Abstract
Purpose
According to international guidelines, selective lymph node dissection can be performed on patients with early-stage endometrial cancer. However, some patients at early stage have already occurred lymph node metastasis at the time of diagnosis. This study was aimed to find a method to predict the risk of lymph node metastasis in this part of patient.
Methods
We collected data from 571 patients as training cohort and 351 patients as validation cohort for this study. Then we performed univariate and multivariate analyses to confirm the correlation of frequently used factors and lymph node metastasis. Combined analysis of four commonly indicators (ERα, PR, P53 and Ki67) from pathological parameter sources was mainly carried out, and the combined ratio is defined as (ERα + PR)/(Ki67 + P53). Then the accuracy of the combined ratio and other factors in prediction were compared by AUC value. Also, the optimal truncation value was searched. Finally, patients followed up for more than two years were divided into groups by the threshold value, and their difference in survival was explored.
Results
This study showed that CA125, grade, LVSI, ERα, PR, P53, Ki67 have statistical significance (P-value <0.05). The AUC value of combined ratio is 0.876, which is the best. The best cutoff value of combined ratio is 1.38
Conclusion
The combined ratio cutoff value of 1.38 in this study can be used for prediction of risk of lymph node metastasis in early-stage endometrial cancer patients and provide a reference for therapeutic planning.
Introduction
Endometrial cancer is a common malignant cancer in women, especially for perimenopausal and postmenopausal women. The incidence of endometrial cancer is growing in both developed and underdeveloped regions. With changes in fertility concept and economic development, the incidence in East Asia and South Asia showed a rapid growth trend [Citation1]. According to the recommendations of the international guidelines [Citation2], low-risk patients (low- and medium-grade endometrial cancer, without deep muscle infiltration, which means infiltration depth was less than 1/2, or cervical stromal infiltration) usually did not undergo lymph node resection (including lymph node biopsy, pelvic lymph node dissection, and para-aortic lymph node dissection). However, lymph node metastasis had already occurred in some of these patients [Citation3]. Biopsy of sentinel lymph node has been generally proven to be effectual [Citation4, Citation5], but its low sensitivity may lead to missing out some patients with lymph node metastasis [Citation3]. The occurrence of lymph node metastasis without proper treatment will result in increasing risk of recurrence and poor prognosis. However, patients who had undergone lymph node resection (including lymph node biopsy, pelvic and para-aortic lymph node ressection) would probably have lower limb lymphedema [Citation6], urinary incontinence [Citation7], and other complications after surgery. Accurately determining whether a patient needs lymph node resection will strongly influence the survival and life quality of patient with endometrial cancer after surgery.
There are many studies focusing on predicting the risk of lymph node metastasis in patients with endometrial cancer at early-stage. They attempted to define the risk of lymph node metastasis in these patients by age, presence of lymphatic vascular invasion, tumor grade, carbohydrate antigen 125 (CA125), estrogen receptor α (ERα), progesterone receptor (PR), Ki67, and P53 [Citation8–11].It is of great significance for surgeons to identify patients with relative high risk of lymph node metastasis in low-risk patients, but there is still controversy over whether to perform lymph node resection or further enlarged the area of lymph node removal during surgery [Citation12].Our retrospective study attempted to establish a simple and easy-to-use ratio model of lymph node metastasis risk, which was comprehensively using of common immunohistochemical indicators - ERα, PR, Ki67, P53, to identify patients with a higher risk of lymph node metastasis in low-risk patients. Therefore, in the hope that the combined ratio model in this study can be used to provide a reference for perioperative surgery planning and postoperative therapeutic schedule.
1. Materials and methods
1.1. Research objects
All methods were performed in accordance with relevant guidelines and were in line with World Medical Association Declaration of Helsinki. 1280 patients who had received primary surgery at the First Affiliated Hospital of Chongqing Medical University from 2013 to 2020 were collected as training cohort, and 873 patients from the Second Affiliated Hospital of Chongqing Medical University from 2016 to June 2020 were also collected as validation group. We used data from another institution as a validation group for external resource to improve the credibility of this study. These patients were all in the FIGO staging [Citation13]I-III, which was established by the International Federation of Obstetrics and Gynecology in 2009. The inclusion criteria were as follows: ① postoperative pathological diagnosis was endometrioid carcinoma and met the FIGO staging diagnosis of I-III; ② complete case data, including age, BMI, whether with hypertension and diabetes, records of surgery, postoperative pathological results (such as grading and histological type, depth of invasion, etc.), percentage of positive cells of 4 immunohistochemical indexes (ERα, PR, Ki67, P53). The following cases in training cohort and validation cohort were excluded: ① patients who have not received standardized surgical treatment; ② patients who haven’t undergo lymph node dissection (including lymph node biopsy, or pelvic lymph node dissection, or para-aortic lymph node dissection); ③ patients accompanied with others malignant tumors; ④ patients with incomplete case data; ⑤ patients who were missing (without any follow-up); ⑥ patients with at least one of these high-risk factors for lymph node metastasis: high grading endometrioid carcinoma, non-endometrioid carcinoma, accompanied by deep muscle layer invasion (the depth of muscle layer infiltration is greater than or equal to 1/2), accompanied by cervical stroma infiltration. Finally, the number of cases for the training cohort in this study met the criteria was 571, and 389 of them had a follow-up time of at least two years. 351 cases from the Second Affiliated Hospital of Chongqing Medical University (all of them had a follow-up time of at least two years) who met the same inclusion and exclusion criteria were served as the validation cohort.
1.2. Treatment and follow-up
All patients had received total hysterectomy and bilateral oviduct oophorectomy, with or without lymph node dissection (sentinel lymph node, pelvic lymph node, and para-aortic lymph node), which was performed by senior surgeons. After surgery, other postoperative adjuvant therapy (chemotherapy or radiotherapy), as well as the dose, frequency and cycle of adjuvant therapy should be determined according to the guidance of multidisciplinary consultation and international guidelines [Citation2]. The postoperative follow-up methods were mainly outpatient service and telephone follow-up. Follow-up would be conducted every 3 months in the first 2 years after the operation, every 6 months for the following three-years, and once a year after 5 years. The content mainly includes a physical examination, necessary imaging examination, and blood biochemical examination. According to physical examination and imaging examination, recurrence was confirmed, including X-ray, CT, PET/CT, MRI, and other special examinations. Recurrence was divided into vaginal stump recurrence, peritoneal metastasis (peritoneal cancer), distant metastasis [Citation14], according to the location of recurrence. Recurrence-free survival is defined as the time between the date of surgery and the date of recurrence (proved by imaging or histology) [Citation15].Death is defined as death due to postoperative recurrence. And overall survival is defined as the time between the date of surgery and the patient’s date of death [Citation16].
1.3. Histological and pathological procedure
We used formalin to fix all postoperative specimens in specified time and then transported them to the department of pathology of Chongqing Medical University for the following process. The specimens were processed in accordance to the same standards [Citation17] which was the working guidelines of pathological slices staining and immunohistochemistry in the department of pathology. In brief, we used paraffin to fix the tissue specimens. After that, the tissue would be made into 2-3 micrometers slices. The representatively cancerous parts were confirmed and chosen by H&E staining. The cancer tissue histological type, size of lesion, grade, and invasion depth and area were firstly judged by the junior pathologists, then rechecked by superior pathologist. Immunohistochemistry (IHC) of four indicators (ER, PR, Ki67 and P53) was performed by an automated Immuno-Stainer (manufactured by Leica Bond-Max) according to the instruction [Citation18]. The slices were dried for 12 hours at 60 °C. Then xylene was used to deparaffinized. After that the slices was hydrated. The slices were performed epitope retrieval at a temperature of 100 °C for about twenty minutes, cooled down to 20 °C, and then bathed in 0.3% H2O2 solution of methanol for 5 minutes for blocking endogenous peroxidase activity. After antigen retrieval, the slices would be performed antibody incubation respectively: ER (SP1, in 1:50), PR (MX009, in 1:500), Ki67 (MX006, in 1:300), and P53 (MX008, in 1:200) (Maixin Biotech, China). The samples were incubated for about half an hour at 20-25 °C with an anti-mouse secondary antibody (manufactured by Leica). 3,3′-diaminobenzidine tetrahydrochloride (DAB Substrate System, DAKO) and hematoxylin were used to color the slices. The slices were washed by phosphate-buffered saline (PBS) during each step.
Immunohistochemical results of ERα, PR, Ki67 and P53 were independently assessed by two qualified pathologists. The ultimate results were considered as the average percentage of positively stained tumor cells (0–100%) in five random microscope fields. Pathologists’ assessments for the percentage of positively stained tumor cells were considered identical if the results differed ≤10%; Otherwise, the primary evaluation was considered to inaccurate (as the results differed >10%), then the two pathologists would reevaluate (unblinded) and reached a consensus toward the result. The average proportion of two pathologists’ results represented the result of the slices.
1.4. Statistical analysis
Statistical analysis was performed by using SPSS 26.0 for data processing. Firstly, we conducted univariate and multivariate analysis of the variables that may affect lymph node metastasis by logistic regression. The results of 4 indicators (ERα, PR, Ki67, P53) were recorded as the percentage. The combined ratio model of the four indicators was defined as (ERα + PR)/(Ki67 + P53) [Citation19],which had been raised in other studies. And then the receiver operating characteristic curve (ROC curve) and the Yorden index were used for determining the optimal cutoff value of the combined ratio. The area under the ROC curve (AUC value) were calculated for comparing different accuracy of combined ratio model and each single-factor model for prediction of the risk of lymph node metastasis. The patients were divided into low- and high-risk groups by the cutoff value. The different survival rates were identified by Kaplan-Meier (KM) analysis. P < 0.05 (two-sided) was considered to have statistical difference.
2. Results
2.1. Clinical data characteristics
We compared the clinical characteristics of the two cohorts. We excluded 709 patients with common high-risk factors of lymph node metastasis according to the inclusion and exclusion criteria, and the remaining 571 patients were served as the training cohort of this study. Among them, 389 cases were followed up for more than two years. At the same time, we have collected 873 cases and 522 of them were excluded according to the same criteria. The remaining 351 cases (all of them have followed up for at least two years) were served as the validation cohort. The details of inclusion and exclusion were shown in . The median follow-up time in the training cohort was 48 months, with a range of 9-79 months. While in the validation cohort it was 46 months, with a range of 7-79 months. records the basic clinical data characteristics of both training and validation cohorts. The median age of the patients was 54.49, and the median BMI was 24.90 kg/m2 in training cohort. While in validation cohort, the median age was 53.46, and the median BMI was 25.04 kg/m2. 53.4% of cases were Grade 1, and 46.6% were Grade 2 in training cohort. While in validation cohort, 59.3% cases were Grade 1 and 40.7% were Grade 2. There were 22.4% and 28.2% cases had a serum CA125 of greater than 35 U/ml, and 19.8% and 18.5% cases had lymphatic vascular invasion in training and validation cohorts, respectively. The median positive percentages of ERα, PR, Ki67 and P53 in training cohort were 90%, 90%, 30% and 20%, respectively. And the median positive percentages of ERα, PR, Ki67 and P53 in validation cohort were 85%, 75%, 30% and 20%, respectively. The results indicated that there was no statistical significance except CA125 between training and validation cohorts.
Table 1. The clinical features of cases which were included in training cohort and validation cohort.
2.2. Lymph node resection and lymph node metastasis
shows the numbers and extent of lymph node resection confirmed by pathological examination after surgery, and whether lymph node metastasis had occurred. In training cohort, 571 patients all had received at least pelvic lymph node dissection, of which 155 patients received pelvic lymph node dissection as well as para-aortic lymph node dissection at the same time. The median number of total dissected lymph nodes was 32, the median number of dissected pelvic lymph nodes was 30, and the median of dissected para-aortic lymph nodes was 10. According to the results of postoperative pathological examination, 480 of them had no lymph node metastasis, 79 of them had pelvic lymph node metastasis, and 12 of them had pelvic lymph node as well as para-aortic lymph node metastasis.
Table 2. Clinical data of lymph node metastasis and lymph node resection.
While in validation cohort, 351 patients all had received at least pelvic lymph node dissection as well, of which 81 patients received pelvic lymph node dissection and para-aortic lymph node dissection. The median of total dissected lymph nodes was 31, the median of dissected pelvic lymph nodes was 29, and the median of dissected para-aortic lymph nodes was 8. According to the results of postoperative pathological examination, 304 patients had no lymph node metastasis, 28 patients had pelvic lymph node metastasis, and 9 patients had pelvic lymph node and para-aortic lymph node metastasis. There was no case of only para-aortic lymph node metastasis in this study.
2.3. Univariate and multivariate analysis
We used logistic regression to analyze the variables that may significantly impact on lymph node metastasis in training cohort. Univariate analysis showed that Age (P = 0.123), BMI (P = 0.397) had no statistical significance. Therefore, the remaining factors (P < 0.05): CA125, grade, lymphatic vascular invasion (LVSI), ERα, PR, P53 and Ki67 were further analyzed by multivariate analysis. Age, BMI, ER, PR, P53, Ki67 were served as continuous variables. CA125, Grade, LVSI were served as categorical variables. The results were shown in .
Table 3. Result of univariate and multivariate analysis for risk of lymph node metastasis.
2.4. Comparison of prediction accuracy and cutoff value
We further tested the accuracy of the model based on the combined ratio which is defined as the value of (ERα + PR)/(Ki67 + P53) and tried to identify the best cutoff value for this model. The ROC curve was used to compare the combined ratio and other single-factor models’ inaccuracy of prediction, which were shown in , and the AUC value were recorded in . The AUC value of the combined ratio is 0.876, which is greater than the AUC value of other single factors. It was obvious that the combined ratio has the best accuracy in predicting lymph node metastasis. We used Youden index to find the best cutoff value of combined ratio. According to the definition of the Youden index (which was defined as sensitivity + specificity −1), the best threshold of the combined ratio was 1.38 in training cohort which was shown in . While in validation cohort, the prime cutoff value of the combined ratio could be obtained as 1.40, which also proved the universality of the cutoff values we obtained.
Figure 1. The procedure of inclusion and exclusion.
Description: Both training cohort (n = 571) and validation cohort (n = 351) met the same criteria.
Figure 2. ROC curve of combined ratio and other single-factor models.
Description: ROC curve suggests that the area under the curve (AUC) of combined ratio which defined as the value of (ERα + PR)/(Ki67 + P53) is the largest. The values are recorded in . The area under the curve (AUC) value at the black dot is the highest. And the dot stands for the value here is the best threshold of the combined ratio for predicting lymph node metastasis. The best cutoff value of the combined ratio can be obtained as 1.38 in training cohort, while in validation cohort can be obtained as 1.40. Abbreviations: CA125, carbohydrate antigen 125; LVSI, lymphatic vascular invasion; ERα, estrogen receptor α; PR, progesterone receptor.
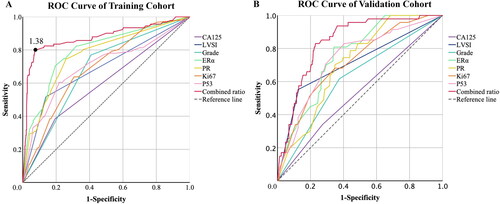
Table 4. Area under the ROC curve of models constructed with combined ratio and other single factors in prediction of lymph node metastasis.
2.5. Analysis of differences in survival of patients stratified by the cutoff value
The patients were grouped according to the threshold we obtained, and we compared the survival difference between the two groups. According to the cutoff value obtained in the training cohort, patients with a cutoff value less than 1.38 were divided into the high-risk group of lymph node metastasis, while patients with a cutoff value greater than or equal to were divided into the low-risk group of lymph node metastasis. KM analysis was performed to analyze the different survival between the two groups of patients (314 cases as the low-risk group and 75 cases as the high-risk group, 389 cases in total as training cohort; 266 cases as the low-risk group and 85 cases as the high-risk group, 351 cases in total as training cohort, all of these cases had a follow-up time of at least two years). Log-rank was used to test the difference. The result is shown in and .
Figure 3. Kaplan-Meier analysis of differences in survival of patients stratified by the cutoff value.
Description: We used the combined ratio which obtained in training cohort to divide patients with a follow-up times at least two years into low-risk group and high-risk group. The training cohort: 314 cases in low-risk group and 75 cases in high-risk group. The validation cohort: 266 cases in low-risk group and 85 cases in high-risk group. (A) RFS curve of cohorts grouped by the combined ratio in training cohort. (B) OS curve of cohorts grouped by the combined ratio in training cohort. (C) RFS curve of cohorts grouped by the combined ratio in validation cohort. (D) OS curve of cohorts grouped by the combined ratio in validation cohort.
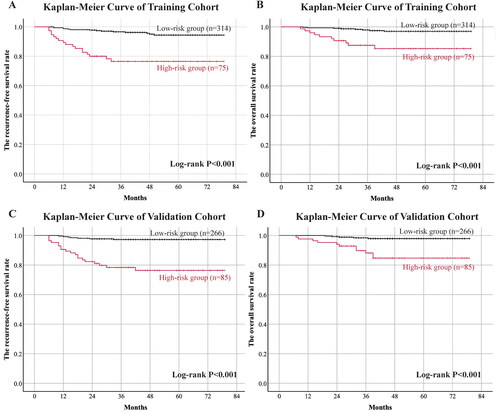
Table 5. The survival rates of high-risk and low-risk group in training and validation cohorts.
By the end of the follow-up time in training cohort, there were 14 recurrence cases (4.5%) in the low-risk group, with a three-year recurrence free survival rate of 96.7% and 95% confidence interval of 94.7% to 98.7%. There were 17 relapsed cases in the high-risk group, accounting for 22.7%, and the three-year recurrence-free survival rate was 76.5%, with a 95% confidence interval (CI) of 66.7%-86.3%, P < 0.001. 8 of them appeared to death (2.5%) in the low-risk group, with a three-year overall survival rate of 97.9% (95% CI 96.1% to 99.7%). There were 10 deaths (13.3%) in the high-risk group, and the three-year overall survival rate was 87.5% (95% CI 79.9% to 95.1%), P < 0.001.
While in validation cohort, there were 7 recurrence cases (2.6%) in the low-risk group, with a three-year recurrence free survival rate of 97.3% and 95% confidence interval of 95.3% to 99.3%. There were 19 relapsed cases in the high-risk group, accounting for 22.4%, and the three-year recurrence-free survival rate was 78.4%, with a 95% confidence interval of 69.6%-87.2%, P < 0.001. There appeared 5 death cases (1.9%) in low-risk group, account for a three-year overall survival rate of 98.4% (95% confidence interval 96.8% to 99.9%). There were 11 deaths (12.9%) in the high-risk group, with a three-year overall survival rate of 88.2% (95% CI 80.8% to 95.6%), P < 0.001.
3. Discussion
The NCCN guideline still retains the recommendation that lymph node dissection is an option for patients with endometrial cancer in early stage. As for lymph node metastasis has already occurred in some of these patients, accurate surgical staging and suitable adjuvant therapy play an important role in the prognosis of these patients. Patients with lymph node metastasis have a high risk of postoperative recurrence without accurate assessment, surgical staging, and appropriate adjuvant therapy. At present, CA125, lymphatic vascular invasion, grade, ERα, PR, Ki67, P53 and other methods are often used clinically for identifying the hazard of lymph node metastasis. We performed univariate and multivariate analysis of the current mainstream factors which may indicate the occurrence of lymph node metastasis in patients which were low risk of lymph node metastasis in traditional sense. These following factors had statistical differences: CA125, lymphatic vascular invasion, grade, ERα, PR, Ki67, and P53. Both in other experiments and our study, it has been confirmed that the lack of ERα and PR and the high expression of Ki67 and P53 are risk factors of lymph node metastasis in patients with endometrial cancer. Clinical applications of ERα, PR, Ki67, and P53 [Citation20–23] have been widespread, and they have the advantages of easy operation and low cost. However, the application of these indicators is still controversial. There may be patients with the separation of four indicators (for example, a patient with 80% of ERα and PR positive cells and 80% of Ki67 and P53 positive cells). If we only use ERα or PR as a single factor for analysis and prediction, the result suggests that this patient is at low risk of lymph node metastasis. While the result based on Ki67 or P53 suggests that the risk of lymph node metastasis for this patient is high. Therefore, it is difficult for the surgeon to determine whether to perform lymph node resection, extend the area of lymph node resection or more aggressive adjuvant therapies for this case. We defined the combined ratio as the value of (ERα + PR)/(Ki67 + P53) [Citation19], which had been confirmed by other studies [Citation19, Citation24]. These studies claimed that comprehensive usage of these four indicators was obviously superior to the single indicator, which was undoubtedly very practical and interesting.
To facilitate the clinical application, we needed to find a cutoff value to guide the surgeon to make judgments. With the combination ratio as the continuous variable and the appearance of lymph node metastasis as the state variable, the ROC curve was used to find the highest dot of the Yorden index as the cutoff value, which was 1.38 in training cohort, with a sensitivity of 80.2% and specificity of 92.7%. We also obtained similar cutoff value in validation cohort. The result also showed that the combined ratio has the largest area under the curve in two cohorts, which meant it has the best accuracy in predicting lymph node metastasis. It reminded us that we should take note of the use of the combined ratio in clinical application rather than a single indicator. To verify the effectiveness of this cutoff value, patients in two cohorts in this study who were followed up for more than two years were divided into groups by this cutoff value, and the recurrence-free survival rates and overall survival rates of the two groups were calculated respectively. Results indicated that patients below this cutoff value (defined as the high-risk group) had more postoperative recurrence and lower overall survival than those in the low-risk group. This suggested that we could use this cutoff value to identify the risk of lymph node metastasis for patients who were low risk of lymph node metastasis in traditional sense, providing a new reference standard for surgeons before surgery. Expanding the scope of lymph node dissection or appropriate adjuvant therapies can more effectively prevent early-stage patients from recurrence due to inadequate treatment in or after the surgery; it can also prevent a series of postoperative complications due to excessive surgical treatment as well as reduce the cost of treatment for patients. Based on our model, the combined ratio of the patient that we mentioned in the previous part is calculated as 1, which indicates that the patient belongs to the high-risk group of lymph node metastasis; Therefore, expanding the scope of lymph node dissection or more aggressive postoperative adjuvant therapies should be performed for this patient.
Current studies have shown that non-endometrioid carcinoma, pathological grade 3, deep muscular invasion, tumor diameter (≥2 cm), and cervical infiltration probably stands for a high risk of lymph node metastasis [Citation25]. There seems to be no controversy about these patients should receive systematic lymph node dissection. Therefore, patients with these factors were excluded. Studies have indicated that no more than 15% of patients with early-stage disease have lymph node involvement [Citation26]. While these patients still showed differences in prognosis. Thus, accurate staging of these patients and risk prediction are particularly important to decide the treatment strategy. Some of these pathological factors (for example: lymph vascular invasion) can only be determined by the postoperative specimen. And such categorical variables were hard to be included in this model, as other four indicators were served as continuous variables to give full function of its prediction potential. And serum CA125 has no direct correlation with pathological indicator. Therefore, these factors are not included in the model. While ERα, PR, Ki67, and P53 can be obtained by preoperative endometrial curettage. Despite differences in preoperative and postoperative histopathology, a study by Corr, Bradley R showed that discrepancy of preoperative and postoperative histopathology which would adjust the perioperative decision for lymph node dissection occurs in only 0.7% of cases [Citation27]. Another study noted that the continuous innovation of technology had greatly improved the accuracy of preoperative histopathology [Citation28]. Even if there is doubt about the preoperative application, this model can also be used to guide surgeon after surgery whether they should conduct more active adjuvant treatment or even secondary surgery to patients. Several studies [Citation19, Citation24] have taken the combined ratio as a vital factor for prediction of the recurrence or metastasis of endometrial cancer patients and have also confirmed its effectiveness and accuracy. Many studies have reported the role of sentinel lymph node mapping (SNM), which is also a promising method [Citation29, Citation30]. SNM were more sensitive in detecting low-volume disease, also had an advantage of reducing the risk of post-operative complications [Citation31]. While the combined ratio can be obtained before surgery. Thus, the cutoff value derived in this study can guide the operator in preoperative whether to perform lymph node dissection or not and, more importantly, predict the prognosis of patient (whether to apply with appropriate adjuvant therapy etc.) Patients with combined ratio below the cutoff value should be considered for intraoperative SNM or SNM back-up lymph node dissection, although lymph node dissection has not been proven to improve prognosis. The decision to perform lymph node dissection is not the most critical point, but rather we should focus on the accurate staging of the patient and the prediction of risk in recurrence or death. Hence, as a preoperative indicator, the combined ratio can be used in cooperate with intraoperative sentinel lymph node mapping to more accurately stage and predict the prognosis of patients and thereby guide the treatment planning. Further research is also required. Compared with others, our study conducted a more detailed stratification of patients, focusing on low-risk endometrial cancer patients, so that the cutoff value obtained is more practical and meaningful.
There are also some limitations to this experiment. First, this experiment is a retrospective experiment, and there may be some deviations due to data collection or follow-up. Second, we used the data of postoperative pathology to construct our model for its completeness and accuracy. The consistency of preoperative and postoperative samples is also a controversial issue. Again, the number of cases in this study can be further expanded due to the limited number of follow-up years. Finally, although immunohistochemistry has become a common method in postoperative medical examinations for patients, and its practicality has been confirmed, the interpretation of immunohistochemistry still needs to develop a wide range of international standards to avoid misinterpretation caused by subjective factors, which can better promote the wide application of this model.
Nevertheless, the combined ratio cutoff value proposed in this study has a high reference value in prediction of the risk of lymph node metastasis for patients at early-stage. Surgeons can use the preoperative endometrial diagnosing and curettage results as a reference. The combined ratio cutoff value is used to preoperatively judge patients who were low risk in traditional sense: patients have a combined ratio lower than 1.38 are considered high-risk groups. So, lymph node dissection should be performed, or the area of lymph node dissection can be expanded, or more aggressive postoperative adjuvant treatment can be considered for these patients; those higher than 1.38 are considered as a low-risk group. For these patients in low-risk group, if there are no obvious signs of lymph node metastasis, lymph node dissection may not be required, or the area of the dissection may be limited.
Author contributions
Research design, data collection and analysis were conducted by Yuzhen Huang, Peng Jiang, Wei Kong, Yuan Tu, Ning Li, Jinyu Wang, Qian Zhou and Rui Yuan. The draft was written by Yuzhen Huang and the authors reached to consensus toward final manuscript.
Ethics approval
Approved by Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (2020-192).
Acknowledgments
We would like to thank the gynecology department of the First Affiliated Hospital of Chongqing Medical University for subject enrollment and data interpretation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978–2013. J Natl Cancer Inst. 2018;110(4):1–9. doi:10.1093/jnci/djx214.
- NCCN. NCCN Guidelines for Uterine Neoplasms Version 1.2022. NCCN Guidelines www.nccn.org. (2022).
- Ballester M, Dubernard G, Lécuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011;12(5):469–476. doi:10.1016/S1470-2045(11)70070-5.
- Abu-Rustum NR. The increasing credibility of sentinel lymph node mapping in endometrial cancer. Ann Surg Oncol. 2013;20(2):353–354. doi:10.1245/s10434-012-2685-8.
- Cormier B, Rozenholc AT, Gotlieb W, Communities of Practice (CoP) Group of Society of Gynecologic Oncology of Canada (GOC), et al. Sentinel lymph node procedure in endometrial cancer: a systematic review and proposal for standardization of future research. Gynecol Oncol. 2015;138(2):478–485. doi:10.1016/j.ygyno.2015.05.039.
- Franchi M, Ghezzi F, Riva C, et al. Postoperative complications after pelvic lymphadenectomy for the surgical staging of endometrial cancer. J Surg Oncol. 2001;78(4):232–237. discussion 237240, doi:10.1002/jso.1158.
- Lipetskaia L, Sharma S, Johnson MS, Ostergard DR, Francis S. Urinary incontinence and quality of life in endometrial cancer patients after robotic-assisted laparoscopic hysterectomy with lymph node dissection. J Obstet Gynaecol. 2019;39(7):986–990. doi:10.1080/01443615.2019.1584887.
- Espiau Romera A, Cuesta Guardiola T, Benito Vielba M, et al. HE4 tumor marker as a predictive factor for lymphatic metastasis in endometrial cancer. Int J Gynaecol Obstet. 2020;149(3):265–268. doi:10.1002/ijgo.13140.
- Han S-S, Lee SH, Kim DH, et al. Evaluation of preoperative criteria used to predict lymph node metastasis in endometrial cancer. Acta Obstet Gynecol Scand. 2010;89(2):168–174. doi:10.3109/00016340903370114.
- Taşkın S, Şükür YE, Varlı B, et al. Nomogram with potential clinical use to predict lymph node metastasis in endometrial cancer patients diagnosed incidentally by postoperative pathological assessment. Arch Gynecol Obstet. 2017;296(4):803–809. doi:10.1007/s00404-017-4477-7.
- Wakayama A, Kudaka W, Matsumoto H, et al. Lymphatic vessel involvement is predictive for lymph node metastasis and an important prognostic factor in endometrial cancer. Int J Clin Oncol. 2018;23(3):532–538. doi:10.1007/s10147-017-1227-6.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109(1):11–18. doi:10.1016/j.ygyno.2008.01.023.
- Amant F, Mirza MR, Koskas M, Creutzberg CL. Cancer of the corpus uteri. Int J Gynecol Obstet. 2018;143:37–50. doi:10.1002/ijgo.12612.
- Ouldamer L, Bendifallah S, Body G, et al. Predicting poor prognosis recurrence in women with endometrial cancer: a nomogram developed by the FRANCOGYN study group. Br J Cancer. 2016;115(11):1296–1303. doi:10.1038/bjc.2016.337.
- Huijgens AN, Mertens HJ. Factors predicting recurrent endometrial cancer. Facts Views Vis Obgyn. 2013;5(3):179–186.
- Ouldamer L, Bendifallah S, Body G, Groupe de Recherche FRANCOGYN, et al. Change in hazard rates of recurrence over time following diagnosis of endometrial cancer: an age stratified multicentre study from the FRANCOGYN group. Eur J Surg Oncol. 2018;44(12):1914–1920. doi:10.1016/j.ejso.2018.07.053.
- Jiang P, Jia M, Hu J, et al. Prognostic value of Ki67 in patients with stage 1-2 endometrial cancer: Validation of the cut-off value of Ki67 as a predictive factor. Onco Targets Ther. 2020;13:10841–10850. doi:10.2147/OTT.S274420.
- Yang B, Shan B, Xue X, et al. Predicting lymph node metastasis in endometrial cancer using serum CA125 combined with immunohistochemical markers PR and Ki67, and a comparison with other prediction models. PLoS One. 2016;11(5):e0155145. doi:10.1371/journal.pone.0155145.
- Gulseren V, et al. Do estrogen, progesterone, P53 and Ki67 receptor ratios determined from curettage materials in endometrioid-type endometrial carcinoma predict lymph node metastasis? Curr Probl Cancer. 100498;2020;44 doi:10.1016/j.currproblcancer.2019.07.003.
- Di Donato V, Iacobelli V, Schiavi MC, et al. Impact of hormone receptor status and Ki-67 expression on disease-free survival in patients affected by high-risk endometrial cancer. Int J Gynecol Cancer. 2018;28(3):505–513. doi:10.1097/IGC.0000000000001191.
- Li M, Zhao L, Shen D, et al. Clinical implications and prognostic value of single and combined biomarkers in endometrial carcinoma. Chin Med J (Engl). 2014;127(8):1459–1463.
- Markova I, Duskova M, Lubusky M, et al. Selected immunohistochemical prognostic factors in endometrial cancer. Int J Gynecol Cancer. 2010;20(4):576–582. doi:10.1111/IGC.0b013e3181d80ac4.
- Tomica D, Ramić S, Danolić D, et al. Impact of oestrogen and progesterone receptor expression in the cancer cells and myometrium on survival of patients with endometrial cancer. J Obstet Gynaecol. 2018;38(1):96–102. doi:10.1080/01443615.2017.1328591.
- Jia M, Jiang P, Huang Z, et al. The combined ratio of estrogen, progesterone, Ki-67, and P53 to predict the recurrence of endometrial cancer. J Surg Oncol. 2020;122(8):1808–1814. doi:10.1002/jso.26212.
- Sari ME, Yalcin I, Sahin H, Meydanli MM, Gungor T. Risk factors for paraaortic lymph node metastasis in endometrial cancer. Int J Clin Oncol. 2017;22(5):937–944. doi:10.1007/s10147-017-1139-5.
- Bogani G, Dowdy SC, Cliby WA, et al. Management of endometrial cancer: issues and controversies. Eur J Gynaecol Oncol. 2016;37(1):6–12.
- Corr BR, Carrubba A, Sheeder J, Cheng G, Guntupalli SR. Histopathology discrepancy of preoperative endometrial sampling and final specimen: How does this influence selective lymph node dissection? Int J Gynecol Cancer. 2017;27(2):297–301. doi:10.1097/IGC.0000000000000866.
- Di Spiezio Sardo A, De Angelis MC, Della Corte L, et al. Should endometrial biopsy under direct hysteroscopic visualization using the grasp technique become the new gold standard for the preoperative evaluation of the patient with endometrial cancer? Gynecol Oncol. 2020;158(2):347–353. doi:10.1016/j.ygyno.2020.05.012.
- Abu-Rustum NR. Sentinel lymph node mapping for endometrial cancer: a modern approach to surgical staging. J Natl Compr Canc Netw. 2014;12(2):288–297. doi:10.6004/jnccn.2014.0026.
- Bogani G, Casarin J, Leone Roberti Maggiore U, et al. Survival outcomes in endometrial cancer patients having lymphadenectomy, sentinel node mapping followed by lymphadectomy and sentinel node mapping alone: Long-term results of a propensity-matched analysis. Gynecol Oncol. 2020;158(1):77–83. doi:10.1016/j.ygyno.2020.04.691.
- Bogani G, Di Donato V, Papadia A, et al. Evaluating long-term outcomes of three approaches to retroperitoneal staging in endometrial cancer. Gynecol Oncol. 2022;166(2):277–283. doi:10.1016/j.ygyno.2022.06.007.
