Abstract
Background: Granulomatous lobular mastitis (GLM) is a rare, benign, chronic inflammatory illness of the mammary gland with an unknown cause. Many scholars believe that the pathogenesis of GLM is mediated by autoimmunity. This article reviews the progress of the role of CD4+ T lymphocyte subsets in the development of GLM to explore potential therapeutic targets.
Methods: Original articles from inception to October 2021 were systematically searched by two members on PubMed and China National Knowledge Infrastructure.
Results: Current studies have confirmed the presence of disorders of several immune molecules in the serum and tissue microenvironment of GLM patients, including interleukin (IL) -2, IL-4, IL-6, and IL-10. This may be related to the dysregulation of Th1/Th2 and Th17/Treg balance.
Conclusions: Altered expression and the malfunctioning of Th, Treg, and associated cytokines may contribute to GLM pathogenesis. Immune molecules and immune-related pathways may be potential targets and breakthroughs for future GLM treatment.
Introduction
Granulomatous lobular mastitis (GLM) is an uncommon, nonmalignant, chronic breast illness of unknown etiology. It is commonly encountered in women with a reproductive history within 5 years. Patients with GLM principally experience the following symptoms: unilateral, firm, and discrete mass formation; pain; and local skin alterations such as erythema, abscess development, skin ulceration, and sinus or fistula formation [Citation1]. It is often difficult to differentiate GLM from breast carcinoma both clinically and radiographically. Histological examination is the cornerstone of a definitive diagnosis. Pathologically, GLM is defined as granulomatous inflammation that focuses on terminal mammary lobules and involves the infiltration of the afflicted region by a variety of inflammatory cells [Citation2]. Recently, the global prevalence of GLM has increased, whereas an optimal treatment remains ambiguous due to the unclear etiology and pathogenesis of the disease. Adverse effects of clinical treatment and the occurrence of protracted and recurring disease episodes are responsible for immense pain in patients with GLM. Therefore, it is an urgent problem to clarify the pathogenesis of GLM and find effective therapeutic targets.
The hypothesis that there is an immunological foundation for GLM has received much attention. When Kessler and Woollock [Citation3] originally described GLM in 1972, they reported that its histological appearance was comparable with that of autoimmune disorders such as granulomatous thyroiditis and granulomatous orchitis. Brown and Tang [Citation4] suggested that localized immune responses evoked by intraductal residual milk during lactation were crucial to the pathogenesis of GLM. According to certain studies, patients with GLM respond well to steroid therapy [Citation5, Citation6]. Hovanessian et al. [Citation7] suggested that the clinical response rate to steroid therapy in patients with GLM is greater than 70%. Additionally, immunosuppressive agents, such as methotrexate, have proven to be effective for treatment-resistant patients [Citation8]. Erythema nodosum is reportedly an extramammary symptom of GLM [Citation9, Citation10]. Autoantibody profiles of patients with GLM have been reported in the literature; however, relevant data are inconsistent. In an investigation of eight cases of GLM conducted by Ozel et al. [Citation11], anti-nuclear and anti-double-stranded DNA antibodies were found to be within normal range in two individuals, and six patients tested positive for rheumatoid factor. Conversely, in a study by Asoglu et al. [Citation12], eighteen patients tested negative for autoantibodies.
Although definitive evidence of a correlation between autoantibodies and GLM has not yet been produced, a consensus regarding the existence of immune disorders in GLM patients has been reached. Erhan et al. [Citation13] identified an inflammatory microenvironment consisting predominantly of T lymphocytes in individuals with GLM via immunohistochemical staining, a finding that was confirmed by Ren et al. [Citation14] and Huang et al. [Citation15], suggesting that a T lymphocyte-mediated immunological response underlies the pathophysiology of GLM. Subsequently, researchers have used enzyme-linked immunosorbent assays (ELISA) and immunohistochemical staining to examine serum- or tissue-specific expression levels of T lymphocyte-related cytokines in patients with GLM, some of which have correlated with GLM severity [Citation16–18]. The discoveries mentioned above reveal the possible etiopathogenesis of GLM. CD4+ T cells contribute to the body’s adaptive immune response. Therefore, naive T cells interact with homologous antigens on antigen-presenting cells before differentiating into a range of the following effector cell subsets: helper T cells (Th) and regulatory T cells (Treg). This review summarizes what is presently known regarding the role of CD4+ T cell subsets in the pathophysiology of GLM.
Differentiation of CD4+ T Cells
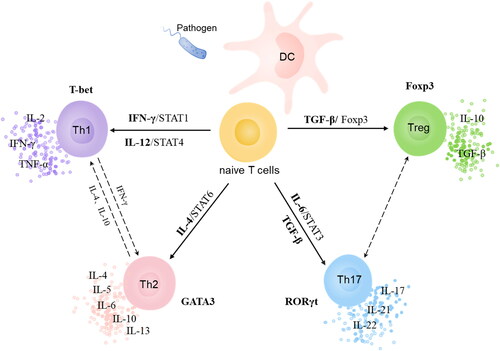
When CD4+ T lymphocytes are stimulated by interleukin 12 (IL-12) and interferon-gamma (IFN-γ), they primarily differentiate into T helper 1 cells (Th1) [Citation19] (). Innate immune cells secrete IL-12, whereas natural killer cells and T cells secrete IFN-γ. Through the signal transducer and activator of transcription (STAT) 4, STAT1, and T box transcription factor T-bet, they cause naive T cells to differentiate into Th1 [Citation20–22]. After IL-12 binds to its receptor, it phosphorylates STAT4 via the Janus kinase (JAK) pathway, which, in turn, promotes the expression of a downstream factor, T-bet, generating a large quantity of IFN-γ and establishing a positive feedback loop with a cascade-amplification effect. T-bet is the regulatory factor that is most vital to Th1 differentiation [Citation23]. Its biological activity depends on both IL-12/STAT4 and IFN-γ/STAT1 signaling pathways. Th1 secretes cytokines such as tumor necrosis factor-α (TNF-α), IFN-γ, and IL-2, which promote cellular immunity against infection by intracellular pathogens. Additionally, Th1 mediates inflammatory responses, delayed-type hypersensitivity, and some autoimmune diseases.
Differentiation of CD4+ T cells to T helper 2 cells (Th2) requires the action of IL-4, STAT6, and downstream GATA binding protein 3 (GATA3) (). IL-4 phosphorylates and activates the JAK pathway, which phosphorylates receptor-bound STAT6. Homodimers of phosphorylated STAT6 translocate from cytoplasm to nucleus, initiating Th2 differentiation [Citation20]. The transcription factor most important for Th2 differentiation is GATA3. The biological activity of GATA3 relies heavily on the IL-4/STAT6 signaling pathway. Existing literature suggests that IL-2 and its downstream signaling pathway (STAT5) are essential for differentiation of CD4+ T cells into Th2 [Citation24, Citation25]. Th2 secrete IL-4, IL-5, IL-6, IL-10, and IL-13 and contribute to humoral immunity against infection by worms and other extracellular pathogens.
Differentiation of CD4+ T cells to T helper 17 cells (Th17) and Treg is predominantly determined by transforming growth factor β (TGF-β) and IL-6 (). Naive T cells differentiate into Th17 when both factors are present. In the absence of IL-6, naive T cells exposed to TGF-β differentiate into Treg cells, which induce the expression of forkhead box protein 3 (Foxp3) and retinoic acid-related orphan receptor gamma (RORγt). IL-6 enhances RORγt expression and inhibits Foxp3 expression by activating STAT3. The transcription factors that are most important for differentiation to Th17 and Treg are STAT3 and Foxp3, respectively [Citation26]. Furthermore, RORγt is a Th17-specific transcription factor that may function in tandem with STAT3 to control IL-17 production [Citation24]. IL-21 and IL-22 can be autocrinally secreted by Th17, acting as positive feedback mechanism for STAT3. Previously, it was reported that the expression of IL-6 and pSTAT3 proteins in GLM foci was higher than that of adjacent normal breast tissue. Further, IL-6 expression was correlated with levels of STAT3 phosphorylation [Citation17]. Th17 mainly generates Th17 cytokines including IL-17, IL-21, and IL-22, facilitating its participation in the inflammatory response against extracellular bacteria and fungi. Treg has an immunosuppressive impact, reducing autoimmunity and tissue damage caused by over- or under-activation of the immune system. Treg primarily maintains immune tolerance in the following ways: (a) secreting IL-10, TGF-β, and other inhibitory cytokines; (b) detrimentally affecting immunity by attaching to surface CD25 and IL-2 with high affinity; and (c) directly killing immune cells by generating granzyme and perforin [Citation27].
Th1/Th2 and GLM
Th1/Th2 Balance
Cross-regulatory of cytokines and signaling pathways occurs in Th1 and Th2 differentiation. For example, GATA3 can downregulate STAT4 expression, thereby inhibiting differentiation to Th1. STAT5 can promote Th2 differentiation and inhibit T-bet expression. Furthermore, IL-4 and IL-10 downregulate differentiation into Th1 by inhibiting IL-12 secretion [Citation19]. In contrast, Th1 cytokines can block Th2 development via a feedforward mechanism. IFN-γ increases the level of T-bet, which in turn induces the expression of Runt-related transcription factor 3 (Runx3). Runx3 expression boosts IFN-γ production while suppressing the expression of IL-4 [Citation24, Citation28]. Th1 and Th2 are at dynamic equilibrium under physiological conditions. However, pathological states may cause a subgroup to dominate, a phenomenon known as Th1/Th2 drift. This may mediate the onset and progression of conditions such as rheumatoid arthritis and systemic lupus erythematosus [Citation29, Citation30].
Xia and Chen [Citation31] studied the differences in serum levels of IFN-γ and IL-4 before and after liver-discharging and heat-clearing therapy (a therapy used in traditional Chinese medicine). Their work revealed that the IFN-γ expression levels in those with GLM were lower than those in controls. Further, IL-4 levels were significantly reduced after treatment. In addition, IFN-γ and IFN/IL-4 ratios tended to increase after treatment. The study suggested that a Th1/Th2 drift toward Th2 dominance may underlie the pathogenesis of non-lactating mastitis. After treatment, the balance between Th1 and Th2 was gradually restored as disease symptoms resolved. Although no further analyses of the different pathological types were performed in the above literature, these results are suggestive that Th1/Th2 drift might mediate the occurrence and development of GLM.
Yang [Citation16] found that IL-2 expression levels increased significantly in GLM foci in the mass stage and that IL-4 was overexpressed in foci after skin ulceration. Throughout the disease course, expression levels of IL-2 tended to decrease, while those of IL-4 tended to increase. This phenomenon was confirmed in a recent study (that reported a correlation between the expression levels of IL-2 and IL-4 within 60 GLM foci) [Citation32], which suggests that Th1/Th2 drift may mediate the chronicity of GLM. Additionally, Liu [Citation20] found that Th1-mediated cytokine expression levels (IFN-γ and IL-12A) in GLM foci were significantly higher than those in benign, noninfectious breast disease, while Th2-mediated cytokine expression levels (IL-4 and IL-10) did not differ significantly. Therefore, the author concluded that GLM was related to Th1-mediated immune response. However, Liu did not conduct a subgroup analysis of disease stages and failed to assess dynamic changes in Th1/Th2 drift. Wang [Citation33] reported evidence that before treatment with traditional Chinese medicine combined with methylprednisolone, the content of IL-6 and IL-1β in GLM foci in the mass stage was higher, while that of IFN-γ was lower. After treatment, IL-6 and IL-1β in GLM foci decreased, while the expression of IFN-γ increased, suggesting that Th2 may be relatively dominant during the pathogenesis of GLM and, thus, contributes to its chronicity. Furthermore, the balance between Th1 and Th2 cytokines tends to be restored after effective treatment [Citation34].
TNF-α
TNF-α, formerly known as cachectin, is a pro-inflammatory cytokine generated by macrophages, natural killer and T cells, and others. Its biological function is complicated. TNF-α participates in normal immune functioning by mediating cell death versus survival signaling and plays a role in immune stimulation, anti-infection and anti-tumor functions, and sleep regulation [Citation35, Citation36]. The ability of TNF-α to induce mammary epithelial cell apoptosis and necroptosis (programmed necrosis) in mice has been shown [Citation37]. TNF-α is critical for granuloma formation, which closely correlates with its chemotactic effect on T cells [Citation38, Citation39]. Additionally, it promotes the production of cytokines such as IL-2, IL-6, and IL-8 to aggravate local inflammation [Citation38, Citation40]. Therefore, elevated serum TNF-α levels are often indicative of increased levels of inflammation. A study by Koksal et al. [Citation18] revealed no great difference in serum levels of TNF-α between patients with and without GLM, a finding that failed to confirm a correlation between TNF-α levels and the course of GLM. Conversely, Liu et al. [Citation41] observed that overexpression of TNF-α and its receptor, p55, occurred in GLM foci in the mass stage rather than the abscess or ulcer stage. Further, TNF-α levels were negatively correlated with the progression of disease. T lymphocytes can produce TNF-α, which in turn acts on T lymphocytes to induce the expression of other cytokines capable of collectively contributing to disease development.
IL-6
IL-6 is a glycoprotein with a molecular weight of approximately 21–28 kDa. After IL-6 binds to its receptor, the complex binds to gp130 and initiates the JAK/STAT, extracellular signal regulated kinase, and phosphatidylinositol 3 kinase (PI3K) signaling pathways [Citation42]. Multiple studies have demonstrated that serum levels of IL-6 are greater in patients with GLM than in healthy individuals [Citation17, Citation43]. Furthermore, IL-6 levels are elevated in severe cases compared with in those non-severe cases, and decreases are associated with disease remission. Therefore, these findings indicate that IL-6 is a biomarker of GLM severity [Citation2].
Hyperprolactinemia caused by pituitary tumors or antipsychotic drugs is associated with the pathogenesis of GLM. In a study by Yuan [Citation44], levels of prolactin (PRL) increased in those with GLM compared with those in healthy individuals. Hyperprolactinemia triggers a Th1/Th2-driven autoimmune response. Pro-inflammatory cytokines, including IL-1, IL-2, and IL-6, enhance the synthesis of PRL [Citation45], thereby triggering the nuclear factor-κB (NF-κB) signaling pathway. This results in the production of pro-inflammatory mediators such as IL-1, IL-6, TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) [Citation46, Citation47]. These findings may indicate a possible mechanism for granuloma formation in the breasts of patients with GLM.
IL-6 likely drives the onset and progression of GLM and is a useful biomarker of disease severity. At present, a variety of drugs targeting IL-6 and its receptor such as tocilizumab, siltuximab, and sarilumab have been approved for the treatment of diseases such as rheumatoid arthritis and idiopathic angiofollicular lymph node hyperplasia [Citation48]. Tocilizumab has also been included in the immunotherapy regimen used to treat patients with severe and critical coronavirus disease 19 [Citation49]. Further studies are needed to confirm whether a link exists between IL-6 and GLM. Therapies that target IL-6 and its signaling pathways have the potential to revolutionize GLM treatment.
Th17/Treg and GLM
The balance between Th17 and Treg is maintained via a mutually antagonistic mechanism that maintains immune stability. Th17 predominates when TGF-β and IL-6 are both elevated. Many illnesses are linked to Th17/Treg axis imbalance. For instance, the predominance of Treg in a tumor increases the risk of metastasis and recurrence [Citation50]. Treg expression is associated with reduction in the expression levels of Foxp3. This results in differentiation to Th17, a process that contributes to the pathophysiology of rheumatoid arthrtitis [Citation51].
IL-17 is the key effector secreted by Th17. By activating the mitogen-activated protein kinase and NF-κB pathways, it stimulates the production of IL-6, IL-8, GM-CSF, and cell adhesion molecule-1 by T cells and epithelial cells, resulting in strong pro-inflammatory effects [Citation52]. In 1989, Fiorentino et al. [Citation53] found that Th2 secrete IL-10, which suppresses Th1. Thereafter, it was revealed that IL-10 may be produced by a variety of CD4+ T cells [Citation54]. Inhibition of inflammation by Treg is mediated by IL-10. By limiting the capacity of macrophages to present antigens and generate cytokines, IL-10 prevents Th1 from secreting IFN-γ.
Although a correlation between Th17/Treg-related cytokines and the pathogenesis of GLM has not yet been clarified, existing research shows that GLM-mediated inflammation is likely influenced by an imbalance in Th17/Treg. No obvious difference in the levels of expression of IL-17A was observed when 26 GLM foci and healthy controls were compared via immunohistochemistry performed by Liu [Citation20]. Koksal et al. [Citation18] found that the levels of IL-17 and IL-8 in the peripheral blood of patients with GLM were considerably greater than those in the peripheral blood of healthy individuals. Furthermore, they found that levels of IL-10 expression in serum were elevated in patients with GLM, especially those whose disease was in remission. This finding suggested that IL-10 may be produced to control GLM by limiting the release of pro-inflammatory factors. In a different investigation, the researchers reported that levels of IL-22 and IL-23 were upregulated in patients with GLM. However, no change in IL-17 was observed [Citation55].
The immune-regulatory function of Treg affects immune-mediated disorders such as transplantation, autoimmunity, and auto-inflammation. Therefore, Treg is a viable therapeutic target for GLM treatment [Citation56]. The transcription factor Foxp3 is specifically expressed in Treg cells. Demethylation of Foxp3 is directly related to the inhibitory activity of Treg. Foxp3+Treg expresses CD25 and CTLA-4 on the cell surface, which can contribute to a variety of immune diseases, if irregularly expressed. Flow cytometry was performed by Ucaryilmaz et al. [Citation55, Citation57] to show that Foxp3 expression and the proportion of CD3+CD4+CD45RA-Foxp3lowTreg (non-suppressive Treg, non-Treg) in the peripheral blood of patients with GLM was lower than that in the blood of healthy persons. Patients with active GLM had greater levels of CD3+CD4+CD45RA-Foxp3+ Treg compared with those in remission, and additional examination of Treg subsets revealed that the levels of CD3+CD4+CD45RA-Foxp3highTreg (activated Treg [aTreg]) and non-Treg were higher in patients with active GLM compared with those with inactive GLM. As seen in the above research, it was confirmed that there are changes in the expression levels of different Treg subsets in patients with GLM. According to the findings of previous studies, there were variations in the levels of distinct Treg subsets in patients with GLM.
Foxp3 regulates Treg metabolism and activity through the PI3K/protein kinase B (PKB/AKT)/mammalian target of rapamycin (mTOR) pathway, which facilitates the growth and metastasis of breast carcinoma by promoting cell proliferation, inhibiting cell apoptosis, and contributing to neovascularization [Citation58]. According to Zhao et al. [Citation43], ELISA revealed a considerable increase in the expression levels of PI3K, AKT, and mTOR in the serum of patients with GLM, when compared with those in healthy controls. This robust positive connection implies that aberrant expression levels and the malfunctioning of Treg, particularly Treg cell subgroups, may be implicated in the pathogenesis GLM, although the mechanism by which this occurs requires additional exploration.
MicroRNAs are short, endogenous, non-coding RNA molecules with lengths approximately 20–25 nucleotides. MicroRNA can suppress the translation of messenger RNA or induce its cleavage for gene regulation. This type of regulation may be used to influence immune cell proliferation and differentiation, and alter the incidence and progression of numerous autoimmune disorders [Citation59]. MiR-155 is found within the third exon of a non-coding transcribed region of human chromosome 21 and is strongly expressed by activated B and T cells and monocytes/macrophages. It continuously enhances phosphorylation of STAT3 by blocking suppressor of cytokine signaling 1 and upregulating RORγt expression levels, thereby promoting Th17 differentiation [Citation60]. MiR-21 can suppress Treg expression via the TGF-1/SMAD signaling pathway, reducing the level of Foxp3 expression and inducing a Th17/Treg imbalance [Citation59]. Serum levels of miR-155 and miR-21 in patients with GLM have been shown to be substantially different from those in healthy controls [Citation61], indicating that microRNA may affect the Th17/Treg balance in GLM.
Conclusion
Immune disturbances are thought to contribute to the pathophysiology of GLM, even though the etiology and pathogenesis of GLM remains a mystery. Many researchers have examined the immunological microenvironment of foci or sera of patients with GLM. This work has demonstrated that altered expression and the malfunctioning of Th, Treg, and associated cytokines may contribute to GLM pathogenesis. Current work has limitations. Foremost is the limited quantity of published papers on this topic and the narrow sample size. In addition, the stage of GLM is an important factor to consider in studies on this topic. Further prospective study with a large sample size will be needed if we wish to determine the precise mechanism by which immune response contributes to the pathophysiology of GLM. Targeted therapy, based on CD4+ T cell subsets, has the potential of being useful in the treatment of GLM, and immunomodulatory therapy may provide a new avenue for tailored GLM treatment.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Velidedeoglu M, Umman V, Kilic F, et al. Idiopathic granulomatous mastitis: introducing a diagnostic algorithm based on 5 years of follow-up of 152 cases from Turkey and a review of the literature. Surg Today. 2022;52(4):668–680. doi:10.1007/s00595-021-02367-6.
- Huang YM, Lo C, Cheng CF, Lu CH, Hsieh SC, Li KJ. Serum C-reactive protein and interleukin-6 levels as biomarkers for disease severity and clinical outcomes in patients with idiopathic granulomatous mastitis. JCM. 2021;10(10):2077. doi:10.3390/jcm10102077.
- Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol. 1972;58(6):642–646. doi:10.1093/ajcp/58.6.642.
- Brown KL, Tang PH. Postlactational tumoral granulomatous mastitis: a localized immune phenomenon. Am J Surg. 1979;138(2):326–329. doi:10.1016/0002-9610(79)90397-0.
- Karanlik H, Ozgur I, Simsek S, et al. Can steroids plus surgery become a first-line treatment of idiopathic granulomatous mastitis. Breast Care (Basel). 2014;9(5):338–342. doi:10.1159/000366437.
- Mizrakli T, Velidedeoglu M, Yemisen M, et al. Corticosteroid treatment in the management of idiopathic granulomatous mastitis to avoid unnecessary surgery. Surg Today. 2015;45(4):457–465. doi:10.1007/s00595-014-0966-5.
- Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193(2):574–581. doi:10.2214/AJR.08.1528.
- Papila Kundaktepe B, Velidedeoğlu M, Mete B. The effect of methotrexate monotherapy on treatment-resistant idiopathic granulomatous mastitis patients. Surgeon. 2021;20(3):e13–e19. doi:10.1016/j.surge.2021.03.001.
- Luo W, Xu B, Wang L, et al. Clinical characteristics and predictive factors of erythema nodosum in granulomatous lobular mastitis. Aust J Dermatology. 2021;62(3):342–346. doi:10.1111/ajd.13640.
- Parperis K, Achilleos S, Costi E, Vardas M. Granulomatous mastitis, erythema nodosum and arthritis syndrome: case-based review. Rheumatol Int. 2021;41(6):1175–1181. doi:10.1007/s00296-021-04820-8.
- Ozel L, Unal A, Unal E, et al. Granulomatous mastitis: is it an autoimmune disease? Diagnostic and therapeutic dilemmas. Surg Today. 2012;42(8):729–733. doi:10.1007/s00595-011-0046-z.
- Asoglu O, Ozmen V, Karanlik H, et al. Feasibility of surgical management in patients with granulomatous mastitis. Breast J. 2005;11(2):108–114. doi:10.1111/j.1075-122X.2005.21576.x.
- Erhan Y, Veral A, Kara E, et al. A clinicopthologic study of a rare clinical entity mimicking breast carcinoma: idiopathic granulomatous mastitis. Breast. 2000;9(1):52–56. doi:10.1054/brst.1999.0072.
- Ren XC, Huang YP, Wu LJ, Qiu LL, Zhao ZQ. Clinical pathology and cytological analysis of granulomatous lobular mastitis. Zhejiang Clinical Medical Journal. 2004;6:905–906. doi:10.3969/j.issn.1008-7664.2004.10.060. (In Chinese)
- Huang B, Han L, Zhao YY. The clinical pathobiology feature of granulomatous lobular mastitis. J Mod Oncol. 2007;15:334–335. doi:10.3969/j.issn.1672-4992.2007.03.014. (In Chinese)
- Yang XH. The relative study of expression of IL-2-IL-4 in the tissue of GM [Thesis]. [Shandong, China]: Shandong University of Traditional Chinese Medicine; 2010.
- Diao Y, Shan CY, Zhao Y, et al. Role of IL-6/STAT3 signaling pathway in the granulomatous mastitis. Prog Mod Biomed. 2018;18:4486–4488. doi:10.13241/j.cnki.pmb.2018.23.020. (In Chinese)
- Koksal H, Vatansev H, Artac H, Kadoglou N. The clinical value of interleukins-8, -10, and -17 in idiopathic granulomatous mastitis. Clin Rheumatol. 2020;39(5):1671–1677. doi:10.1007/s10067-020-04925-8.
- Zhang Y, Zhang Y, Gu W, Sun B. TH1/TH2 cell differentiation and molecular signals. Adv Exp Med Biol. 2014;841:15–44. doi:10.1007/978-94-017-9487-9_2.
- Liu L. The investigation of the etiology and antituberculous therapy for non-puerperal matitis [Dissertation]. [Shandong, China]: Shandong University; 2017.
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi:10.1016/S0092-8674(00)80702-3.
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi:10.1016/j.immuni.2009.05.001.
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. Pillars article: a novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000. 100: 655–669. J Immunol. 2015;194(7):2961–2975. doi:10.1016/S0092-8674(00)80702-3.
- Schmitt N, Ueno H. Regulation of human helper T cell subset differentiation by cytokines. Curr Opin Immunol. 2015;34:130–136. doi:10.1016/j.coi.2015.03.007.
- Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14(3):205–215. doi:10.1016/S1074-7613(01)00103-0.
- Barbi J, Pardoll D, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259(1):115–139. doi:10.1111/imr.12172.
- Shevyrev D, Tereshchenko V. Treg heterogeneity, function, and homeostasis. Front Immunol. 2019;10:3100. doi:10.3389/fimmu.2019.03100.
- Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate IFNG and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8(2):145–153. doi:10.1038/ni1424.
- Bao Y, Peng J, Yang KL, et al. Therapeutic effects of Chinese medicine Di-Long (Pheretima vulgaris) on rheumatoid arthritis through inhibiting NF-κB activation and regulating Th1/Th2 balance. Biomed Pharmacother. 2022;147:112643. doi:10.1016/j.biopha.2022.112643.
- Muhammad Yusoff F, Wong KK, Mohd Redzwan N. Mohd Redzwan N. Th1, Th2, and Th17 cytokines in systemic lupus erythematosus. Autoimmunity. 2020;53(1):8–20. doi:10.1080/08916934.2019.1693545.
- Xia Y, Chen H. The investigation of liver-discharging and heat-clearing therapy to regulate the balance of Th1/Th2 cells in non-lactation mastitis. Medical Information. 2012;25:103–104. doi:10.3969/j.issn.1006-1959.2012.02.096. (In Chinese)
- Yang XH, Shi Y, Shi H, Yang TZ. Expression and clinical significance of IL-2 and IL-4 in pathological tissues of granulomatous mastitis. Chin Rem Clin. 2021;21:908–910. doi:10.11655/zgywylc2021.06.003. (In Chinese)
- Wang YY. To Investigate the Expression of IL-6, IL-1β, and IFN-Γ in the Effect of Yiqiheying on the Immune Imbalance in the Mass Stage [Thesis]. [Shandong, China]: Shandong University of Traditional Chinese Medicin; 2020.
- Ding XB. Efficacy of lesions resection and conservative antibiotics in granulomatous mastitis and effect on serum interleukin-2 and interleukin-4 levels. Matern Child Health Care China. 2017;32(21):5290–5291. doi:10.7620/zgfybj.j.issn.1001-4411.2017.21.38. (In Chinese)
- Idriss HT, Naismith JT. TNFα and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech. 2000;50(3):184–195. doi:10.1002/1097-0029(20000801)50:3 < 184::AID-JEMT2 > 3.0.CO;2-H.
- Ruterbusch M, Pruner KB, Shehata L, Pepper M. In vivo CD4(+) T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev Immunol. 2020;38:705–725. doi:10.1146/annurev-immunol-103019-085803.
- Yang C, Yang M, Wang G, Li Y, Lei Z, Li L. TNF-α induced apoptosis and necroptosis in mouse mammary epithelial cells. J Nanjing Agric Univ. 2022;45:1–10. doi:10.7685/jnau.202102008. (In Chinese)
- Silva DAAd, Silva MVd, Barros CCO, et al. TNF-α blockade impairs in vitro tuberculous granuloma formation and down modulate Th1, Th17 and Treg cytokines. PLoS ONE. 2018;13(3):e0194430. doi:10.1371/journal.pone.0194430.
- Amber KT, Bloom R, Mrowietz U, Hertl M. TNF-α: a treatment target or cause of sarcoidosis. J Eur Acad Dermatol Venereol. 2015;29(11):2104–2111. doi:10.1111/jdv.13246.
- Zhang CJ, Kong C. Immunological studies about the progress of non-lactating mastitis. J Dalian Med Univ. 2014;36:307–313. doi:10.11724/jdmu.2014.04.01. (In Chinese)
- Liu X, Wang N, Li F, Song A, Zhang L. Expressions and clinical significance of immune-related factors in different TCM syndrome patterns of granulomatous mastitis. Acta Chin Med Pharmacol. 2020;48:23–28. doi:10.19664/j.cnki.1002-2392.200063. (In Chinese)
- Guo Y, Wang B, Wang T, et al. Biological characteristics of IL-6 and related intestinal diseases. Int J Biol Sci. 2021;17(1):204–219. doi:10.7150/ijbs.51362.
- Zhao Y, Wang X, Zhang S, Kang HF, Guan HT, et al. Study of PI3K/AKT/mTOR pathway and immunoglobulin in the pathogenesis of granulomatous lobular mastitis. China Med Her. 2018;15:8–10. doi:CNKI:SUN:YYCY.0.2018-30-003. (In Chinese)
- Yuan S, Li T, Tang R, Zhang S, Li C, Zhang W. Clinical study of inflammation causing effect of prolactin on granulomatous lobular mastitis patients. Chongqing Med J. 2021;50:30–33. doi:10.3969/j.issn.1671-8348.2021.01.007. (InChinese)
- Vieira Borba V, Shoenfeld Y. Prolactin, autoimmunity, and motherhood: when should women avoid breastfeeding. Clin Rheumatol. 2019;38(5):1263–1270. doi:10.1007/s10067-018-04415-y.
- Nikolaev A, Blake CN, Carlson DL. Association between hyperprolactinemia and granulomatous mastitis. Breast J. 2016;22(2):224–231. doi:10.1111/tbj.12552.
- Borba VV, Zandman-Goddard G, Shoenfeld Y. Prolactin and autoimmunity. Front Immunol. 2018;9:73. doi:10.3389/fimmu.2018.00073.
- Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16(6):335–345. doi:10.1038/s41584-020-0419-z.
- Gupta S, Wang W, Hayek SS, STOP-COVID Investigators, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41–51. doi:10.1001/jamainternmed.2020.6252.
- Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target. Cancer Sci. 2019;110(7):2080–2089. doi:10.1111/cas.14069.
- Komatsu N, Okamoto K, Sawa S, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20(1):62–68. doi:10.1038/nm.3432.
- Amatya N, Garg AV, Gaffen SL. IL-17 signaling: the Yin and the Yang. Trends Immunol. 2017;38(5):310–322. doi:10.1016/j.it.2017.01.006.
- Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170(6):2081–2095. doi:10.1084/jem.170.6.2081.
- Saraiva M, Vieira P, Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020;217(1):e20190418. doi:10.1084/jem.20190418.
- Saydam M, Yilmaz KB, Sahin M, et al. New findings on autoimmune etiology of idiopathic granulomatous mastitis: serum IL-17, IL-22 and IL-23 levels of patients. J Invest Surg. 2021;34(9):993–997. doi:10.1080/08941939.2020.1725190.
- Hoeppli RE, Wu D, Cook L, Levings MK. The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front Immunol. 2015;6:61. doi:10.3389/fimmu.2015.00061.
- Ucaryilmaz H, Koksal H, Emsen A, Kadoglou N, Dixon JM, Artac H. The role of regulatory T and B cells in the etiopathogenesis of idiopathic granulomatous mastitis. Immunol Invest. 2022;51(2):357–367. doi:10.1080/08820139.2020.1832114.
- Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016;35(4):515–524. doi:10.1007/s10555-016-9637-x.
- Li S, Fan Q, He S, Tang T, Liao Y, Xie J. MicroRNA-21 negatively regulates Treg cells through a TGF-β1/Smad-independent pathway in patients with coronary heart disease. Cell Physiol Biochem. 2015;37(3):866–878. doi:10.1159/000430214.
- Chen L, Gao D, Shao Z, Zheng Q, Yu Q. miR-155 indicates the fate of CD4(+) T cells. Immunol Lett. 2020;224:40–49. doi:10.1016/j.imlet.2020.05.003.
- Aksan H, Kundaktepe BP, Sayili U, et al. Circulating miR-155, let-7c, miR-21, and PTEN levels in differential diagnosis and prognosis of idiopathic granulomatous mastitis and breast cancer. Biofactors. 2020;46(6):955–962. doi:10.1002/biof.1676.