Abstract
Objective
The link between inflammation and cancer survival has been the subject of substantial research. The goal of this review is to summarize the evidence on the prognostic value of systemic inflammation score (SIS) in esophageal cancer patients undergoing surgical intervention.
Methods
PubMed, Scopus, Embase, and Web of Science were searched for relevant articles published until 30th June 2022. We pooled adjusted data on overall survival (OS) and disease-free survival (DFS) using a random-effects meta-analysis model. The review was pre-registered on PROSPER (No. CRD42022340717).
Results
Eight studies were included. All studies were conducted either in China or Japan. Six studies showed that patients with SIS of 1-2 had poor OS as compared to those with scores of 0 (HR:1.42 95% CI: 1.24, 1.62 I2=25%). SIS of 1 (HR:1.45 95% CI: 1.18, 1.78 I2=0%) and 2 (HR:1.94 95% CI: 1.49, 2.53 I2=0%) were also associated with poor OS. Two studies compared the SIS score of 2 vs 0-1. Meta-analysis indicated that poor OS was associated with SIS of 2 (HR:1.80 95% CI: 1.25, 2.58). Data from three studies showed that the SIS score did not predict DFS (HR:1.40 95% CI: 0.82, 2.39 I2=91%).
Conclusion
SIS can be a novel prognostic indicator for esophageal cancer patients undergoing surgical intervention. Higher SIS is associated with a poor OS, but it does not predict DFS. Future studies are needed to strengthen the current evidence.
Introduction
Esophageal cancer is considered the eighth most common cancer and the sixth most common cause of cancer-related deaths worldwide. Recent global data indicates that approximately 500,000 deaths every year are attributable to esophageal cancer [Citation1]. High mortality rates associated with this malignancy are primarily due to late diagnosis. Data from the USA shows that only about 18% of patients are diagnosed in an early stage of the esophageal cancer, and 40% of patients have distant metastasis at the time of diagnosis [Citation2]. While recent advances in diagnostic and treatment of esophageal cancer have led to improved outcomes, the survival rate of esophageal cancer patients is still poor due to the complex anatomy of the region combined with high recurrence rates [Citation3]. Therefore, an important part of treatment planning for esophageal cancer is recognizing and targeting patients with worse outcomes. Accurate prognostic indicators can provide valuable information on long-term outcomes. Moreover, patients with such indicators can be provided additional more aggressive therapies to improve survival [Citation4].
Malignant conditions are often associated with inflammation that may cause cellular damage, angiogenesis, alteration of extracellular matrix, and development of premetastatic niches due to the release of inflammatory mediators. The inflammatory state produced by cancer changes the microenvironment leading to increased risk of recurrence and metastasis [Citation5, Citation6]. However, there is still no consensus on the standard inflammatory biomarkers or an accepted scoring system that can be utilized in clinical practice to predict the prognosis of cancer patients based on the inflammation status.
Recently, the systemic inflammation score (SIS), derived from a combination of serum albumin and lymphocyte-monocyte ratio (LMR) scores, has been proposed as a marker of inflammation and a predictor of the prognosis in several malignancies including oral, gastric, pancreatic, and hepatic cancer [Citation7–10]. Numerous studies have assessed the prognostic ability of SIS for esophageal cancer [Citation11–13]. However, the results of individual studies have been inconclusive, and there is still no consensus on whether SIS can be used in routine practice to assess patients with esophageal cancer. The aim of this study is to summarize the evidence and to conduct the first systematic review and meta-analysis on the prognostic ability of SIS in esophageal cancer patients undergoing surgical intervention.
Material and methods
Search and eligibility
The review was pre-registered on PROSPERO (No. CRD42022340717) and was conducted based on the guidelines of the PRISMA statement [Citation14].
PubMed, Scopus, Embase, and Web of Science databases were searched for articles published untill 30th June 2022. No language restriction was applied to the search. All study types examining the relationship between SIS and the prognosis of esophageal cancer patients were eligible for inclusion.
Only studies that were conducted on a target population of esophageal cancer patients undergoing surgical intervention, and reporting outcomes that were separated based on the SIS score, were included. The reported outcomes were either overall survival (OS) or disease-free survival (DFS) as effect size with 95% confidence intervals (CI). The exclusion criteria were as follows: (1) Studies reporting duplicate or overlapping data (2) Studies not reporting outcomes of esophageal cancer separately (3) SIS was not based on albumin and LMR values (4) Case reports, reviews, and editorials.
A combination of the following free-text and MeSH search terms were used for the search: “esophageal cancer”, “esophageal carcinoma”, “esophageal malignancy”; “systemic inflammation score”, “inflammation”; “prognosis”, “survival”; and “cancer”. The PubMed search strategy is demonstrated in Supplementary Table 1. Similar search strings were used for other databases. After duplicates removal, titles and abstracts of the identified papers were screened independently by the two reviewers based on the eligibility criteria. Full texts of eligible studies were further screened for the papers that meet all eligibility criteria. Any disagreements were resolved by consensus. The references of the included studies were further cross-checked for additional relevant articles.
Table 1. Details of included studies.
Data management and study quality
Two reviewers independently extracted data that included study authors, publication date, study location and eligible population, tumor stage included, SIS definition and time of measurement, sample size, age, male gender, histology, vascular and lymphatic invasion, use of adjuvant chemotherapy, follow-up and ratios of OS/DFS.
The Newcastle Ottawa Scale (NOS) [Citation15] was chosen for assessing the risk of bias due to the observational nature of the studies. The articles were judged and scored on three domains: study population, comparability, and outcomes, with the final score ranging from 0 to 9.
Statistical analysis
OS and DFS were reported as hazard ratios (HR) with 95% CI which was combined to calculate the meta-analysis result as HR. We chose to perform the analysis in a random-effects model. Inter-study heterogeneity was assessed using the I2 statistic. I2=25–50% meant low, 50–75% meant medium, and more than 75% meant substantial heterogeneity. Due to the limited number of studies, funnel plots were not used to detect the publication bias. “Review Manager” (RevMan, version 5.3; Nordic Cochrane Center (Cochrane Collaboration), Copenhagen, Denmark; 2014) was used for the meta-analysis.
Results
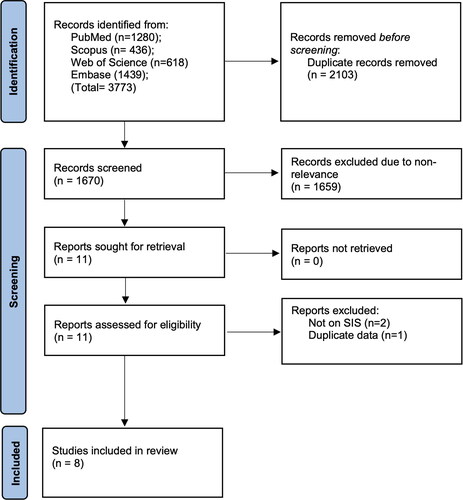
The search results are shown in . A total of 1670 records were screened. Of them, 1659 were found not to be related to the topic of the review. Eleven studies were selected for the full text analysis and eight studies met the eligibility criteria and were included in the review [Citation11–13, Citation16–20].
All studies were conducted either in China or Japan and published between 2015 and 2022. All except one study included patients with stage I-IV esophageal cancer. There were some variations in the cutoff of albumin and LMR values used to calculate SIS. Albumin cutoff values used were 4 g/dl or 3.7 g/dl while LMR values ranged from 3.3 to 4.44. All studies measured SIS before surgical intervention. Follow-up duration was more than 3 years in all studies. All studies were of good quality with NOS scores of 7 or 8 ().
Overall survival
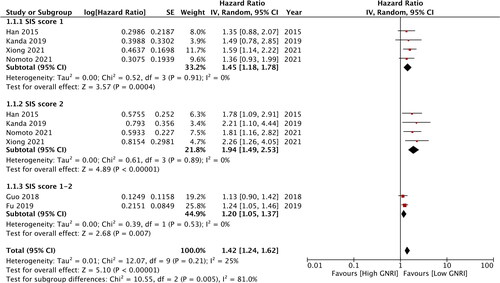
All studies reported data on OS, however the cutoff values and the reference groups differed between the studies. To segregate the data, separate analysis was conducted for homogenous data. Our analysis showed that SIS = 1 was associated with worse OS (HR:1.45 95% CI: 1.18, 1.78) compared to score 0 (). There was no heterogeneity in the meta-analysis, with I2=0%. Similarly, data from four studies indicated that the SIS = 2 was also associated with poor OS as compared to the score of 0 (HR:1.94 95% CI: 1.49, 2.53) (). There was no inter-study heterogeneity (I2=0%). Two studies reported combined data of SIS of 1-2 vs a score of 0. Meta-analysis results showed that poor OS was associated with higher SIS scores (HR:1.20 95% CI: 1.05, 1.37 I2=0%) (). Combining data from all six studies, our results demonstrated that patients with SIS of 1-2 had poor OS (HR:1.42 95% CI: 1.24, 1.62 I2=25%) ().
Two studies compared the SIS = 2 vs SIS if 0-1. Meta-analysis indicated that poor OS correlated with the SIS = 2 (HR:1.80 95% CI: 1.25, 2.58) (). There was minimal inter-study heterogeneity (I2=11%).
Disease-free survival
Data on DFS was reported only by three studies. Pooled analysis demonstrated no difference in DFS between patients with SIS of 1-2 as compared to those with SIS = 0 (HR:1.40 95% CI: 0.82, 2.39) (). The heterogeneity for this meta-analysis was high with I2=91%.
Discussion
The ability to accurately predict prognosis in a high-morality malignancies such as esophageal cancer can significantly aid in the treatment planning and targeted delivery of advanced therapeutic modalities. Like any other malignancies, esophageal cancer is primarily staged based on the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system [Citation21]. While this system is easy to use and is accepted worldwide, it has certain limitations. TNM staging is a broad classification system and does not holistically encompass all factors related to patient prognosis. Patients with similar pathological staging scores may have different OS as well as DFS [Citation22]. Additionally, TNM staging system does not take into account the level of host- and cancer-related inflammation and is only based on the biological features of the tumor [Citation23]. Research has shown that malignancies lead to the release of several pro-inflammatory cytokines, including interleukins, transforming growth factor-β, eicosanoids, proteases, that contribute to creating a tumor-promoting inflammatory environment. Such changes stimulate growth and multiplication of tumor cells and blood vessels, promote metastasis, downgrade adaptive immunity, and may also alter the response to treatment. Considering the important role of inflammation in tumorigenesis, numerous studies explored the prognostic value of blood-borne markers in esophageal cancer. Yodying et al [Citation24] performed a pooled analysis of seven studies and showed that neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were predictive of poor OS but not DFS. Recently, Powell et al [Citation25] explored the prognostic value of several different inflammatory markers like serum hemoglobin and albumin, C-reactive protein, modified Glasgow Prognostic Score, and differential NLR in a cohort of 330 esophageal cancer patients undergoing surgery. The authors noted that only NLR was an independent prognostic factor for both OS and DFS in esophageal cancer patients. Another study has shown that both LMR and NLR are predictive of OS in esophageal cancer patients [Citation26].
However, studies comparing the prognostic abilities of various inflammatory factors in cases of esophageal cancer are still scarce. Fu et al [Citation18] showed that SIS is a better prognostic marker for esophageal cancer compared to NLR. Chen et al [Citation27] reported that in a cohort of gastric cancer patients, SIS was superior to the modified Glasgow Prognostic Score and lymphocyte C-reactive protein ratio in predicting prognosis of the patients. To the best of our knowledge, our study is the first review and meta-analysis that combined data to assess the ability of SIS to predict outcomes in esophageal cancer.
Our pooled analysis demonstrated that high SIS scores were predictive of poor OS in patients with esophageal cancer. A limitation we faced while pooling data was the difference in the scores and reference groups used by the studies. Nevertheless, we noted that both SIS scores of 1 and 2 were associated with poor OS, and a score of 2 had higher predictive value as compared to a score of 1. The predictive value of SIS was maintained even when we combined the data of all six studies, using 0 as a reference group. Our results showed that SIS scores of either 1-2 vs 0 were associated with poor OS. Importantly, there was minimal heterogeneity in the meta-analysis which adds to the credibility of the results. Two studies used a SIS of 1 as a reference group. Combining the data of these studies also demonstrated that the score of 2 was associated with the poor survival. However, our analysis failed to demonstrate the prognostic ability of SIS for DFS due to the scarce data from just three studies.
The results of our meta-analysis are in agreement with the previous studies. Eltohami et al [Citation7] have found that higher SIS were associated with poor OS in patients with oral cancer. Zhang et al [Citation8] performed a retrospective study of 126 patients with intrahepatic cholangiocarcinoma and showed that SIS is a powerful prognostic indicator predicting clinical outcomes in such patients. Markus et al [Citation9] have demonstrated similar results for SIS in patients with metastatic pancreatic cancer. Ma et al [Citation28] have shown that SIS is a good prognostic indicator in patients with gastric cancer undergoing resection.
The good prognostic ability of SIS could be due to the fact that it combines two singular important indicators: LMR and albumin. Separately, both these variables can predict outcomes in cancer patients, but the predictive value of SIS is better than that of individual values [Citation12]. Low albumin levels are related to malnutrition and cachexia and reflect the baseline inflammatory and nutritional levels of the patient [Citation10]. Lymphocyte levels on the other hand represent the innate and adaptive immunity of the cancer patient and have anti-malignancy properties by limiting the multiplication, invasion, and metastasis of tumor cells [Citation29]. Higher levels of tumor-infiltrating lymphocytes are associated with better outcomes in cancer patients [Citation30]. In contrast, high monocyte counts are associated with the proliferation of cancer, and tumor-monocyte-endothelial interaction has been linked to higher chances of metastasis [Citation31, Citation32]. Therefore, the combination of albumin, lymphocytes, and monocytes in SIS could result in its better prognostic value in esophageal cancer patients. However, the efficiency of SIS as compared to the established TNM system is still unclear. Additionally, it is not established yet whether combining SIS with TNM will potentially increase the predictability of outcomes. There is a need for further studies on the prognostic value of SIS alone and in combinations with the TNM system to generate evidence on the best prognostic indicators for esophageal cancer.
The clinical significance of our results is that SIS, as an easy-to-calculate score, could be routinely used to generate a broad overview of the prognosis of esophageal cancer patients. The laboratory values used to calculate SIS are routinely available in all healthcare setups worldwide allowing a global use of the score. Secondly, it is also important to establish whether modifying SIS using interventions such as nutritional supplements can improve the prognosis of esophageal cancer patients. Future randomized studies should compare targeted interventions in low SIS patients with a placebo to generate high-quality evidence.
The primary strength of our review is that it is the first study that summarizes data on the predictive ability of SIS in patients with esophageal cancer. Secondly, we reported low heterogeneity for OS, potentially because a similar homogenous cohort was analyzed across all included studies. All studies in our review were conducted in Asian patients with stage I-IV cancer and undergoing surgical intervention. This homogeneity combined with differences in data presentation precluded a subgroup analysis in our review.
However, there were some limitations to our study. The retrospective nature of the included studies introduced a potential important source of bias. Additionally, the cutoffs used in different studeis to classify patients under SIS were not consistent. Also, there was a high heterogeneity in the meta-analysis of DFS, which suggests the need for further investigation into potential sources of variation. A formal assessment of publication bias was not conducted, which could have influenced our results. Lastly, the current data is only from Asian studies which affect the generalizability of our results. Further studies that include Western populations of patients are needed to establish the predictive value of SIS.
Conclusions
SIS can be a novel prognostic indicator for esophageal cancer patients undergoing surgical intervention. Higher SIS scores are associated with poor OS but do not predict DFS. Future studies are needed to strengthen the current evidence.
Author contributions
L.S. analyzed the data and was the major contributor in writing the manuscript; X.W. and L.S. were contributors in the data analysis; C.Y. was responsible for reviewing and editing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
| List of Abbreviations | ||
| SIS | = | systemic inflammation score |
| OS | = | overall survival |
| DFS | = | disease-free survival |
| LMR | = | lymphocyte-monocyte ratio |
| CI | = | confidence intervals |
| NOS | = | Newcastle Ottawa Scale |
| AJCC | = | American Joint Committee on Cancer |
| TNM | = | tumor-node-metastasis |
| NLR | = | neutrophil-to-lymphocyte ratio |
| PLR | = | platelet-to-lymphocyte ratio |
Supplemental Material
Download PDF (78.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of data and materials
The data that support the findings of this study are openly available in [PROSPERO] at [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022340717], reference number [No CRD42022340717].e
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):1–7. doi:10.3322/CAAC.21492.
- Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13(6):1010–1021. doi:10.1007/S12328-020-01237-X.
- Kelly RJ. Emerging Multimodality Approaches to Treat Localized Esophageal Cancer. J Natl Compr Canc Netw. 2019;17(8):1009–1014. doi:10.6004/JNCCN.2019.7337.
- Hong L, Han Y, Zhang H, Fan D. Prognostic markers in esophageal cancer: from basic research to clinical use. Expert Rev Gastroenterol Hepatol. 2015;9(7):887–889. doi:10.1586/17474124.2015.1041507.
- Hernández M, Martín R, García-Cubillas MD, Maeso-Hernández P, Nieto ML. Secreted PLA2 induces proliferation in astrocytoma through the EGF receptor: another inflammation-cancer link. Neuro Oncol. 2010;12(10):1014–1023. doi:10.1093/NEUONC/NOQ078.
- Liu S, Li C, Zhang P. Enhanced recovery after surgery for hip fractures: a systematic review and meta-analysis. Perioper Med (London, England). 2021;10(1):31. doi:10.1186/S13741-021-00201-8.
- Eltohami YI, Kao HK, Lao WWK, et al. The prediction value of the systemic inflammation score for oral cavity squamous cell carcinoma. Otolaryngol Head Neck Surg. 2018;158(6):1042–1050. doi:10.1177/0194599817751678.
- Zhang Y, Shi SM, Yang H, et al. Systemic inflammation score predicts survival in patients with intrahepatic cholangiocarcinoma undergoing curative resection. J Cancer. 2019;10(2):494–503. doi:10.7150/JCA.26890.
- Markus M, Abendroth A, Noureddine R, et al. Combined systemic inflammation score (SIS) correlates with prognosis in patients with advanced pancreatic cancer receiving palliative chemotherapy. J Cancer Res Clin Oncol. 2021;147(2):579–591. doi:10.1007/S00432-020-03361-0.
- Liu C, Li X. Stage-Dependent changes in albumin, NLR, PLR, and AFR are correlated with shorter survival in patients with gastric cancer. Clin Lab. 2019;65(09/2019):1623–1633. doi:10.7754/Clin.Lab.2019.190132.
- Aoyama T, Ju M, Komori K, et al. The systemic inflammation score is an independent prognostic factor for esophageal cancer patients who receive curative treatment. Anticancer Res. 2022;42(5):2711–2717. doi:10.21873/ANTICANRES.15749.
- Xiong J, Kang W, Ma F, et al. Modified systemic inflammation score is an independent predictor of long-term outcome in patients undergoing surgery for adenocarcinoma of the esophagogastric junction. Front Surg. 2021;8:622821. doi:10.3389/fsurg.2021.622821.
- Nomoto D, Baba Y, Akiyama T, et al. Adapted systemic inflammation score as a novel prognostic marker for esophageal squamous cell carcinoma patients. Ann Gastroenterol Surg. 2021;5(5):669–676. doi:10.1002/AGS3.12464.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi:10.1016/j.ijsu.2021.105906.
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 30, 2020.
- Tsuchiya N, Kunisaki C, Sato S, et al. Feasibility of esophagectomy for esophageal cancer in elderly patients: a case-control study. Langenbecks Arch Surg. 2021;406(8):2687–2697. doi:10.1007/S00423-021-02271-0.
- Kanda M, Koike M, Tanaka C, et al. Modified Systemic Inflammation Score is Useful for Risk Stratification After Radical Resection of Squamous Cell Carcinoma of the Esophagus. Ann Surg Oncol. 2019;26(13):4773–4781. doi:10.1245/S10434-019-07914-7.
- Fu X, Li T, Dai Y, Li J. Preoperative systemic inflammation score (SIS) is superior to neutrophil to lymphocyte ratio (NLR) as a predicting indicator in patients with esophageal squamous cell carcinoma. BMC Cancer. 2019;19(1):721. doi:10.1186/s12885-019-5940-6.
- Guo XW, Liu YC, Gao F, et al. Pretreatment NRS-2002 scores combined with hematologic inflammation markers are independent prognostic factors in patients with resectable thoracic esophageal squamous cell carcinoma. Cancer Manag Res. 2018;10:2409–2418. doi:10.2147/CMAR.S167179.
- Han L, Song Q, Jia Y, et al. The clinical significance of systemic inflammation score in esophageal squamous cell carcinoma. Tumour Biol. 2016;37(3):3081–3090. doi:10.1007/S13277-015-4152-1.
- Mullaney PJ, Wadley MS, Hyde C, et al. Appraisal of compliance with the UICC/AJCC staging system in the staging of gastric cancer. Union Internacional Contra la Cancrum/American Joint Committee on Cancer. Br J Surg. 2002;89(11):1405–1408. doi:10.1046/J.1365-2168.2002.02262.X.
- Zhang G, Zhang C, Wang L, et al. The prognostic value of tumor deposits and the impact on the TNM classification system in esophageal cancer patients. J Surg Oncol. 2021;123(4):891–903. doi:10.1002/JSO.26376.
- McMillan DC. Cancer and systemic inflammation: stage the tumour and stage the host. Br J Cancer. 2013;109(3):529. doi:10.1038/BJC.2013.418.
- Yodying H, Matsuda A, Miyashita M, et al. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann Surg Oncol. 2016;23(2):646–654. doi:10.1245/S10434-015-4869-5.
- Powell AGMT, Eley C, Chin C, et al. Prognostic significance of serum inflammatory markers in esophageal cancer. Esophagus. 2021;18(2):267–277. doi:10.1007/S10388-020-00772-3.
- Shang QX, Yang YS, Hu WP, et al. Clinical and prognostic significance of preoperative lymphocyte-monocyte ratio, neutrophil-lymphocyte ratio and neutrophil-monocyte ratio on esophageal squamous cell carcinoma patients. Transl Cancer Res. 2020;9(6):3903–3914. doi:10.21037/TCR-19-2777.
- Chen YR, Chen YL, Ouyang SS, et al. Prognostic efficacy of preoperative mGPS, SIS and LCS in patients with gastric cancer. Clin Chim Acta. 2020;511:81–89. doi:10.1016/J.CCA.2020.09.027.
- Ma M, Weng M, Chen F, et al. Systemic inflammation score is a prognostic marker after curative resection in gastric cancer. ANZ J Surg. 2019;89(4):377–382. doi:10.1111/ANS.15103.
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi:10.1016/J.IMMUNI.2004.07.017.
- Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30(21):2678–2683. doi:10.1200/JCO.2011.37.8539.
- Evani SJ, Prabhu RG, Gnanaruban V, Finol EA, Ramasubramanian AK. Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow. FASEB J. 2013;27(8):3017–3029. doi:10.1096/FJ.12-224824.
- Augier S, Ciucci T, Luci C, Carle GF, Blin-Wakkach C, Wakkach A. Inflammatory blood monocytes contribute to tumor development and represent a privileged target to improve host immunosurveillance. J Immunol. 2010;185(12):7165–7173. doi:10.4049/JIMMUNOL.0902583.