Abstract
Objective
Cervical cancer is linked with the constitutive activation of growth factors and gene mutations-induced pro-survival signaling pathways. Herein, we purposed to explore the possible molecular mechanism of Foxo3a-mediated DNMT3B in the proliferation and migration of cervical cancer cells via mediating the PTEN promoter methylation.
Methods
Foxo3a expression in cervical cancer was tested by qRT-PCR and western blot experiments. The cervical cancer cell biological functions with overexpression of Foxo3a were evaluated by CCK-8 assay, Transwell experiment, and flow cytometry, respectively. MS-PCR was utilized for testing the PTEN methylation levels, and ChIP experiment was implemented for evaluating the enrichment of DNMT3B in the PTEN promoter region and the binding of Foxo3a and DNMT3B. The PTEN methylation and interference with Foxo3a expression were performed in cervical cancer cells, and then their impacts on cervical cancer cell biological functions were observed.
Results
FOXO3a was expressed at a low level in cervical cancer, and its overexpression contributed to a reduction in cell proliferative, migratory and invasive capabilities, and an elevation in apoptosis rate. Foxo3a blocked its methylation with the PTEN promoter by repressing DNMT3B activity. Upon treatment with methyltransferase inhibitor (5-aza-dc), the malignant phenotypes of cervical cancer cells were diminished. 5-aza-dc neutralized the impacts of silencing Foxo3a on malignant phenotypes.
Conclusion
This research underlines that Foxo3a blocks its methylation with the PTEN promoter by inhibiting DNMT3B activity, which subsequently impedes cervical cancer cell progression.
Keywords:
Introduction
Cervical cancer is one of the most frequent gynecological diseases all over the world, and most of the cases occur in these middle-and low income countries [Citation1]. Cervical cancer is attributed to various risk factors, including sexually transmitted infections, socioeconomic factors, family history, bad living habits, as well as prolonged usage of oral contraceptives [Citation2]. Cervical cancer is both preventable and curable with early detection and adequate treatment [Citation3]. At present, the primary treatment approaches for cervical cancer consist of radiotherapy, surgical resection, immunotherapy, as well as local targeted therapy [Citation4]. These therapies only show effective performances for early-stage cervical cancer, while for locally staged and metastatic ones, they have limited efficacy because of their drug resistance, severe side effect, recurrences, and metastases [Citation5]. Consequently, there is an urgent demand to seek novel biomarkers for better diagnosis and treatment of cervical cancer.
Like most other cancers, cervical cancer is also linked with the constitutive activation of growth factors and gene mutations-induced pro-survival signaling pathways [Citation6]. Epigenetic changes exert functions in cervical cancer development, and DNA methylation is an epigenetic modification in which cells modulate gene transcription and expression [Citation7]. Forkhead/winged helix box (FOXO)3a, belongs to the class of FOXO transcription factors, acts as an anti-tumor factor implicated in different cellular processes [Citation8]. FOXO3a is modified by acetylation, ubiquitination, and phosphorylation, which, on the contrary, impacts the transcriptional activity and stability, and unclear/cytoplasm shuttling [Citation9,Citation10]. As previously demonstrated, the suppressive role of FoxO3a is realized through controlling the gene expression involved in oxidative stress, apoptosis, as well as autophagy [Citation11,Citation12]. It is reported that FOXO3a plays a part in cervical cancer cells [Citation13], while its inner mechanism remains to be undefined. Yang et al. have supported that FOXO3a negatively modulates the DNA methyltransferase 3 beta (DNMT3B) promoter activity [Citation13]. More interestingly, Li et al. in their work have demonstrated that DNMT3B expression has a correlation with PTEN level, and there is a positive relationship between DNMT3B overexpression and PTEN loss [Citation14]. Based on this, we conducted this work to elucidate the possible molecular mechanism of Foxo3a-mediated DNMT3B in the proliferation and migration capabilities of cervical cancer cells via mediating the PTEN promoter methylation.
Materials and methods
Ethical approval
All patients and family members in this study gave informed consent, and this work was reviewed and approved by the ethics committee of Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology (approval number: 20170412).
Participants
Forty-five cervical cancer tissues and corresponding adjacent normal tissue specimens were harvested in the Obstetrics and Gynecology Department of Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology from May 2017 to December 2018. These patients were included if they had complete case data, were pathologically diagnosed with cervical cancer post-surgery, received no preoperative chemoradiotherapy, or no hormonal medication was taken within six months before surgery. Nevertheless, these patients who were combined with other malignant tumors, systemic infectious diseases, or severe liver and renal insufficiency, were excluded. All tissues were immediately placed in liquid nitrogen.
Cell culture
Human cervical epithelial immortalized cell lines H8 and human cervical cancer cell lines (HeLa, Caski, SiHa, C33A, and ME-180), from American Type Culture Collection (ATCC, Rockville, MD, USA), were cultivated in 10% fetal bovine serum (FBS)-contained DMEM at 37 °C with 5% CO2. These cells were subsequently passaged every 2 to 3 days, and those in the logarithmic phase were obtained for later experimental studies. For the overexpression experiments, pCMV-FOXO3a (oe-FOXO3a) and the control vector were used, and for the silencing experiments, FOXO3a shRNA and the control sequence were used. The above vectors and shRNA were synthesized by RIBOBIO (Guangzhou, China). Cervical cancer cell transfection was implemented using the Lipofectamine 3000 reagent (Invitrogen, Carlsbad, USA) as per the manufacturer’s requirements. The 5-aza-dc (a DNA methyltransferase inhibitor that causes re-expression of genes inactivated by CpG island methylation through demethylation) was purchased from Sigma (A3656, Beijing, China), and cervical cancer cells were cultivated for 48 hours with medium supplemented with 20 μM 5-aza-dc or dimethyl sulfoxide (DMSO). The cells in different groups upon transfection were cultivated for 48 hours at 37 °C with 5% CO2 for the subsequent correlation detection experiments.
RNA isolation and quantitative RT-PCR (qRT-PCR) assay
Trizol Reagent (15596026, Invitrogen) was utilized for extracting the total RNAs from cells and tissues. Under the PrimeScript RT reagent Kit (Takara, Japan) instructions, the RNAs were subjected to reverse transcription to cDNA. The synthesized cDNA was tested by qRT-PCR with the SYBR Green Premix Ex Taq II kit (Takara) and the ABI PRISM 7500 qRT-PCR system (Applied biosystems, Foster City, USA). Three replicates were set for each well. With GAPDH as an internal reference, gene expression was analyzed by the 2-ΔΔCt method. The primer design is listed in Supplementary Table 1.
Western blot
Total protein from the tissues and cells was extracted, and its oncentration was assessed using a BCA kit (AmyJet Scientific, Wuhan, Hubei, China). Next, the extracted proteins were blended with the 5× loading buffer (pH = 6.8), and bathed in boiling water for 5 minutes, followed by ice bathing and centrifugation. After that, the proteins were separated by 10% polyacrylamide gel electrophoresis, followed by transferring onto a nitrocellulose membrane. After that, the membrane was blocked for 1 hour with 5% skim milk, followed by incubation overnight with Foxo3a (ab109629; 1: 1000, Abcam, Cambridge, UK) and GAPDH (ab181602; 1: 10000, Abcam) at 4 °C, and horseradish peroxidase-labeled IgG (ab97051; 1:5000, Abcam) secondary antibody at 37 °C for l hour. The membrane was transferred to the ECL reaction solution (Pierce, USA) at room temperature for 1 minute, and the Bio-rad Gel Dol EZ Imager (GEL DOC EZ IMAGER, Bio-rad, California, USA) was implemented for development. The western blotting images of proteins were processed by ImageJ2x software,
Methylation-specific PCR (MS-PCR)
The methylated condition of the PTEN promoter was examined by MS-PCR. In detail, the genomic DNA was extracted under the requirements of the Genomic DNA extraction kit (TIANGEN BIOTECH CO., LTD., Beijing, China). The DNA concentration and purity were tested by UV spectrophotometry. The methylation level of the PTEN promoter region was evaluated using the DNA Methylation-GoldTM kit (D5005, Zymo Research, Irvine, CA, USA), with the methylated and nonmethylated primers for PTEN genes as described in Supplementary Table 2. gDNA (1 µg) was taken for bisulfite modification and kept at −80 °C for no more than 1 month. Using the reaction column for desulfurization and purification, the purified DNA was utilized for the next PCR reaction. The reaction products were subjected to agarose gel electrophoresis, and the image analysis was performed with gel electrophoresis imaging and an image analysis system. Only the methylated primers could amplify the target band if the CpG island in the promoter region of the PTEN gene was fully methylated; only the unmethylated primers could amplify the target band if completely unmethylated; both primer pairs could amplify the target bands if partially methylate. Partial methylation was assigned to the methylation category [Citation15].
Cell viability assay
Cells were inoculated onto 96-well plates at 2 × 103 cells/well and routinely cultured. At the 24th, 48th, and 72nd hour post-inoculation, each well was appended with 20 μL cell counting kit (CCK)-8 reagent, with subsequent 3-hours cultivation. Next, the optical density (OD) value at 450 nm wavelength was then examined using a microplate reader. Three replicate wells were set at each time point to calculate the mean value.
Cell apoptosis measurement
Corresponding treated cells were collected, and then appended to 500 μL pre-cooled 1× binding buffer. Next, the cell suspension was supplemented with 5 μL Annexin-V-FITC and 5 μL PI, gently mixed, and tested on flow cytometry (BD FACSArial I cell sorter). On the scatter plot, the lower left quadrant (Q4) reflected healthy living cells (FITC-/PI-); the lower right quadrant (Q3) represented early apoptotic cells (FITC+/PI-), the upper right quadrant (Q2) corresponded to necrotic and late apoptotic cells (FITC+/PI+). Lastly, the apoptosis rate was counted with the percentage of early apoptosis (Q3) + the percentage of late apoptosis (Q2).
Cell migration and invasion assay
Forty-eight hours post-transfection, the cells were digested in a serum-free medium for 24 hours. Upon washing, the cells in serum-free DMEM (5 × 104) were seeded in the upper chamber (50 mg/L Matrigel for invasion assay), while the 600 μL of complete medium was put in the lower chamber. Post 24 hours of incubation, the remaining cells on the upper surface were discarded, and the migrated or invaded cells were subjected to fixing with formaldehyde, dying with 0.1% crystal violet solution, followed by photographing and counting.
ChIP assay
The ChIP assay was implemented following the requirements of the ChIP kit (Millipore, Temecula, CA, USA). Reaching 70–80% cell confluence, the cells were fixed for 10 minutes in 1% formaldehyde for cross-linking the cellular DNA and protein. Subsequently, an ultrasonic breaker was set to 2 seconds for each ultrasonic cycle, with 5-second intervals, and 15 cycles were performed to break the chromatin at 120 w. Afterward, the supernatant was harvested post-centrifugation (4 °C, 13,000 rpm), and then sub-packed into two tubes. The tube was respectively probed with rabbit anti-IgG (6 µg/mL, ab6757, Abcam) and rabbit anti-DNMT3B (1:500, ab227883, Abcam) at 4 °C overnight. Next, the endogenous DNA-protein complex was precipitated with Protein Agarose/Sepharose. The supernatant was abandoned after transient centrifugation, and the nonspecific complex was rinsed and de-crosslinked at 65 °C overnight. Lastly, the phenol/chloroform was utilized for extracting and purifying the DNA fragments. Enrichment of DNMT3B in the PTEN promoter fragments was assessed by qRT-PCR using the PTEN promoter fragment-specific primers [Citation16]. The experiment was repeated 3 times.
Statistics
All data, expressed in the form of mean ± standard deviation, were processed with SPSS 21.0 Statistics software (SPSS, Inc., Chicago, IL, USA). The data between cancerous tissues and adjacent normal tissues were analyzed using the paired t-test, the other two groups, the independent sample t-test, and the multiple groups, the one-way ANOVA and Tukey’s multiple comparisons test. The data at different time points was performed by repeated measures ANOVA with the Bonferroni post hoc test. A p-value less than 0.05 was considered significant difference.
Results
FOXO3a is lowly expressed in cervical cancer tissues and cells
First, we found through the TCGA database that FOXO3a was lowly expressed in cervical cancer tissues (), and further qRT-PCR experiments verified FOXO3a expression in clinical samples of cervical cancer tissues and corresponding adjacent normal tissues. We observed that () FOXO3a in cervical cancer tissues showed a low expression versus adjacent normal tissues. Meanwhile, the FOXO3a expression of human cervical epithelial immortalized cell line H8 and human cervical cancer cell lines (Caski, HeLa, SiHa, C33A, and ME-180) was evaluated, with the findings disclosed that FOXO3a expression was downregulated in the aforesaid cervical cancer cells in comparison to H8 cells. Among these, HeLa cells, exhibited the most significant difference, were obtained for subsequent experiments (). The above outcomes unveil that FOXO3a shows a low expression in cervical cancer.
Figure 1. FOXO3a is differentially expressed in cervical cancer tissues and cells. (A) The TCGA database for predicting FOXO3a expression in cervical cancer tissues, and FOXO3a expression was high in cervical cancer tissues. (B) qRT-PCR test for verifying FOXO3a expression in cervical cancer tissues and adjacent normal tissues, and there was increased FOXO3a expression in cervical cancer tissues in comparison to adjacent normal tissues. (C and D) qRT-PCR and western blot experiments for testing FOXO3a expression in human cervical epithelial immortalized cell line H8 and human cervical cancer cell lines (Caski, HeLa, SiHa, C33A, and ME-180), and FOXO3a expression was downregulated in the aforesaid cervical cancer cells in comparison to H8 cells. *p < 0.05; **p < 0.01.
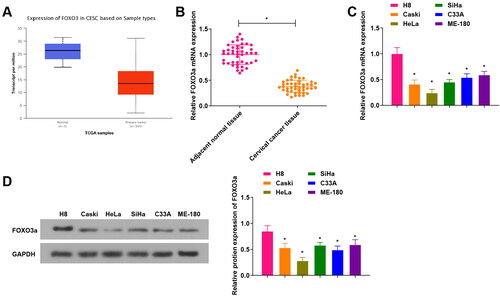
With the median value of FOXO3a mRNA expression level in the cervical cancer tissues, 45 cervical cancer patients were assigned into a FOXO3a low expression group (n = 22 cases) and a FOXO3a high expression group (n = 23 cases) to further elaborate the correlation between Foxo3a expression and clinicopathological features of cervical cancer (Supplementary Table 3). It was suggested that Foxo3a expression was not related to the patient’s age, but had a relationship with the International Federation of Gynecology and Obstetrics (FIGO) stage, differentiation degree, tumor diameter, as well as lymph node metastasis (LMN).
Overexpression of FOXO3a impedes malignant phenotypes of cervical cancer cells
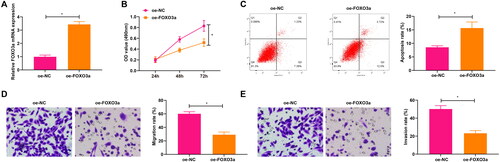
Overexpression of FOXO3a was processed and the transfection efficiency was validated by qRT-PCR experiments, with the findings demonstrating that FOXO3a expression was elevated in cells transfected with oe-FOXO3a in contrast to those transfected with oe-NC (). The CCK-8 assay unveiled that oe-FOXO3a transfection contributed to a diminished cell proliferation capacity in comparison to oe-NC transfection (). Obtained by flow cytometry results, the elevated cell apoptosis rate was witnessed upon oe-FOXO3a transfection in comparison to oe-NC transfection (). Results from the Transwell assay revealed suppression in cell migratory and invasive capabilities in response to oe-FOXO3a transfection when compared with oe-NC transfection (). It is concluded that overexpression of FOXO3a impedes malignant phenotypes of cervical cancer cells.
Figure 2. Overexpression of FOXO3a impacts the cervical cancer cell biological functions. (A) FOXO3a transfection efficiency in cells was measured by qRT-PCR, and FOXO3a expression was elevated in cells transfected with oe-FOXO3a in contrast to those transfected with oe-NC. (B) CCK-8 assay for evaluating the cell proliferation capacity upon transfection of oe-FOXO3a and oe-NC. (C) The cell apoptosis rate upon transfection of oe-FOXO3a and oe-NC was measured by flow cytometry. (D and E) The cell migratory and invasive capabilities upon transfection of oe-FOXO3a and oe-NC were assessed by Transwell assay. *p < 0.05.
FOXO3a promotes PTEN expression by inhibiting DNMT3B activity and then blocking its methylation with the PTEN promoter
As previously reported, FOXO3a negatively mediates the DNMT3B promoter activity through interacting with the binding element FOXO3a-E of the DNMT3B promoter [Citation17]. Besides, DNMT3b can repress PTEN expression through methylation [Citation18]. We speculated that Foxo3a may affect its occurrence of methylation with PTEN promoter by modulating DNMT3B activity.
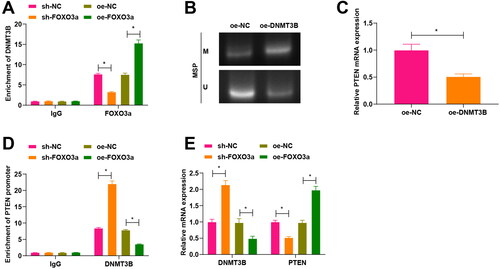
To prove the above conjecture, we conducted a ChIP experiment to figure out whether Foxo3a could bind to DNMT3B, and the obtained findings demonstrated that sh-FOXO3a treatment had a reduced enrichment of DNMT3B than sh-NC treatment, while oe-FOXO3a treatment had an elevated enrichment of DNMT3B than oe-NC treatment, indicating that Foxo3a could enrich for DNMT3B (). Meanwhile, the methylation status of the PTEN gene in cervical cancer cells was evaluated by MS-PCR, which revealed higher PTEN promoter methylation levels in cervical cancer cells with oe-DNMT3B treatment in comparison to those cells with oe-NC treatment (). The further qRT-PCR test also revealed a decrease in PTEN expression upon oe-DNMT3B treatment in contrast to oe-NC treatment ().
Figure 3. Foxo3a blocks its methylation with the PTEN promoter by inhibiting DNMT3B activity. (A) The binding of Foxo3a to DNMT3B was verified by ChIP test, and sh-FOXO3a treatment had a reduced enrichment of DNMT3B than sh-NC treatment, while oe-FOXO3a treatment had an elevated enrichment of DNMT3B than oe-NC treatment. (B) The methylation level of cells in each group was evaluated by MS-PCR experiment, and there were higher PTEN promoter methylation levels in cervical cancer cells with oe-DNMT3B treatment in comparison to those cells with oe-NC treatment. (C) PTEN mRNA expression after DNMT3B overexpression was tested by qRT-PCR, and decreased PTEN mRNA expression was observed upon oe-DNMT3B treatment in contrast to oe-NC treatment. (D) ChIP assay for examining DNMT3B enrichment in the PTEN promoter region after overexpression and silencing of Foxo3a, and DNMT3B enrichment in the PTEN promoter region was reduced after overexpression of Foxo3a, while there exhibited elevated DNMT3B enrichment in the PTEN promoter region after Foxo3a interference. E. DNMT3B and PTEN expression levels were assessed by qRT-PCR, and there were a decreased DNMT3B expression and an increased PTEN expression after Foxo3a overexpression, while there were an increased DNMT3B expression and a reduced PTEN expression after Foxo3a reduction. *p < 0.05.
For further investigating whether Foxo3a impacted DNMT3B enrichment in the PTEN promoter region, we performed a ChIP assay after overexpression and silencing of Foxo3a in cervical cancer cells. Experimental results reflected that DNMT3B enrichment in the PTEN promoter region was reduced after overexpression of Foxo3a, while after Foxo3a interference, there exhibited elevated DNMT3B enrichment in the PTEN promoter region (). Furthermore, the qRT-PCR results unveiled that there were a decreased DNMT3B expression and an increased PTEN expression after Foxo3a overexpression, while there were an increased DNMT3B expression and a reduced PTEN expression after Foxo3a reduction (). The aforementioned findings imply that Foxo3a inhibits its methylation with the PTEN promoter through blocking the DNMT3B activity, thereby enhancing PTEN expression.
Suppression of PTEN methylation impedes cervical cancer cell malignant phenotypes
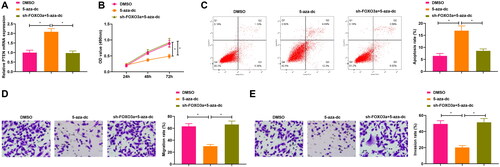
To next investigate the mechanism by which Foxo3a restricted the biological activities of cervical cancer cells, we demethylated PTEN and observed that PTEN expression was elevated upon treatment with a methyltransferase inhibitor (5-aza-dc) in comparison to DMSO treatment, and PTEN expression was reduced after treatment of sh-Foxo3a and 5-aza-dc compared with 5-aza-dc treatment alone (). The further functional experiments disclosed that the treatment with 5-aza-dc in cervical cancer cells contributed to a reduction in cell proliferative, migratory and invasive capabilities, and an elevation in the apoptosis rate of cervical cancer in comparison to DMSO treatment. The cell proliferative, migratory, and invasive capabilities increased, and the apoptosis rate decreased in cervical cancer after treatment of sh-Foxo3a and 5-aza-dc versus those upon 5-aza-dc treatment alone (). These results indicate that suppression of PTEN methylation impedes cervical cancer cell malignant phenotypes.
Figure 4. Suppression of PTEN methylation impacts the cervical cancer cell biological functions. A. PTEN expression in cells upon sh-Foxo3a and 5-aza-dc treatment, and 5-aza-dc treatment alone was measured by qRT-PCR. B. CCK-8 assay for evaluating the cell proliferation capacity upon sh-Foxo3a and 5-aza-dc treatment, and 5-aza-dc treatment alone. C. The cell apoptosis rate was measured by flow cytometry upon sh-Foxo3a and 5-aza-dc treatment, and 5-aza-dc treatment alone. D-E. The cell migratory and invasive capabilities were assessed by Transwell assay upon sh-Foxo3a and 5-aza-dc treatment, and 5-aza-dc treatment alone. *p < 0.05.
Discussion
Cervical cancer is a gynecological disease with higher morbidity and mortality although it is preventable and curable in an early stage [Citation3]. Therefore, identifying the cellular and molecular mechanisms that induce cervical cancer development is a major concern for researchers at home and abroad [Citation19]. In our study, we purposed to figure out the possible molecular mechanism of Foxo3a-mediated DNMT3B in the proliferation and migration of cervical cancer cells via mediating the PTEN promoter methylation. Collectively, this research underlines that Foxo3a blocks its methylation with the PTEN promoter by inhibiting DNMT3B activity, which subsequently impedes cervical cancer cell progression.
To date, many articles reveal an anti-tumor function of FOXO3a on human diseases. For instance, upregulation of FOXO3a impairs tumor growth in breast cancer cells [Citation20] and prostate cancer cells [Citation21]. Meanwhile, inhibition of FOXO3a transcriptional activity in tumor cells contributes to the enhancement of tumor progression [Citation22]. More importantly, FOXO3a has been considered to be a potent biomarker in cervical cancer [Citation23–25]. Certain traditional factors such as FIGO stage, LMN, deep stromal infiltration as well as lymph-vascular space invasion are usually considered to be the prognostic factors of cervical cancer [Citation26]. Here, the correlation between Foxo3a level and characteristics of cervical cancer suggested that Foxo3a level had a relationship with FIGO stage, differentiation degree, tumor diameter, as well as lymph node metastasis. Meanwhile, in our work, we observed that FOXO3a was expressed at a low level in cervical cancer, and its overexpression contributed to a reduction in cell proliferative, migratory, and invasive capabilities, and an elevation in apoptosis rate. Accordingly, upregulation of FOXO3a by demethylating agents can retard tumor growth in breast cancer cells [Citation20]. Also, Foxo3a’s low expression reflects a poor prognosis of ovarian cancer patients, which could be an effective prognostic target in ovarian cancer [Citation27]. All these findings confirmed the suppressive function of Foxo3a in gynecological diseases.
As reported, FOXO3a may transcriptionally inhibit the DNMT genes [Citation28]. Meanwhile, FOXO3a represses the DNMT3B expression and FOXO3a negatively modulates the DNMT3B promoter activity through binding to its putative binding sites [Citation17]. Besides, DNMT3b can repress PTEN expression through methylation [Citation18]. We speculated that Foxo3a may affect its occurrence of methylation with PTEN promoter by modulating DNMT3B activity. To verify our hypothesis, we performed a ChIP experiment for evaluating the enrichment of DNMT3B in the PTEN promoter region and the binding of Foxo3a and DNMT3B, with the findings demonstrating that Foxo3a blocked its methylation with the PTEN promoter by repressing DNMT3B activity. 5-Aza-dc, as a DNA methyltransferase inhibitor, can restore the expression of tumor suppressors to antagonize cancer progression [Citation29]. In fact, 5-aza-dc has been regarded as a supplemental factor for other anticancer agents to treat diverse diseases via the mitigation of DNA hyper-methylation [Citation30]. Specifically, 5-aza-dc exhibits an anti-tumor effect in cervical cancer via restricting cell proliferation and stimulating cell apoptosis [Citation7]. In our research, we observed that the treatment with 5-aza-dc in cervical cancer cells contributed to a reduction in cell proliferative, migratory, and invasive capabilities, and an elevation in apoptosis rate. Similarly, cervical cancer cell viability is diminished upon treatment with 5-aza-dc, and the inhibiting rate of cell growth is reflected by the elevated concentration of 5-aza-dc [Citation31]. Moreover, DNA methyltransferase inhibitor drugs could be a useful therapy for cervical cancer treatment [Citation32]. Our study also indicated that 5-aza-dc neutralized the impacts of silencing Foxo3a on cervical cancer cell malignant phenotypes.
In summary, this work highlights that Foxo3a blocks its methylation with the PTEN promoter by inhibiting DNMT3B activity, which subsequently impedes cervical cancer cell progression. Joint detection of the levels of Foxo3a, DNMT3B, and PTEN in cervical cancer might be potential targets for prognosis indicators. Therefore, advances in cervical cancer pathogenesis can contribute to the understanding of new anti-cancer therapies.
Supplemental Material
Download PDF (136.6 KB)Disclosure statement
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Funding
References
- Guzel C, van Sten-Van’t Hoff J, de Kok I, et al. Molecular markers for cervical cancer screening. Expert Rev Proteomics. 2021;18(8):1–8. doi:10.1080/14789450.2021.1980387.
- Francies FZ, Bassa S, Chatziioannou A, Kaufmann AM, Dlamini Z. Splicing genomics events in cervical cancer: insights for phenotypic stratification and biomarker potency. Genes. 2021;12(2):130. doi:10.3390/genes12020.
- Dorji T, Tshomo U, Gyamtsho S, Tamang ST, Wangmo S, Pongpirul K. Gender-neutral HPV elimination, cervical cancer screening, and treatment: experience from Bhutan. Int J Gynaecol Obstet. 2022;156(3):425–429. doi:10.1002/ijgo.13728.
- He M, Xia L, Li J. Potential mechanisms of plant-derived natural products in the treatment of cervical cancer. Biomolecules. 2021;11(10):1539. doi:10.3390/biom1110.
- Rogers L, Siu SS, Luesley D, Bryant A, Dickinson HO. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst Rev. 2012;5(5):CD007583. doi:10.1002/14651858.CD007583.pub3.
- Burmeister CA, Khan SF, Schafer G, et al. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022;13:200238. doi:10.1016/j.tvr.2022.200238.
- Chen GD, Qian DY, Li ZG, Fan GY, You KL, Wu YL. Down-regulation of p16 and MGMT promotes the anti-proliferative and pro-apoptotic effects of 5-Aza-dC and radiation on cervical cancer cells. Cell Biochem Funct. 2017;35(8):488–496. doi:10.1002/cbf.3282.
- Schmidt M, Fernandez de Mattos S, van der Horst A, et al. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22(22):7842–7852. doi:10.1128/MCB.22.22.7842-7852.2002.
- Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7(11):847–859. doi:10.1038/nrc2223.
- Fernandez de Mattos S, Villalonga P, Clardy J, Lam EW. FOXO3a mediates the cytotoxic effects of cisplatin in colon cancer cells. Mol Cancer Ther. 2008;7(10):3237–3246. doi:10.1158/1535-7163.MCT-08-0398.
- Shiota M, Yokomizo A, Kashiwagi E, et al. Foxo3a expression and acetylation regulate cancer cell growth and sensitivity to cisplatin. Cancer Sci. 2010;101(5):1177–1185. doi:10.1111/j.1349-7006.2010.01503.x.
- Khatri S, Yepiskoposyan H, Gallo CA, Tandon P, Plas DR. FOXO3a regulates glycolysis via transcriptional control of tumor suppressor TSC1. J Biol Chem. 2010;285(21):15960–15965. doi:10.1074/jbc.M110.121871.
- Yung MM, Chan DW, Liu VW, Yao KM, Ngan HY. Activation of AMPK inhibits cervical cancer cell growth through AKT/FOXO3a/FOXM1 signaling cascade. BMC Cancer. 2013;13:327. doi:10.1186/1471-2407-13-327.
- Li W, Wang Y, Fang X, et al. Differential expression and clinical significance of DNA methyltransferase 3B (DNMT3B), phosphatase and tensin homolog (PTEN) and human MutL homologs 1 (hMLH1) in endometrial carcinomas. Med Sci Monit. 2017;23:938–947. doi:10.12659/msm.902267.
- Li W, Huang K, Wen F, et al. LINC00184 silencing inhibits glycolysis and restores mitochondrial oxidative phosphorylation in esophageal cancer through demethylation of PTEN. EBioMedicine. 2019;44:298–310. doi:10.1016/j.ebiom.2019.05.055.
- Lin N, Yao Z, Xu M, et al. Long noncoding RNA MALAT1 potentiates growth and inhibits senescence by antagonizing ABI3BP in gallbladder cancer cells. J Exp Clin Cancer Res. 2019;38(1):244. doi:10.1186/s13046-019-1237-5.
- Yang YC, Tang YA, Shieh JM, Lin RK, Hsu HS, Wang YC. DNMT3B overexpression by deregulation of FOXO3a-mediated transcription repression and MDM2 overexpression in lung cancer. J Thorac Oncol. 2014;9(9):1305–1315. doi:10.1097/JTO.0000000000000240.
- Zhou W, Xu S, Chen X, Wang C. HOTAIR suppresses PTEN via DNMT3b and confers drug resistance in acute myeloid leukemia. Hematology. 2021;26(1):170–178. doi:10.1080/16078454.2021.1880733.
- Qin G, Li P, Xue Z. Triptolide induces protective autophagy and apoptosis in human cervical cancer cells by downregulating Akt/mTOR activation. Oncol Lett. 2018;16(3):3929–3934. doi:10.3892/ol.2018.9074.
- Liu H, Song Y, Qiu H, et al. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2020;27(3):966–983. doi:10.1038/s41418-019-0389-3.
- Habrowska-Górczyńska DE, Kozieł MJ, Kowalska K, Piastowska-Ciesielska AW. FOXO3a and its regulators in prostate cancer. IJMS. 2021;22(22):12530. doi:10.3390/ijms2222.
- Potente M, Urbich C, Sasaki K-i, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115(9):2382–2392. doi:10.1172/JCI23126.
- Tang X, Liu S, Ding Y, et al. Serum circular FoxO3a serves as a novel prognostic biomarker in squamous cervical cancer. Cancer Manag Res. 2020;12:2531–2540. doi:10.2147/CMAR.S243329.
- Liu Z, Li Y, She G, et al. Resveratrol induces cervical cancer HeLa cell apoptosis through the activation and nuclear translocation promotion of FOXO3a. Pharmazie. 2020;75(6):250–254. doi:10.1691/ph.2020.0386.
- Qi L, Wang Y, Su S, et al. Sodium selenite inhibits cervical cancer growth via ROS mediated AMPK/FOXO3a/GADD45a axis. Chem Biol Interact. 2022;367:110171. doi:10.1016/j.cbi.2022.110171.
- Leonardi S, Buttarelli M, De Stefano I, et al. The relevance of prelamin A and RAD51 as molecular biomarkers in cervical cancer. Oncotarget. 2017;8(55):94247–94258. doi:10.18632/oncotarget.21686.
- Fei M, Zhao Y, Wang Y, et al. Low expression of Foxo3a is associated with poor prognosis in ovarian cancer patients. Cancer Invest. 2009;27(1):52–59. doi:10.1080/07357900802146204.
- Lees-Murdock DJ, Lau HT, Castrillon DH, De Felici M, Walsh CP. DNA methyltransferase loading, but not de novo methylation, is an oocyte-autonomous process stimulated by SCF signalling. Dev Biol. 2008;321(1):238–250. doi:10.1016/j.ydbio.2008.06.024.
- Stresemann C, Brueckner B, Musch T, Stopper H, Lyko F. Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines. Cancer Res. 2006;66(5):2794–2800. doi:10.1158/0008-5472.CAN-05-2821.
- Shames DS, Minna JD, Gazdar AF. DNA methylation in health, disease, and cancer. Curr Mol Med. 2007;7(1):85–102. doi:10.2174/156652407779940413.
- Wu Y, Meng L, Wang H, et al. Regulation of DNA methylation on the expression of the FHIT gene contributes to cervical carcinoma cell tumorigenesis. Oncol Rep. 2006;16(3):625–629.
- Woo HJ, Kim SJ, Song KJ, et al. Hypermethylation of the tumor-suppressor cell adhesion molecule 1 in human papillomavirus-transformed cervical carcinoma cells. Int J Oncol. 2015;46(6):2656–2662. doi:10.3892/ijo.2015.2945.
