Abstract
Background
Perifascial areolar tissue (PAT) is an areolar layer over the muscle fascia. PAT has been shown to be resistant to ischemia and prone to survival even in ischemic conditions. PAT grafts provide a vascular tissue layer on necrotic bone and tendons where skin grafting is not possible. The effect of PAT grafting on burn reconstruction has not yet been reported. Thus, in this study, we aimed to present our experience and discuss the role of PAT grafting in extremity burn reconstruction.
Methods
Between January 2019 and December 2020, 16 PAT grafting procedures were performed in 11 patients. All patients had second- or third-degree burns in the upper and lower extremities, with exposed bone or tendon. PAT grafts were harvested from the abdominal region and were used for the upper extremity in 7 patients and the lower extremity in 4 patients. Immediate skin grafting was performed during the same session.
Results
The patients’ mean age was 50.7 years; defect size, 3.3 × 3 cm2; and follow-up time, 11.8 months. The survival rates of the PAT and skin grafts were 93.8% and 68.6%, respectively. Partial skin graft losses were encountered in 4 patients, and total skin graft loss was seen in 1 patient.
Conclusion
PAT grafting is an alternative method to the use of dermal substitutes and flap surgery in small-to-medium-sized defects with exposed bone and tendon in burn patients.
Introduction
Deep extremity burns represent a major clinical challenge for reconstructive surgeons. The skin is thin, and tendons are more superficial in the distal extremities. Thus, deep extremity burns can lead to tendon or bone exposure [Citation1]. Flap surgery is the most frequently used method to cover these structures. However, it is challenging to perform using local or free flaps when the patient has a high comorbidity burden, poor vascular condition, and diminished skin quality over surrounding tissues due to burn trauma. Skin grafting after settled granulation and dermal substitutes are other alternatives to flap surgery. However, these methods are associated with prolonged healing time and high costs [Citation2]. Therefore, new options for covering exposed bones and tendons in extremity burns are needed.
Perifascial areolar tissue (PAT) is a well-vascularized connective tissue between the muscle fascia and the subcutaneous deep fat layer [Citation3]. This rich vascularity enables PAT grafts to survive even in ischemic conditions, which require graft elevation [Citation4]. The graft can be harvested from the abdomen, temporal region, posterior trunk, and lateral femoral region. The mechanism of graft survival is explained as revascularization from surrounding granulation tissue with the bridging phenomenon [Citation5]. The thin and sticky morphology of the PAT graft allows surgeons to fill concave defects or perform graft placement through tiny fistula openings [Citation6]. As the PAT graft requires placement between healthy granulation tissues, it provides a prepared wound bed for skin grafting. Thus, successful results have been reported for the use of PAT grafts to cover exposed tendons, bones, or plates have been reported [Citation7]. Various indications for PAT grafting have been reported in the literature, but the efficacy and role of PAT grafting in extremity burn reconstruction have not been reported.
In this study, we aimed to report our experience in using PAT grafts in the reconstruction of exposed tendons and bones after burn trauma.
Materials and methods
Patient characteristics and study protocol
This retrospective single-center study was conducted from January 2019 to December 2020 using data from our university burn unit database. It was approved by the institutional review board of Başkent University (project No. KA22/463) and supported by the Başkent University Research Fund. The principles of the Helsinki Declaration were followed in this study, which included 11 patients.
Patients with exposed tendon or bone caused by second- or third-degree burns in the lower and upper extremities were included in the study, whereas those with burns in areas other than the extremities were excluded. All PAT grafts were harvested from the abdominal region. Simultaneous skin grafting was performed over the PAT grafts in all patients. The patients’ characteristics (sex, age, and comorbidities), type of burn injury, defect distribution and size, complications, and follow-up time were noted ().
Table 1. Patient characteristics and clinical features.
Surgical technique
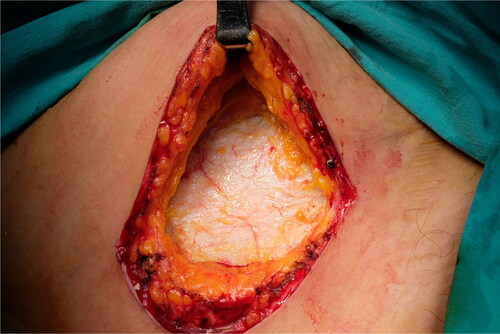
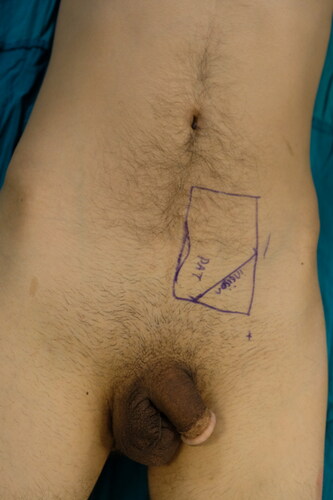
A 5-cm-long incision was made 2 to 3 cm superior to the inguinal crease from the medial to the lateral side. Superior and deep fascial layers were dissected until the rich areolar vascular layer over the rectus fascia was visualized (). An incision was made to the PAT without lacerating the rectus and external oblique muscle fascia. PAT grafts can be elevated superiorly or inferiorly depending on the defect. Surgical retractors were placed according to the direction of harvesting. Nontraumatic forceps were used to handle the PAT. Metzenbaum scissors or low-energy cautery was used to harvest the PAT graft. Perforator vessels raised from the fascia were carefully cauterized. Meticulous dissection was performed to harvest the PAT graft en bloc. Harvesting from the opposite direction without making an excessive skin incision was performed when a bigger PAT graft was needed. A suction drain was placed, and the skin was closed in layers after the graft was harvested.
Case examples
Case 1
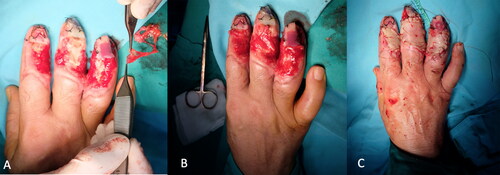
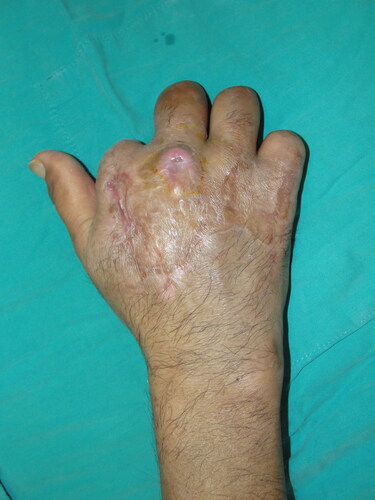
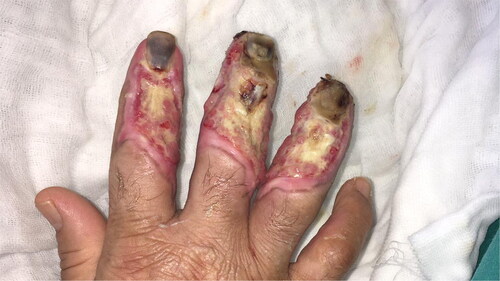
A 58-year-old woman with exposed tendon and bone in her second, third, and fourth digits was referred to our burn unit. She had an epileptic seizure and fell while holding a boiling kettle. On physical examination, she had full-thickness burn defects in the middle of the distal and middle phalanges, surrounded by granulation tissue (). A PAT graft was harvested from the abdomen over the external oblique muscle fascia. It was applied on the exposed tendon and phalanges while maintaining full contact with the surrounding granulation tissue (). A full-thickness skin graft was simultaneously harvested from the abdominal incision. The PAT and skin grafts completely survived. However, she developed donor site seroma, which regressed gradually over 1 month. A late postoperative image is shown in .
Figure 2. Preoperative image of the patient who had scald burn. Extensor tendons were exposed and distally with surrounding granulation tissue.
Case 2
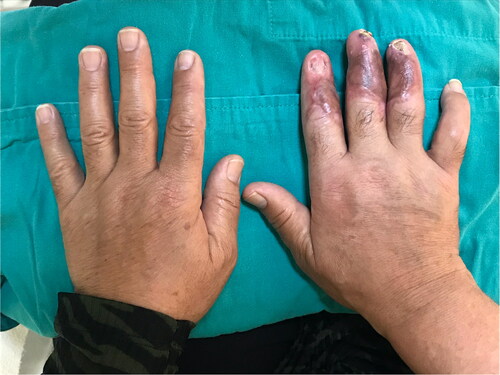
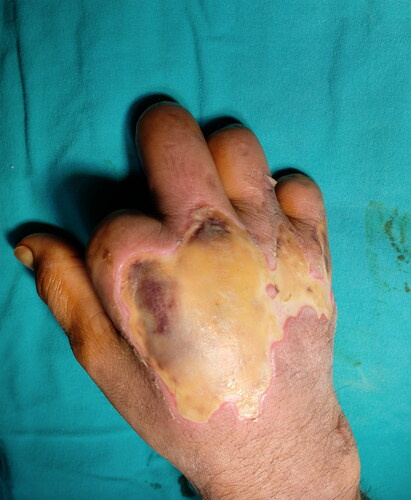
A 55-year-old man with a hand burn in the dorsum of his right hand was admitted to our burn unit. His hand was exposed to an infrared heater for 30 minutes from a 45-cm distance while trying to warm his hand. His medical history included diabetes mellitus, severe peripheral arterial disease, renal transplant rejection, and regular hemodialysis three times a week. He had a history of amputation of 4 digits at different levels and bilateral below-the-knee amputations. On physical examination, he had deep second-degree burns on the dorsum of his right hand (). After debridement, the second extensor tendon was exposed. A PAT graft was harvested from the abdomen and applied over the exposed second extensor tendon. A split-thickness skin graft was used for immediate closure of the dorsal defect (). No early or late-term complications were encountered during graft harvesting. A late postoperative image is shown in .
Figure 5. Full-thickness burns of the patient in his hand dorsum after exposure to the radiant heater. The patient had multiple amputations in different levels in his four extremities due to severe peripheral artery disease.
Case 3
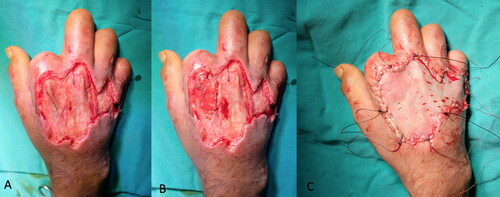
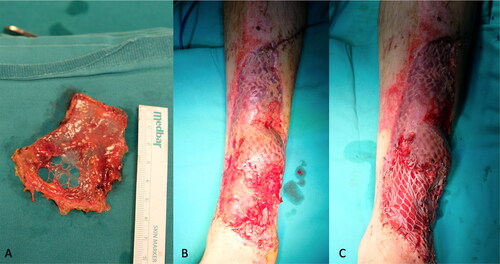
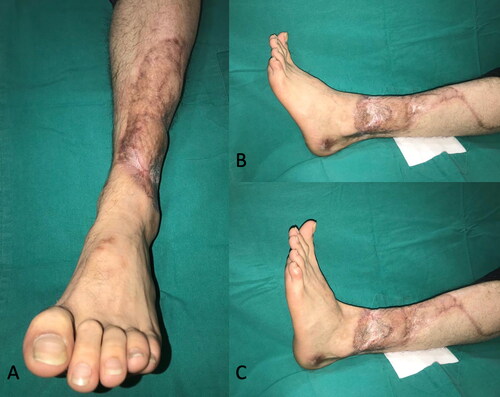
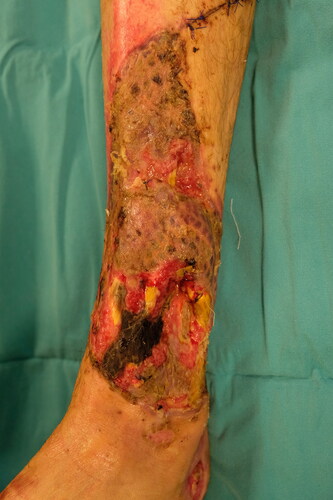
A 35-year-old man sustained high-voltage electrical burns. He had third-degree lower and upper extremity burns on 8% of the total body surface. He underwent immediate debridement and peroneus brevis muscle flap reconstruction to cover the exposed lower one-third of the defect. The tibialis anterior tendon and distal one-third of the fibula became exposed 2 weeks after the operation (). In a second operation, a PAT graft was harvested from the abdomen and applied on the exposed tibialis anterior tendon. The PAT graft was laid over the exposed tendon, with full contact to the surrounding granulation tissue. It was simultaneously covered with a meshed skin graft at a meshing ratio of 3:1 (). The PAT grafts completely survived. The skin graft showed partial necrosis. The patient was followed up with conventional wound care, and the defects were fully epithelized in 1 month after the operation. An image of the patient taken 6 months after the operation is shown in .
Figure 8. The patient sustained from high-voltage electric burns in his lower extremity and right hand. Although he had a muscle flap reconstruction and skin grafting previously, there was still anterior tibial muscle tendon and tibial bone exposure at the injured site.
Results
The patients’ mean age was 50.7 years, ranging from 32 and 67 years. The mean defect size was 3.3 × 3 cm2 (range, 1 × 1 to 5 × 5 cm2). High-voltage electrical, scald, and flame burns were seen in 36%, 27%, and 27% of the patients, respectively. The mean follow-up time was 11.8 months (range, 6–18 months).
Eleven PAT grafts were used for the upper extremity, and 5 PAT grafting procedures were performed for the lower extremity to cover exposed bones or tendons. Any tendon adhesion was not observed in the late postoperative period. The survival rates of the PAT and skin grafts were 93.8% and 68.6%, respectively. The PAT graft failed to engraft in the ankle region in 1 patient. PAT and skin regrafting were performed 2 weeks later, with an uneventful healing. Partial skin graft necrosis was found in 4 patients, of whom 3 achieved healing with conservative wound care. The remaining patient required regrafting over the PAT graft. All patients older than 53 years (7/11 patients) had mild to severe comorbidities. Partial skin necrosis was found in 2 patients with diabetes mellitus. No complications were found in the patients with severe comorbidities such as chronic kidney failure and peripheral arterial disease. Seroma formation was found in 1 patient but was resolved within 1 month with a compression dressing.
Discussion
Exposed tendons, bones, and vital structures such as neurovascular bundles generally require flap reconstruction surgery. However, this is generally harmful to adjusting tissues, and the blood circulation in local flaps might be unreliable because of the burn injury, which may lead to flap necrosis and result in increased defect size. Although free microvascular transfers can provide sufficient tissue in one stage for large defects, the success of free flap surgery depends on the patient’s general health, vascular risk factors, and type of burn injury. Thus, patients should be well evaluated for their suitability as candidates for microvascular tissue transfer. Interpolation flaps can also be used as an alternative to free flaps [Citation8]. However, the use of these flaps has several disadvantages such as joint stiffness and pressure sores due to prolonged immobility [Citation9, Citation10]. For patients with small- to medium-sized tendon or bone exposures after undergoing primary microvascular reconstruction, surgical correction with a new free or interpolation flap is not preferred. The current treatment strategy for these patients is skin grafting on dermal substitutes or delayed skin grafting.
Dermal substitutes are indicated for small- to medium-sized defects in burn reconstruction [Citation11–13]. However, their high costs constitute a problem for developing countries [Citation2]. Delayed closure is associated with the risk of infections such as osteomyelitis or tenosynovitis, which may lead to permanent loss of exposed tissues. In these clinical scenarios, PAT grafting can be an alternative reconstructive option for upper and lower extremity burns.
In the literature, successful results have been reported in chronic ischemic ulcers or fistulas [Citation4, Citation14]. In these studies, PAT grafts were used as allogenic dermal substitutes to provide a basal layer for skin grafting. The mechanism of PAT engraftment was later demonstrated in histopathological tissue specimens. A previous study found that fibroadipose PAT is surrounded by granulation tissue, which is a supportive finding for the phenomenon of graft survival [Citation6]. Hayashi et al. revealed a well-organized collagen deposition and endothelial cell infiltration around PAT graft-based vessels. These findings support the mechanisms underlying revascularization and rapid intake of PAT grafts [Citation15]. The authors of a recent experimental study suggested that the rapid intake of PAT grafts may be attributed to the presence of stem cells [Citation16]. Another hypothesis is that skin graft intake is attributed to the texture of the PAT graft, which allows for easy nutrient diffusion in the imbibition phase [Citation16].
In this study, we demonstrated the usefulness of PAT grafting in upper and lower extremity burns with exposed tendon/bone. In these patients, we observed several advantages of PAT grafting. First, the donor site did not constitute a problem in our series. The PAT lies over the deep muscle fascia such as the rectus abdominis and tensor fascia lata, which are generally not affected even in third-degree burn traumas. These donor sites can provide sufficient PAT grafts. The commonly preferred route for PAT graft harvesting is through a transverse incision over the inguinal crease [Citation17]. However, this incision carries a risk of damage to superior circumflex iliac artery (SCIA)-based workhorse free flaps, which might be needed in burn treatments such as contracture release or defect reconstruction [Citation18, Citation19]. We modified the traditional harvest site to a more superior and medial position from the inguinal crease to protect the SCIA and its branches (). Second, PAT graft harvesting has a short learning curve. Third, the grafting procedure did not require microvascular anastomosis or frequent postoperative follow-up. As a consequence, the operation time was shorter than that required for microvascular tissue transfers. Fourth, we successfully reconstructed the extremity defects of the patients with poor vascular conditions who were not candidates for free microvascular transfers. The main drawbacks of PAT grafting are the unpredictable intake rate of the skin graft applied simultaneously and donor site-related complications.
Figure 11. Different from the literature, we advocate more superior incision for PAT graft harvesting in order to protect superficial circumplex epigastric artery based flaps which may be needed in later stages of burn reconstruction.
The timing of skin grafting has been controversial. Kouzimi advocated delayed skin grafting after total PAT graft intake [Citation6]. Miyanaga et al. delayed split-thickness skin graft placement for 3 weeks and achieved a skin graft intake rate higher than 95% [Citation20]. Dressings such as hydrogels should be preferred for PAT graft viability, as the PAT is fragile and prone to dehydration. In a second stage, a PAT graft can be applied on a vascularized PAT graft. However, the two-stage operation and coverage of the PAT grafts with artificial dermis are the downsides of this method because of their high costs. Abe et al. used negative-pressure wound therapy (NPWT) to increase the intake rate of the skin graft applied simultaneously [Citation21]. After 5–7 days of NPWT at low pressures, a 75% success rate of PAT and skin grafting was achieved. The authors concluded that NPWT helped eliminate shear forces and increased blood flow, which led to better outcomes than those previously reported in the literature. In our case series, we applied skin grafts with PAT grafts simultaneously. The success rate of skin graft intake in our series was 68.8%, which could be due to the fat tissue included in the PAT graft, the loss of granulation tissue due to compression or infection, and shear stress. Abe et al. used NPWT to reduce shear forces and increase vascularization to mitigate the low graft intake rates [Citation21]. The amount of fat tissue included in the graft during harvesting can be minimized with a more delicate dissection. We did not use NPWT in any of our patients. For maximum contact with the surrounding granulation tissue, we harvested slightly larger PAT grafts than the defects with exposed bone or tendon. PAT grafts are prone to desiccation due to the fibroadipose structure of PAT. Thus, we used sheet full-thickness skin grafts instead of meshed grafts in the dorsum of the fingers. We observed skin graft intake with white graft reaction in the following 4 to 5 days, with a 10% intake failure rate. Total regrafting was needed in two patients with skin graft rejection. The thickness of the perifascial loose areolar tissue was less than 0.5 mm. We observed that the fat included in the graft during harvesting might have contributed to the intake rates. In some cases, although the skin grafts failed to heal, we did not encounter any tendon or bone exposure. The reason for PAT graft failure in medial ankle defects can be the anatomy of the medial ankle or meshed skin grafting. Medial ankle defects are deep, and tendons lie superficially and just lateral to the defect. Thus, filling the defect and achieving contact with granulation tissue with the PAT graft were difficult. We think that local muscle flaps such as peroneus brevis and free flaps might be more promising options than PAT grafting for covering defects with exposed tendons in the ankle region [Citation22, Citation23]. Donor site seroma and pain were encountered in 2 patients. During PAT graft elevation, the cutaneous nerves should be preserved and perforators from the deep fascia should be cauterized. To prevent these complications, we also recommend the application of drains after harvesting.
Various application areas of the PAT graft have been mentioned in the literature [Citation15, Citation17, Citation20, Citation21]. However, its use in burn reconstruction has not been reported yet. In this respect, our work is unique.
Our study has several limitations. A retrospective data search was performed. With PAT grafting, we managed to obtain acceptable results for small- to medium-sized defects but not for large defects with exposed tendon or bone. Another limitation of this study was the limited case series with a few types of defects. Larger case series with various burn defects are needed to estimate the effectiveness of PAT grafting in burn reconstruction.
In conclusion, this is the first case series study to report on the usefulness of PAT grafting for upper and lower extremity burns. On the basis of our findings, PAT grafting is an alternative method to dermal substitutes and flap surgery for burn patients with small- to medium-sized defects with exposed bone and tendon. Our experience shows that skin grafts can be applied in the same session when adjacent tissues are well granulated. We believe that our study provides insights to guide future studies.
Acknowledgments
There is no contributor who does not meet the criteria for authorship. Hence, the section of acknowledgment is none.
Disclosure statement
The authors declare that they have no conflict of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Smith MA, Munster AM, Spence RJ. Burns of the hand and upper limb—a review. Burns. 1998;24(6):1–8.
- Ryssel H, Radu CA, Germann G, Otte M, Gazyakan E. Single‐stage Matriderm® and skin grafting as an alternative reconstruction in high‐voltage injuries. Int Wound J. 2010;7(5):385–392. doi:10.1111/j.1742-481X.2010.00703.x.
- Nakajima H, Imanishi N, Minabe T, Kishi K, Aiso S. Anatomical study of subcutaneous adipofascial tissue: a concept of the protective adipofascial system (PAFS) and lubricant adipofascial system (LAFS). Scand J Plast Reconstruct Surg Hand Surg. 2004;38(5):261–266. doi:10.1080/02844310410029543.
- Kouraba S. Perifascial areolar tissue graft: novel autologous graft material and its clinical application for coverage of exposed bone and tendon. AHZ J Surg. 2003;73(2):A260.
- Gondo M, Matsumura H, Watanabe K. Clinical application of subdermal areolar tissue and superficial fascia graft as a new material for coverage of small exposure of bone cortex or orthopaedic fixation device. J Plast Surg Hand Surg. 2017;51(4):223–227. doi:10.1080/2000656X.2016.1223088.
- Koizumi T, Nakagawa M, Nagamatsu S, et al. The versatile perifascial areolar tissue graft: adaptability to a variety of defects. J Plast Surg Hand Surg. 2013;47(4):276–280. doi:10.3109/2000656X.2012.759955.
- Ito T, Akazawa S, Ichikawa Y, et al. Exposed artificial plate covered with perifascial areolar tissue as a nonvascularized graft. Plast Reconstruct Surg Global Open. 2019;7(2):e2109.
- Hundeshagen G, Warszawski J, Tapking C, et al. Concepts in early reconstruction of the burned hand. Ann Plast Surg. 2020;84(3):276–282. doi:10.1097/SAP.0000000000002019.
- Irmak F, Mo Y. Interpolation flaps and determination of pedicle separation time. Hand Microsurg. 2019;8(2):102–108.
- Barillo DJ, Arabitg R, Cancio LC, Goodwin CW. Distant pedicle flaps for soft tissue coverage of severely burned hands: an old idea revisited. Burns. 2001;27(6):613–619. doi:10.1016/S0305-4179(01)00014-6.
- Cuadra A, Correa G, Roa R, et al. Functional results of burned hands treated with Integra®. J Plast Reconstruct Aesthet Surg. 2012;65(2):228–234. doi:10.1016/j.bjps.2011.09.008.
- Ryssel H, Gazyakan E, Germann G, Öhlbauer M. The use of MatriDerm® in early excision and simultaneous autologous skin grafting in burns—a pilot study. Burns. 2008;34(1):93–97. doi:10.1016/j.burns.2007.01.018.
- Bozkurt M, Tatar BE, Karakol P, et al. Matriderm and platelet-rich plasma combination in the treatment of recurrent carpal tunnel syndrome: a new approach. Eur J Plast Surg. 2022;45:299–306.
- Koizumi T, Nakagawa M, Nagamatsu S, Kayano S, Akazawa S. Perifascial areolar tissue graft as a nonvascularized alternative to flaps. Plast Reconstruct Surg. 2010;126(4):182e–183e.
- Hayashi A, Komoto M, Tanaka R, et al. The availability of perifascial areolar tissue graft for deep cutaneous ulcer coverage. Journal of Plastic. Reconstruct Aesthet Surg. 2015;68(12):1743–1749. doi:10.1016/j.bjps.2015.08.008.
- Oshima J, Sasaki K, Shibuya Y, Sekido M. A Novel Model of Perifascial Areolar Tissue Transplant in Rats. Indian J Plast Surg. 2022;55(03):268–271.
- Choi WY, Sung KW, Kim YS, et al. Reconstruction for skull base defect using fat-containing perifascial areolar tissue. Ann Plastic Surg. 2017;78(6):673–679. doi:10.1097/SAP.0000000000000909.
- Bedreag OH, Papurica M, Rogobete AF, et al. New perspectives of volemic resuscitation in polytrauma patients: a review. Burns Trauma. 2016;4:1747–1759. doi:10.1186/s41038-016-0029-9.
- Gentileschi S, Servillo M, De Bonis F, et al. Radioanatomical study of the pedicle of the superficial circumflex iliac perforator flap. J Reconstruct Microsurg. 2019;35(09):669–676.
- Miyanaga T, Haseda Y, Daizo H, et al. A Perifascial areolar tissue graft with topical administration of basic fibroblast growth factor for treatment of complex wounds with exposed tendons and/or bones. J Foot Ankle Surg. 2018;57(1):104–110.
- Abe Y, Hashimoto I, Ishida S, Mineda K, Yoshimoto S. The perifascial areolar tissue and negative pressure wound therapy for one‐stage skin grafting on exposed bone and tendon. J Med Invest. 2018;65(1.2):96–102. doi:10.2152/jmi.65.96.
- Yang X, Fang Z, Liu M, et al. Reconstruction of deep burn wounds around the ankle with free fascia flaps transfer and split-thickness skin graft. J Burn Care Res. 2019;40(6):763–768. doi:10.1093/jbcr/irz078.
- Koski EA, Kuokkanen HO, Tukiainen EJ. Distally-based peroneus brevis muscle flap: a successful way of reconstructing lateral soft tissue defects of the ankle. Scand J Plast Reconstr Surg Hand Surg. 2005;39(5):299–301.