Abstract
Background
Micropercutaneous nephrolithotomy (microperc) is the least invasive among percutaneous nephrolithotripsy (PCNL) procedures. Although microperc has a high stone-free rate and certain advantages over other methods, modifications may be needed to improve the technique. We describe our experience performing microperc using a self-assembled visual needle and ureteral access sheath (UAS).
Methods
Between June 2016 and April 2019, the data of 30 patients with kidney stones undergoing microperc with our self-assembled 4.8 Fr visual needle combined with a UAS was retrospectively analyzed. Patients were placed in an obilique spine lithotomy position.
Results
Two cases were excluded: one due to conversion to mini PCNL and the other required flexible ureteroscopy during microperc. The remaining 28 cases included 18 men and 10 women, age 38.4 ± 7.5 years, stone size 1.7 ± 0.4 cm, and stone density on CT 969 ± 233 HU. Operative time was 47 ± 9.9 minutes, visual analogue scale score of tract pain on postoperative day 1 was 2.5 ± 1.0, hemoglobin decrease was 6.4 ± 1.0 g/L, and hospital stay was 3.1 ± 0.8 days. There was 1 case of fever and urinary infection, 2 cases of hematuria, and 1 case of flank pain. All symptoms resolved after conservative or antibiotic treatment. On postoperative day 1, 12 (42.9%) caseswere stone-free. The stone-free rates at postoperative month 1 and 3 were 92.9% (26/28) and 100% (28/28), respectively.
Conclusions
Our self-assembled visual needle and UAS instrument is effective for microperc. Use of the UAS may improve the operative outcome.
Introduction
Percutaneous nephrolithotomy (PCNL) is the first-line option for >2 cm diameter kidney stones and complex renal stones. PCNL has a high stone-free rate (SFR), however, one of its major complications is hemorrhage, which is associated with the size of the percutaneous renal tract.Citation1–4 Efforts to decrease complications have focused on instrument size. Thus, less invasive and more refined instruments have been developed, such as those used during mini-PCNL and ultramini-PCNL.Citation2–6 Bader et al.Citation3 established percutaneous renal tracts using a 16-gauge optical puncture system in 2011. Desai et al.Citation7 first reported on the puncture and disintegration of urinary stones in a single step without tract dilation and named this innovative PCNL “microperc.” Thereafter, microperc went on to be used to treat moderate-size renal stones in an effort to reduce tract size and tract-related complications.Citation4,Citation5,Citation8–12 Advantages of the single-step procedure without tract dilation are minimal tract bleeding, high likelihood of terminating the procedure without tubes, and reduced postoperative pain and hospitalization.Citation5,Citation8,Citation13
Nevertheless, microperc has drawbacks. First, it is performed without an Amplatz sheath, which is intended to facilitate controlling intrapelvic pressure(IPP) and removing fragments.Citation5–8,Citation11,Citation14–17 Irrigation fluid may not be actively drained and pressurized irrigation may increase IPP, requiring aspiration through the ureteral catheter during the procedure.Citation7,Citation8,Citation18 High IPP can cause fluid absorption, intravasation, extravasation, and even renal perforation.Citation5,Citation8,Citation13,Citation14 Second, similar to extracorporeal shock wave lithotripsy (ESWL) and flexible ureteroscopy, PCNL is a “break and leave” process with stone fragments left to discharge spontaneously after the surgery.Citation2,Citation6–8,Citation10,Citation14 However, fragment discharge may be difficult, such as in patients with an abnormal lower calyx and anatomy.Citation6,Citation7,Citation10,Citation19–21 Furthermore, residual stone fragments can cause renal colic and postoperative steinstrasse.Citation5,Citation6,Citation8,Citation14,Citation16,Citation22–24 Third, stone fragments and powder may impede operator vision and thus reduce efficiency and prolong operative time.Citation5,Citation20 Fourth, even minimal bleeding can be a concern for vision interference due to a small irrigation channel and in case a suction device is lacking.Citation1,Citation5,Citation7,Citation13,Citation14,Citation19,Citation20
Tepeler et al.Citation25 reported that during all phases of microperc IPP can be significantly higher than conventional PCNL, and the highest levels of IPP during microperc were also markedly higher in the two groups of patients they studied (30.3 ± 3.9 mmHg and 20.1 ± 3.1 mmHg). A 14-gauge intravenous cannula has been used as a micro-Amplatz sheath and another cannula to establish a second tract for relieving intrapelvic pressure.Citation26 However, the extra tract often carries a risk of trauma. Microperc is still in early development and technical improvements are needed to perfect it to establish its place among ESWL, PCNL, and ureteroscopy.Citation4,Citation7,Citation8,Citation14,Citation17,Citation27,Citation28
PCNL can be successfully performed on patients in the oblique supine lithotomyposition.Citation29,Citation30 During retrograde intrarenal surgery, a ureteral access sheath (UAS) is typically used to maintain low IPP. During flexible uteroscopy, when a UAS is utilized and irrigation flow is <100 cm H2O, IPP remains lower than 30 mmHg.Citation31 Hence, we hypothesize that UAS may facilitate controlling IPP during microperc, flushing out the turbid irrigation fluid, and providing access for flexibleureterscopy in some scenarios, such as stone fragment migration, to improve the success rate.
In this article, we describe the results of applying a self-assembled visual puncture needle combined with UAS to manage kidney stones.
Materials and methods
Patients and study design
We reviewed the case records of 30 patients with urinary calculi who underwent microperc using a self-assembled visual puncture needle between June 2016 and April 2019. This study was conducted in accordance with the Declaration of Helsinki. Inclusion criteria: single stone or multiple stones parallel to the puncture tract that could be fragmented via a single tract;Citation12 stone size ranged 1.0-3.0 cm in diameter;Citation12 ≥2 cm nonobstructive pelvic stones; failed ESWL or flexible ureteroscopy due to calyceal neck stricture.Citation10 Exclusion criteria: multiple stones distributed in different calyces requiring multiple (≥2) puncture tracts;Citation12,Citation15 other procedure during the same admission, such as flexible ureteroscopy lithotripsy (FURSL) or mini PCNL;renal insufficiency.
Preoperative evaluation included a urine test, urine culture, ultrasound, computed tomography (CT), intravenous pyelogram (IVP), and kidney-ureter-bladder (KUB) study. Stone burden was calculated based on the maximum diameter of a single stone or the sum of maximum diameters of multiple stones.Citation8 Surgery was deferred for patients with urinary tract infection (UTI) and positive urine culture until the urine test and culture were both negative. Preoperative, intraoperative, and postoperative data were collected.
Visual needle
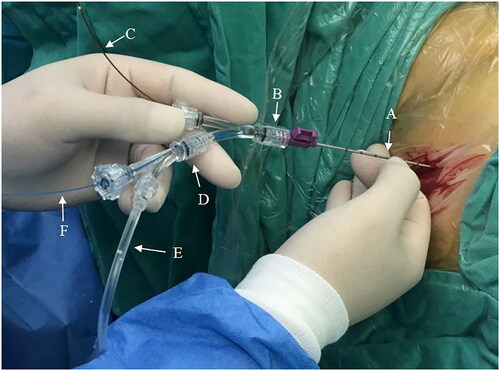
The self-assembled visual needle consisted of a 16 G puncture needle (outer diameter 1.6 mm, or 4.85 Fr) (Magnum, MN1616; Becton, Dickinson and Company, Franklin Lakes, NJ, USA), integrated fiber optic microbundle (Fiber Endoscope, YC-LF-A;Youkang Company, Wuhan, China), and two ureteroscopy Y-adapters (Gateway Advantage Y-Adapter; Boston Scientific, Natick, MA, USA) (). The diameter of the highly flexible fiber optic bundle was 0.8 mm, with an optic resolution of 10 000 pixels. The two Y-adapters were screwed together: the stem of Y-adapter #2 was screwed into one of the ports of Y-adapter #1. The stem of Y-adapter #1 was then attached to the needle hub. The fiber optic bundle was inserted through the other port of Y-adapter #1 and threaded through the needle shaft until it was flush with the needle tip.Citation7 An irrigation tube and a 272 μm holmium:yttrium-aluminum-garnet (Ho:YAG) laser fiber were each introduced through a port of Y-adapter #2. The proximal end of the irrigation tube was connected to a 50 ml syringe to pump irrigation fluid. The proximal end of the fiber optic bundle was connected to anendoscopic camera system (CV-180 CLV-180 Evis Exera II Endoscopy System; Olympus, Tokyo, Japan) and xenon light source.
Surgical methods
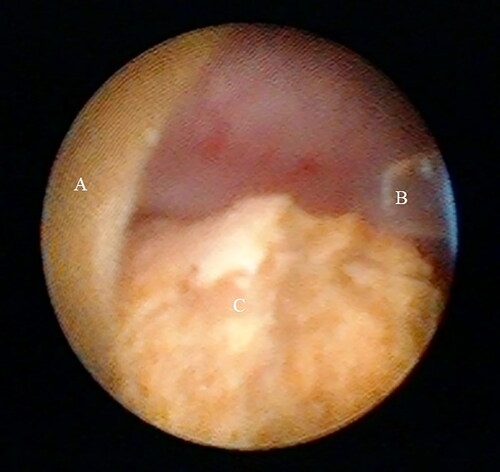
After general anesthesia with laryngeal mask or tracheal intubation, patients were placed in the oblique supine lithotomy position, and the perineum and kidney areas were disinfected and draped. For antibiotic prophylaxis cefoxitinor levofloxacin was initiated 30 minutesprior to surgery and did not exceed 24 hours in uneventful cases. A 0.038-inch hydrophilic guide wire (ZIP wire; Boston Scientific) was inserted into the affected ureter and ipsilateral ureteroscopy was performed with 8.0/9.8 Fr semirigid ureteroscope(model #8703.534; Richard Wolf, Knittlingen, Germany) to evaluate ureter diameter and the length from the ureteral orifice to the ureteropelvic junction. Based on the examination, an appropriate UAS (11/13 Fr, 12/14 Fr, or 13/15 Fr) (Navigator; Boston Scientific) was inserted into the ureter along the guidewire. Ultrasound confirmation ensured UAS tip placement was at the ureteropelvic junction or proximal ureter. The UAS core was retained and normal saline was infused to delineate the calyceal anatomy. Puncture was performed under ultrasound guidance. Stone fragmentation with one access was the key for successful puncture.Citation4,Citation15,Citation16,Citation28 For stones located in a single calyx, the visual needle was advanced directly into the calyx. Stones distributed in different calyces were also managed with one access.Mid-posterior or lower-calyceal puncture was used for pelvic stones. During needle advancement, a surgical assistant continuously perfused normal saline with a 50 ml syringe. The puncture needle allowed visualization of the entire tract in real time during percutaneous access tract establishment and verified the correct pelvicalyceal system. Successful puncture meant that the target calyx was observed and stones were visualized. After successful puncture, a 272 µm laser fiber (Shanghai Raykeen Laser Technology, Shanghai, China) was threaded into the needle through a port of Y-adapter #2. Holmium laser energy was set to high frequency and low energy, such as 30 Hz, 0.5j (). During irrigation, if the fluid flowed too slowly from the UAS or the upper ureter mucosa blocked the UAS outlet as observed under direct visualization, the guide wire was inserted to carefully separatethe mucosa. The stones were fragmented into dust or fine particles that were washed out through the UAS and collected for composition analysis (). After disintegration, ultrasound confirmed the absence or presence of residual stones. A 5.5 Fr ureteral stent was indwelled transurethrally to prevent urinary tract obstruction due to blood clots or possible residual stones.
Postoperative evaluation
On postoperative day 1, patients underwent renal ultrasonography and KUB to evaluate the absence or presence of peritoneal effusion and hematoma and stent position. Tract pain was assessed using a visual analogue scale (VAS). Postoperative hemoglobin decline was also evaluated. Postoperative temperature >38.5 °C was diagnosed as fever. Complications were classified according to the Clavien-Dindo system.Citation32,Citation33 The ureteral stent was removed by cystoscopy at postoperative week 2-4 during an outpatient visit. Non-enhancing CT was used to evaluate patients at 1-month and 3-month follow-up. Clinically insignificant residual fragments (CIRFs) were defined as asymptomatic non-obstructing residual fragments <4 mm on CT.Citation2,Citation7,Citation12
Results
Preoperative data
Two patients were excluded from our analysis: one case was converted to mini PCNL and the second required flexible ureteroscopy during the microperc procedure (). There were 19 cases of flank pain, 8 gross hematuria, 5 urinary tract infection (UTI), and 5 patients had no symptoms. Five cases had a history of failed ESWL and 4 cases had a history of ureteroscopic lithotripsy. Preoperative urine screening was positive for white blood cells and nitrites in 5 patients, and urine cultures in 4 patients were positive for Escherichia coli, which turned negative after antibiotic treatment.
Table 1. Demographic data and stone characteristics.
Intraoperative results
In our cohort, all microperc procedures were performed unilaterally. Two cases were excluded from our analysis. One case was converted to mini PCNL because the visual field was obscured due to intraoperative bleeding. In the second case, flexible ureteroscopy was required during the microperc procedure because large stone fragments had migrated, which precluded use of the visual needle. The fragments were retrieved using a Zero Tip nitinol stone retrieval basket (Boston Scientific) and moved to a better location for laser disintegration. Microperc was successfully performed on the remaining 28 cases using a single tract. In 2 cases, each of the target calyces was filled with stones. Both the calyx and stones were clearly observed on ultrasound when the needle tip entered the target calyx. But the calyx and stones were not seen with the visual system. The needle was then advanced further to penetrate through the calyceal mucosa into the calyx at which point the stones were clearly visualized. Under direct visualization, the stones were pushed aside to confirm calyx entry. After fragmentation, the stone fragments and dust were flushed out through the UAS.
Operative time for microperc was defined as the duration between initial retrograde ureterscopy to placement of indwelling stent and urethral catheter. Operative time for our cohort was 47 ± 9.9 minutes.
Postoperative results
Postoperative tract pain VAS was 2.5 ± 1.0 (range of 1-6) (). Hospital stay was 3.1 ± 0.8 days. Patients were discharged on postoperativeday 1, except for 2 cases of hematuria (Clavien I), 1 case of urinary infection and fever (Clavien II), and 1 case of flank pain (Clavien I). Hematuria and flank pain resolved on postoperative days 2–3 after conservative treatment and fever on postoperative day 3 following antibiotic therapy. Ultrasound examination did not show obvious peritoneal or pleural effusion, or retroperitoneal hematoma. There were no cases of urine or blood exudate at the skin puncture site. Hemoglobin decline was 6.4 ± 1.0 g/L on postoperative day 1. There were no residual stone fragments in 12 (42.9%) cases on postoperative day 1 (). At postoperative month 1, the stone-free rate was 92.9% (26/28). In the two cases with residual stones, their diameters were 2 and 4 mm. These fragments disappeared by the 3-month follow-up visit.
Figure 4. Pre-and post-operative CT and abdominal plain film (KUB) of left low calyceal stone treated with microperc: (A) preoperative CT; (B) KUB; (C) stone and fragments disappeared on postoperative day 1.
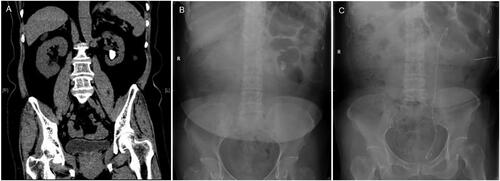
Table 2. Intra-and postoperative Findings.
Stone analysis showed 15 cases of calcium oxalate monohydrate, 4 cases of uric acid, and 3 cases of ammonium magnesium phosphate hexahydrate, 6 case of a mixture of calcium oxalate monohydrate and calcium oxalate dihydrate.
Discussion
In this study, we successfully performed microperc on 28 patients using a self-assembled visual needle combined with a ureteral access sheath (UAS). Results, such as stone-free rate (SFR), were comparable to conventional microperc. Patients placed in the oblique spine lithotomy position allowed for both transurethral and percutaneous renal access without repositioning.315.0-7.0 UAS facilitateda clear operative field of vision, controlled IPP, and combined with flexible ureteroscopy during microperc resulted in an improved postoperative SFR compared with microperc alone.
Conventional PCNL, including mini and ultramini PCNL, comprises three steps: tract establishment, tract dilation, and nephroscopy.Citation14 Their common shortcomings include: the procedures require multiple steps, tract bleeding, calyceal neck tearing, and pelvic perforation.Citation7,Citation13,Citation14,Citation22 However, reducing the size of the percutaneous renal tract does decrease injury and associated complications.Citation23 Microperc using a 4.85 Fr needle has the smallest tract size, avoids tract dilation during stone disintegration, and the fragments are left to pass spontaneously.Citation7–9,Citation12,Citation22 In this study, ureteral stents were not placed preoperatively as describedin our previous study,Citation29 since after ureteroscopy the appropriate UAS was selected and placed successfully under ultrasound guidance. However, flexible ureteroscopy and a stone retrieval basket were used in one case to move the stone fragments to a better location for lithotripsy without repositioning the patient.
Similar to flexible ureterscopy, bleeding and stone powder are major factors affecting the operative field of vision during microperc, necessitating conversion to mini PCNL,Citation1,Citation8,Citation17,Citation34,Citation35 However, when a UAS is utilized during microperc, fragments and blood clots are easily flushed out through the sheath than through a 5.0-7.0 Fr ureter catheter,Citation4,Citation7,Citation10,Citation16 thus facilitating a clear operative field of vision and a reduced postoperative stone burden. Pressurized irrigation may improve the postoperative stone-free rate,Citation7–9,Citation20,Citation36 however, this can increase IPP and related complications such as renal pelvic-venous reflux and even calyceal perforation.Citation1,Citation5,Citation7,Citation8 Using a UAS, a low IPP is easily maintained, whereas intermittent aspiration is needed to decrease IPP if a ureteral catheter is used.Citation1,Citation4,Citation7,Citation16 With a Holmium laser, stones of different compositions can be powdered into fragments <1-2 mm, which can be easily flushed out through a UAS.Citation16 In fact, even fragments as large as 3-4 mm can pass througha 13-14 Fr UAS. In the past, conversion to mini PCNL was a solution to remove displaced stones during microperc.Citation13 Now, UAS may provide another access during flexible ureteroscopy to reposition and remove fragments.Citation29
Our self-assembled visual needle is disposable and less costly than the all-seeing needle. The latter is of narrow caliber and is prone to bending and breakage during lithotripsy.Citation4,Citation10 Our visual needle is easily assembled just prior to skin puncture and requires only the insertion of the laser fiber into the needle sheath once the tract is established. In comparison, the all-seeing needle necessitates multiple steps during assembly and usage: after skin puncture and tract establishment, the stylet is removed and the 3-way connector is attached, followed by the telescope, the irrigation system, and the laser fiber.Citation3,Citation7,Citation19 Manipulation of our needle is simple and efficient. Another advantage of our setup is that all tissue layers are displayed on the camera during puncture,Citation7,Citation20 which permits accurate tract establishment under direct vision, thus improving the success rate of surgery.Citation7,Citation20,Citation24 In two cases that had calyces full of stones, ultrasound showed that the needle tip had reached the target calyx and stones, and the operator even felt the needle tip contact the stones. However, the calyx and stones were not observed with the visual system. After advancing the needle further, the tip of needle penetrated through the calyceal mucosa and entered the calyx at which point the calyx and stones were visualized. Thus, in this scenario, direct visualization helped to accurately establish the tract while under ultrasound and x-ray the calyceal mucosa was unable to be identified.
In this study, the mean operative time including ureterosopy was 47 minutes, which was consistent with the duration of 40-104 minutes in previous reports.Citation2,Citation6,Citation12,Citation14–16,Citation22,Citation37 Thus, better irrigation and visualization contributed to the high efficiency. Postoperative tract pain was VAS 2.5, which is in line with previous reports.Citation7,Citation15 Mean hospital stay was 3.1 days, which was also similar to other studies.Citation7,Citation37 In the absence of nephrostomy tube insertion, postoperative pain is significantly less and hospital stay is shortened.Citation1 Hemoglobin decrease was 6.4 g/L on postoperative day 1, which was comparable with the previously reported 6.3-14 g/L.Citation2,Citation7,Citation8,Citation13,Citation15,Citation16,Citation24,Citation37 In our study, fever occurred in 1 case (Clavien II) and disappeared after antibiotic treatment. Insertion of ureteral stent due to renal colic (Clavien IIIa) after microperchas been reported.Citation8,Citation13,Citation14,Citation16,Citation22–24,Citation37 In our cohort, this complication did not occur, which could be related to ureteral stent placement and fewer residual fragments. SFR at postoperative month 1 was 92.9% (26/28), which was similar to previous results of 80.9-95.7%.Citation2,Citation6–8,Citation13,Citation14,Citation16,Citation22,Citation24,Citation37 Furthermore, 12 cases were fragment-free on postoperative day 1. Therefore, our method appears to render patients stone-free earlier in the postoperative period. In addition, we thought that fragment clearance was affected by the location of the UAS. However, in 1 case the UAS outlet was obstructed by the upper ureter wall, which was then nudged out of the way by inserting the guidewire through the sheath, thus allowing for smooth drainage. Therefore, drainage should be continually monitored during the procedure.
Formal guidance on application of microperc is still lacking. Most reports on its use are for moderate size stones (10-20 mm) and lower calyceal stones that are difficult to handle using extracorporeal shock wave lithotripsy (ESWL) or retrograde intrarenal surgery (RIRS).Citation4,Citation6–8,Citation10,Citation12,Citation13,Citation15,Citation16,Citation20,Citation23,Citation38–40 Microperc is also performed for pediatric renal stones,Citation1,Citation7,Citation18,Citation20,Citation40 pelvic ectopic kidney stones,Citation4,Citation7,Citation8,Citation20,Citation40 kidney stones following partial nephrectomy,Citation21 kidney stones in severe kyphoscoliosis,Citation14,Citation26 bladder stones,Citation6 stones in the isthmus of a horseshoe kidney,Citation7,Citation21 and awkward lower calyceal anatomy.Citation7,Citation21 The ideal scenario for microperc is a single calyceal stone, pelvic stone, or multiple stones parallel to the percutaneous renal tract so that lithotripsy can be completed with a single tract and multiple punctures avoided.Citation1,Citation4,Citation7,Citation13,Citation15,Citation16,Citation22,Citation37 The upper limit of stone burden for microperc has not been identified,Citation1,Citation6,Citation8,Citation14 although stone burden ≥30 mm has been successfully treated.Citation1,Citation13,Citation26 However, large stones require a longer lithotripsy time and carry more risk of complications,Citation7,Citation20 and can even necessitate conversion to mini PCNL.Citation8 Stones that completely obstruct the urinary tract, such as obstructive ureter due to ureteral stones and impacted pelvic stones, are predisposing factors for a higher IPP, therefore microperc should be avoid in these scenarios.Citation1,Citation5,Citation8,Citation13,Citation14,Citation24 Patients with marked hydronephrosis were not included in our cohort due to the risk of converting to mini-PCNL or other procedures since lithotripsy might be difficult because of poor operative field of vision or displacement of stones.Citation4,Citation6,Citation14,Citation15,Citation26
There are several limitations of this study. First, it was a retrospective case series analysis with a small sample size. Second, our results were not compared with those of the commercial all-seeing needle (PolyDiagnost, Pfaffenhofen, Germany) and other methods. Third, all patients in our cohort were indwelled with ureteral stents. This may have offset the advantage of microperc, which is intended to accomplish a totally tubeless kidney stone management procedure without a nephrostomy tube or double-J stent.Citation5,Citation6,Citation13–15,Citation21,Citation40,Citation41 However, some cases of >20.0 mm calculus that necessitate long operative times or result in more residual fragments are also indwelled with ureteral stents.Citation1,Citation4,Citation6,Citation7,Citation12,Citation14,Citation15,Citation19,Citation37,Citation38 In addition, stent insertion can be required due to postoperative renal colic and steinstrasse.Citation10,Citation13,Citation14,Citation23 Therefore, indications for ureteral stent placement warrants further validation.Citation6 Fourth, use of UAS leads to increased cost, ureteral spasms, swelling, obstruction, pain due to removal of the stent, and even future stricture.Citation6,Citation34 Fifth, ureteroscopy performed prior to UAS insertion may increase the complexity, duration, and cost of surgery. Finally, this was an exploratory study, and as such we did not measure IPP during our procedures because irrigation flow was constant, which allowed for continual flushing of fragments and powder through the UAS. This likely decreased IPP and improved SFR.
In summary, the self-assembled visual puncture needle combined with UAS in the oblique lithotripsy position integrates visualized puncture, stone disintegration, and intraoperative stone discharge. Furthermore, it has the advantage of combining flexible ureteroscopy at any time during the procedure. This modification of microperc reduces postoperative stone burden and complications, allows for intraoperative acquisition of stone samples for composition analysis, and increases SFR. The self-assembled visual puncture needle is technically simple and is effective for performing microperc. Furthermore, it is less costly compared with the commercial all-seeing needle. However, its widespread application needs to be validated in large prospective clinical trials.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jiqing Zhang, Ning Kang, and Yuguang Jiang. Jiqing Zhang and Junhui Zhang provided the concept and design the instrument and perform the surgery. The first draft of the manuscript was written by Jiqing Zhang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgment
The authors thank Nissi S. Wang for developmental editing of this manuscript. We thank all the cases for their participation in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Pérez-Fentes D, Blanco-Gómez B, García-Freire C. Micropercutaneous nephrolithotomy. A new therapeutic option for pediatric renal lithiasis. Actas Urol Esp. 2014;38(7):483–487. doi:https://doi.org/10.1016/j.acuro.2014.02.010.
- Ölçücüoğlu E, Kasap Y, Ölçücüoğlu E, et al. Micropercutaneous nephrolithotripsy: initial experience. WIITM. 2015;3(3):368–372. doi:https://doi.org/10.5114/wiitm.2015.54223.
- Bader MJ, Gratzke C, Seitz M, Sharma R, Stief CG, Desai M. The "all-seeing needle": initial results of an optical puncture system confirming access in percutaneous nephrolithotomy. Eur Urol. 2011;59(6):1054–1059. doi:https://doi.org/10.1016/j.eururo.2011.03.026.
- Sabnis RB, Ganesamoni R, Ganpule AP, et al. Current role of microperc in the management of small renal calculi. Indian J Urol. 2013;29(3):214–218. doi:https://doi.org/10.4103/0970-1591.117282.
- Akbulut F, Ucpinar B, Savun M, et al. A major complication in micropercutaneous nephrolithotomy: upper calyceal perforation with extrarenal migration of stone fragments due to increased intrarenal pelvic pressure. Case Rep Urol. 2015;2015:792780. doi:https://doi.org/10.1155/2015/792780.
- Piskin MM, Guven S, Kilinc M, Arslan M, Goger E, Ozturk A. Preliminary, favorable experience with microperc in kidney and bladder stones. J Endourol. 2012;26(11):1443–1447. doi:https://doi.org/10.1089/end.2012.0333.
- Desai MR, Sharma R, Mishra S, Sabnis RB, Stief C, Bader M. Single-step percutaneous nephrolithotomy (microperc): the initial clinical report. J Urol. 2011;186(1):140–145. doi:https://doi.org/10.1016/j.juro.2011.03.029.
- Silay MS, Tepeler A, Atis G, Sancaktutar AA, et al. Initial report of microperc in the treatment of pediatric nephrolithiasis. J Pediatr Surg. 2013;48(7):1578–1583. doi:https://doi.org/10.1016/j.jpedsurg.2013.06.015.
- Desai M, Mishra S. ‘Microperc’ micro percutaneous nephrolithotomy: evidence to practice. Curr Opin Urol. 2012;22(2):134–138. doi:https://doi.org/10.1097/MOU.0b013e32834fc3bb.
- Armagan A, Karatag T, Buldu I, et al. Comparison of flexible ureterorenoscopy and micropercutaneous nephrolithotomy in the treatment for moderately size lower-pole stones. World J Urol. 2015;33(11):1827–1831. doi:https://doi.org/10.1007/s00345-015-1503-x.
- Penbegul N, Utangac MM, Daggulli M, et al. A novel drainage technique during micropercutaneous nephrolithotomy in pediatric patients: double angiocath. J Pediatr Surg. 2016;51(6):1051–1053. doi:https://doi.org/10.1016/j.jpedsurg.2016.03.003.
- Kandemir A, Guven S, Balasar M, Sonmez MG, Taskapu H, Gurbuz R. A prospective randomized comparison of micropercutaneous nephrolithotomy (Microperc) and retrograde intrarenal surgery (RIRS) for the management of lower pole kidney stones. World J Urol. 2017;35(11):1771–1776. doi:https://doi.org/10.1007/s00345-017-2058-9.
- Hatipoglu NK, Tepeler A, Buldu I, et al. Initial experience of micro-percutaneous nephrolithotomy in the treatment of renal calculi in 140 renal units. Urolithiasis. 2014;42(2):159–164. doi:https://doi.org/10.1007/s00240-013-0631-2.
- Armagan A, Tepeler A, Silay MS, et al. Micropercutaneous nephrolithotomy in the treatment of moderate-size renal calculi. J Endourol. 2013;27(2):177–181. doi:https://doi.org/10.1089/end.2012.0517.
- Sabnis RB, Ganesamoni R, Doshi A, Ganpule AP, Jagtap J, Desai MR. Micropercutaneous nephrolithotomy (microperc) vs retrograde intrarenal surgery for the management of small renal calculi: a randomized controlled trial. BJU Int. 2013;112(3):355–361. doi:https://doi.org/10.1111/bju.12164.
- Karatag T, Buldu I, Inan R, Istanbulluoglu MO. Is micropercutaneous nephrolithotomy technique really efficacicous for the treatment of moderate size renal calculi? Yes. Urol Int. 2015;95(1):9–14. doi:https://doi.org/10.1159/000368373.
- Penbegul N, Bodakci MN, Hatipoglu NK, et al. Microsheath for microperc: 14-gauge angiocath. J Endourol. 2013;27(7):835–839. doi:https://doi.org/10.1089/end.2012.0737.
- Sancaktutar AA, Adanur S, Ziypak T, Hatipoglu NK, et al. Micropercutaneous nephrolithotomy in the management of bilateral renal stones in a 7-month-old infant: the youngest case in the literature. Urol Int. 2016;96(2):238–240. doi:https://doi.org/10.1159/000360645.
- Thuroff JW. Words of wisdom: Re: Micropercutaneous nephrolithotomy (Microperc) vs retrograde intrarenal surgery for the management of small renal calculi: a randomized controlled trial. Eur Urol. 2014;65(3):668–669.
- Kaynar M, Sümer A, Şalvarcı A, Tekinarslan E, Cenker A, Istanbulluoğlu MO. Micropercutaneous nephrolithotomy (microperc) in a two-year-old with the ‘all-seeing needle’. Urol Int. 2013;91(2):239–241. doi:https://doi.org/10.1159/000345056.
- Karatag T, Buldu I, Kaynar M, Taskapu H, Tekinarslan E, Istanbulluoglu MO. Treatment of symptomatic lower pole stones of a kidney with partial nephrectomy using micropercutaneous nephrolithotomy technique. Case Rep Urol. 2015;2015:456714. doi:https://doi.org/10.1155/2015/456714.
- Bodakçi MN, Penbegul N, Dağgülli M, et al. Ultrasound-guided micropercutaneous nephrolithotomy in pediatric patients with kidney stones. Int J Urol. 2015;22(8):773–777. doi:https://doi.org/10.1111/iju.12817.
- Tok A, Akbulut F, Buldu I, et al. Comparison of microperc and mini-percutaneous nephrolithotomy for medium-sized lower calyx stones. Urolithiasis. 2016;44(2):155–159. doi:https://doi.org/10.1007/s00240-015-0804-2.
- Ganpule A, Chhabra JS, Kore V, Mishra S, Sabnis R, Desai M. Factors predicting outcomes of micropercutaneous nephrolithotomy: results from a large single-centre experience. BJU Int. 2016;117(3):478–483. doi:https://doi.org/10.1111/bju.13263.
- Tepeler A, Akman T, Silay MS, et al. Comparison of intrarenal pelvic pressure during micro-percutaneous nephrolithotomy and conventional percutaneous nephrolithotomy. Urolithiasis. 2014;42(3):275–279. doi:https://doi.org/10.1007/s00240-014-0646-3.
- Dağgülli M, Penbegül N, Dede O, Utanğaç MM. Bilateral microperc in a severe kyphoscoliosis. Turk J Urol. 2016;42(1):41–43. doi:https://doi.org/10.5152/tud.2015.03789.
- Cepeda M, Amón JH. MicroPerc: Fashion or reality. Arch Esp Urol. 2017;70(1):217–225.
- Ganpule AP, Chabra J, Desai MR. "Microperc" micropercutaneous nephrolithotomy: a review of the literature. Urolithiasis. 2018;46(1):107–114. doi:https://doi.org/10.1007/s00240-017-1021-y.
- Zhang JQ, Wang Y, Zhang JH, Zhang XD, Xing NZ. Retrospective analysis of ultrasound-guided flexible ureteroscopy in the management of calyceal Diverticular Calculi. Chinese Med J. 2016;129(17):2067–2073. doi:https://doi.org/10.4103/0366-6999.189060.
- McCahy P, Rzetelski-West K, Gleeson J. Complete stone clearance using a modified supine position: initial experience and comparison with prone percutaneous nephrolithotomy. J Endourol. 2013;27(6):705–709. doi:https://doi.org/10.1089/end.2012.0650.
- Tokas T, Skolarikos A, Herrmann TRW, Nagele U, Training and Research in Urological Surgery and Technology (T.R.U.S.T.)-Group. Pressure matters 2: intrarenal pressure ranges during upper-tract endourological procedures. World J Urol. 2019;37(1):133–142. doi:https://doi.org/10.1007/s00345-018-2379-3.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:https://doi.org/10.1097/01.sla.0000133083.54934.ae.
- Xue W, Pacik D, Boellaard W, CROES PCNL Study Group, et al. Management of single large nonstaghorn renal stones in the CROES PCNL global study. J Urol. 2012;187(4):1293–1297. doi:https://doi.org/10.1016/j.juro.2011.11.113.
- Rapoport D, Perks AE, Teichman JM. Ureteral access sheath use and stenting in ureteroscopy: effect on unplanned emergency room visits and cost. J Endourol. 2007;21(9):993–997. doi:https://doi.org/10.1089/end.2006.0236.
- Karatag T, Tepeler A, Buldu I, et al. Is micro-percutaneous nephrolithotomy surgery technically feasible and efficient under spinal anesthesia?Urolithiasis. 2015;43(3):249–254. doi:https://doi.org/10.1007/s00240-015-0752-x.
- Tepeler A, Armagan A, Sancaktutar AA, Silay MS, et al. The role of microperc in the treatment of symptomatic lower pole renal calculi. J Endourol. 2013;27(1):13–18. doi:https://doi.org/10.1089/end.2012.0422.
- Bagcioglu M, Demir A, Sulhan H, Karadag MA, Uslu M, Tekdogan UY. Comparison of flexible ureteroscopy and micropercutaneous nephrolithotomy in terms of cost-effectiveness: analysis of 111 procedures. Urolithiasis. 2016;44(4):339–344. doi:https://doi.org/10.1007/s00240-015-0828-7.
- Kiremit MC, Guven S, Sarica K, et al. Contemporary management of medium-sized (10-20 mm) renal stones: A Retrospective Multicenter Observational Study. J Endourol. 2015;29(7):838–843. doi:https://doi.org/10.1089/end.2014.0698.
- Karatag T, Buldu I, Kaynar M, Inan R, Istanbulluoglu MO. Does the presence of hydronephrosis have effects on micropercutaneous nephrolithotomy?Int Urol Nephrol. 2015;47(3):441–444. doi:https://doi.org/10.1007/s11255-014-0907-7.
- Tepeler A, Silay MS, Armagan A, et al. Laparoscopic-assisted "microperc" of a stone in a pelvic kidney of a 3-year-old girl. J Laparoendosc Adv Surg Tech A. 2013;23(2):174–176. doi:https://doi.org/10.1089/lap.2012.0270.
- Huusmann S, Nagele U, Herrmann TR, on behalf Training and Research in Urological Surgery and Technology (T.R.U.S.T.)-Group. Miniaturization of percutaneous nephrolithotomy Smaller, but better?Curr Opin Urol. 2017;27(2):161–169. doi:https://doi.org/10.1097/MOU.0000000000000375.