Abstract
Background: Patients with gastrointestinal cancer often suffer from malnutrition during tumor progression. Malnutrition is associated with postoperative complications and decreased quality of life. Supporting cancer patients with proper nutrition is vital for improving their prognoses.
Method: Google scholar and PubMed database searches were performed. Selection criteria included gastrointestinal cancer, surgery, ω − 3 fatty acids, randomized clinical trials from 2007 to August 2022.
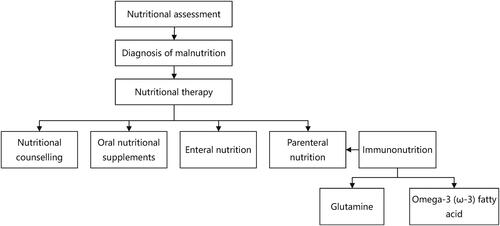
Conclusion: Nutritional therapy includes nutritional counseling, enteral nutrition, parenteral nutrition, and oral nutritional supplements. Immune nutrients like glutamine and ω-3 fatty acid have been demonstrated with benefits in reducing inflammatory responses and postoperative complications, regulating immune function and improving prognosis.
1. Introduction
Cancers of the stomach and colon are the fifth and third most common types, respectively, and the third and fourth leading causes of cancer-related deaths worldwide [Citation1]. Surgery is currently the mainstay of treatment for patients gastrointestinal(GI) cancer without distant metastases [Citation2]. Patients with locally advanced GI cancer are treated with postoperative chemotherapy. However, because of inadequate nutritional intake caused by primary anorexia or GI symptoms, systemic inflammation syndrome and alternative substance metabolism caused by cancer, cancer patients have a malnutrition rate of 20%–70%, and that in upper GI cancer patients is up to 60.2% [Citation3, Citation4]. Among patients receiving postoperative chemotherapy, gastrointestinal toxicity such as nausea and vomiting is a common complication. Treatment-induced GI mucositis and changes in the composition of the microflora could result in absorption and other intestinal function dysregulation and increase the rate and severity degree of malnutrition [Citation5]. For GI cancer patients, malnutrition contributes to loss of weight and muscle [Citation6], reduced immune function, more postoperative infections [Citation7], increased chemotherapy toxicity [Citation5], dose reductions and delays or cessation of chemotherapy [Citation5], lower quality of life [Citation8], prolonged length of stay (LOS) and higher mortality [Citation7]. However, 25.5%–34.0% of patients who are well-nourished received improper nutritional therapy while 41.8%–59.1% of those with malnutrition received no nutritional support at all according to a survey [Citation9, Citation10]. Delivery of proper nutrition support to patients with malnutrition can increase energy intake and body weight, reduce of mortality and nonelective hospital readmission rates, improve survival and prognosis and save the cost of disease as well [Citation11–13].
2. Method
Google scholar and PubMed database searches were performed. Selection criteria included gastrointestinal cancer, surgery, ω − 3 fatty acids, randomized clinical trials from 2007 to August 2022.
3. Definition of malnutrition
Malnutrition can be diagnosed by the following two criteria according to ESPEN: 1) BMI < 18.5 kg/m2; 2) Weight loss (unintentional)>10% indefinite of time, or > 5% over the past three months combined with either BMI < 20kg/m2 if < 70years old, or FFMI < 15 and 17 kg/m2 in women and men, respectively, or BMI < 22kg/m2 if > 70 years old [Citation6]. It is important to conduct a nutritional assessment by any validated malnutrition screening tools, such as Nutrition Risk Screening 2002 (NRS-2002) [Citation14], Malnutrition Universal Screening Tool (MUST), Patient-Generated Subjective Global Assessment (PG-SGA), Mini Nutritional Assessment (MNA) [Citation6]. The goal of nutritional therapy is to enhance immune function and nutritional status, which will ultimately improve the prognosis for cancer.
4. Therapy for GI cancer patients underwent surgery
The forms of nutritional support preoperatively include nutrition counseling, oral nutritional supplements (ONS), artificial nutrition includes enteral nutrition (EN) and parenteral nutrition (PN). The dosage of nutrition therapy should depend on energy requirements and nutrition elements. Total energy requirement of cancer patients is suggested ranging from 25 to 30 kcal/kg/day. The dosage of protein intake should be 1.0–1.5 g/kg/day, 1.2–1.5 g/kg/day for chronically ill older patients [Citation15]. All energy and protein intakes through oral, EN, and PN should be calculated. Besides, micronutrient is at a risk of deficiency in malnutrition patients. According to ESPEN recommendations, minerals and vitamins should be given to cancer patients in proportions that roughly correspond to the recommended dietary allowance (RDA), but avoid using high-dose micronutrients unless there are specific deficiencies [Citation15].
4.1. Nutritional counselling
Nutrition counseling is the first and the most common choice of nutrition support which involves nutritional history, diagnosis, and nutrition treatment to relieve symptoms and increase energy intake in order to keep up with or work on healthful nutritional status [Citation15]. Critical elements of nutrition counseling include communicating to patients the reasons and goals for dietary advice, and motivating them to adjust to their disease’s changing nutritional demands [Citation3].
4.2. Oral nutritional supplements
ONS are made to provide nutrient-dense, high-energy liquids that can be prepared as drinks or added to drinks and foods [Citation16]. For all patients identified as malnourished or could not meet their energy needs by normal diet, ONS is suggested. What’s more, for patients who are about to undergo surgery, ONS enhanced with immune-modulating elements such arginine, nucleotides, and omega-3 fatty acids is recommended for 5–7 days before to surgery to improve patients’ nutritional condition, boost immune function, minimize postoperative inflammatory response, and improve cancer prognosis [Citation16–18]. It can significantly reduce hospital (re)admissions as well, especially in older patients [Citation19]. While significant differences in postoperative complications and body weight did not be found in the recent meta-analysis, which is consistent with Reece et al. [Citation16, Citation20].
Most of GI cancer patients underwent nutritional treatment were unable to achieve their energy requirements via normal oral diet for a long period, putting them at risk of postoperative malnutrition after discharge [Citation17]. For these patients, they are suggested to continue nutritional follow-up after discharge [Citation17]. We conducted six randomized controlled trials (RCTs) [Citation21–26] to investigate the use of ONS for 6–12 weeks in postoperative gastric and colorectal cancer patients. The outcomes demonstrated that ONS administration following surgery can lessen body weight loss [Citation21–23], improve nutritional outcomes such as body mass index (BMI) and skeletal muscle index (SMI) [Citation24–26], lessen the occurrence of sarcopenia and chemotherapy modifications like delay, dosage reduction, or termination [Citation25, Citation26].
4.3. Enteral nutrition
Patients who are unable to eat for more than a week or whose estimated energy intake is less than 60% of their demand for more than 1–2 weeks are deemed to have inadequate food intake and artificial nutrition is recommended [Citation15]. Enteral nutrition is more consistent with physiological processes. So, if possible, it is preferred during perioperative in major abdominal surgery.
Early enteral nutrition contributes to more increase in the level of nutritional and immune indicators and body weight, shortener of the LOS, reduction of the risk of postoperative complications and promotion of the functional recovery of the digestive system as well [Citation27, Citation28].
Recent years, early oral feeding attracted attention, and many studies were conducted. Results of most studies suggested that early oral feeding can shorten the LOS, save cost, improve clinical outcomes, and have no negative effects on healing of anastomoses in patients underwent colorectal [Citation17, Citation29–31]. Even after partial or total gastrectomy, early oral feeding can be safe, feasible and associated with several potential benefits without increasing clinical complications [Citation32–36]. However, a multicenter RCT found that early oral feeding did not show an advantage in shortening the LOS in patients underwent distal gastrectomy (DG) and it increased postoperative complications rate in DG (P = 0.045) on the contrary [Citation37]. More conclusive study is necessary to firmly prove the potential benefits of early oral eating for GI surgery patients. Post-discharge enteral feeding is helpful in boosting calorie intake in patients undergoing upper GI cancer surgery, but there is no discernible benefit in tiredness, quality of life, or health economics [Citation38].
Early oral feeding and EN has been suggested by ESPEN and ERAS, while the early feeding intolerance and the poor compliance resulted by it are the main barriers to administration of early enteral nutrition [Citation17, Citation39]. Patients who have undergone a radical gastrectomy have a feeding intolerance rate of 49.3%, which is the dominant independent factor for postoperative complications in colorectal [Citation40, Citation41]. Measures such as choosing appropriate feeding routes, maintaining good oral hygiene, and proper positioning for tube feeding can reduce the occurrence of feeding intolerance [Citation42]. A recent study attempted to improve early feeding intolerance with ONS for 7 days preoperatively, but the results were negative [Citation43]. Further researches are need to identify definitive mechanisms of feeding intolerance, propose more effective ways and more appropriate nutritional preparations to reduce its incidence.
4.4. Parenteral nutrition
Parenteral nutrition can provide adequate macronutrients and micronutrients through intravenous route which may allow for improving nitrogen balance efficiently and immune function, decreasing postoperative complications [Citation44]. The effects of total parenteral nutrition on the gastrointestinal tract, however, such as a reduction in brush-border hydrolase, microvillus height and nutrient transporter activity, are associated with bacterial translocation (BT) and the recovery of intestinal function [Citation45]. There are many studies have demonstrated that EN has more benefits over PN both in pre- and post-of operation [Citation46–48]. But when oral/EN could not achieve adequate energy or there are contraindications to EN such as intestinal obstruction and intestinal ischemia, parenteral nutrition is needed to be administration as soon as possible.
Appropriate parenteral nutrition prior to surgery is vital. A period of 7–14 days of preoperative PN is advised, especially for patients who have significant nutrition risk or malnutrition [Citation17]. A substantial decrease in postoperative overall problems (RR 0.64, 95%CI 0.46 to 0.87) was seen in a Cochrane study of preoperative PN in patients having gastrointestinal surgery [Citation49]. 10 days preoperatively and 9 days postoperatively of PN were able to lower the mortality rate and the complication risk by almost one third in severely malnourished, GI cancer patients [Citation50].
5. Immunonutrition
Immunonutrition, which includes glutamine, arginine, nucleotides and omega-3 fatty acids, has attracted more and more attention, especially for perioperative nutrition management between cancer patients in recent years. It can modulate host immune systems and inflammatory response [Citation51]. The advantages of oral/enteral formula enhanced with immunonutrients have been demonstrated in several researches. Data on supplementing arginine or nucleotides as a single item are scarce for arginine and nucleotides are mainly mixed in enteral nutrition preparations. Supplementations of glutamine and omega-3 fatty acids are easily available recently and mainly administrated through parenteral route.
5.1. Glutamine
Glutamine is a conditionally necessary amino acid that can improve nitrogen balance, enhance muscle protein synthesis and decrease protein loss. A retrospective study of 1950 patients found that parenteral glutamine supplementation of 0.05–0.49 g/kg/day postoperatively resulted in a reduced drop in serum albumin levels (−0.6 vs. −1.1 g/dL; P < 0.001) [Citation52]. The significance of glutamine to cell survival and proliferation in vitro was first described by Ehrensvard et al. [Citation53] Glutamine can enhance immunome function, such as regulate T-lymphocyte proliferation and facilitate the differentiation of B-lymphocytes into cells that produce and secrete antibodies [Citation54]. A RCT conducted glutamine with a dose of 0.5 g/kg/day from 1 day preoperatively to the 3rd postoperatively revealed that perioperative parenteral nutrition enriched with dipeptide glutamine meliorates postoperative immunodepression and reduces the LOS [Citation55]. An animal study found that total parenteral nutrition (TPN) enriched glutamine can significantly attenuate the TPN-associated loss of epithelial barrier function (EBF) and TPN-associated intestinal mucosal atrophy, which is confirmed in a clinical research investigation of patients with advanced gastric cancer [Citation56, Citation57]. Wang et al. reported that patients with advanced gastric cancer accepted parenteral nutrition support enhanced with glutamine of 0.4 g/(kg·day) can effectively protect the intestinal mucosal barrier function and improve the level of MMP-2 and MMP-9 during perioperative chemotherapy [Citation57]. It should be emphasized that MMP-2 and MMP-9 can affect the progression of gastric cancer and there is a positive correlation between intestinal permeability and the levels of MMP-2 and MMP-9 in the intestinal mucosa according to animal researches [Citation57, Citation58]. Besides, glutamine may function as a motility-recovery agent following gastrectomy according to Mochiki et al. [Citation59] More high-quality researches are required to determine the exact effect and optimal dosage of glutamine after gastrointestinal surgery. Currently, no definite recommendation for the oral glutamine supplementation has been proposed.
5.2. Omega-3 (ω-3) fatty acid
Omega-3 (ω-3) fatty acid is one of polyunsaturated fatty acids (PUFAs), which mainly includes α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). ALA is rich in nuts and seeds, and EPA and DHA are mainly found in fish oil [Citation60]. ω-3 fatty acid can modulate immune system and reduce inflammatory reaction. In the initiation of acute inflammatory response, it modulate inflammatory reaction mainly through decreasing the secretion of pro-inflammatory factors like IL-1β, IL-6 and TNF-α, increasing the production of anti-inflammatory cytokine IL-10 and prostaglandin D3 which can reduce the neutrophil adhesion and transmigration [Citation60]. During the post-acute phase, ω-3 fatty acid can be used to produce maresins, protectins and resolvins, which are called resolution specialized pro-resolving mediators(SPMs) [Citation61]. Besides, ω-3 fatty acid can optimize the omega-6/omega-3 balance. Omega-6 PUFAs, such as arachidonic acid (AA) and linoleic acid (LA), can be metabolized to proinflammatory factors, specifically prostaglandin E2, thromboxanes, leukotriene B4, lipoxins [Citation62, Citation63]. A high ratio of omega-6/omega-3 is considered as a critical condition with proinflammatory potential. A recent high quality meta-analysis investigated about omega-6 sparing benefits of parenteral lipid emulsions on critically sick patients showed that omega-6 FA reducing lipid emulsions in PN can decrease the hospital and ICU LOS, 28-day mortality, and mechanical ventilation [Citation64]. What’s more, they demonstrated that lipid emulsions with fish oil and lower omega-6 FA decrease the occurrence of nosocomial infections. Omega-3 PUFA also can regulate intestinal inflammatory response and enhance anti-stress ability, resulting in reducing the occurrence of diarrhea, bloating and diarrhea and improving postoperative enteral feeding tolerance [Citation65].
Cause the benefits of ω-3 fatty acids in reducing the inflammatory response and regulating the immune response, several researches have been done to observe the effect of fish oil on clinical outcomes in GI cancer patients underwent surgery. Studies demonstrating parenteral fish oil’s benefit for GI cancer patients are summarized in . The advantages of omega-3 fish oil are related to the dosage and time point of use. In previous studies, the dose of omega-3 fish oil was 0.08–0.20 g/kg/day. A multi-center study with 661 patients have shown that when 0.10–0.20g/kg/d omega-3 fish oil was provided, the prognosis of severe patients can be significantly improved [Citation77]. More high-quality studies are required to demonstrate the effectiveness in GI cancer patients and determine more precise optimal dosages and time point of use.
Table 1. Effectiveness of parenteral fish oil on gastrointestinal cancer patients.
6. Conclusion
Patients with gastrointestinal cancer often suffer from malnutrition which associated with worse prognosis. Nutritional therapy includes nutritional counseling, enteral nutrition, parenteral nutrition, and oral nutritional supplements. Immune nutrients like glutamine and ω-3 fatty acid have been demonstrated with benefits in reducing inflammatory responses and postoperative complications, regulating immune function and improving prognosis. This review summarizes the current nutritional therapy and various nutritional issues in GI cancer surgery (). More well-developed studies are demanded to be demonstrated to explore more efficient and meaningful nutritional treatment options.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):1–7. doi:10.3322/caac.21492
- Wang F-H, Shen L, Li J, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019;39(1):10. doi:10.1186/s40880-019-0349-9
- Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187–1196. doi:10.1016/j.clnu.2017.06.017
- Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. 2014;38(2):196–204. doi:10.1177/0148607113502674
- Boussios S, Pentheroudakis G, Katsanos K, Pavlidis N. Systemic treatment-induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann Gastroenterol. 2012;25(2):106–118.
- Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition – an ESPEN consensus statement. Clin Nutr. 2015;34(3):335–340. doi:10.1016/j.clnu.2015.03.001
- Pressoir M, Desne S, Berchery D, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer. 2010;102(6):966–971. doi:10.1038/sj.bjc.6605578
- Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153–159. doi:10.7762/cnr.2015.4.3.153
- Sun H, Zhang L, Zhang P, et al. A comprehensive nutritional survey of hospitalized patients: Results from nutritionDay 2016 in China. PLoS One. 2018;13(3):e0194312. doi:10.1371/journal.pone.0194312
- Guo ZQ, Yu JM, Li W, Investigation on the Nutrition Status and Clinical Outcome of Common Cancers (INSCOC) Group, et al. Survey and analysis of the nutritional status in hospitalized patients with malignant gastric tumors and its influence on the quality of life. Support Care Cancer. 2020;28(1):373–380. doi:10.1007/s00520-019-04803-3
- Tyler R, Barrocas A, Guenter P, ASPEN Value Project Scientific Advisory Council, et al. Value of nutrition support therapy: impact on clinical and economic outcomes in the United States. JPEN J Parenter Enteral Nutr. 2020;44(3):395–406. doi:10.1002/jpen.1768
- Pimiento JM, Evans DC, Tyler R, ASPEN Value Project Scientific Advisory Council, et al. Value of nutrition support therapy in patients with gastrointestinal malignancies: a narrative review and health economic analysis of impact on clinical outcomes in the United States. J Gastrointest Oncol. 2021;12(2):864–873. doi:10.21037/jgo-20-326
- Gomes F, Baumgartner A, Bounoure L, et al. Association of nutritional support with clinical outcomes among medical inpatients who are malnourished or at nutritional risk: an updated systematic review and meta-analysis. JAMA Netw Open. 2019;2(11):e1915138. doi:10.1001/jamanetworkopen.2019.15138
- Kondrup J, Allison SP, Elia M, Vellas B, Plauth M, Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN Guidelines for Nutrition Screening 2002. Clin Nutr. 2003;22(4):415–421. doi:10.1016/S0261-5614(03)00098-0
- Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. doi:10.1016/j.clnu.2016.07.015
- Chen X, Yang K, Zhang X, Li K. Meta-analysis of preoperative oral nutritional supplements for patients with gastric cancer: East Asian experience. Eur J Clin Nutr. 2020;74(7):991–1000. doi:10.1038/s41430-019-0483-0
- Weimann A, Braga M, Carli F, et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr. 2021;40(7):4745–4761. doi:10.1016/j.clnu.2021.03.031
- Zhao Q, Li Y, Yu B, et al. Effects of preoperative enteral nutrition on postoperative recent nutritional status in patients with Siewert II and III adenocarcinoma of Esophagogastric junction after neoadjuvant chemoradiotherapy. Nutr Cancer. 2018;70(6):895–903. doi:10.1080/01635581.2018.1490780
- Stratton RJ, Hebuterne X, Elia M. A systematic review and meta-analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing Res Rev. 2013;12(4):884–897. doi:10.1016/j.arr.2013.07.002
- Reece L, Hogan S, Allman-Farinelli M, Carey S. Oral nutrition interventions in patients undergoing gastrointestinal surgery for cancer: A systematic literature review. Support Care Cancer. 2020;28(12):5673–5691. doi:10.1007/s00520-020-05673-w
- Hatao F, Chen KY, Wu JM, et al. Randomized controlled clinical trial assessing the effects of oral nutritional supplements in postoperative gastric cancer patients. Langenbecks Arch Surg. 2017;402(2):203–211. doi:10.1007/s00423-016-1527-8
- Kobayashi D, Ishigure K, Mochizuki Y, et al. Multi-institutional prospective feasibility study to explore tolerability and efficacy of oral nutritional supplements for patients with gastric cancer undergoing gastrectomy (CCOG1301). Gastric Cancer. 2017;20(4):718–727. doi:10.1007/s10120-016-0668-3
- Kimura Y, Nishikawa K, Kishi K, et al. Long-term effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients (KSES002). Ann Gastroenterol Surg. 2019;3(6):648–656. doi:10.1002/ags3.12290
- Zhu M-W, Yang X, Xiu D-R, et al. Effect of oral nutritional supplementation on the post-discharge nutritional status and quality of life of gastrointestinal cancer patients after surgery: a multi-center study. Asia Pac J Clin Nutr. 2019;28(3):450–456. Doi: 10.6133/apjcn.201909_28(3).0004
- Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: A randomized clinical trial. Clin Nutr. 2021;40(1):40–46. doi:10.1016/j.clnu.2020.04.043
- Tan S, Meng Q, Jiang Y, et al. Impact of oral nutritional supplements in post-discharge patients at nutritional risk following colorectal cancer surgery: A randomised clinical trial. Clin Nutr. 2021;40(1):47–53. doi:10.1016/j.clnu.2020.05.038
- Nikniaz Z, Somi MH, Nagashi S, Nikniaz L. Impact of early enteral nutrition on nutritional and immunological outcomes of gastric cancer patients undergoing gastrostomy: a systematic review and meta-analysis. Nutr Cancer. 2017;69(5):693–701. doi:10.1080/01635581.2017.1324996
- Shu X-L, Kang K, Gu L-J, Zhang Y-S. Effect of early enteral nutrition on patients with digestive tract surgery: A meta-analysis of randomized controlled trials. Exp Ther Med. 2016;12(4):2136–2144. doi:10.3892/etm.2016.3559
- Feo CV, Romanini B, Sortini D, et al. Early oral feeding after colorectal resection: a randomized controlled study. ANZ J Surg. 2004;74(5):298–301. doi:10.1111/j.1445-1433.2004.02985.x
- Wang Y, Zhang Y, Hu X, et al. Impact of early oral feeding on nasogastric tube reinsertion after elective colorectal surgery: a systematic review and meta-analysis. Front Surg. 2022;9:807811. doi:10.3389/fsurg.2022.807811
- Jochum SB, Ritz EM, Bhama AR, Hayden DM, Saclarides TJ, Favuzza J. Early feeding in colorectal surgery patients: safe and cost effective. Int J Colorectal Dis. 2020;35(3):465–469. doi:10.1007/s00384-019-03500-1
- Mahmoodzadeh H, Shoar S, Sirati F, Khorgami Z. Early initiation of oral feeding following upper gastrointestinal tumor surgery: a randomized controlled trial. Surg Today. 2015;45(2):203–208. doi:10.1007/s00595-014-0937-x
- Jang A, Jeong O. Early postoperative oral feeding after total gastrectomy in gastric carcinoma patients: a retrospective before-after study using propensity score matching. JPEN J Parenter Enteral Nutr. 2019;43(5):649–657. doi:10.1002/jpen.1438
- Lu YX, Wang YJ, Xie TY, et al. Effects of early oral feeding after radical total gastrectomy in gastric cancer patients. World J Gastroenterol. 2020;26(36):5508–5519. doi:10.3748/wjg.v26.i36.5508
- Shinohara T, Maeda Y, Koyama R, Minagawa N, Hamaguchi J, Hamada T. Feasibility and safety of early oral feeding in patients with gastric cancer after radical gastrectomy. Indian J Surg Oncol. 2020;11(1):47–55. doi:10.1007/s13193-019-00999-2
- He H, Ma Y, Zheng Z, Deng X, Zhu J, Wang Y. Early versus delayed oral feeding after gastrectomy for gastric cancer: A systematic review and meta-analysis. Int J Nurs Stud. 2022;126:104120. doi:10.1016/j.ijnurstu.2021.104120
- Shimizu N, Oki E, Tanizawa Y, et al. Effect of early oral feeding on length of hospital stay following gastrectomy for gastric cancer: a Japanese multicenter, randomized controlled trial. Surg Today. 2018;48(9):865–874. doi:10.1007/s00595-018-1665-4
- Froghi F, Sanders G, Berrisford R, et al. A randomised trial of post-discharge enteral feeding following surgical resection of an upper gastrointestinal malignancy. Clin Nutr. 2017;36(6):1516–1519. doi:10.1016/j.clnu.2016.10.022
- Mortensen K, Nilsson M, Slim K, Enhanced Recovery After Surgery (ERAS®) Group, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. 2014;101(10):1209–1229. doi:10.1002/bjs.9582
- Slim K, Reymond T, Joris J, Paul S, Pereira B, Cotte E. Intolerance to early oral feeding in enhanced recovery after colorectal surgery: an early red flag? Colorectal Dis. 2020;22(1):95–101. doi:10.1111/codi.14785
- Xiaoyong W, Xuzhao L, Deliang Y, et al. Construction of a model predicting the risk of tube feeding intolerance after gastrectomy for gastric cancer based on 225 cases from a single Chinese center. Oncotarget. 2017;8(59):99940–99949. doi:10.18632/oncotarget.21966
- Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi:10.1016/j.clnu.2018.08.037
- He FJ, Wang MJ, Yang K, et al. Effects of preoperative oral nutritional supplements on improving postoperative early enteral feeding intolerance and short-term prognosis for gastric cancer: a prospective, single-center, single-blind, randomized controlled trial. Nutrients. 2022;14(7):1472. doi:10.3390/nu14071472
- Lakananurak N, Gramlich L. The role of preoperative parenteral nutrition. Nutrients. 2020;12(5):1320. doi:10.3390/nu12051320
- Jiang XH, Li N, Li JS. Intestinal permeability in patients after surgical trauma and effect of enteral nutrition versus parenteral nutrition. World J Gastroenterol. 2003;9(8):1878–1880. doi:10.3748/wjg.v9.i8.1878
- Li J, Li S, Xi H, et al. Effect of preoperative nutrition therapy type and duration on short-time outcomes in gastric cancer patient with gastric outlet obstruction. Chin J Cancer Res. 2021;33(2):232–242. doi:10.21147/j.issn.1000-9604.2021.02.10
- Li K, Wang D, Zhang X, Yang J, Chen X. Efficacy of early enteral nutrition versus total parenteral nutrition for patients with gastric cancer complicated with diabetes mellitus: A systematic review and meta-analysis. Nutr Diet. 2022;79(1):129–139. doi:10.1111/1747-0080.12721
- Xin F, Mzee SAS, Botwe G, et al. Short-term evaluation of immune levels and nutritional values of EN versus PN in gastric cancer: a systematic review and a meta-analysis. World J Surg Oncol. 2019;17(1):114. doi:10.1186/s12957-019-1658-9
- Burden S, Todd C, Hill J, Lal S. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev. 2012;11:Cd008879. doi:10.1002/14651858.CD008879.pub2
- Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr. 2000;24(1):7–14. doi:10.1177/014860710002400107
- Jayarajan S, Daly JM. The relationships of nutrients, routes of delivery, and immunocompetence. Surg Clin North Am. 2011;91(4):737–753, vii. vii. doi:10.1016/j.suc.2011.04.004
- Wu JM, Ho TW, Lai IR, Chen CN, Lin MT. Parenteral glutamine supplementation improves serum albumin values in surgical cancer patients. Clin Nutr. 2021;40(2):645–650. doi:10.1016/j.clnu.2020.06.015
- Ehrensvard G, Fischer A, Stjernholm R. Protein metabolism of tissue cells in vitro; the chemical nature of some obligate factors of tissue cell nutrition. Acta Physiol Scand. 1949;18(2–3):218–230. doi:10.1111/j.1748-1716.1949.tb00614.x
- Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr. 2001;131(9 Suppl):2515S–2522S. discussion 2523S-4S. doi:10.1093/jn/131.9.2515S
- Yao GX, Xue XB, Jiang ZM, Yang NF, Wilmore DW. Effects of perioperative parenteral glutamine-dipeptide supplementation on plasma endotoxin level, plasma endotoxin inactivation capacity and clinical outcome. Clin Nutr. 2005;24(4):510–515. doi:10.1016/j.clnu.2005.04.002
- Nose K, Yang H, Sun X, et al. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: a potential mechanism for the preservation of epithelial barrier function. J Interferon Cytokine Res. 2010;30(2):67–80. doi:10.1089/jir.2009.0046
- Wang J, Li Y, Qi Y. Effect of glutamine-enriched nutritional support on intestinal mucosal barrier function, MMP-2, MMP-9 and immune function in patients with advanced gastric cancer during perioperative chemotherapy. Oncol Lett. 2017;14(3):3606–3610. doi:10.3892/ol.2017.6612
- Jakubowska K, Pryczynicz A, Iwanowicz P, et al. Expressions of Matrix Metalloproteinases (MMP-2, MMP-7, and MMP-9) and Their Inhibitors (TIMP-1, TIMP-2) in Inflammatory Bowel Diseases. Gastroenterol Res Pract. 2016;2016:2456179. doi:10.1155/2016/2456179
- Mochiki E, Ohno T, Yanai M, Toyomasu Y, Andoh H, Kuwano H. Effects of glutamine on gastrointestinal motor activity in patients following gastric surgery. World J Surg. 2011;35(4):805–810. doi:10.1007/s00268-011-0962-5
- Gutierrez S, Svahn SL, Johansson ME. Effects of Omega-3 fatty acids on immune cells. Int J Mol Sci. 2019;20(20). doi:10.3390/ijms20205028
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi:10.1038/nature13479
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–379. doi:10.1016/s0753-3322(02)00253-6
- Freitas RDS, Campos MM. Protective effects of Omega-3 fatty acids in cancer-related complications. Nutrients. 2019;11(5):945. doi:10.3390/nu11050945
- Notz Q, Lee ZY, Menger J, et al. Omega-6 sparing effects of parenteral lipid emulsions-an updated systematic review and meta-analysis on clinical outcomes in critically ill patients. Crit Care. 2022;26(1):23. doi:10.1186/s13054-022-03896-3
- Shao F, Xin F-Z, Yang C-G, et al. The impact of microbial immune enteral nutrition on the patients with acute radiation enteritis in bowel function and immune status. Cell Biochem Biophys. 2014;69(2):357–361. doi:10.1007/s12013-013-9807-1
- Ma C-J, Wu J-M, Tsai H-L, et al. Prospective double-blind randomized study on the efficacy and safety of an n-3 fatty acid enriched intravenous fat emulsion in postsurgical gastric and colorectal cancer patients. Nutr J. 2015;14:9. doi:10.1186/1475-2891-14-9
- Wei Z, Wang W, Chen J, Yang D, Yan R, Cai Q. A prospective, randomized, controlled study of ω-3 fish oil fat emulsion-based parenteral nutrition for patients following surgical resection of gastric tumors. Nutr J. 2014;13:25. doi:10.1186/1475-2891-13-25
- de Miranda Torrinhas RSM, Santana R, Garcia T, et al. Parenteral fish oil as a pharmacological agent to modulate post-operative immune response: a randomized, double-blind, and controlled clinical trial in patients with gastrointestinal cancer. Clin Nutr. 2013;32(4):503–510. doi:10.1016/j.clnu.2012.12.008
- Makay O, Kaya T, Firat O, et al. ω-3 Fatty acids have no impact on serum lactate levels after major gastric cancer surgery. JPEN J Parenter Enteral Nutr. 2011;35(4):488–492. doi:10.1177/0148607110386611
- Jiang ZM, Wilmore DW, Wang XR, et al. Randomized clinical trial of intravenous soybean oil alone versus soybean oil plus fish oil emulsion after gastrointestinal cancer surgery. Br J Surg. 2010;97(6):804–809. doi:10.1002/bjs.6999
- Liang B, Wang S, Ye Y-J, et al. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol. 2008;14(15):2434–2439. doi:10.3748/wjg.14.2434
- Wang D, Zhang H, Zhang Y, et al. [Effects of omega-3 polyunsaturated fatty acids on postoperative inflammatory reaction and clinical efficacy]. Zhonghua Wei Chang Wai Ke Za Zhi. 2015;18(7):651–655.
- Wang J, Yu JC, Kang WM, Ma ZQ. Superiority of a fish oil-enriched emulsion to medium-chain triacylglycerols/long-chain triacylglycerols in gastrointestinal surgery patients: a randomized clinical trial. Nutrition. 2012;28(6):623–629. doi:10.1016/j.nut.2011.08.004
- Ma CJ, Sun LC, Chen FM, et al. A double-blind randomized study comparing the efficacy and safety of a composite vs a conventional intravenous fat emulsion in postsurgical gastrointestinal tumor patients. Nutr Clin Pract. 2012;27(3):410–415. doi:10.1177/0884533611436115
- Xu LN, Xu YY, Li GP, Yang B. Effect of postoperative ω-3 fatty acid immunonutritional therapy on NK cell gene methylation in elderly patients with gastric cancer. Curr Med Sci. 2022;42(2):373–378. doi:10.1007/s11596-022-2567-7
- Yang J, Zhang X, Li K, et al. Effects of EN combined with PN enriched with n-3 polyunsaturated fatty acids on immune related indicators and early rehabilitation of patients with gastric cancer: A randomized controlled trial. Clin Nutr. 2022;41(6):1163–1170. doi:10.1016/j.clnu.2022.03.018
- Heller AR, Rössler S, Litz RJ, et al. Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit Care Med. 2006;34(4):972–979.