Abstract
Purpose
Readmission is one of the measures of quality of care and potential costs. This study aimed to determine whether lactate dehydrogenase (LDH) is associated with an increased risk of 30-day readmission in gastric cancer.
Methods
We performed a retrospective study of patients who underwent radical gastrectomy for gastric cancer at our institution between July 2014 and May 2018. Balanced cohorts were created by propensity score matching (PSM) with a 1:1 ratio to generate the elevated LDH (ELDH) group (n = 151) and the low LDH group (Control) (n = 302). To determine the incidence, causes, and risk factors of 30-day readmission, subgroup analyzes were performed and used to develop an efficient prediction model.
Results
A total of 788 patients met the criteria to be included in the study. The cutoff value for serum LDH was 215.5. After PSM, a total of 302 patients were matched in pairs (ELDH group, n = 151, Control group, n = 151). ELDH levels had a higher risk of readmission (p = 0.005, Odds ratio 3.768, 95% confidence interval 1.493–9.510). The pre-match 30-day readmission rate was 7.2 percent, and common causes of post-match readmission included infection-related symptoms, gastrointestinal symptoms, and gastrointestinal bleeding.
Conclusions
Patients with preoperative ELDH levels, postoperative complications, and high preoperative American Society of Anesthesiologists Scores had a higher risk of readmission 30 days after surgery.
Introduction
Gastric cancer has become the third most common cause of social tumor burden in China, with about 480,000 new cases per year [Citation1]. For gastric cancer, surgical resection remains one of the main treatments. With the development of rapid recovery, the time from admission to discharge for surgical patients has been decreasing. This situation has led to concerns that patients leaving the hospital in a vulnerable state will cause unplanned readmissions. Readmissions can significantly increase the medical burden for families and patients, and may impact patient’s postoperative recovery and quality of life [Citation2]. For governments, readmissions add to the financial burden on the health care system. Therefore, for surgical patients, we need appropriate patient perioperative care during hospitalization, as well as assessment, education, and associated follow-up of the patient upon discharge. The United States required a reduction in readmissions as part of the Affordable Care Act of 2010, although the act did not take effect [Citation3, Citation4]. However, the readmission rate of gastric cancer patients is still 6.6% to 8.7%, which is elevated compared to other tumors [Citation5].
Lactate dehydrogenase (LDH) is closely related to the glycolysis and mitochondrial defects required for tumor growth [Citation6]. The Warburg effect exists in tumor cells indicating that cancer cells preferentially use the anaerobic pathway of glycolysis to generate energy despite the presence of oxygen [Citation7]. LDH is one of the crucial enzymes for anaerobic glycolysis and gluconeogenesis, which catalyzes the redox reaction between propionic acid and L-lactic acid. In addition, LDH is typically cleared by receptor-mediated macrophage endocytosis [Citation8]. When a tumor grows, the immune system within its microenvironment changes accordingly, resulting in insufficient LDH clearance from the tumor [Citation9]. Therefore, patients with high lactate dehydrogenase levels have relatively low overall survival and disease-free survival, and correlation analysis suggests that elevated serum LDH has a negative effect on the prognosis of gastric cancer [Citation10–12], and some scholars recommend LDH as an objective indicator of tumor prognosis [Citation13, Citation14]. At the same time, some studies have shown that patients with gastric cancer have a higher readmission rate after surgery, and the prognosis associated with it is also worse [Citation15]. Therefore, we believe that LDH is also related to hospital readmissions. However, to the best of our knowledge, although there have been numerous studies on LDH and gastric cancer prognosis, no study has analyzed the correlation between LDH and the 30-day readmission of gastric cancer patients. Therefore, we included LDH in our retrospective study to investigate the association of readmission with LDH in gastric cancer patients.
Materials and methods
Patient
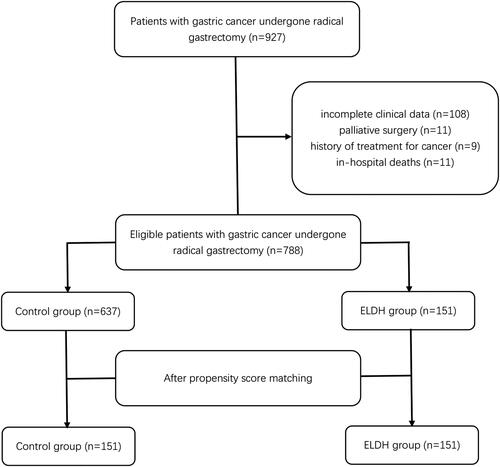
The First Affiliated Hospital of Wenzhou Medical University is a comprehensive hospital integrating medical treatment, teaching, scientific research, and preventive health care. It is responsible for the medical care and treatment of critical and intractable diseases for nearly 30 million people in southern Zhejiang, southern Fujian, and northeastern China. We retrospectively included 927 patients who presented to the Department of Gastrointestinal Surgery of the First Affiliated Hospital of Wenzhou Medical University between July 2014 and May 2018. Our inclusion criteria were: 1) age ≥ 18 years; 2) pathological diagnosis of gastric cancer; 3) surgical treatment at our research center; and 4) readmission at our research center within 30 days of discharge. The exclusion criteria were: 1) patients who died during the initial hospital stay after surgery or within 30 days of discharge; 2) palliative surgery; and 3) history of treatment for cancer. As shown in , after examining inclusion and exclusion criteria, we identified 788 eligible patients. After propensity matching, 302 patients were eventually enrolled in the study. All procedures in this study were standard open and laparoscopic gastrectomy performed by experienced surgeons according to guidelines [Citation16, Citation17]
Data collection
We collected patient data on three aspects: (1) Basic clinical characteristics: age, gender, Body Mass Index (BMI), American Society of Anesthesiologists (ASA) grade, preoperative comorbidities (using the Charlson Comorbidity Index (CCI) score), preoperative hemoglobin concentration, preoperative plasma albumin concentration, preoperative nutrition risk screening (NRS) score, preoperative serum LDH level, and history of abdominal surgery and sarcopenia; (2) Surgical characteristics: including TNM stage (Tumor Node Metastasis), operation method (laparoscopic or open), whether total gastrectomy was performed, whether combined resection was performed during the operation, operation time (≥210 min), and surgical anastomosis method (Billroth I, Billroth II, Roux-en -Y); and (3) Postoperative outcomes: postoperative complications, postoperative hospital stay, hospitalization costs, readmission within one month after discharge, and readmission causes.
Evaluated parameters
To control for deviations in the study, all subjective assessments were performed by two specially trained independent researchers. They were blinded to basic patient information and other data. Preoperative hypoalbuminemia was defined as preoperative plasma albumin <35 g/L. Preoperative anemia was determined according to the hemoglobin concentration in peripheral blood (hemoglobin concentration <120 g/L in men and <110 g/L in women). The NRS was assessed by NRS 2002 [Citation18], which was completed within 24 hours after the patient was admitted to the hospital, and a score of ≥ 3 was judged as having nutritional risk. The diagnosis of sarcopenia was based on previous studies and defined as: (1) low muscle mass (L3 skeletal muscle index <40.8 cm2/m2 in males and <34.9 cm2/m2 in females); (2) low muscle strength (handgrip strength <26 kg for males or <18 kg for females); and (3) low muscle performance (usual gait speed <0.8 m/s over 6 m). According to the Clavien-Dindo classification [Citation19], major complications were defined as those that met the criteria of grade II or higher. Readmission was defined as readmission within 30 days of discharge. We chose the 30-day period because the 30-day readmission rate has long been the standard rate reported in previous studies. When a patient was readmitted for more than one reason, the most prominent problem was considered as the reason for readmission.
Propensity score analysis
To reduce the bias due to nonrandom assignment, we used propensity score matching (PSM) to balance the elevated LDH (ELDH) group with the baseline characteristics of the control group. We selected the following factors as covariates for PSM: sex, age, BMI, CCI score, ASA grade, history of abdominal surgery, preoperative NRS score, TNM, preoperative hemoglobin, preoperative albumin. Propensity scores were calculated by a binary logistic regression model using a 1:1 matching of patients using a neighborhood matching algorithm (a caliper size of 0.05). After matching, 302 patients were included in the study and the data was analyzed by univariate and multivariate analysis.
Statistical analysis
Continuous data were tested for normal distribution using the Kolmogorov-Smirnov method. Continuous data that conformed to a normal distribution are represented by their mean and standard deviation. Continuous data that did not conform to a normal distribution are represented using the median and quarterback distance [Citation20]. Comparisons between normally distributed continuous variables were performed using the chi-square test or Fisher’s exact test. Conversely, comparisons between non-normally distributed continuous variables used the Mann-Whitney U test.
LDH was analyzed by receiver operating characteristic curve and the truncated value of 215.5 U/L was obtained, which was higher than the cutoff value in the ELDH group. Using PSM, 788 patients were matched 1:1 at the LDH cutoff to balance potential covariables between the two groups. Clinically relevant parameters were assessed using univariate analysis to identify potential risk factors associated with outcomes. Variables with p values < 0.10 in univariate analysis were included in multivariate analysis. Differences at p < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS software for Windows (IBM version 25.0; Armonk, USA) and PSM was analyzed using the R Software for Windows (version 4.2.1).
Results
Patient characteristics
As shown in , we included a total of 788 patients before matching. According to the ROC curve analysis, the critical value of LDH was 215.5 U/L, and two groups were obtained, the ELDH group (n = 151) and the Control group (n = 637). We first compared the clinical characteristics of the two matched groups (). Univariate analysis showed that BMI index (p = 0.039) and sex (p = 0.045) were significantly different between the two groups. There were no statistically significant differences between the two groups in other characteristics, including age, albumin level, hemoglobin level, CCI score, ASA grade, NRS score, history of abdominal surgery, and TNM stage. The statistical difference between the two groups disappears after PSM analysis. Through this method, we obtained the matched data shown in for the ELDH group (n = 151) and the Control group (n = 151). In the matched population, the median age of the ELDH group was 66 years, and the proportion of males was 67.5 percent. The median age of the control variable group was 67 years, and the proportion of males was 58.3%. After matching, there was no significant difference between the two groups by univariate analysis, thus removing the bias to some extent.
Table 1. The comparison of patient clinical characteristics before and after propensity score matching.
Situations related to readmission
Before matching, the overall 30-day readmission rate for the 788 patients was 7.2 percent, compared with 13.9 percent in the ELDH group and 5.7 percent in the control group. In the matched population, readmissions were counted, and as shown in , we demonstrate that 28 of the 302 patients in the analysis were readmitted within 30 days. Most of the readmissions were due to infection-related symptoms (n = 12, 45.9%), including intra-abdominal infection, incision infection, fever, and anastomotic leakage. The second most common readmission cause was gastrointestinal symptoms (n = 9, 32.1%), such as intestinal obstruction, nausea and vomiting, and gastroparesis.
Table 2. Reasons for readmission of gastric cancer patients.
Univariate and multivariate analyses of risk factors for 30-day readmission
We performed univariate and multivariate analyses of readmissions in matched groups. Factors with p < 0.10 in univariate analysis were included in multivariate analysis. Univariate analysis showed between-group differences in LDH level (p = 0.005), ASA score (p = 0.007), and postoperative complications (p = 0.001) (). Moreover, multivariate logistic regression analysis adjusted for potential confounders showed that LDH (p = 0.005, odds ratio (OR) 3.768, 95% confidence interval (CI) 1.493–9.510), ASA score (p = 0.035, OR 2.784, 95%CI 1.074–7.214), and postoperative complication (p = 0.001, OR 4.156, 95%CI 1.802–9.583) were independent risk factors for 30-day readmission. In terms of expenditures, we compared the readmission group with the non-readmission group and found a statistical difference between the two groups (p = 0.010, OR 2.904, 95%CI 1.257–6.711).
Table 3. Univariate and multivariate analysis associated with 30-day readmission.
Subgroup analysis for distinct subsets of patients
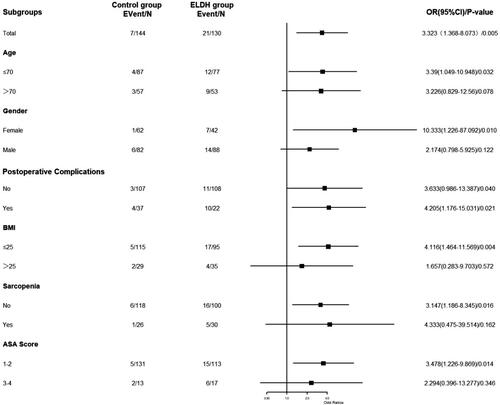
We performed a subgroup analysis to explore potential heterogeneity, as shown in . We found no statistically significant differences between ELDH and controls in subgroups that included older age, males, obesity, sarcopenia, and high ASA scores. There were no differences among the other subgroups.
Analysis of postoperative outcomes
After univariate and multivariate analyses, postoperative complications were also associated with readmission. Therefore, we compared the postoperative outcomes between the ELDH group and the control group, as shown in . Postoperative complications were not the same as readmissions, and the same patient may have had multiple postoperative complications. Postoperative complications occurred in 41 of 302 patients. In the post-surgery complication group, 26 had infection-related complications. There was a correlation between ELDH and incision infection in 17 cases (p = 0.015). The length of hospital stay was significantly different between the ELDH group and the control group (p = 0.017, OR 2.180, 95%CI 1.136–4.182), which meant that the postoperative hospital stay in the ELDH group was more than twice as long as the control group. There was no statistically significant difference in the cost of first admission between the two groups.
Table 4. Postoperative complications and short-term outcomes.
Nomogram for readmission
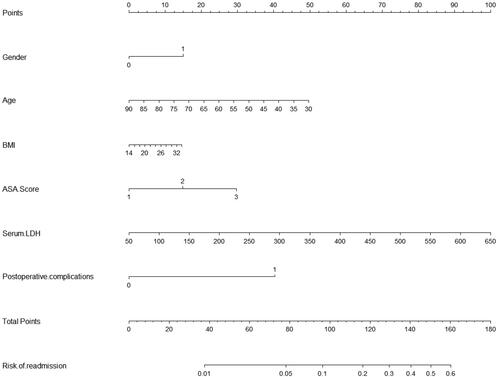
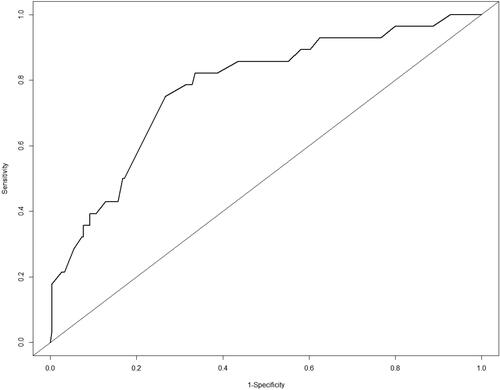
A score system for accurately predicting readmission was established by multivariate logic analysis of 302 patients to identify independent risk factors, as shown in . The nomogram had a C-index of 0.75. The ROC analysis in shows that the combined score had a higher value, and the area under the curve (AUC) was 0.75.
Discussion
Gastric cancer is treated by upper gastrointestinal tract surgery, a relatively complex procedure with a high mortality rate. In our pre-matching data, the readmission rate at our study center was 7.2%, which is consistent with our prior study [Citation21], as well as a related readmission study in Asian gastric cancer patients, which ranged from 6.6% to 8.7% [Citation5]. By comparison, we show that the ELDH group has a steep readmission rate, 13.9% in the group with ELDH and 5.7% in the control group, and a multivariate logistic analysis after PSM demonstrated that there is a statistical difference. In addition, related studies suggested that increased mortality is associated with the occurrence of readmission [Citation22], thus requiring early intervention and readmission. However, in the post-PSM data, we found no statistical difference in mean hospitalization costs between the ELDH group and the control group (57392.26 yuan and 57199.52 yuan, respectively). Patients at high risk of readmission will be under greater financial stress than those at low risk due to the possibility of a second hospitalization, especially in today’s pandemic environment. Thus, we believe that the financial pressures will be higher for the ELDH group. A growing number of countries and hospitals now include readmissions in their hospital quality management indicators, as higher readmission rates imply higher financial support. In particular, the readmission rates in European and American countries are significantly higher than those in Asia and may be related to the differences in Western social and cultural habits and the treatment of gastric cancer [Citation23, Citation24]. The Affordable Care Act of 2010 in the United States, for example, was designed to reduce the number of readmission procedures in order to reduce insurance-related costs [Citation4]. Thus, we can also reduce the burden on social care by analyzing readmissions while intervening for patients at risk of readmission.
In this study, we found that although the LDH level has an impact on the overall hospitalization period (p = 0.017), the two groups did not have a significantly different postoperative hospitalization time of more than 13 days (p = 0.566). There was no difference in the average hospitalization costs, but we found that higher LDH levels were an independent risk factor for readmission within 30 days after discharge of gastric cancer surgery patients (OR 3.768, 95%CI 1.493–9.510). LDH, a product of neoplastic necrosis due to hypoxia, may be a sign of high tumor burden [Citation25]. Correlation analysis showed that high LDH levels indicate aggressive tumor biology and are a metabolic biomarker and immune-monitoring prognostic biomarker [Citation26]. The risk of readmission associated with high LDH levels may be related to the aggressive tumor growth and metastasis [Citation27]. When the tumor needs anaerobic glycolysis to generate more energy, the serum LDH levels rise accordingly. In addition, lactate produced by anaerobic glycolysis can further lead to a pH decrease at the cellular level, activating pathways that stimulate tumor growth and invasion [Citation28–30]. Therefore, when the preoperative LDH level is high, the patient’s tumor cells may be in an active growth and invasion state, resulting in a poor short-term prognosis. When these patients with unnoticed poor prognoses are discharged, the risk of readmissions increases. Regarding the short-term outcomes, patients with higher preoperative serum LDH levels were more likely to have incision infections and readmission events. As shown in , the most frequent reasons for readmission were gastrointestinal and infectious symptoms. LDH is closely associated with inflammation [Citation26, Citation31], having even been proposed in some disciplines as an indicator of early organ damage or inflammation [Citation32, Citation33]. When tissues become infected, LDH is released, especially in patients with lung infections. Therefore, gastrointestinal and associated infectious symptoms were more likely to occur in the ELDH group. Gastrointestinal symptoms may lead to diaphragmatic dysfunction, increasing the burden on respiratory muscles and oxygen consumption, especially in patients with intestinal obstruction [Citation34]. When oxygen consumption increases, anaerobic respiration also increases, especially when the patient’s oxygen intake does not increase or improve due to their symptoms, and serum LDH also increases accordingly. Patients with high LDH levels before surgery may be in this inflammatory state, and when they are discharged home after surgery, the inflammatory response of the disease itself is not entirely relieved, resulting in the worsening of inflammatory symptoms after discharge and readmission events.
In addition, we conducted an analysis of the sub-group, as shown in . We demonstrate that LDH and readmission were statistically significant in most sub-groups. However, in the groups of elderly, men, obesity, sarcopenia, and high ASA scores, LDH and readmission were not statistically significant. With increased age and obesity, human LDH levels gradually decrease [Citation35]; thus, we suggest that this situation may be affected by LDH distribution. Of course, another possibility is that the amount of data decreased after sub-group analysis, which leads to bias.
In summary, our findings suggest that LDH has great potential to be an effective indicator for assessing unplanned readmissions, which clinicians can use to examine patients for readmission and address the underlying etiology of readmission.
Unlike the patients in internal medicine, surgery patients are facing planned surgical procedures and have known surgical risks, which makes them at a high risk of readmission [Citation36]. This was confirmed by our research showing a positive association between complications and readmissions (OR 4.156, 95%CI 1.802–9.583). Patients with complications during their first hospital stay were more likely to have readmissions after discharge. However, the reasons for readmission were more dependent on symptoms after discharge [Citation37]. After PSM, a total of 73 patients developed postoperative complications, but only 19.2% (n = 14) of patients with postoperative complications were readmitted, with few readmissions for the same symptoms. In a large study of readmissions in general surgery patients, this view was echoed by Merkow et al. (2015) [Citation38]. Therefore, we should conduct early interventions before discharging patients with complications after surgery, thereby improving the transition process of inpatient patients to outpatient patients.
The ASA score in the study represented the patient’s preoperative baseline status. In this study, we examined the association between the ASA score and readmission. Patients with an ASA score ≥3 have a higher probability of unplanned readmission after discharge. In the study by AC Meyer et al. (2021) [Citation39], they concluded that patients with higher ASA scores were also at higher risk for cardiovascular complications. Meanwhile, in the analysis of Ahmad et al. (2009), preoperative cardiac disease was independently associated with readmission [Citation13]. Combining both viewpoints, we postulate it might be th`e association between the ASA score and heart disease that leads to the association between the ASA score and readmission. However, no relevant analysis of cardiovascular disease was performed in this study, so we believe that future research needs examine this possibility.
Readmission will bring economic, physical, and psychological burdens to patients. Therefore, we obtained these independent risk factors for readmission through PSM analysis and established a scoring model to evaluate the risk probability of readmission. In , we assigned the risk factors numerical values, multiplied each value by a coefficient, and added them together to calculate the probability. By ROC analysis, we found that the model area under the curve (AUC) of 0.78 was significantly larger than the AUC of a single independent factor. These data are easily available for patients. Therefore, we believe that this model can be used to evaluate the 30-day readmission probability of gastric cancer patients. Through our promoter diagram, the accurate probability of readmission for patients with gastric cancer patients can be provided to surgeons. The Nuo model is composed of the patient’s basic characteristics and related clinical features, which can be used after surgery. When the patients with a high risk of readmission are recognized using the promoter diagram, we can reduce the patient’s readmission likelihood by strengthening the relevant treatment and hospitalization.
TNM stage was not found to be a risk factor in gastric cancer studies [Citation21], as we found in our study. A relationship between the TNM stage and readmission was found in readmission studies related to colorectal tumors [Citation40]. The novelty of this study is that LDH is closely related to unplanned readmission after one month in gastric cancer patients through PSM and has great potential to become an effective indicator for evaluating unplanned readmission within one month for gastric cancer patients.
This study has some limitations. First, this is a single-center study, and there may be local bias, since when some patients develop symptoms after discharge, they may be directly hospitalized in local hospitals for reasons of travel or customs. Second, some patients were admitted due to a variety of complex factors. We chose the most obvious symptoms as the reason for readmission, which may lead to some reasons for readmission being ignored.
Conclusion
Patients with elevated preoperative LDH levels have a higher risk of readmission 30 days after surgery. LDH was an independent risk factor for 30-day postoperative readmission in gastric cancer patients, and it has the potential to become an evaluation index.
Author contributions
Wei-Sheng Chen: analyzed and interpreted the data, drafted and revised the manuscript; Ze-Xin Huang and Hui-Hui Zhang: acquired data; Yi-Qi Cai: carried out the statistical analysis; Xiao-Dong Chen: analyzed and revised the manuscript; Wen-Jing Chen: interpreted the data and revised the manuscript; Yun-Shi Huang and Guan-Bao Zhu: contributed toward the conception of the study, designed the study, analyzed and interpreted the data. All the authors read and approved the final manuscript.
Ethical Standards
The ethics committee of The First Affiliated Hospital of Wenzhou Medical University approved this study (approval number: 2014 No. 063) and the need for individual informed consent was waived.
| Abbreviations | ||
| LDH | = | Lactate dehydrogenase |
| BMI | = | body mass index |
| ASA | = | American Society of Anesthesiologists |
| NRS | = | nutritional risk screening |
| TNM | = | tumor node metastasis |
| SD | = | standard deviations |
| IQR | = | interquartile range |
| PSM | = | propensity score matching |
Acknowledgments
The authors would like to thank Editage (https://www.editage.com) for language editing support. There are no other commercial interests or sources of financial or material support to declare.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134(7):1–11. doi:10.1097/CM9.0000000000001474.
- Wu WW, Zhang WH, Zhang WY, Yang L, Deng XQ, Zhu T. Risk factors of the postoperative 30-day readmission of gastric cancer surgery after discharge: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2019;98(10):e14639. doi:10.1097/MD.0000000000014639.
- Asaoka R, Kawamura T, Makuuchi R, et al. Risk factors for 30-day hospital readmission after radical gastrectomy: a single-center retrospective study. Gastric Cancer. 2019;22(2):413–420. doi:10.1007/s10120-018-0856-4.
- Seidman L. The Affordable Care Act versus medicare for all. J Health Polit Policy Law. 2015;40(4):911–921. doi:10.1215/03616878-3150160.
- Dan Z, YiNan D, ZengXi Y, XiChen W, JieBin P, LanNing Y. Thirty-day readmission after radical gastrectomy for gastric cancer: a meta-analysis. J Surg Res. 2019;243:180–188. doi:10.1016/j.jss.2019.04.076.
- Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9(6):425–434. doi:10.1016/j.ccr.2006.04.023.
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi:10.1126/science.123.3191.309.
- Smit MJ, Duursma AM, Bouma JM, Gruber M. Receptor-mediated endocytosis of lactate dehydrogenase M4 by liver macrophages: a mechanism for elimination of enzymes from plasma. Evidence for competition by creatine kinase MM, adenylate kinase, malate, and alcohol dehydrogenase. J Biol Chem. 1987;262(27):13020–13026.
- Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017;19(4):353–363. doi:10.3233/CBM-160336.
- Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 2015;54(7):961–970. doi:10.3109/0284186X.2015.1043026.
- Jin HF, Wang JF, Shao M, Zhou K, Ma X, Lv XP. Down-regulation of miR-7 in gastric cancer is associated with elevated LDH-a expression and chemoresistance to cisplatin. Front Cell Dev Biol. 2020;8:555937. doi:10.3389/fcell.2020.555937.
- Zhang Y, Lin S, Chen Y, Yang F, Liu S. LDH-Apromotes epithelial-mesenchymal transition by upregulating ZEB2 in intestinal-type gastric cancer. Onco Targets Ther. 2018;11:2363–2373. doi:10.2147/OTT.S163570.
- Arkenau HT, Barriuso J, Olmos D, et al. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol. 2009;27(16):2692–2696. doi:10.1200/JCO.2008.19.5081.
- Fussenich LM, Desar IM, Peters ME, et al. A new, simple and objective prognostic score for phase I cancer patients. Eur J Cancer. 2011;47(8):1152–1160. doi:10.1016/j.ejca.2010.12.028.
- Merchant SJ, Ituarte PH, Choi A, et al. Hospital readmission following surgery for gastric cancer: frequency, timing, etiologies, and survival. J Gastrointest Surg. 2015;19(10):1769–1781. doi:10.1007/s11605-015-2883-3.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. doi:10.1007/s10120-016-0622-4.
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network. 2016;14(10):1286–1312. doi:10.6004/jnccn.2016.0137.
- Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Ad Hoc EWG. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. Jun. 2003;22(3):321–336. doi:10.1016/S0261-5614(02)00214-5.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2.
- Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi:10.1001/jamaoncol.2019.2996.
- Zhuang CL, Wang SL, Huang DD, et al. Risk factors for hospital readmission after radical gastrectomy for gastric cancer: a prospective study. PLoS One. 2015;10(4):e0125572. doi:10.1371/journal.pone.0125572.
- Ansari MZ, Collopy BT, Booth JL. Hospital characteristics associated with unplanned readmissions. Aust Health Rev. 1995;18(3):63–75.
- Ueno T, Iida M, Yoshino S, et al. East versus west: differences in surgical management in Asia compared with Europe and North America. Surg Clin North Am. 2017;97(2):453–466. doi:10.1016/j.suc.2016.12.002.
- Markar SR, Karthikesalingam A, Jackson D, Hanna GB. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: comparison between West and East. Ann Surg Oncol. 2013;20(7):2328–2338. doi:10.1245/s10434-012-2862-9.
- Lukacova S, Sorensen BS, Alsner J, Overgaard J, Horsman MR. The impact of hypoxia on the activity of lactate dehydrogenase in two different pre-clinical tumour models. Acta Oncol. 2008;47(5):941–947. doi:10.1080/02841860701644086.
- Bai L, Lin ZY, Lu YX, et al. The prognostic value of preoperative serum lactate dehydrogenase levels in patients underwent curative-intent hepatectomy for colorectal liver metastases: a two-center cohort study. Cancer Med. 2021;10(22):8005–8019. doi:10.1002/cam4.4315.
- Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol. 2008;15(8):2336–2344. doi:10.1245/s10434-008-9955-5.
- Stubbs M, McSheehy PM, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today. 2000;6(1):15–19. doi:10.1016/s1357-4310(99)01615-9.
- Maxwell PH. The HIF pathway in cancer. Semin Cell Dev Biol. 2005;16(4–5):523–530. Aug doi:10.1016/j.semcdb.2005.03.001.
- Li G, Gao J, Tao YL, et al. Increased pretreatment levels of serum LDH and ALP as poor prognostic factors for nasopharyngeal carcinoma. Chin J Cancer. 2012;31(4):197–206. doi:10.5732/cjc.011.10283.
- Martinez-Outschoorn UE, Prisco M, Ertel A, et al. Ketones and lactate increase cancer cell "stemness," driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via metabolo-genomics. Cell Cycle. 2011;10(8):1271–1286. doi:10.4161/cc.10.8.15330.
- Uramoto H, Nakanishi R, Fujino Y, et al. Prediction of pulmonary complications after a lobectomy in patients with non-small cell lung cancer. Thorax. 2001;56(1):59–61. doi:10.1136/thorax.56.1.59.
- Turna A, Solak O, Cetinkaya E, et al. Lactate dehydrodgenase levels predict pulmonary morbidity after lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2004;26(3):483–487. doi:10.1016/j.ejcts.2004.05.041.
- Ball J, Venn R, Williams G, Forni L. 22nd International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium, 19-22 March 2002. Crit Care. 2002;6(3):264–270. doi:10.1186/cc1502.
- Johansen MJ, Gade J, Stender S, et al. The effect of overweight and obesity on liver biochemical markers in children and adolescents. J Clin Endocrinol Metab. 2020;105(2). doi:10.1210/clinem/dgz010.
- Sweeney JF. Postoperative complications and hospital readmissions in surgical patients: an important association. Ann Surg. 2013;258(1):19–20. Jul doi:10.1097/SLA.0b013e318297a37e.
- Tevis SE, Kohlnhofer BM, Weber SM, Kennedy GD. Postdischarge complications are an important predictor of postoperative readmissions. Am J Surg. 2014;208(4):505–510. doi:10.1016/j.amjsurg.2014.05.013.
- Merkow RP, Ju MH, Chung JW, et al. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015;313(5):483–495. doi:10.1001/jama.2014.18614.
- Meyer AC, Eklund H, Hedstrom M, Modig K. The ASA score predicts infections, cardiovascular complications, and hospital readmissions after hip fracture - A nationwide cohort study. Osteoporos Int. 2021;32(11):2185–2192. doi:10.1007/s00198-021-05956-w.
- Quintana JM, Anton-Ladislao A, Lazaro S, et al. Predictors of readmission and reoperation in patients with colorectal cancer. Support Care Cancer. 2020;28(5):2339–2350. doi:10.1007/s00520-019-05050-2.