocol for premedication and induction was used for each surgery as well as terminal evaluation prior to euthanasia.
Surgical procedures
Group A
Dogs in Group A underwent a single surgery in which the bladder vesicle was created and concomitantly omentalized () in order to determine if viability could be achieved with a single procedure. A standard midline approach to the abdomen was performed. The urinary bladder was exteriorized and isolated. The ureters were identified and a curved Doyen intestinal clamp was placed horizontally across the bladder immediately cranial to the ureterovesicular junctions. The bladder was transected along the cranial aspect of the Doyens with a scalpel. The transected bladder was oversewn using 4-0 PDS in a simple continuous pattern creating a separate vesicle no longer attached to a neurovascular pedicle or the remaining urinary tract. Using a #10 scalpel blade the seromuscular layers of the bladder vesicle were scored with superficial incisions extending circumferentially over the surface to encourage omental adhesion and vascular ingrowth. The omentum was isolated, wrapped over the vesicle, and tacked multifocally to the vesicle wall. The omentum-wrapped vesicle was tacked to the left ventrolateral body wall. The remaining trigone, with intact ureterovesicular junctions, was oversewn using 4-0 PDS in a simple continuous pattern. A leak test confirmed closure, and periurethral fat was draped and tacked over the incision. The abdominal incision was closed routinely.
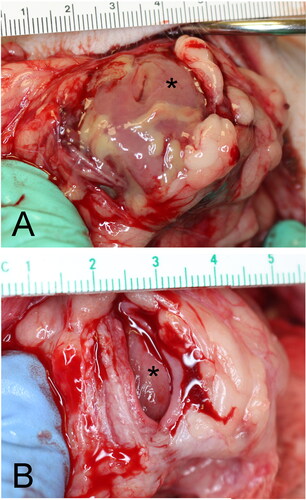
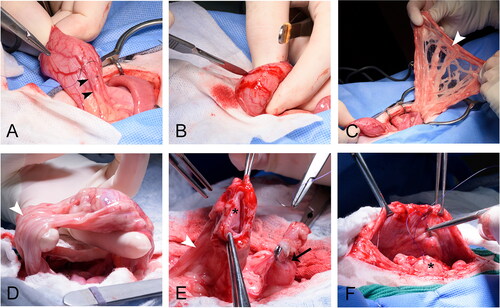
Figure 2. Dogs in Group A underwent a single surgical procedure in order to determine if viability could be achieved with a single procedure. The bladder vesicle was created and omentalized in the same procedure. The body of the urinary bladder was (A) isolated and (B) transected and oversewn. (A) The ureters (outlined in white) and ureterovesicular junctions were left intact draining into the trigone, which was oversewn to allow for continued urination. (C) The oversewn vesicle (asterisk) was then excoriated and wrapped in omentum (white arrowhead) prior to tacking to the body wall.
All three dogs were recovered and cared for until their terminal dates of 3, 7, or 14 days postoperatively. Because the trigone and ureteral insertions were spared, all dogs urinated freely. A terminal evaluation was performed with patients under general anesthesia. The abdomen was re-approached through the same incision. The vesicle was identified and omental adhesion assessed. The vesicle was ultrasonographically examined for vascularity and then incised mid-body to assess for active bleeding throughout the bladder wall layers. The vesicle and attached omentum were subsequently excised from the peritoneal wall, placed in 10% neutral buffered formalin, and submitted for histopathologic analysis. Euthanasia was carried out under general anesthesia using Fatal-Plus (390 mg/ml pentobarbital sodium, 1 ml/10lbs IV).
Group B
Dogs in Group B underwent a staged surgical procedure to assess if this improved viability by allowing omental adhesions more time to provide vascular support. The first surgery was dedicated to omentalization of the bladder and the second to creation of the bladder vesicle (). During the first surgery a standard laparotomy with exteriorization of the bladder was performed as previously described. The ureters were identified along with the caudal vesicular arteries. Just cranial to each ureterovesicular junction larger branches of the caudal vesicular artery were ligated using 4-0 PDS. The seromuscular layers of the bladder were scored and the bladder omentalized as previously described. The omentalized bladder was returned to the abdomen, and no other procedures were performed. The abdominal incision was closed routinely.
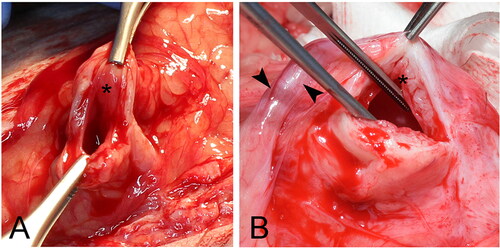
Figure 3. Group B was a staged procedure to assess if a staged technique improved viability of the bladder vesicle. During the first surgery (A-C) branches of the caudal vesicular artery (black arrowheads) were ligated (A) to stress the bladder and encourage neovascularization. The serosal layer of the bladder was then excoriated (B) and omentalized (white arrowhead). Dogs recovered over 2 weeks prior to the second surgery. During the second surgery (D-F) the bladder vesicle was created as in Group A. (D) Omental adhesions were present (white arrowhead) circumferentially over the bladder. (E) The omentalized (white arrowhead) body of the bladder (asterisk) was transected from the trigone (black arrow). Each was oversewn again preserving the trigone with intact ureters. (F) The omentalized bladder vesicle (asterisk) was tacked to the body wall.
Fourteen days post-operatively a second laparotomy was performed for creation of the bladder vesicle as described for Group A. During surgery the bladder and omental adhesions were visually assessed.
All Group B dogs were recovered and cared for until terminal evaluation 3, 7, or 14 days post-second surgery as determined by previous random assignment. As in Group A, all dogs urinated freely. Terminal evaluation and euthanasia was performed as described for Group A.
Group C
Group C was designed to represent a clinical scenario of primary tumor removal and continent neoreservoir creation. The results obtained from Groups A and B dogs regarding bladder viability aided in determining the surgical procedure for Group C. A staged surgical approach was implemented for dogs in Group C following a protocol similar to that described for Group B with the addition of ureteral reimplantation (). During the first surgery just prior to omentalization, bilateral neoureterocystostomy was performed in preparation for future distal bladder/urethral transection. For neoureterocystostomy the ureters were identified and the distal 2/3 isolated to decrease tension. The previously described vesicular arteries were also identified and major branches ligated proximal to the ureterovesicular junctions. The ureters were sequentially ligated using 4-0 PDS and transected 3-4mm proximal to the ureterovesicular junctions. Residual fat was dissected from the free ureteral ends and the ureters spatulated. An extravesicular technique as previously described [Citation36] was performed using 6-0 PDS in two simple continuous patterns for reimplantation bilaterally along the left and right ventroapical aspects of the bladder. A 3.5 Fr red rubber catheter was placed into the bladder lumen via a separate stab incision and passed into each ureter during suturing to prevent inadvertent catching of the opposite wall during ureterostomy. After neoureterocystostomy and closure of the stab incision, bladder omentalization was performed as previously described for Group B. The abdomen was closed routinely.
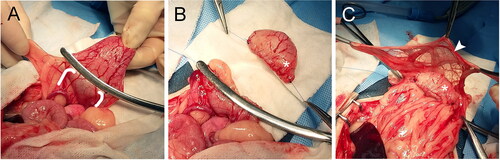
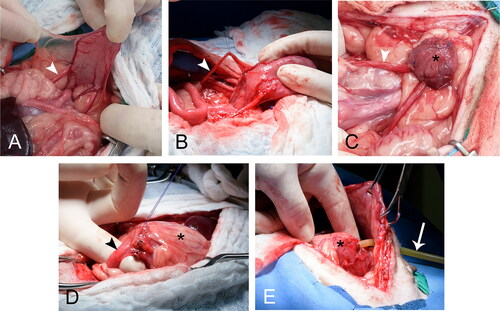
Figure 4. A staged surgical approach was implemented for Group C. This group was meant to represent the more clinically applicable technique of trigono-urethral resection and neoreservoir creation with cystostomy tube. In clinical cases neoureterocystostomy would be required so this was performed in our study. During the first surgery (A-C) the ureters (white arrowhead) were identified and isolated (A-B). Branches of the caudal vesicular artery were ligated as performed in Group B. (C) Ureters were reimplanted into the body of the bladder (asterisk), and the bladder was superficially excoriated and omentalized. Dogs recovered for 2 weeks before the second surgery. During the second surgery (D-E) the omentalized bladder (asterisk) was isolated and transected at the neck (black arrowhead). (E) A cystostomy tube (white arrow) was placed through the neck of the bladder into the newly created neoreservoir. Reimplanted ureters (not visible) remained attached to the neoreservoir. The cystostomy tube exited the lateral body wall and the omentalized neoreservoir (asterisk) was tacked to the body wall.
The second surgical procedure was carried out 14 days later. The abdomen was re-approached and the bladder assessed. The bladder neck and proximal urethra were exposed, and any remaining vasculature was ligated and transected. In preparation for transection of the bladder from the urethra, two circumferential purse string sutures were placed, one in the bladder neck and another 1 cm distal to the first in the proximal urethra. The trigone/urethra was transected between the two purse strings. The purse string associated with the urethra was tied and the urethral stump oversewn. A 16 Fr Pezzer cystostomy tube was placed through the opening in the neck of the bladder, and the pre-placed purse string was tied. A second purse string was placed just proximal to the first. The cystostomy tube was passed bluntly through the left abdominal wall and a 1 cm skin incision. A box-lock suture pattern was then placed to secure and pexy the neoreservoir to the abdominal wall around the cystostomy tube. A flange and stockinette were placed externally to help further secure the tube. The abdomen was closed routinely.
Two months later dogs from Group C were re-anesthetized for terminal evaluation. The urinary tract, including kidneys, ureters, and bladder, were similarly assessed ultrasonographically and visually for evidence of viability and subsequently excised for histopathology. Euthanasia was carried out as described above.
Postoperative care
Dogs in all groups received analgesic medications after non-terminal procedures including a carprofen injection (4.4 mg/kg SQ once) immediately postoperative followed by twice daily oral administration (2-2.5 mg/kg) for 5 days postoperatively. A full physical exam and pain assessment was performed every 8 hours in the first 48 hours, and daily thereafter. Buprenorphine 0.02 mg/kg SQ q8 hours was given during the first 48 hours. Additional buprenorphine was available for patients having a pain score of greater than or equal to two out of four based on the Colorado State University Canine Acute Pain Scale [Citation37].
Dogs in each group were cared for and assessed daily until their predetermined terminal timepoints. Immediate postoperative care was similar in all groups with the exception that Group C dogs had cystostomy tubes requiring maintenance. The cystostomy tube was utilized to empty their bladder four times daily for the first three days and then transitioned to three times daily for the duration of the study. Urine volumes were recorded for each timepoint. Amoxicillin/sulbactam (Clavamox, 12-14mg/kg PO q12h) was available for administration if indicated based on clinical findings or diagnostic results.
Diagnostic imaging
Prior to each surgery all dogs had focused abdominal ultrasounds of the urinary bladder performed while under general anesthesia. All ultrasounds were carried out by a single board-certified radiologist. For all 9 dogs, bladder wall vascularity (via SMI Doppler) along with anatomic characteristics were assessed. In Group C the entire urinary tract was assessed via ultrasound prior to the first surgery; baseline values for ureteral and renal pelvis dimensions were established along with presence of vascular flow through the bladder wall. Ultrasounographic assessment of the urinary tract was repeated prior to the second surgery, bimonthly, and prior to termination to assess for changes in ureteral or renal pelvis dimension as well as vascularity of the surgically created reservoir.
Histopathology
Following gross evaluation of the urinary tract at terminal timepoints, tissues were collected for histopathology and placed in 10% neutral buffered formalin. In Groups A and B, the bladder vesicles with adhered omentum were collected and submitted for histopathology. In Group C the entire urinary tract including kidneys, ureters, neoreservoirs, and cystostomy tube stoma sites were collected and submitted. All samples were routinely processed, Paraffin-embedded, and sectioned at 5 µm, mounted on frosted glass slides, and routinely stained with hematoxylin and eosin (H&E). Sections of the urinary bladder wall were also stained with Gomori’s trichrome. All sections were evaluated by one pathology resident and one board-certified pathologist. Urinary bladders were assessed histologically for evidence of ischemic tissue damage (degeneration and/or necrosis), inflammation, and repair. The urothelium, where intact, was evaluated for changes consistent with hydropic degeneration (cytoplasmic swelling and pallor). All wall layers (urothelium, lamina propria, muscularis propria, and serosa/adventitia) were evaluated for evidence of necrosis and/or repair. Necrosis was characterized by nuclear pyknosis, karyorrhexis, or karyolysis accompanied by cytoplasmic eosinophilia with or without partial (erosion) or full-thickness (ulceration) sloughing of the epithelium into the bladder lumen. For each urinary bladder, the extent of wall necrosis was assigned a score ranging from 0 to 4, with a score of 0 assigned to bladders in which all wall layers were histologically devoid of evidence of active necrosis and a score of 4 assigned to urinary bladders in which there was necrosis of all wall layers including the mucosa, submucosa, and serosa/adventitia. Inflammation was characterized as either neutrophil-predominate, or lymphoplasmacytic in nature.
Results
Group A
All 3 dogs successfully underwent surgery for bladder vesicle creation and omentalization. Vitals were within normal limits at all checked timepoints, and pain scores were 0 out of 4 for all. Urine production was consistently appreciated in 2 of the 3 dogs. Dog A3 urinated within the first 24 hours after surgery; however, further urine production was questionable in the following 48 hours. The dog was subjectively less interactive and lethargic compared to counterparts 48 hours after surgery, but vitals and pain scores were normal. Vomitus was noted 60 hours postoperatively, and, as the patient was slated for terminal evaluation at the 3-day postoperative timepoint, planned blood tests, surgical assessment, and euthanasia were performed on schedule.
Complete blood count and chemistry for all three dogs were unremarkable prior to the first surgery. Repeat blood tests were unremarkable for Dog A7 and A14. Dog A3 developed a severe azotemia with electrolyte imbalances on terminal serum biochemistry evaluation, characterized by a marked elevation of BUN (189 mg/dL, ref 11-27) and creatinine (10.4 mg/dL, ref 0.5-1.4), hyperphosphatemia (17.4 mg/dL, ref 2.6-5.3), and hyperkalemia (9.5 mg/dL, ref 3.6-5.3).
Abdominal ultrasound evaluation
Prior to surgery, all three dogs had normal urinary bladders with normal wall thickness (1.5 mm) and layering on ultrasound examination. All showed subjectively moderate intermittent blood flow within the serosal layer and mild intermittent flow within the submucosal/mucosal layer on SMI Doppler assessment.
On reevaluation moderate to marked bladder wall thickening was appreciated at each terminal evaluation, greatest in A3. Wall layering was completely lost in A3 and A14, while layering in A7 was visible but poorly defined with blurred margins. On Doppler of A3 the serosal signal was maintained with an increased submucosal/mucosal signal which had a pulsatile quality. For all layers at later timepoints the signal was absent (A7) to reduced (A14) with only a mild intermittent signal of the serosal layer in A14. In all cases the omentum had moderate signal adjacent to the vesicle’s serosal margin and was hyperechoic.
Gross evaluation
Bladder vesicles from each timepoint were appreciated tacked to the body wall with omentum well-adhered circumferentially. On incision of the vesicle, hemorrhage was noted from only the serosal layer in A3. No bleeding from any layer was appreciated in A7, and only slight hemorrhage was noted from the serosal and muscularis layers of A14. As seen in , the mucosal layer was cyanotic to necrotic in appearance at all timepoints with one having evident sloughing and a putrid odor (A7). The unaltered ureters of A3 were bilaterally dilated presumably from unintentional iatrogenic obstruction at the level of the ureterovesicular junctions.
Histopathologic evaluation
In Group A, neoreservoir creation with concomitant omentalization resulted in complete loss (necrosis) of the mucosa at all timepoints (). There was progressive loss of normal urinary bladder wall architecture, with ongoing replacement by granulation tissue and, ultimately, fibrosis. The character of the inflammatory infiltrate progressed from mild neutrophilic (A3) to robust neutrophilic (A7) to mild, chronic, perivascular, lymphoplasmacytic (A14). Intraluminal, mucosa-associated cocci bacteria were identified histologically in one dog (A7).
Table 1. Necrosis scores of bladder vesicles/neoreservoirs of each group.
Group B
All three dogs underwent successful abdominal surgery for omentalization of the bladder with an uncomplicated 2-week recovery. Physical exams were unremarkable with normal vital signs and pain scores of 0/4 at all timepoints during recovery. Patients continued to urinate normally with no change in frequency or volume. After two weeks each dog underwent a successful second surgery for vesicle creation at which point the bladder was assessed as normal in appearance with well-adhered omentum circumferentially. Normal hemorrhage from the incised bladder wall layers during vesicle creation was appreciated. Recovery was again unremarkable for each dog, and urine production was appreciated from each.
Complete blood count and chemistry results for all three dogs were unremarkable prior to both surgeries and at terminal evaluation.
Abdominal ultrasound evaluation
Prior to surgery all three dogs had normal urinary bladders. As with Group A, wall thickness and layering was normal. All showed subjectively moderate intermittent blood flow within the serosal layer. Dogs B3 and B14 showed mild to moderate intermittent submucosal/mucosal layer blood flow whilst Dog B7 showed mild intermittent submucosal/mucosal layer blood flow. Dog B3 also showed intermittent linear signal traversing the muscularis layer.
Repeat ultrasound of the bladder was performed 2 weeks after the initial omental wrapping surgery, immediately prior to the 2nd surgery in which the bladder vesicle was created. At this evaluation all dogs had a normal bladder wall thickness. Doppler signals were similar to pre-surgery for all layers in each dog with only B14 having a subjectively mildly decreased submucosal/mucosal signal compared to prior to surgery. There was moderate SMI Doppler signal within the omentum immediately adjacent to the serosa in Dogs B14 and B7.
Prior to termination, ultrasound examinations were repeated on days 3, 7, and 14 post-creation of the bladder vesicle. Each bladder vesicle had mild to moderate wall thickening with only B3 having loss of normal wall layering. Serosal signal was maintained in all dogs. Submucosa/mucosal signal was reduced (trace) in B3 but mildly improved in B7 and B14 compared to prior assessment. A moderate omental signal was present adjacent to the serosa.
Gross evaluation
The neoreservoir was attached to the peritoneal wall on reexamination with well adhered omentum circumferentially in all cases. Dog B3 was noted to have active mild hemorrhage from the serosal and submucosal layers. The mucosal layer was pink in coloration and intact though subjectively edematous. At the day 7 and 14 timepoints, Dogs B7 and B14 were found to have hemorrhage from all layers of the bladder wall, including the mucosa which was pink and normal in appearance (). Overall the bladder at these later timepoints was considered grossly normal in appearance.
Histopathologic evaluation
Group B dogs retained all bladder wall layers (). Mature fibrosis of the serosal/adventitial surface was evident at all timepoints and progressed in maturity over time. Inflammation was primarily lymphoplasmacytic (chronic) in nature and showed evidence of resolution at the later timepoint (B14). Patent vascular profiles were consistently captured within all examined layers at all timepoints.
Group C
All dogs successfully underwent staged surgical procedures and were followed for approximately 2 months thereafter. All dogs recovered from the first surgery (neoureterocystostomy and omentalization) with only minor complications. Temporary dysuria, hematuria, and inappetence were noted in all dogs. Two dogs had a single episode of vomiting over the 2-week recovery. Vitals were normal at all checkpoints with pain scores of 0/4 in all dogs. Each incision healed without complication.
During the second surgery a neoreservoir with cystostomy tube was successfully created in all 3 dogs. Gross assessment during surgery revealed omental adhesions circumferentially on the bladder in all dogs. The ureters were difficult to visualize due to the omentalization in two dogs but palpated as mildly to moderately dilated in both with no other remarkable findings. The third dog’s (C2) ureters were more easily visualized despite omentalization. The right ureter was moderately distended with a notable narrowing/kinking of the ureter approximately 2 cm proximal to the ureteral insertion. This kinking was caused by an adhesion from the right uterine horn and the associated mesovarium causing a focal constriction of the ureter. The uterus and associated adhesion were dissected away from the ureter. The left ureter in this dog was mildly to moderately dilated but otherwise unremarkable. The bladder walls from all 3 dogs had active bleeding from all layers during the second surgery.
After the second surgery two dogs were followed until the set study endpoint of 8 weeks postoperatively. One dog (C2) in group C had to be euthanized 6 days prior to the end of the study (7 weeks post-second surgery vs 8 weeks) due to COVID-19 research regulations. All terminal data for that dog was collected 6 days early. All 3 dogs did well overall during the follow-up period. Pain scores were 0/4 for all timepoints. Vitals were assessed as normal during all physical exams except for pyrexia documented in one dog at 4 and 7 weeks after the second surgery. Minor complications were noted during the recovery/follow-up period including temporary hyporexia (3/3 dogs), intermittent vomiting (2/3), seroma formation (1/3), minimal superficial incisional dehiscence (1/3), urinary tract infections (UTIs in 3/3), and inadvertent cystostomy tube removal (2/3). Adjustments to food and water bowl access and alterations in type of Elizabethan collar improved patients’ demeanor, activity, and appetite thereafter. All complications resolved spontaneously or were easily treated.
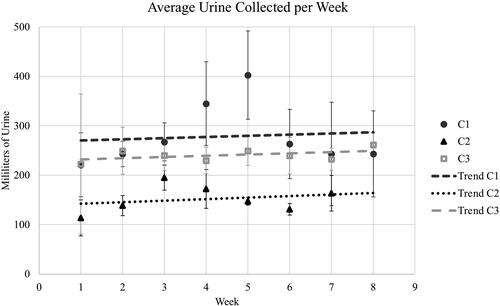
Based on urine collected via cystostomy tube, the average daily urine production for all 3 dogs was 21.4 ml/kg/day. The highest quantity of urine collected over 8 hours was 260 ml. A slight upward trend in urine volume was noted through the follow-up period (). Two dogs pulled their cystostomy tubes during this period; these were easily replaced through stoma sites with Foley catheters.
Figure 6. Urine collected from neoreservoirs at 8-hour intervals was recorded, and weekly volume averages were calculated for each week during the 2-month follow-up period. Standard deviations for each week are applied. The trendlines for each dog over time reflect mild increases in urine capacity of the neoreservoir.
Baseline complete blood count and serum biochemical tests were unremarkable in all 3 dogs. Prior to the second surgery (2 weeks after ureteral reimplantation), two dogs had mild increases in serum creatinine although the values were still within normal range, from 0.4 mg/dL baseline to 0.9 and 0.7 for dogs C2 and C3 respectively (ref 0.5 − 1.4 mg/dL). Otherwise blood test results prior to the second surgery and every 2 weeks thereafter were unremarkable in all dogs except for one dog at a single timepoint. Dog C1 developed azotemia 7 weeks post-second surgery with an inflammatory leukogram. All values returned to normal by the next evaluation (terminal 8-week sample). Results are summarized in .
Table 2. Group C diagnostic results at various timepoints.
Urinalyses and urine cultures were unremarkable prior to both the first and second surgeries. Some degree of hematuria and pyuria were noted on all analyses made after cystostomy tube placement. All dogs had additional urinalyses during the follow-up period in response to clinical signs (e.g., cloudy urine, inappetence). Within 2-4 weeks of cystostomy tube placement, all dogs developed a UTI. A single organism was isolated in each dog and included Streptococcus gallolyticus, Enterococcus faecalis, and Staphylococcus pseudintermedius. At 7 weeks post-second surgery, Dog C1 had urine submitted for culture which grew both the above Streptococcus and Staphylococcus species for that timepoint only. All cultured bacteria remained susceptible to amoxicillin/sulbactam, but treatment was only initiated if clinical signs became apparent. Dog C1 had three, 5-day courses of this antibiotic with the last course discontinued 2 days prior to study-end, and Dog C2 a single 14-day course which was discontinued 2 weeks prior to termination. Dog C3 had a 10-day course initiated 3 days after the second surgery to treat an incisional infection/dehiscence, but treatment specifically for UTI was never deemed necessary. This dog (C3) was the only dog that had a positive urine culture from the neoreservoir at terminal evaluation. Methenamine (33 mg/kg PO BID) was given to a single dog (C1) for 1 week up to terminal evaluation. All cultures from renal pelves were negative at the terminal timepoint.
Abdominal ultrasound evaluation
Prior to surgery, all three dogs in Group C had normal kidneys and normal bladder walls on ultrasound examination with moderate serosal signal and mild to moderate intermittent submucosal/mucosal signal on SMI Doppler interrogation. Trace renal pelvis dilation in Dog C2 was noted bilaterally but considered within normal limits. provides a reference for all measurements taken in each dog at various timepoints as described below.
Table 3. Ultrasound measurements of renal pelves and ureters for Group C dogs.
Dog C1: Two weeks after neoureterocystostomy and omentalization there was bilateral pyelectasia with mildly dilated ureters. Peristalsis was identified in both ureters. Thickened hyperechoic tissue was identified at each ureteral implantation site, protruding approximately 8-9mm into the lumen with suture material visible within the wall. The bladder wall was otherwise normal in thickness and layering with Doppler signal similar to that prior to surgery.
At the 2-week recheck following creation of the neoreservoir, the renal pelves returned to normal with no evidence of pyelectasia or ureteral dilation. Neither ureter could be traced to the insertion sites. The ureteral implantation sites were less proliferative. Doppler signal within the wall was maintained and similar to that prior to surgery other than mildly increased mucosal signal around the cystotomy site.
At the 4-week recheck the renal pelves were again mildly dilated along with the ureters but this improved slightly by the 6-week recheck. The bladder wall was normal in thickness with maintained Doppler signal at both rechecks.
On terminal evaluation at 8 weeks there was mildly progressive renal pyelectasia bilaterally and similarly mildly dilated ureters. Appropriate ureteral peristalsis was noted bilaterally. Bladder wall thickness was static. There was mildly decreased serosal signal and mildly increased mucosal signal compared to prior.
Dog C2: Two weeks post initial surgery the left and right renal pelvis was mildly and moderately dilated respectively. The ureters were mildly dilated bilaterally. The left ureteral insertion site was mildly proliferative. Approximately 2 cm proximal to the right ureteral insertion site there was a focal narrowing and kinking of the right ureter where the wall was thickened with echogenic debris within the lumen distal to this narrowing. The ureter was mildly dilated distal to this narrowing and the ureteral insertion site was moderately proliferative into the lumen. The bladder wall was otherwise normal with similar mural Doppler signal compared to pre-surgery.
At the 2-week recheck the left renal pelvis and ureter were similar to baseline. The right renal pelvis was progressively markedly dilated. The right ureter was similarly mildly dilated proximal to the previously identified stricture. Distal to the stricture the ureter focally dilated before narrowing toward the insertion site with a trace amount of luminal echogenic debris. The wall was mildly thickened and hyperechoic surrounding the cystotomy site and adjacent the left ureteral insertion. The wall was otherwise normal with appropriate Doppler signal.
The 4 and 6-week recheck ultrasounds were similar to previous with the exception of progressive marked dilation of the right renal pelvis with only a thin rim of cortical parenchyma visible at the latter timepoint. The focal wall thickening near the left ureteral insertion site remained moderately thickened which appeared associated with where the tip of the cystotomy tube abutted the bladder wall.
At terminal evaluation 7 weeks following neoreservoir creation, the left renal pelvis and left ureter were normal to minimally distended. The right renal pelvis was again markedly dilated measuring 44.6 mm x 69.3 mm, with minimal cortical parenchyma peripherally. The right ureter was similar to previous with no change to the stricture or focal dilation. The left ureteral insertion was similarly effaced by the focal bladder wall thickening with blurring of wall layers. The urinary bladder wall was otherwise normal with appropriate signal on Doppler.
Dog C3: Two weeks after neoureterocystostomy and omentalization there was mild bilateral pyelectasia and ureteral dilation. There was mildly proliferative hyperechoic tissue protruding into the lumen at each ureteral insertion site. The bladder wall had normal layering and thickness with moderate serosal and submucosal/mucosal signal.
At the 2-week recheck there was mildly progressive pyelectasia bilaterally. The ureters were mildly dilated similar to previous. The urinary bladder wall was mildly thickened surrounding the cystotomy site. The ureteral insertion site remained mildly proliferative into the lumen on the right, and the ureter adjacent to the insertion was focally mildly dilated and bulged slightly into the lumen. The proliferative tissue at the left ureteral insertion was mildly progressive. The bladder was otherwise normal in thickness and wall layering with similar blood flow on Doppler as prior.
At the 4-week recheck the renal pelvis dilation was mildly improved on the left, but mildly progressive on the right. The ureters were similarly mildly dilated with static proliferative tissue at the insertion site on the left and improvement on the right though the ureter immediately proximal remained mildly dilated with focal convex bulging of the bladder wall. The urinary bladder wall was otherwise normal with similar moderate serosal and submucosal/mucosal signal on Doppler. The 6-week recheck was similar but with distinct progressive dilation of the right renal pelvis.
At the final recheck the left renal pelvis dilation was improved, and the right renal pelvis was progressively markedly dilated, measuring 34.8 mm. The left ureter was less dilated with a ureteral jet identified. The right ureter was similarly dilated with a similar focal dilation (5 mm x 9.6 mm) at the insertion site, suspected to represent a small ureterocele secondary to ureteral implantation. There was subjectively weak peristalsis along the right ureter.
Gross evaluation
Neoreservoirs had a well-developed cystostomy tube stoma site in all 3 dogs. Omentum was adhered circumferentially to all 3 neoreservoirs. On incision, bleeding was noted from all layers in all cases. One neoreservoir (C1) was noted to have a thicker muscularis layer (). Subjective assessment of ureteral and renal size reflected that of ultrasound findings. All ureters were assessed as having some degree of dilation. Two dogs (C2 and C3) had marked hydronephrosis of 1 kidney each (Figure 8). The ureters associated with these kidneys were subjectively more dilated. The adhesion induced stricture previously noted in Dog C2 after the first surgery was still present at the end of the study. Despite attempts to release the adhesion and prevent stricture, a 3.5 Fr red-rubber catheter was unable to be passed through this stricture at termination. Five out of 6 ureterocystostomy junctions were patent with the ability to pass a 3.5 Fr Red Rubber retrograde into the ureters. For Dog C3 the red rubber was unable to be passed through the ureterocystostomy junction. The remaining ureters were patent enough to pass the red rubber to the level of the renal pelvis.
Histopathologic evaluation
Group C neoreservoir viability was characterized by the retention of the mucosa and muscularis propria as represented in . Wall layering was disrupted by fibrosis only at reimplantation sites, the transection site, and the cystostomy site. In one case (C3) the urothelium at the site of right neoureterocystotomy was ulcerated and this ulceration was associated with low numbers of superficial cocci bacteria. In this case (C3), the associated ureter was dilated and there was moderate nephrosis of the right renal pelvis, consistent with ureteral obstruction. Sequela of ureteral obstruction was identified in one other kidney (C2). In this dog (C2) there was dilation of the right ureter and renal pelvis, and the adhesion of the urinary bladder/ureter to the uterus was captured histologically. The urothelium at the neoureterocystotomy site was intact and the orifice appeared patent. In all Group C dogs, there appears to be active extrusion of suture material into the urinary bladder lumen at the sites of ureterocystostomy.
Discussion
The results of this study indicate that a continent neoreservoir can be created from the body of the urinary bladder in dogs undergoing resection of the trigone and urethra. In patients with trigonal/urethral UC, primary tumor resection may result in devascularization of the urinary bladder. Our results support previous literature that the omentum is capable of providing vascular support to contacting organs lacking an intrinsic blood supply [Citation31, Citation34, Citation35], as it was able to maintain bladder/neoreservoir viability in our study dogs. While previous studies have indicated that autogenous cell culture-seeded neobladders were able to survive implantation with the omentum serving of as the sole source of blood supply [Citation16, Citation38], immediate reliance on the omentum by our bladder vesicles was not reliable as demonstrated in Group A. Neovascularization of the urinary bladders in our study was more successful if a staged surgical approach was implemented, allowing sufficient time for vascular ingrowth from the omental adhesions intentionally created in Groups B and C.
As neobladders of cell cultures have survived acute implantation with omentalization, it seemed possible that resection of the primary tumor, neoureterocystostomy, and creation of the omentalized continent reservoir could potentially be performed during a single operative procedure [Citation16, Citation38]. However, we have shown that definitive surgery would need to be performed after a period of omental neovascularization of at least 2 weeks. Results from Group A indicate that neoreservoir viability cannot be maintained with a single procedure. Bladder vesicles in this group exhibited visual and histopathologic evidence of necrosis with mural thickening, loss of wall layering, and reduced blood flow (or vascularity) on ultrasound. One dog in Group A developed azotemia in relation to the procedure; however, in clinical cases the trigone would not be saved and oversewn, so iatrogenic obstruction in clinical cases would not occur at this location.
Results from Group B indicate that a two-week delay between omentalization and transection of the neoreservoir from intrinsic blood supply was sufficient to maintain reservoir viability. Group C dogs underwent a procedure proposed for patients with trigonourethral UC. The typical trigonal location of UC often requires ureteral reimplantation if the primary tumor is to be completely excised. To mimic this scenario, neoureterocystostomy was performed in conjunction with the omentalization. Ureteral reimplantation was performed first so that the omentum would not obscure the surface of the bladder during neoureterocystostomy.
All Group C dogs tolerated the procedure well based on clinical progression over the 2-month follow-up period. The temporary hyporexia noted in all cases was perceived to be secondary to environmental factors, primarily the necessity to wear Elizabethan collars. The intermittent vomiting was suspected to be secondary to postoperative nausea, UTI, and/or antibiotic administration, as dogs were otherwise systemically well. The additional laboratory analyses 7 weeks after the second surgery in dog C1 were performed due to pyrexia documented over more than one checkup. A second bacterial organism was identified on urine culture with systemic evidence of inflammation and azotemia suggesting an active urinary tract infection as the source of clinical signs. Methenamine was started in this dog due to the repeatable pyrexia despite antibiotics and the multi-bacterial urinary tract infection. While evidence of Methenamine’s effect remains anecdotal in veterinary medicine, safety and efficacy has been reported in human literature [Citation39, Citation40]. No side effects were noted while the dog was on the short course.
After ureteral reimplantation in 2 dogs, increases in serum creatinine concentrations were noted, although values remained within reference range. This was likely secondary to swelling at the ureteral reimplantation sites. These values returned to close to baseline in both cases during the follow-up period.
Patients with cystostomy tubes commonly test positive for bacteria in their urine likely due to skin or fecal contamination through the stoma [Citation20, Citation41]. Our study reflected this finding and indicates that UTIs may be an expected complication. In order to limit the development of antibiotic resistant bacteria over the long-term, treatment was only initiated if patients had systemic signs of an active UTI. Antibiotic administration during our short follow-up period did not result in resistance, nor did a complete change/shift in bacterial species occur for any dog.
Neoreservoirs in this study allowed for continence but required intermittent drainage via a cystostomy tube. As the dogs in this study were completely healthy, transection of the bladder from the urethra was performed at the trigone. In clinical cases it is possible that more of the trigone will need to be removed than performed in this study resulting in a smaller neoreservoir. During the follow-up period substantial amounts of urine could be held within the neoreservoir. This would indicate that the neoreservoirs are distensible and can increase in capacity with time as reflected in .
Anatomic changes to the urinary tract were monitored ultrasonographically with variability in results. After neoureterocystostomy all ureters developed some degree of dilation. This is consistent with other literature that reports ureteral/renal dilation after reimplantation [Citation7, Citation14, Citation36, Citation42]. Luminal diameters were variable at each subsequent recheck. No well-defined trend in ureteral measurements was identified except for the 2 progressively dilated ureters associated with hydronephrosis in 2 dogs. For causes of ureteral obstruction, stenosis of the ureteral reimplantation site is a reported complication of ureteral surgery and the apparent cause in C3 [Citation36, Citation43], but stricture due to uterine adhesion was not expected as a possible complication as seen in C2. While ureteral dilation did not consistently correlate with lack of patency, minor subclinical ureteral obstruction, ureteral reflux, and/or pressure transmitted from the neoreservoir should be considered as causes. Some degree of ureteral dilation should be expected following the proposed procedure, and ureteral stricture/stenosis should be monitored for as a potential complication. Despite the documented cases of unilateral hydroureter and hydronephrosis (2/6), neither dog developed biochemical or clinical changes associated with this finding. Renal function remained adequate with no systemic repercussions of the procedure over the 2-month follow-up period.
Compared to other surgical procedures currently available for treating UC, the proposed procedure results in limited negative side-effects. While gastrointestinal reservoirs can result in continence, they have been abandoned in dogs due to their marked systemic side effects [Citation12, Citation15]. Other described surgical procedures result in incontinence or frequent urination that can be unacceptable to owners of household pets. With the proposed procedure incontinence is eliminated, although maintenance of a cystostomy tube is required. Urinary tract infections remain a possible complication as with other reported surgical techniques used in clinical patients.
A previous study of 2 dogs with trigonal neoplasia showed that the trigone could be resected and the remaining bladder anastomosed to the urethra while preserving the vesicular blood supply [Citation7]. Damage to either vesicular artery during this procedure could lead to bladder necrosis. For more extensive tumors viability may be difficult to maintain while still achieving appropriate surgical margins. A benefit of this procedure is that a cystostomy tube would not be required, but it is not clear if patients would maintain continence. The procedure that we have evaluated would eliminate the risk of bladder/neoreservoir necrosis.
An important consideration prior to clinical implementation of the studied technique is the possibility of tumor recurrence within the neoreservoir. High recurrence rates (78-90%) have been reported for UC despite implementation of surgical and medical therapies [Citation1, Citation44–46]. Various studies looking at partial cystectomy as a treatment have reported recurrence after surgery. In one study of 10 dogs with UC, 6 dogs had no known residual tumor and clean surgical margins after partial cystectomy; 5 of those dogs experienced recurrence of a bladder mass and euthanasia, 4 of which were confirmed to have UC recurrence (noted in trigone, urethra, and ureter) on necropsy [Citation44]. In another study of 36 dogs, 3 out of 4 dogs with known complete margins after partial cystectomy were euthanized for complications related to UC; two for mass recurrence in the bladder [Citation46].
The previously described “field effect” has been suggested as a potential cause for local recurrence [Citation4, Citation9]. In addition, exosomes released from histologically unaffected bladder urothelium may also contribute to metastasis or recurrence [Citation47]. As such, tumor could recur within the neoreservoir or at the stoma despite complete surgical excision. Therefore, continued monitoring of the neoreservoir via ultrasound and BRAF analysis would be prudent.
Regardless of high recurrence rates historically reported after surgery, surgery is still considered of benefit due to improve survival rates [Citation1, Citation4, Citation44, Citation46]. Median survival times (MST) of 1 year have been reported after complete surgical excision of the primary tumor with or without chemotherapy [Citation1, Citation44]. In a review of 37 dogs with partial cystectomy, MST was 348 days for all cases regardless of adjuvant therapy; however, those with partial cystectomy and at least daily piroxicam with/without chemotherapy reached MST of 772 [Citation46]. Although “field cancerization” may result in recurrence even with the proposed technique, prognosis is suspected to still be improved. Adjuvant chemotherapy would be recommended after the proposed procedure. In addition to systemic chemotherapy, local adjuvant therapy administered via the cystostomy tube could be considered.
The main limitation to this study is that a small number of normal dogs were used. The procedure’s effect on prognosis, recurrence, and metastasis remains unknown. In addition, the neoreservoir size that can be achieved is unknown as this will likely vary based on the patient’s tumor size and location. While the procedure was successful in creating a continent neoreservoir vascularized solely by the omentum, it did require two surgical procedures, which could pose an issue for clients who are financially restricted or patients that are acutely obstructed.
The results of this study support our hypotheses that 1) a continent reservoir can be made from the body of the urinary bladder and that 2) vascularization of this neoreservoir can be supplied solely by omentum, as long as a sufficient period of vascular ingrowth (two weeks) is provided. This novel approach for creation of a neoreservoir could be utilized for patients requiring trigono-urethral resection of UC with minimal complications. Achieving primary tumor resection may improve survival times and improve quality of life. Monitoring of the neoreservoir would be indicated, as would clinical testing and appropriate therapy for urinary tract infections.
Acknowledgements
The authors would like to thank the clinicians, technicians, and staff of the North Carolina State University College of Veterinary Medicine Laboratory Animal Resources Department for their care of these dogs as well as the Histopathology Laboratory for their technical assistance. They would also like to thank John Joyner for photography and Baxter Fernandez for assistance. This project was made possible by funding from the Dean and Marilyn Green Urologic Research Fund.
Disclosure statement
The authors report no conflicts of interest.
References
- Norris AM, Laing EJ, Valli VEO, et al. Canine bladder and urethral tumors: a retrospective study of 115 cases (1980-1985). J Vet Intern Med. 1992;6(3):145–153. doi:https://doi.org/10.1111/j.1939-1676.1992.tb00330.x.
- Knapp DW, McMillan SK. Tumors of the urinary system. In: Withrow SJ, Vail DM, Page RL (eds). Withrow and MacEwen’s small animal clinical oncology. 5th ed. St. Louis (Missouri): W.B. Saunders; 2013: 572–582.
- Mutsaers AJ, Widmer WR, Knapp DW. Canine transitional cell carcinoma. J Vet Intern Med. 2003;17(2):136–144. doi:https://doi.org/10.1111/j.1939-1676.2003.tb02424.x.
- Knapp DW, Glickman NW, Denicola DB, Bonney PL, Lin TL, Glickman LT. Naturally-occurring canine transitional cell carcinoma of the urinary bladder: a relevant model of human invasive bladder cancer. Urol Oncol. 2000;5(2):47–59. doi:https://doi.org/10.1016/S1078-1439(99)00006-X.
- Choy K, Fidel J. Tolerability and tumor response of a novel low-dose palliative radiation therapy protocol in dogs with transitional cell carcinoma of the bladder and urethra. Vet Radiol Ultrasound. 2016;57(3):341–351. doi:https://doi.org/10.1111/vru.12339.
- Boston S, Singh A. Total cystectomy for treatment of transitional cell carcinoma of the urethra and bladder trigone in a dog. Vet Surg. 2014;43(3):294–300. doi:https://doi.org/10.1111/j.1532-950X.2014.12104.x.
- Saulnier-Troff F-G, Busoni V, Annick H. A technique for resection of invasive tumors involving the trigone area of the bladder in dogs: preliminary results in two dogs. Vet Surg. 2008;37(5):427–437. doi:https://doi.org/10.1111/j.1532-950X.2008.00406.x.
- Blackburn AL, Berent AC, Weisse CW, Brown DC. Evaluation of outcome following urethral stent placement for the treatment of obstructive carcinoma of the urethra in dogs: 42 cases (2004-2008)). J Am Vet Med Assoc. 2013;242(1):59–68. doi:https://doi.org/10.2460/javma.242.1.59.
- Griffin M, Culp W, Rebhun R. Lower urinary tract neoplasia. Vet Sci. 2018;5(4):96. doi:https://doi.org/10.3390/vetsci5040096.
- Walker M, Breider M. Intraoperative radiotherapy of canine bladder cancer. Vet Radiol. 1987;28(6):200–204. doi:https://doi.org/10.1111/j.1740-8261.1987.tb00053.x.
- Azémar M-D, Comperat E, Richard F, Cussenot O, Rouprêt M. Bladder recurrence after surgery for upper urinary tract urothelial cell carcinoma: frequency, risk factors, and surveillance. Urol Oncol. 2011;29(2):130–136. doi:https://doi.org/10.1016/j.urolonc.2009.06.003.
- Stone EA, Withrow SJ, Page RL, Schwarz PD, Wheeler SL, Seim III. Ureterocolonic anastomosis in ten dogs with transitional cell carcinoma. Vet Surg. 1988;17(3):147–153. doi:https://doi.org/10.1111/j.1532-950x.1988.tb00293.x.
- Saeki K, Fujita A, Fujita N, Nakagawa T, Nishimura R. Total cystectomy and subsequent urinary diversion to the prepuce or vagina in dogs with transitional cell carcinoma of the trigone area: a report of 10 cases. Can Vet J. 2015;56(1):73–80.
- Fries CL, Binnington AG, Valli VE, Connolly JG, Holmberg DL, Pennock P. Enterocystoplasty with cystectomy and subtotal intracapsular prostatectomy in the male dog. Vet Surg. 1991;20(2):104–112. doi:https://doi.org/10.1111/j.1532-950x.1991.tb00316.x.
- McCarthy RJ, Lipowitz AJ, O'Brien TD. Continent jejunal reservoir (Kock pouch) for urinary diversion in dogs. Vet Surg. 1992 May-Jun;21(3):208–216. doi:https://doi.org/10.1111/j.1532-950x.1992.tb00048.x.
- Kwon TG, Yoo JJ, Atala A. Local and systemic effects of a tissue engineered neobladder in a canine cystoplasty model. J Urol. 2008;179(5):2035–2041. doi:https://doi.org/10.1016/j.juro.2008.01.005.
- Fulkerson CM, Knapp DW. Management of transitional cell carcinoma of the urinary bladder in dogs: a review. Vet J. 2015;205(2):217–225. doi:https://doi.org/10.1016/j.tvjl.2015.01.017.
- Weisse C, Berent A, Todd K, Clifford C, Solomon J. Evaluation of palliative stenting for management of malignant urethral obstructions in dogs. J Am Vet Med Assoc. 2006;229(2):226–234. doi:https://doi.org/10.2460/javma.229.2.226.
- McMillan SK, Knapp DW, Ramos-Vara JA, Bonney PL, Adams LG. Outcome of urethral stent placement for management of urethral obstruction secondary to transitional cell carcinoma in dogs: 19 cases (2007-2010)). J Am Vet Med Assoc. 2012;241(12):1627–1632. doi:https://doi.org/10.2460/javma.241.12.1627.
- Smith JD, Stone EA, Gilson SD. Placement of a permanent cystostomy catheter to relieve urine outflow obstruction in dogs with transitional cell carcinoma. J Am Vet Med Assoc. 1995;206(4):496–499.
- Henry CJ, McCaw DL, Turnquist SE. Clinical evaluation of mitoxantrone and piroxicam in a canine model of human invasive urinary bladder carcinoma. Clin Cancer Res. 2003;9(2):906–911.
- Arnold EJ, Childress MO, Fourez LM, et al. Clinical trial of vinblastine in dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 2011;25(6):1385–1390. doi:https://doi.org/10.1111/j.1939-1676.2011.00796.x.
- Knapp DW, Ramos-Vara JA, Moore GE, Dhawan D, Bonney PL, Young KE. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014;55(1):100–118. doi:https://doi.org/10.1093/ilar/ilu018.
- Li Z, Liu Z, Yao K, et al. An improved ileal conduit surgery for bladder cancer with fewer complications. Cancer Commun. 2019;39(1):19. doi:https://doi.org/10.1186/s40880-019-0366-8.
- Stephenson WT, Holmes FF, Noble MJ, Gerald KB. Analysis of bladder carcinoma by subsite. Cystoscopic location may have prognostic value. Cancer. 1990;66(7):1630–1635. doi:https://doi.org/10.1002/1097-0142(19901001)66:7<1630::AID-CNCR2820660730>3.0.CO;2-7.
- Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (Stages Ta, T1, and Tis): 2007 Update. J Urol. 2007;178(6):2314–2330. doi:https://doi.org/10.1016/j.juro.2007.09.003.
- Spiess PE, Agarwal N, Bangs R, et al. Bladder cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(10):1240–1267. doi:https://doi.org/10.6004/jnccn.2017.0156.
- Svatek RS, Siefker-Radtke A, Dinney CP. Management of metastatic urothelial cancer: The role of surgery as an adjunct to chemotherapy. Can Urol Assoc J. 2009;3(6 Suppl 4):S228–S31. doi:https://doi.org/10.5489/cuaj.1203.
- Hautmann RE, Hautmann SH, Hautmann O. Complications associated with urinary diversion. Nat Rev Urol. 2011;8(12):667–677. doi:https://doi.org/10.1038/nrurol.2011.147.
- Platell C, Cooper D, Papadimitriou JM, Hall JC. The omentum. World J Gastroenterol. 2000;6(2):169–176. doi:https://doi.org/10.3748/wjg.v6.i2.169.
- Di Nicola V. Omentum a powerful biological source in regenerative surgery. Regen Ther. 2019;11:182–191. doi:https://doi.org/10.1016/j.reth.2019.07.008.
- Konturek SJ, Brzozowski T, Majka I, Pawlik W, Stachura J. Omentum and basic fibroblast growth factor in healing of chronic gastric ulcerations in rats. Dig Dis Sci. 1994;39(5):1064–1071. doi:https://doi.org/10.1007/BF02087559.
- Williams JK, Carlson GW, Austin GE, Austin ED, Rand RP, Jurkiewicz MJ. Short gut syndrome: treatment by neovascularization of the small intestine. Ann Plast Surg. 1996;37(1):84– 90. doi:https://doi.org/10.1097/00000637-199607000-00013.
- Shoshany G, Mordohovich D, Lichtig H, Bar-Maor J. Preserved viability of the isolated bowel segment, created by omentoenteropexy: a histological observation. J Pediatr Surg. 1995;30(9):1291–1293. doi:https://doi.org/10.1016/0022-3468(95)90487-5.
- Shoshany G, Cohen E, Mordohovich D, Hayari L, Har-Shai Y, Bar-Maor JA. Creation of the isolated bowel segment in animals by omentoenteropexy. J Pediatr Surg. 1994;29(10):1344–1347. doi:https://doi.org/10.1016/0022-3468(94)90112-0.
- Mehl ML, Kyles AE, Pollard R, et al. Comparison of 3 techniques for ureteroneocystostomy in cats. Vet Surg. 2005;34(2):114–119. doi:https://doi.org/10.1111/j.1532-950X.2005.00008.x.
- Mich PM, Hellyer PW, Kogan L, Schoenfeld-Tacher R. Effects of a pilot training program on veterinary students' pain knowledge, attitude, and assessment skills. J Vet Med Educ. 2010;37(4):358–368. doi:https://doi.org/10.3138/jvme.37.4.358.
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. doi:https://doi.org/10.1016/S0140-6736(06)68438-9.
- Olin SJ, Bartges JW. Urinary Tract infections: treatment/comparative therapeutics. Vet Clin North Am Small Anim Pract. 2015;45(4):721–746. doi:https://doi.org/10.1016/j.cvsm.2015.02.005.
- Chwa A, Kavanagh K, Linnebur SA, Fixen DR. Evaluation of methenamine for urinary tract infection prevention in older adults: a review of the evidence. Ther Adv Drug Saf. 2019;10:1–9.
- Beck AL, Grierson JM, Ogden DM, Hamilton MH, Lipscomb VJ. Outcome of and complications associated with tube cystostomy in dogs and cats: 76 Cases (1995-2006). J Am Vet Med Assoc. 2007;230(8):1184–1189. doi:https://doi.org/10.2460/javma.230.8.1184.
- El-Hammady S, Shokeir AAA. A novel technique of ureteroneocystostomy (extravesical seromuscular tunnel): an experimental study in dogs. I. Preliminary results. Urology. 1995;45(2):339–343. doi:https://doi.org/10.1016/0090-4295(95)80031-x.
- Hardie EM, Kyles A. Management of ureteral obstruction. Vet Clin North Am - Small Anim Pract. 2004;34(4):989–1010.
- Stone EA, George TF, Gilson SD, Page RL. Partial cystectomy for urinary bladder neoplasia: surgical technique and outcome in 11 dogs. J Small Anim Pract. 1996;37(10):480–485. doi:https://doi.org/10.1111/j.1748-5827.1996.tb01745.x.
- Hamaide A. Surgery of the urinary bladder. In: Dominique Griffon AH (ed). Complications in small animal surgery. 1st ed. Ames (Iowa): John Wiley & Sons, Inc.; 2016: 492–496.
- Marvel SJ, Séguin B, Dailey DD, Thamm DH. Clinical outcome of partial cystectomy for transitional cell carcinoma of the canine bladder. Vet Comp Oncol. 2017;15(4):1417–1427. doi:https://doi.org/10.1111/vco.12286.
- Hiltbrunner S, Mints M, Eldh M, et al. Urinary exosomes from bladder cancer patients show a residual cancer phenotype despite complete pathological downstaging. Sci Rep. 2020;10(1).