Abstract
Background
To determine the effect of 131I treatment on postoperative parathyroid function and the timing of recovery of parathyroid function (RPF) in patients with protracted hypoPT.
Methods
260 patients with papillary thyroid cancer (PTC) were retrospectively analyzed, including 166 patients treated with radioactive iodine-131 (131I) classified into the 131I group and 94 patients without 131I treatment classified into the control group. Data on clinicopathological characteristics, demographics, dose and interval time of 131I treatment, number of parathyroid glands remaining in situ (PGRIS), occurrence of hypoPT, duration of RPF, preoperative and postoperative levels of Ca and PTH were collected.
Results
The patients in the 131I group showed a higher persistent hypoPT rate than those in the control group (p = 0). The PGRIS and total number of PG were significantly higher in patients who recovered from protracted HypoPT (p = 0.02; p = 0.03). PGRIS and 131I treatment [1 ∼ 2 VS 0, p = 0.03, OR 3.19; 3 ∼ 4 VS 0, p = 0.02, OR3.62; p = 0.02, OR 1.98, respectively] were independent factors influencing postoperative persistent hypoPT. The timing of RPF differed significantly for patients in the control group compared to those in the 131I group [p = 0.00].
Conclusions
We found that 131I treatment significantly prolonged the RPF of patients with protracted hypoPT and caused late RPF (even beyond 12 months). The diagnosis of “permanent” hypoPT should be cautiously made at least 12 months after surgery, especially in patients who receive 131I treatment.
Introduction
Hypoparathyroidism (hypoPT) after thyroidectomy is considered the most common complication of total thyroidectomy, caused by intraoperative injury to the parathyroid glands by compromising vascular supply, thermic injury, or inadvertent excision [Citation1]. Accidental resection, thermal injury, and devascularization of the parathyroid glands are generally considered to be the common causes of HypoPT after thyroid surgery [Citation2].
Radioactive iodine-131 (131I) cleared postoperative residual thyroid tissue, and it is considered an effective, safe, and simple treatment for patients with differentiated thyroid carcinoma (DTC). However, previous reports suggest that the parathyroid may also suffer from radiation damage, leading to hypoPT and disorders of related electrolytes [Citation3,Citation4].
Approximately 90% of adult patients, who develop parathyroid failure immediately after total thyroidectomy, recover within 4–6 weeks after surgery (transient HypoPT). The rest develop protracted HypoPT characterized by low serum PTH levels and the need for continued treatment at least one month after surgery. In adults, HypoPT for more than six months is usually diagnosed as permanent hypoPT, but it is debated [Citation5,Citation6].
Some anecdotal reports have shown that recovery of parathyroid function (RPF) could occur six months or even more than 12 months after thyroidectomy [Citation7–14]. The most relevant factors that lead to late RPF (>6 months) include the number of functioning parathyroid glands that remain in situ and parathyroid autotransplantation.
Some studies suggested that HypoPT could also be related to 131I treatment [Citation3,Citation4, Citation15,Citation16]; however, most of those studies had a limited sample size and/or a short observation time after surgery. Furthermore, it is not yet determined whether 131I treatment influences the duration of RPF after total thyroidectomy.
Given that 131I treatment could adversely affect parathyroid function and could prolong the duration of RPF in patients with protracted hypoPT, this study aimed to determine the effect of 131I treatment on parathyroid function, with special emphasis on 131I treatment and the time required for RPF in patients with protracted hypoPT.
Materials and methods
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was carried out in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was waived by the local Ethics Committee of West China Hospital, Sichuan University, in view of the retrospective nature of the study, and all procedures performed were part of routine care.
Patients and data collection
This was a retrospective study of consecutive patients who were diagnosed with PTC and underwent thyroid surgery from January 2013 to June 2018 in the Thyroid and Parathyroid Surgery, Center of Thyroid & Parathyroid Surgery, West China Hospital, Sichuan University.
A total of 260 patients diagnosed with protracted hypoPT were included in this study; 166 patients who received 131I treatment were categorized into the 131I group, and 94 patients were categorized into the control group.
Clinicopathological characteristics, treatment with 131I, dose and interval time of 131I treatment, the number of parathyroid glands remaining in situ (PGRIS), the occurrence of HypoPT, the duration of RPF, surgical methods of the primary tumor and lymph node metastasis, and preoperative and postoperative levels of Ca and PTH were retrospectively collected. Patients who underwent secondary surgery, diagnosed with other types of malignancies or secondary thyroid carcinoma, and with incomplete data were excluded from the study.
Definition
The definitions of T stages patients and treatment of these were classified according to the 2017 World Health Organization (WHO) Classification of Tumors of Endocrine Organs. The extrathyroidal extension (ETE) detected microscopically only in the histological examination was excluded from stage T disease, tumor >4 cm but limited to the thyroid was designated as T3a, and gross ETE, invading only the strap muscles from a tumor of any size, was designated as T3b [Citation17].
Our study defined postsurgical HypoPT as hypocalcemia and inappropriately low PTH levels. Hypocalcemia was defined as the albumin-adjusted serum total calcium level below the lower limit of the reference interval in our hospital (2.1–2.7 pmol/L). Parathyroid failure is defined as postoperative hypocalcemia within 24 h of surgery and the need for calcium/vitamin D replacement therapy at the time of hospital discharge [Citation15]. Patients with hypocalcemia and inappropriate normal PTH levels were also diagnosed with hypoPT because a specific cutoff limit for PTH levels in the presence of hypoPT is currently not defined [Citation5].
Recovery of parathyroid function (RPF) was defined as a normal range of PTH levels without any symptoms of hypocalcemia or the need for replacement therapy. If RPF was within 4–6 weeks after surgery, the condition was defined as transient HypoPT. Protracted hypoPT was characterized by low serum PTH levels and the need for continued treatment [Citation5]. HypoPT in adults that lasted more than six months was classified as permanent hypoPT.
Considering that parathyroid function recovered after six months in some patients, in this study, we defined hypoPT lasting six months as permanent HypoPT and hypoPT that did not recover at the last follow-up (duration >6 months) as persistent HypoPT.
Surgical and RAI therapy procedures
Total thyroidectomy and at least unilateral central neck dissection (CND) were performed on all patients, irrespective of their groups. CND operations are limited laterally by the common carotid artery and internally by the trachea. The upper and lower boundaries are the hyoid surface and the innominate vessels, including the prelaryngeal, pretracheal, and paratracheal nodal basins. Therapeutic neck dissection was performed only in patients with lymph node metastasis (LNM), confirmed by preoperative fine-needle aspiration biopsy (FNAB) or by examining intraoperative pathologic sections. Some patients with high-risk factors who were suspected of having metastatic lymph nodes in the neck area by preoperative ultrasound or who required more thorough lymph node dissection underwent prophylactic lymph node dissection in the central region [Citation7]. Interoperative nerve monitoring (IONM) and carbon nanoparticles were used to protect the recurrent laryngeal nerve and parathyroid gland.
Carbon nanoparticle (CN) was used to guide cervical node dissection and protect the parathyroid gland. After the front part of the thyroid gland was exposed, CN suspension was slowly injected into the gland using a skin test needle at 1 or 2 selected points in the thyroid (0.1 mL each point). Then, the surgical resection could be started 10 minutes later. The thyroid gland and surrounding lymph nodes were stained black, whereas the parathyroid glands were not. Parathyroid glands devascularized in the surgical field or resected accidentally (regardless of the patients receiving CND or LND) were cut into 1 mm fragments and auto-transplanted to the contralateral sternocleidomastoid muscle after interoperative pathological confirmation.
The risk stratification of the included patients with PTC was used to determine if they would receive radioactive 131I treatment [Citation7]. High-risk and some middle-risk groups require 131I treatment, whereas the low-risk group does not. The final treatment of radioactive 131I is decided by the nuclear medicine specialist. Thyroxine withdrawal has been carried out for 3 weeks before 131I treatment for patients assessed to receive 131I treatment, and thyroxine withdrawal Thyrotropin (TSH) must be greater than 30 mU/L before 131I therapy. Thyroid bed iodide uptake rate was detected before treatment.
The dose of 131I was determined according to the ATA risk stratification. Patients received prednisone (10 mg tid.), macrogol (40 mg qd.), and esomeprazole magnesium enteric-coated tablets (20 mg qd.) orally for 4 days after 131I therapy. Oral administration of levothyroxine was reinstated in all patients on the day after treatment with 131I.
Perioperative management and follow-up
Preoperative and postoperative (24 h after surgery) serum PTH and albumin-adjusted serum total calcium levels were tested in all patients. Prophylactic calcium supplementation (calcium gluconate 4 g, intravenous drip) was administered to all patients regularly after surgery, extraoral administration of 1.5–3 g/day of calcium carbonate and 0.5–1.5 mg/day of calcitriol was performed for patients with abnormally low calcium and/or PTH levels tested 24 h after the operation (normal range: PTH, 1.6–6.9 pmol/L; calcium: 2.1–2.7 pmol/L).
All patients were evaluated by laboratory examinations in the outpatient department in the first month of surgery. The patients whose parathyroid function did not recover in the first month required replacement therapy and were diagnosed with protracted HypoPT. Patients with protracted HypoPT were followed in the outpatient department until their parathyroid function recovered or persistent HypoPT was diagnosed. The follow-up time of the patients with protracted hypoPT ranged from 2 to 39 postoperative months.
Statistical analysis
We used SPSS 26.0 to process the data, and the p-values of <0.05 were considered statistically significant. Categorical variables were presented as absolute, and Pearson’s χ2-test was performed to examine the differences between categorical variables. Continuous variables were presented as median values if they showed a non-normal distribution, and those that showed a normal distribution were expressed as the mean ± standard deviation. The differences among the continuous variables were examined using the Mann-Whitney U test. Multivariate logistic regression analyses were performed to identify the risk factors for persistent HypoPT. The predictive timing to RPF was estimated by the Kaplan-Meier method in patients in the 131I group vs. the control group, N0 vs. N1a vs. N1b, PGRIS 0 vs. PGRIS 0 vs. PGRIS 1 ∼ 2 PGRIS 3 ∼ 4, and any difference in the predictive timing to RPF was evaluated using the log-rank test. SPSS software (version 26) was used for statistical analyses, and a p-value below 0.05 was considered statistically significant.
Results
A total of 260 eligible patients were enrolled and categorized into the 131I group (n = 166) and the control group (n = 94).
Demographics of patients in the two groups
Demographic and clinicopathological characteristics of the patients are presented in . Among the two groups, patients who received 131I treatment reported a higher diagnosis rate of N1 stage and persistent hypoPT than those in the control group (72.89% vs. 25.54%, p = 0; 39.16% vs. 25.53%, p = 0.02), and showed a larger scope of lymph node dissection (p = 0). Patients in the control group showed a higher diagnosis rate if T1 stage (60.64% vs. 45.78%, p = 0.04). Interestingly, although there was a difference in the probability of persistent parathyroids between the two groups, there was no significant difference either in the preoperative or in any postoperative laboratory examination of serum calcium and PTH levels (p > 0.05).
Table 1. Demographic and clinicopathological characteristics of the 260 patients who developed protracted HypoPT with or without 131I treatment.
Other variables, including the occurrence of Hashimoto thyroiditis, nodular goiter, the total number of PG, PG left in situ, and PGRIS, were statistically similar between the two groups.
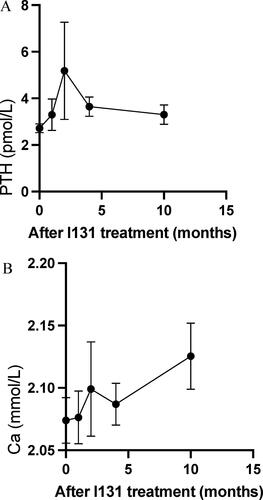
Of the 166 patients who received 131I treatment, serum calcium and PTH levels after the treatment were also examined. The changes of serum calcium and PTH levels following 131I treatment are shown in .
The outcome of parathyroid function recovery
The differences between the patients who recovered or did not recover from protracted HypoPT are shown in ; 172 patients recovered from protracted HypoPT and 88 patients developed persistent HypoPT. Patients who had received 131I treatment showed a lower rate of RPF (58.72% vs.73.86%, p = 0.02). The PGRIS and total number of PG were significantly higher in patients who recovered from protracted HypoPT (p = 0.02; p = 0.03). The results showed that the surgical approach affected the recovery of protracted hypoPT (TT + iCND vs. TT + bCND vs. TT + bCND + iLND vs. TT + bCND + bLND, p = 0.15).
Table 2. Demographic and clinicopathological characteristics of the 260 patients who recovered from protracted HypoPT or developed persistent HypoPT.
The levels of Ca and PTH showed significant differences between the two groups of patients 6 and 12 months after the operation and during the last examination (p < 0.05). However, one month after the operation, only the PTH level was significantly higher in patients who recovered parathyroid function (p = 0.00).
The multivariate analysis () showed that PGRIS (1 ∼ 2 vs. 0, p = 0.03, OR3.19 (1.13 ∼ 8.99); 3 ∼ 4 vs. 0, p = 0.02, OR3.62 (1.29 ∼ 10.2)] and 131I treatment [p = 0.02, OR1.98 (1.11 ∼ 3.51)] were independent influencing factors of postoperative persistent hypoPT.
Table 3. Multivariate analysis for persistent HypoPT vs. recovery from protracted HypoPT.
Timing to RPF
Most of the patients (172) recovered from protracted hypoPT; 100 (58.14%) patients recovered within six months after surgery, 49 (35.10%) patients recovered within 12 months, and 23 (13.37%) patients recovered after 12 months. The timing of the RPF of the 172 patients ranged from 2 to 39 postsurgical months. The demographic variables of the 172 patients who recovered from protracted hypoPT are shown in . Among them, patients who recovered within six months showed a higher diagnostic rate of the N0 stage (50.00% vs. 42.86% vs. 21.74%, p = 0.01). Recovery from protracted hypoPT after 12 months most commonly occurred in patients who received 131I treatment (p = 0.01; 50.00% vs. 69.39% vs.73.91%, p = 0.02). Of the 172 patients, 101 patients received the 131I treatment. In the 101 patients, longer treatment duration and intervals led to a longer duration of RPF (p = 0.00, p = 0.046, respectively). In addition, the results showed that the surgical approach was not related to the timing of RPF (TT + iCND vs TT + bCND vs TT + bCND + iLND vs TT + bCND + bLND, p = 0.31).
Table 4. Demographic variables of 172 patients who recovered from protracted hypoPT within six months, after six months, and/or twelve months after surgery.
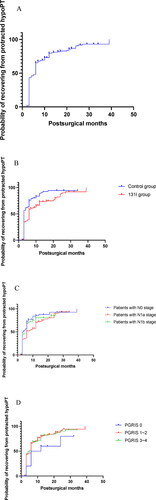
The timing to RPF or the diagnosis of persistent hypoPT in the 260 patients is shown in . The timing to RPF differed significantly based on the log-rank test results in the control group compared to that in the 131I group [95% CI = 1.319–2.895, p = 0.00] (). Interestingly, although the results were statistically similar, the timing to RPF in the patients in the N0 stage approached a significant difference compared to the timing to RPF in the patients in the N1a and N1b stages () (p = 0.05), and that of the patients with PGRIS 0 vs. PGRIS 1 ∼ 2 vs. PGRIS 3 ∼ 4 was not different () (p = 0.22).
Figure 2. Time to recovery of the parathyroid function (RPF) in patients with protracted hypoparathyroidism. (A) Time to RPF for the whole cohort (n = 260). (B) RPF in patients with or without 131I treatment (p = 0.00). (C) RPF in patients with N0 vs. N1a vs. N1b stage (p = 0.05). (D) RPF according to PGRIS (parathyroid glands remaining in situ) (p = 0.22). Horizontal axis expressed in the logarithmic scale (months); hypoPT: hypoparathyroidism; p-values are from the log-rank (Mantel-Cox) test.
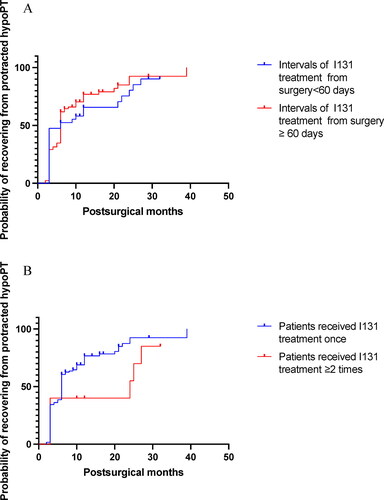
In the 166 patients who received 131I treatment, the timing for RPF or the diagnosis of persistent hypoPT was also analyzed (). No difference was observed either in the patients with different intervals of 131I treatment (<60 days vs. ≥ 60 days), or in the patients with different frequencies of 131I treatment (once vs. ≥ 2 times) (p = 0.08, p = 0.40, respectively).
Figure 3. Time to recovery of parathyroid function (RPF) in patients with protected hypoPT who received 131I treatment. (A) RPF in patients with intervals of 131I treatment <60 days and ≥ 60 days (p = 0.40). (B) RPF in patients treated with 131I once vs. ≥2 times (p = 0.08). The horizontal axis is in the logarithmic scale (months); hypoPT: hypoparathyroidism; p-values are from the log-rank (Mantel-Cox) test.
Discussion
Postoperative hypoPT is a common iatrogenic complication after total thyroidectomy and generally recovers within one month. Studies have shown that the RPF is a dynamic process that can take a long time after surgery [Citation18]; An RPF of 16 years after surgery has also been reported [Citation16]. Therefore, a “permanent hypoPT” diagnosis should be made with caution, as long term replacement therapy can affect patients financially and mentally.
The adverse effect of radioactive iodine on parathyroid function was first reported in 1983 [Citation19]. Because 131I treatment is performed after the operation (>1 month, in most cases) and could be performed multiple times, the continuous damage caused by 131I in the parathyroid could further prolong the duration of RPF in those patients, who could therefore be diagnosed with “permanent hypoPT”, causing psychological distress and economic loss to the patients.
In this study, we reported for the first time that persistent hypoPT might be related to 131I treatment. Similar conclusions were made by Guven et al. [Citation4]. They showed a transient decline in PTH levels in the sixth month following radioactive iodine (RAI) therapy, but the study was only performed with 19 patients and had a short follow-up time (12 months). Other reports suggested that 131I treatment might diminish postoperative parathyroid function [Citation3, Citation20,Citation21]. Interestingly, Gomez et al. [Citation18] found the opposite results. They reported hyperparathyroidism in a patient with PTC after a short latency period after RAI and suggested that serum calcium levels should be included in the routine yearly surveillance of patients who receive RAI therapy. Zhao et al. [Citation22] suggested that 131I treatment did not significantly impair the parathyroid function in a retrospective study that included 160 patients with DTC who received 131I treatment. The levels of PTH, serum calcium, and phosphorus in the patients were compared before treatment and at the end of the follow-up, and no statistically significant difference (p > 0.05) was observed. However, the follow-up time for this study was too short (8.4 ± 1.8 months) to draw a strong conclusion, and the primary diseases were inconsistent.
Additionally, the timing of RPF was not associated with 131I treatment in previous studies. The cutoff time for the diagnosis of permanent hypoPT ranged from 6 to 12 months, and most of the studies had a limited sample size and/or a short observation time after surgery. We first used the concept of “persistent” hypoPT to identify patients who had persistent hypoPT at the end of the follow-up and concluded that 131I treatment prolonged the recovery time of parathyroid function (95% CI = 1.319–2.895, p = 0.00).
The most relevant factors that lead to the prolongation of RPF (>6 months) include the number of PGRIS and parathyroid autotransplantation in patients with protracted HypoPT [Citation23,Citation24]. However, these studies did not consider 131I treatment while estimating the risk of a prolonged duration of RPF.
The results of the current study show that although PGRIS was associated with persistent HypoPT, the RPF timing for patients with PGRIS 0 vs. PGRIS 1 ∼ 2 vs. PGRIS 3 ∼ 4 was similar (p = 0.22). Furthermore, we found that the timing of RPF in patients in the N0 stage approached a significant difference compared to patients in the N1a and N1b stages (p = 0.05). These results were inconsistent with previous studies’ results [Citation11, Citation15, Citation25–27]. Han et al. [Citation11], in a retrospective study with 559 patients, identified PGRIF [PGRIF = number of in situ glands/(total number of identified glands − number of glands in the patient)] as an independent risk factor for transient HypoPT, protracted HypoPT, and postoperative hypocalcemia (OR = 0 177, 0.190, and 0.330, respectively). De Jong MC [Citation26] reported that the number of PGRIS is a key variable of the chances for restoration of the parathyroid function in patients with protracted HypoPT. Luo [Citation11] found that less preserved is the independent risk factor for postoperative HypoPT and hypocalcemia, resulting in a worse function of the parathyroid gland in the long term. These differences might be due to the longer follow-up time of our study and the surgical technique of parathyroid autotransplantation. Furthermore, because indocyanine green angiography (ICG) and autofluorescence are not routine examinations in our hospital, they were not used in this study. Instead, carbon nanoparticle were used intraoperatively to identify and protect parathyroid glands. This may also lead to differences in the results.
Some authors reported that serum Ca, and PTH levels tested 1 month after surgery are associated with RPF [Citation14, Citation28,Citation29]. Sitges-Serra et al. [Citation29] found that higher serum calcium levels at 1 month after total thyroidectomy are associated with recovery of parathyroid function in patients with protracted HypoPT. Kihara et al. [Citation14] reported that high-normal serum calcium and low but detectable iPTH concentrations in 1 month after surgery were associated with a better outcome of protracted HypoPT. Our results are partly consistent with theirs. We found that the the PTH level tested 1 month after surgery was significantly higher in patients who recovered parathyroid function than patients who developed persistent HypoPT (p = 0.00).
Furthermore, we found that the extent of lymph node dissection in the central area of the neck was not related to the occurrence of persistent HypoPT (TT + iCND vs TT + bCND vs TT + bCND + iLND vs TT + bCND + bLND, p > 0.05). This is different from the findings of others according to which CND may be related to the increase in the incidence of permanent parathyroidism after surgery [Citation30,Citation31]. This may be because we only included patients who developed protracted HypoPT postoperatively and excluded the patients whose parathyroid function recovered within one month after the surgery (these patients’ parathyroid function was less affected by surgery and may have a higher proportion of receiving iCND). Therefore, the results indicated that patients receiving iodine 131 treatment showed worse parathyroid function in the follow-up process may be more related to iodine 131 treatment, rather than surgery.
Our study had some limitations. There may be some bias due to the retrospective nature of the study. Additionally, because only patients with protracted hypoPT were included, whether patients with normal parathyroid function within one postoperative month developed hypoPT after 131I treatment remained uncertain.
In conclusion, recovering from postoperative protracted hypoPT in PTC patients might occur even 12 months after the surgery. The 131I treatment significantly prolonged the duration of RPF of patients with protracted hypoPT. The diagnosis of “permanent” hypoPT should be made with caution at least 12 months after surgery, especially in patients receiving 131I treatment. The parathyroid function should be monitored during routine yearly surveillance in PTC patients who receive 131I treatment.
Reporting checklist
The authors have completed the STROBE reporting checklist.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was waived by the local Ethics Committee of West China Hospital, Sichuan University, in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Contributions
(I) Conception and design: Munire Abuduwaili; (II) Administrative support: A Su,; (III) Provision of study materials or patients: A Su (IV) Collection and assembly of data: M Abuduwailia, B Xia, Zhichao Xing,; (V) Data analysis and interpretation: Munire Abuduwaili, Wusiman Baidula; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. These authors contributed equally to this work.
Supplemental Material
Download PDF (2.3 MB)Acknowledgments
We thank director Jingqiang Zhu of Center of Thyroid & Parathyroid Surgery, West China Hospital for providing administrative support.
Data sharing statement
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mihai R, Thakker RV. Postsurgical hypoparathyroidism: current treatments and future prospects for parathyroid allotransplantation. Eur J Endocrinol. 2021;184(5):R165–R175. doi:10.1530/EJE-20–1367.
- Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg. 2014;101(4):307–320. doi:10.1002/bjs.9384
- Glazebrook GA. Effect of decicurie doses of radioactive iodine 131 on parathyroid function. Am J Surg. 1987;154(4):368–373. doi:10.1016/0002–9610(89)90006–8
- Guven A, Salman S, Boztepe H, et al. Parathyroid changes after high dose radioactive iodine in patients with thyroid cancer. Ann Nucl Med. 2009;23(5):437–441. doi:10.1007/s12149–009–0270–4.
- Bollerslev J, Rejnmark L, Marcocci C, et al. European Society of Endocrinology clinical guideline: treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol. 2015;173(2):G1–20. doi:10.1530/EJE-15–0628.
- Orloff LA, Wiseman SM, Bernet VJ, et al. American thyroid association statement on postoperative hypoparathyroidism: diagnosis, prevention, and management in adults. Thyroid 2018;28(7):830–841. doi:10.1089/thy.2017.030
- Haugen BR, Alexander EK, Bible KC, et al. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020
- Wati R. Recovery from permanent hypoparathyroidism after total thyroidectomy. Αγαη 2019;8(5):55.
- Kim S-M, Kim HK, Kim K-J, et al. recovery from permanent hypoparathyroidism after total thyroidectomy. Thyroid 2015;25(7):830–833. doi:http://dx.doi.org/10.1089/thy.2014.0500
- Qiu Y, Xing Z, Xiang Q, Yang Q, Su A, Luo Y. Duration of parathyroid function recovery in patients with protracted hypoparathyroidism after total thyroidectomy for papillary thyroid carcinoma. Front Endocrinol (Lausanne). 2021;12:665190–665198. doi:10.3389/fendo.2021.665190
- Luo H, Zhao W, Yang H, Su A, Wang B, Zhu J. In situ preservation fraction of parathyroid gland in thyroidectomy: a cohort retrospective study. Int J Endocrinol. 2018;2018(1):7493143. doi:10.1155/2018/7493143
- Villarroya-Marquina I, Sancho J, Lorente-Poch L, Gallego-Otaegui L, Sitges-Serra A. Time to parathyroid function recovery in patients with protracted hypoparathyroidism after total thyroidectomy. Eur J Endocrinol. 2018;178(1):103–111. doi:10.1530/EJE-17–0589.
- Lorente-Poch L, Sancho J, Muñoz JL, Gallego-Otaegui L, Martínez-Ruiz C, Sitges-Serra A. Failure of fragmented parathyroid gland autotransplantation to prevent permanent hypoparathyroidism after total thyroidectomy. Langenbecks Arch Surg. 2017;402(2):281–287. doi:10.1007/s00423–016–1548–3.
- Sitges-Serra A, Gómez J, Barczynski M, Lorente-Poch L, Iacobone M, Sancho J. A nomogram to predict the likelihood of permanent hypoparathyroidism after total thyroidectomy based on delayed serum calcium and iPTH measurements. Gland Surg. 2017;6(Suppl 1):S11–S19. doi:10.21037/gs.2017.10.04
- Seo ST, Chang JW, Jin J, Lim YC, Rha KS, Koo BS. Transient and permanent hypocalcemia after total thyroidectomy: early predictive factors and long-term follow-up results. Surg (United States). 2015;158(6):1492–1499. doi:10.1016/j.surg.2015.04.041
- Cusano NE, Anderson L, Rubin MR, et al. Recovery of parathyroid hormone secretion and function in postoperative hypoparathyroidism: a case series. J Clin Endocrinol Metab. 2013;98(11):4285–4290. doi:10.1210/jc.2013–2937.
- Lloyd RO, Kloppel G, Rosai J. 2017. WHO classification of tumours of endocrine organs. 4th ed. Lyon, France: IARC Publications.
- Gomez DL, Shulman DI. Hyperparathyroidism two years after radioactive iodine therapy in an adolescent male. Case Rep Pediatr. 2014;2014(mCi):1–3. doi:10.1155/2014/163848
- Burch WM, Posillico JT. Hypoparathyroidism after I-131 therapy with subsequent return of parathyroid function. J Clin Endocrinol Metab. 1983;57(2):398–401. doi:10.1210/jcem-57–2–398.
- Winslow CP, Meyers AD. Hypocalcemia as a complication of radioiodine therapy. Am J Otolaryngol-Head Neck Med Surg. 1998;19(6):401–403. doi:10.1016/S0196–0709(98)90045-X
- Komarovskiy K, Raghavan S. Hypocalcemia following treatment with radioiodine in a child with graves’ disease. Thyroid 2012;22(2):218–222. doi:10.1089/thy.2011.0094
- Zhao Z-H, Li F-Q, Han J-K, Li X-J. Effect of I131 ‘clear residual thyroid tissue’ after surgery on the function of parathyroid gland in differentiated thyroid cancer. Exp Ther Med. 2015;10(6):2079–2082. doi:10.3892/etm.2015.2812
- Wang B, Zhu CR, Liu H, Wu J. The effectiveness of parathyroid gland autotransplantation in preserving parathyroid function during thyroid surgery for thyroid neoplasms: a meta-analysis. PLoS ONE. 2019;14(8):e0221173. doi:10.1371/journal.pone.0221173
- Lorente-Poch L, Sancho JJ, Ruiz S, Sitges-Serra A. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg. 2015;102(4):359–367. doi:10.1002/bjs.9676
- Kihara M, Miyauchi A, Kontani K, Yamauchi A, Yokomise H. Recovery of parathyroid function after total thyroidectomy: long-term follow-up study. ANZ J Surg. 2005;75(7):532–536. doi:10.1111/j.1445–2197.2005.03435.x
- Sitges-Serra A. The PGRIS and parathyroid splinting concepts for the analysis and prognosis of protracted hypoparathyroidism. Gland Surg. 2017;6(Suppl 1):S86–S93. doi:10.21037/gs.2017.07.16
- de Jong MC, Lorente-Poch L, Sancho-Insenser J, et al. Late recovery of parathyroid function after total thyroidectomy in children and adults: is there a difference? Horm Res Paediatr. 2020;93(9–10):539–547. doi:10.1159/000513768
- Yao XY, Zhou Y, Chen SJ, et al. Is there a regular pattern in the recovery of parathyroid function after thyroid cancer surgery? Cancer Manag Res. 2021;13:6891–6899. doi:10.2147/CMAR.S326705
- Sitges-Serra A, Ruiz S, Girvent M, Manjón H, Dueñas JP, Sancho JJ. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg. 2010;97(11):1687–1695. doi:10.1002/bjs.7219
- White ML, Doherty GM. Level VI lymph node dissection for papillary thyroid cancer. Minerva Chir. 2007;62(5):383–393. http://www.ncbi.nlm.nih.gov/pubmed/17947949.
- Carling T, Long WD, Udelsman R. Controversy surrounding the role for routine central lymph node dissection for differentiated thyroid cancer. Curr Opin Oncol. 2010;22(1):30–34. doi:10.1097/CCO.0b013e328333ac97