Abstract
Background
Our objective is to compare the early outcomes associated with passive (gravity) drainage (PG) and active drainage (AD) after surgery.
Methods
Studies published until April 28, 2022 were retrieved from the PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, Web of Science databases.
Results
Nine studies with 14,169 patients were identified. Two groups had the same intra-abdominal infection rate (RR: 0.55; P = 0.13); In subgroup analysis of pancreaticoduodenectomy, active drainage had no significant effect on postoperative pancreatic fistula (POPF) rate (RR: 1.21; P = 0.26) and clinically relevant POPF (CR-POPF) (RR: 1.05; P = 0.72); Active drainage was not associated with lower percutaneous drainage rate (RR: 1.00; P = 0.96), incidence of sepsis (RR: 1.00; P = 0.99) and overall morbidity (RR: 1.02; P = 0.73). Both groups had the same POPF rate (RR: 1.20; P = 0.18) and CR-POPF rate (RR: 1.20; P = 0.18) after distal pancreatectomy. There was no difference between two groups on the day of drain removal after pancreaticoduodenectomy (Mean difference: −0.16; P = 0.81) and liver surgery (Mean difference: 0.03; P = 0.99).
Conclusions
Active drainage is not superior to passive drainage and both drainage methods can be considered.
Introduction
In extensive and high-risk digestive system surgery, severe procedure-related complications are still common. Low severity complications are often ignored by most surgeons, which leads to high overall morbidity or even deathCitation1,Citation2. To reduce postoperative complications and improve patient outcomes, prophylactic drain insertion has been widely used at the time of surgeryCitation3–5. AD and PG drainages are two of the most commonly used methods in abdominal surgeries to detect the early postoperative intra-abdominal hemorrhage, bile leakage and/or to drain ascites fluid collection. However, there remains controversy on the use of AD compared with PG drainage with limited data on patient outcomes to guide management.
Prophylactic drains are inserted adjacent to the anastomosis or surgical area to detect abdominal collections early on and provide information on the quality of drainage which indirectly allows assessment of the nearby anastomoses, and the drains can also measure the volume of drainage accurately. AD utilize a negative pressure gradient to maintain suction within the abdomen, while PG drainage devices provide a route for fluid extraction due to the differential of pressure between intra-abdominal and atmospheric pressureCitation6. The optimal type of drainage methods after abdominal digestive system surgery remains unclear. There is a belief that the high-pressure gradient generated by AD has the potential to promote the development of a leakage, bowel injury, and increases the rate of intra-abdominal infections and prolonged drainage stayCitation7,Citation8. Conversely, it has also been postulated that AD promotes improved drainage and collapse of the surgical dead space, thereby decreasing the severity of a leakage and intra-abdominal infection if it does occurCitation9,Citation10. The most common reason for using PG drainage is to avoid suction near the anastomosis, which can cause or potentially contribute to the development of a leakage.
Therefore, we conducted a meta-analysis and systematic review to compare the postoperative outcomes between PG drainage and AD among patients undergoing digestive system surgery.
Methods
Search strategy and study selection criteria
This systematic review was performed according to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelinesCitation11. We followed a comprehensive search strategy to identify any relevant articles on the topic, mainly from 4 medical databases including PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE and Web of Science. Only English literature were included. Reference lists of relevant articles were also screened for eligible studies. The PROSPERO registration number was CRD42022328978 (supplementary appendix S1). The complete search strategy is attached as supplementary appendix S2. Inclusion criteria were as follows: (1) observational and interventional studies; (2) study settings and patient characteristics are provided; (3) detailed data on interventions and outcomes are available; (4) Patients received major abdominal surgeries involving digestive system. Drains are typically placed at the time of surgery with one end in close proximity to the anastomosis or parenchymal transection margin, while the other end transverses the abdominal wall and is attached to a device that serves as a reservoir for drained fluid. The reservoir of a PG drainage function as a vessel, allowing fluid to drain by passive gravity. The reservoir of an AD drainage can either collapsed or connect to a negative pressure system, generating a negative pressure, closed suction environment to drain the fluid. Patients undergoing emergency surgery, postoperative reoperation, total pancreatectomy and necrosectomy in the setting of inflammation and necrosis will be excluded, as this represents a group of patients distinctly different from those undergoing elective abdominal surgery. Drains managed with continuous irrigation will not be included, but minimal fluid flushing with the intention of maintaining drain patency is acceptable. Case series (<10), review articles, congress abstract, letters, editorials, or animal studies were also excluded.
Data extraction and assessment of methodological quality
Two investigators (MHZ and TN) independently screened the manuscripts of the potentially eligible studies. Another two investigators (JFP and LGS) checked the results, and disagreement was resolved by consensus. The risks of bias of included studies were evaluated by two independent assessors (MHZ and TN). The Cochrane Collaboration’s tool for assessing risk of bias in RCTs was used to assess interventional trials, and Newcastle-Ottawa Scale (NOS) was used to assess case control studies and cohort studies. A NOS score ≥ 6 were considered high quality.
Data collection and measurement
We used a standard strategy to extract the following data from each study: study characteristics (authors, date of publication, study design, duration of follow-up, and sample size), participants (age and sex) and type of drainage tube. Primary outcome was intra-abdominal infection. Secondary outcomes included requirements for percutaneous drainage, time to drain removal, leakage (pancreatic fistula, gastrointestinal fistula, biliary leakage), sepsis, morbidity as defined by the Clavien-Dindo Classification system. Complications were included based on the definitions used by authors of the individual articles. Data were independently extracted by two investigators (MHZ and TN) and checked by the third investigator (JFP and LGS).
Data synthesis
Missing mean and standard deviation data were calculated from the reported median and range values as reported previouslyCitation12,Citation13. For quantitative synthesis of comparative studies, a random effect meta-analysis was used with standardized mean differences reported for continuous outcomes and RR for binary outcomes, along with 95% confidence intervals (CI) and two-sided P values for each outcome. All the RR with corresponding 95% CI were graphically visualized on forest plots. Heterogeneity across studies was evaluated using the Cochrane Q test and the I2 test. The I2 value of 0% to 49%, 50% to 74%, and higher than 75% indicated low, moderate, and high heterogeneity, respectively.
Results
Study selection
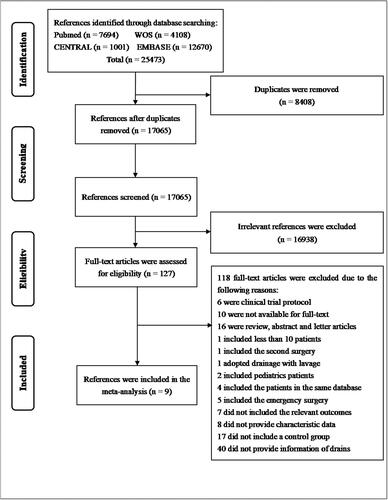
The flow chart of the study selection is presented in (). After searching database, 25473 references were identified. A total of 8408 references were removed as duplicates and 16938 references were excluded after title and abstract screening. Eventually, 127 articles were eligible for full-text review. Finally, 9 articles (4 RCTs, and 5 retrospective studies) were included in the meta-analysisCitation12–20.
Characteristics of the included studies
The main characteristics of the included studies are shown in (). A total of 14108 patients were included, with varied sample sizes ranging from 100 to 9665 patients. Of these, 5 were retrospective and observationalCitation18–20, 3 were Single‑center RCTCitation12,Citation13,Citation15–17 and one dual-center RCT14. Patients enrolled in these studies were at a median age of 38.4 to 68.5 years old.
Table 1. Characteristics of the studies included in the meta‑analysis.
Quality assessment
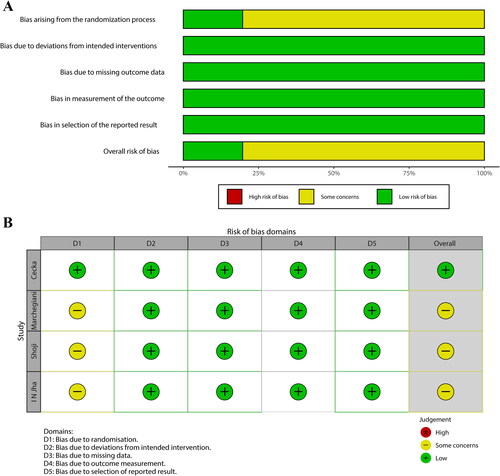
Two authors independently selected trials, extracted data, assessed the risk of bias using the Cochrane risk-of-bias tool 2, and assessed the certainty of the evidence using the Grading of Recommendations, Assessment, Development and Evaluation approach. The quality assessment of the included studies is presented. (). According to the Newcastle Ottawa scale, 5 retrospective studies awarded 3 stars (). Random sequence generation and allocation concealment to prevent selection bias was only provided in one RCT14 and none of retrospective studies specified how patients were assigned to AD group or PG drainage group. Moreover, preventing performance and detection bias by blinding surgeons is impracticable.
Table 2. NOS scores for retrospective studies.
Primary outcome in pooled analysis of RCT studies
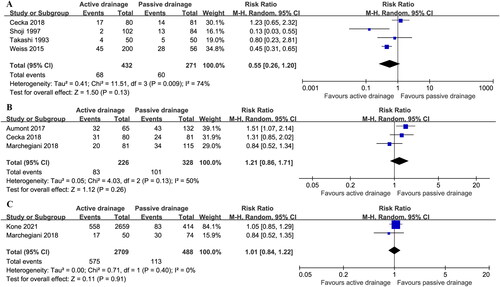
Four RCT studies including 703 patients (432 AD vs. 271 PG drainage) reported incidence of intra-abdominal infecionCitation13,Citation14,Citation16,Citation17. The heterogeneity among studies was high (I2 = 74%), and intra-abdominal infection rate was 15.7% (68/432) in AD group vs. 22.1% (60/271) in PG drainage group. There was no statistically significant difference between two groups (RR: 0.55; 95%CI 0.26 ∼ 1.20; P = 0.13) ().
Secondary outcomes
Postoperative pancreatic fistula
POPF after pancreaticoduodenectomy (PD) was reported in three studies, and POPF rate was 36.7% (83/226) in AD group vs. 30.8% (101/328) in PG drainage group with moderate heterogeneity (I2 = 50%)Citation14,Citation15,Citation18, which was not statistical difference (RR: 1.21; 95%CI 0.86 ∼ 1.71; P = 0.26) ().
POPF after distal pancreatectomy (DP) was reported in two studies, and 21.2% (575/2709) patients in AD group and 23.2% (113/488) patients in PG drainage group developed POPF with low heterogeneity (I2 = 0%)Citation15,Citation20, which did not reach statistical difference (RR: 1.01; 95%CI 0.84 ∼ 1.22; P = 0.91) ().
Clinically relevant POPF
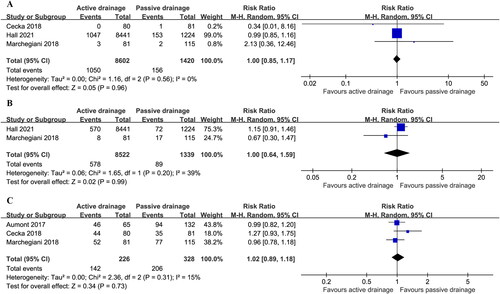
CR-POPF after PD was reported in three studies, and the incident of CR-POPF was 16.4% (1413/8602) in AD group vs. 14.6% (207/1420) in PG drainage group with moderate heterogeneity (I2 = 30%)Citation14,Citation15,Citation19, but there was no statistical difference in the incidence of CR-POPF (RR: 1.05; 95%CI 0.79 ∼ 1.41; P = 0.72) ().
Figure 4. Estimates of the effect of PG drainage and AD use on the incidence of CR-POPF after PD (a) and CR-POPF after DP (b).
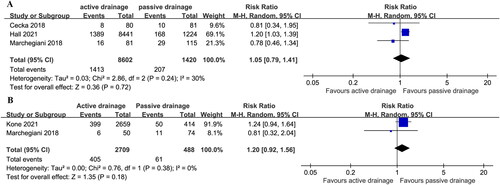
CR-POPF after DP was reported in two studies and appeared to be 15.0% (405/2709) in AD vs. 12.5% (61/488) in PG drainage with low among-study heterogeneity (I2 = 0%)Citation15,Citation20, which was no statistical difference [RR (95%CI) = 1.20 (0.92, 1.56); p = 0.18] ().
Percutaneous drainage
Percutaneous drainage was reported in three studies after PD and did not significantly differ in two groups [12.2% (1050/8602) in AD group vs. 11.0% (156/1420) in PG drainage group] (RR 1.00; 95%CI 0.85 ∼ 1.17); P = 0.96) with low heterogeneity (I2 = 0%)Citation14,Citation15,Citation19().
Sepsis
Sepsis was reported in two studies after PD, and there was no significant difference between two groups [6.8% (578/8522) in AD group vs. 6.6% (89/1339) in PG drainage group] (RR: 1.00; 95%CI 0.64 ∼ 1.59; P = 0.99) with low heterogeneity (I2 = 39%)Citation15,Citation19().
Overall morbidity
Overall morbidity was reported in three studies after PD and did not reach significant difference [62.8% (142/226) in AD group vs. 62.8% (206/328) in PG drainage group] (RR: 1.02; 95%CI 0.89 ∼ 1.18; P = 0.73] with low heterogeneity (I2 = 15%)Citation12,Citation16,Citation18().
Time of drain removal
Two studies reported time of drain removal after PD, without significant difference between AD group and PG drainage group (Mean difference: −0.16; 95%CI −1.49 ∼ 1.17; p = 0.81)Citation14,Citation15. Moderate heterogeneity was observed in included studies (I2 = 23%) ().
Figure 6. Estimates of the effect of PG drainage and AD use on the day of drain removal after PD (a) and liver surgery (b).
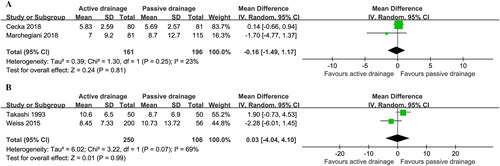
Two studies on liver surgery also reported time of drain removal without significant difference between two groups (Mean difference: 0.03; 95% CI, −4.04 to 4.01; p = 0.99)Citation16,Citation17 and with High heterogeneity (I2 = 69%) ().
Discussion
The main finding of this meta-analysis was that there was no difference in intra-abdominal infection rates, POPF rate, CR-POPF rate, percutaneous drainage rate, the incidence of sepsis, overall morbidity, and time of drain removal between AD group and PG drainage group after abdominal digestive system surgery. Accordingly, active drainage is not superior to passive drainage and both drainage methods can be considered.
Prophylactic drainage after digestive system surgery has been the common practice after abdominal digestive surgery, which is useful in the early recognition of postoperative complications such as bleeding and leakage. Although some clinicians have suggested that abdominal drains might be responsible for pain related to the drain itself, and increase the incidence of infection and hospital stay. But it is undeniable that prophylactic drains are now conventionally placed postoperatively in clinical practiceCitation21. However, the influence of drainage type on postoperative outcomes following digestive system surgery remains short of evidence, very little study has been published comparing gravity and closed-suction drainage in abdominal surgery.
Both AD and PG drainage allow intra-abdominal fluid to drain out of surgical area following surgical interventions, but the risks and benefits of these two drainage methods are unclear, there is no powerful evidence available that indicates which drain system performs betterCitation3,Citation22,Citation23. Bradley et alCitation19 identified 9665 patients with drains following PD and demonstrate that closed-suction drainage was consistently associated with higher rates of multiple pancreatectomy-specific complications, such as CR-POPF, as well as multiple associated complications, such as superficial surgical site infection (SSI), organ-space SSI, and delayed gastric emptying (DGE). However, a retrospective studyCitation10 including 430 patients showed that the drain type was not associated with CR-POPF rate. Similarly, several recent studies also found no differences in outcomes between suction and gravity closed-drainage systemsCitation20. Compared with PG drainage, the risk of retrograde microbial contamination is avoided due to the presence of negative pressure by ADCitation15, so it seems to provide a more effective system by removing fluid from the abdominal cavity, yet it has consistently been associated with higher rates of multiple complicationsCitation24,Citation25. Some clinicians believe that AD may be a potential hazard, because negative pressure might cause the formation of a pancreatic fistulae or leaks in certain types of abdominal digestive system surgeryCitation8.
In clinical practice, the type and diameter of drains used, the day and criteria of drain removal, and the pressure of negative pressure drainage may influence the outcomes. Peter et alCitation26 concluded that the low-pressure suction drain removed an intraperitoneal marker, gentamicin, more effectively than the high-pressure suction drain, but not more effective than the passive drain. However, none of the remaining studies mentioned drainage tube details. It is unknown whether the pressure of drain affects the normal healing process of an already fragile anastomosisCitation8,Citation27. Other studies pointed out that the drainage placement may also had effect on the outcomes. Generally, the inner end of the drainage tube should be placed at the bottom of the surgical area or close to the site where drainage is needed, and it should be placed near the anastomosis in gastrointestinal surgery. Otherwise, it will make the drainages drain insufficiently and leaves a dead cavity. Some studies supported that the drain placement was associated with increased fistula rateCitation24.
Furthermore, the type of anastomosis, anastomosis techniques, and surgical approaches was different among all kinds of operations, which would have a significant impact on the incidence of postoperative complications and study heterogeneityCitation28. Additionally, there is little conclusive scientific evidence on when to utilize drains and what type of drain to use, which are largely based on Surgeon’s experience. Moreover, there is also no standardized system of postoperative evaluation. For example, Filipe et al14used ultrasonographic or computed tomographic examination to detect any fluid collections before discharge, while Sascha et alCitation17 assessed postoperative outcomes through daily abdominal ultrasound examinations in the first postoperative week and computed tomography (CT) scans on clinical suspicion. Thus, the timing and the method of the postoperative complication evaluation is debatable.
This meta-analysis is the first to compare two types of intra-abdominal drainage after abdominal digestive system surgery. We have expanded the scope of surgery to include abdominal surgery, so much heterogeneity between different surgeries may affect the reliability of the result. Consequently, we adopted the subgroup analysis according to different surgeries to try to avoid the heterogeneity. Though the included non-randomized studies were carefully screened for bias, clinical or methodological differences between studies can cause statistical heterogeneity, and observational studies are less reliable than high-quality RCTs. Further, high-quality RCTs are needed to determine whether drainage type affects the incidence of intra-abdominal infection or postoperative complications.
Conclusion
In conclusion, the drainage method did not appear to be associated with intra-abdominal infection rate and other secondary outcomes following abdominal digestive system surgery in the current study. Either AD or PG drainage can be used after abdominal digestive system surgery, but high-quality RCTs are required to provide more reliable evidence.
Authors’ contributions
Minghui zheng, Ting Niu, Junfeng Peng and Ligang Shi contributed equally to this work and share first authorship. Chenghao Shao: conceptualization, methodology, writing-reviewing and editing. Minghui zheng, Ting Niu, Junfeng Peng and Ligang Shi: data curation, writing-original draft preparation. All authors contributed to manuscript revision, read, and approved the submitted version.
| List of abbreviations | ||
| PG | = | passive gravity drainage |
| AD | = | active drainage |
| CENTRAL | = | Cochrane Central Register of Controlled Trials |
| RR | = | risk ratio |
| RCT | = | randomized controlled trial |
| POPF | = | postoperative pancreatic fistula |
| CR-POPF | = | clinically relevant POPF |
| PRISMA | = | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| NOS | = | Newcastle-Ottawa scale |
| CI | = | confidence intervals |
| PD | = | pancreaticoduodenectomy |
| DP | = | distal pancreatectomy |
| SSI | = | surgical site infection |
| DGE | = | delayed gastric emptying |
| CT | = | computed tomography |
Supplemental Material
Download Zip (239 KB)Disclosure statement
The authors declare that they have no competing interests.
Reference
- Staiger RD, Gerns E, Castrejón Subirà M, et al. Can early postoperative complications predict high morbidity and decrease failure to rescue following major abdominal surgery? Ann Surg. 2020;272(5):1–9. doi:10.1097/SLA.0000000000004254.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–2137. doi:10.1056/NEJMsa1010705.
- He S, Xia J, Zhang W, et al. Prophylactic abdominal drainage for pancreatic surgery. Cochrane Database Syst Rev. 2021;12(12):CD010583.
- Van Buren G, Bloomston M, Hughes SJ, et al. A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg. 2014;259(4):605–612.
- Jiang H, Liu N, Zhang M, et al. A randomized trial on the efficacy of prophylactic active drainage in prevention of complications after pancreaticoduodenectomy. Scand J Surg. 2016;105(4):215–222. doi:10.1177/1457496916665543.
- Park LJ, Baker L, Smith H, et al. Passive versus active intra-abdominal drainage following pancreatic resection: does a superior drainage System Exist? A Systematic review and meta-analysis. World J Surg. 2021;45(9):2895–2910. doi:10.1007/s00268-021-06158-5.
- Inoue Y, Imai Y, Kawaguchi N, et al. Management of abdominal drainage after hepatic resection. Dig Surg. 2017;34(5):400–410. doi:10.1159/000455238.
- Grobmyer SR, Graham D, Brennan MF, Coit D. High-pressure gradients generated by closed-suction surgical drainage systems. Surg Infect (Larchmt). 2002;3(3):245–249. doi:10.1089/109629602761624207.
- Gachabayov M, Gogna S, Latifi R, Dong XD. Passive drainage to gravity and closed-suction drainage following pancreatoduodenectomy lead to similar grade B and C postoperative pancreatic fistula rates. A Meta-Analysis. Int J Surg. 2019;67:24–31.
- Lemke M, Park L, Balaa FK, Martel G, Khalil JA, Bertens KA. Passive versus active intra-abdominal drainage following pancreaticoduodenectomy: a retrospective study using the American college of surgeons NSQIP database. World J Surg. 2021;45(2):554–561. doi:10.1007/s00268-020-05823-5.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Jha IN, Singh HB, Prasad N. Clinical trial of drainage following cholecystectomy: suction versus static drainage. J Indian Med Assoc. 1986;84(11):341–342.
- Uetsuji S, Kwon AH, Komada H, et al. Clinical evaluation of closed suction drainage following hepatectomy. Surg Today. 1997;27(4):298–301. doi:10.1007/BF00941801.
- Čečka F, Jon B, Skalický P, et al. Results of a randomized controlled trial comparing closed-suction drains versus passive gravity drains after pancreatic resection. Surgery. 2018;164(5):1057–1063. doi:10.1016/j.surg.2018.05.030.
- Marchegiani G, Perri G, Pulvirenti A, et al. Non-inferiority of open passive drains compared with closed suction drains in pancreatic surgery outcomes: a prospective observational study. Surgery. 2018;164(3):443–449. doi:10.1016/j.surg.2018.04.025.
- Nishizaki T, Matsumata T, Yanaga K, Shimada M, Higashi H, Sugimachi K. Open and closed suction drainage after hepatic resection. Surg Today. 1993;23(10):871–874.
- Weiss S, Messner F, Huth M, et al. Impact of abdominal drainage systems on postoperative complication rates following liver transplantation. Eur J Med Res. 2015;20(1):66. doi:10.1186/s40001-015-0163-z.
- Aumont O, Dupré A, Abjean A, et al. Does intraoperative closed-suction drainage influence the rate of pancreatic fistula after pancreaticoduodenectomy? BMC Surg. 2017;17(1):58. doi:10.1186/s12893-017-0257-3.
- Hall BR, Egr ZH, Krell RW, et al. Association of gravity drainage and complications following Whipple: an analysis of the ACS-NSQIP targeted database. World J Surg Onc. 2021;19(1):118. doi:10.1186/s12957-021-02227-0.
- Kone LB, Maker VK, Banulescu M, Maker AV. Should drains suck? A propensity score analysis of closed-suction versus closed-gravity drainage after pancreatectomy. J Gastrointest Surg. 2021;25(5):1224–1232. doi:10.1007/s11605-020-04613-7.
- Correa-Gallego C, Brennan MF, Dʼangelica M, et al. Operative drainage following pancreatic resection: analysis of 1122 patients resected over 5 years at a single institution. Ann Surg. 2013;258(6):1051–1058. doi:10.1097/SLA.0b013e3182813806.
- Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: enhanced recovery after surgery (ERAS®) society recommendations. Br J Surg. 2014;101(10):1209–1229. doi:10.1002/bjs.9582.
- Kawai M, Tani M, Terasawa H, et al. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006;244(1):1–7.
- El Khoury R, Kabir C, Maker VK, Banulescu M, Wasserman M, Maker AV. Do drains contribute to pancreatic fistulae? Analysis of over 5000 pancreatectomy patients. J Gastrointest Surg. 2018;22(6):1007–1015. doi:10.1007/s11605-018-3702-4.
- Wang Q, Jiang YJ, Li J, et al. Is routine drainage necessary after pancreaticoduodenectomy? WJG. 2014;20(25):8110–8118. doi:10.3748/wjg.v20.i25.8110.
- Loder PB, Smith GH, Morris S, Bambach CP, Smith RC. A randomised comparison of three drainage systems following cholecystectomy. Aust N Z J Surg. 1987;57(8):531–535.
- Shrikhande SV, Sivasanker M, Vollmer CM, et al. Pancreatic anastomosis after pancreatoduodenectomy: a position statement by the international study group of pancreatic surgery (ISGPS). Surgery. 2017;161(5):1221–1234. doi:10.1016/j.surg.2016.11.021.
- Sunagawa M, Yokoyama Y, Yamaguchi J, et al. Is constant negative pressure for external drainage of the main pancreatic duct useful in preventing pancreatic fistula following pancreatoduodenectomy? Pancreatology. 2019;19(4):602–607. doi:10.1016/j.pan.2019.04.002.