Abstract
Background
The main objective of this article is to understand trends in the incidence of renal cancer and to construct a nomogram to predict the prognosis of patients with renal cancer by analyzing clinical parameters.
Methods
We extracted data from the Surveillance, Epidemiology and End Results (SEER) database for patients with renal cancer from 2010 to 2015. The incidence rate was calculated to understand the trend of renal cancer in recent years, and the Kaplan–Meier method was used to analyze the relationship between patients’ clinical variables and overall survival. Nomogram and calibration curves were constructed based on factors predicted by multivariate Cox regression.
Results
Data from 68,496 eligible renal cancer patients were included in the study. The incidence of renal cancer was higher in men than women and tended to stabilize over time. We further found that age, gender, marital status, AJCC stage, histological type, metastatic disease, and surgery were independent parameters for prognosis in renal cancer patients. Finally, a nomogram was constructed based on the above parameters, and its validity was verified with the agreement index and calibration curve.
Conclusion
Renal cancer incidence trend gradually stabilized. Seven independent parameters for renal cancer patients were obtained by analysis and utilized to construct a nomogram that could provide guidance for clinical practice.
Introduction
Renal cancer is the second most common malignancy in the urinary system. The primary histological type is renal cell carcinoma [Citation1]. Primary renal cancer accounts for the fifth and third most common cancers among men and women. Approximately 76,080 renal cancer patients were diagnosed each year, with fewer than 14,000 estimated deaths [Citation2]. The overall incidence of renal cancer is on the rise, but in recent years, the incidence of renal cancer has leveled off, while the mortality rate has decreased significantly [Citation3–5].
Studies have demonstrated that the treatment outcome of advanced renal cancer is not satisfactory [Citation6]. Early detection and treatment of renal cancer is an effective solution to improve overall survival (OS). Traditional tumor staging and grading are effective predictors for various cancers [Citation7, Citation8], but do not take into account the biological characteristics of patients and the influence of socioeconomic factors [Citation9]. Nomogram is a predictive tool based on clinicopathological characteristics and survival probability, which can effectively predict the prognosis of cancer patients [Citation10]. Therefore, in this study, we quantified the standardized incidence rates of renal cancer through the SEER program [Citation11], analyzed the relationship between clinical variables and OS, and used independent parameters s to construct a prognostic nomogram, hoping to predict prognosis of the renal cancer patients effectively.
Methods
Data sources
Data for the analysis in this study were derived from retrospective data in the SEER database (incidence–SEER 18 Registers Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (1975-2016 varying)).
Patient identification
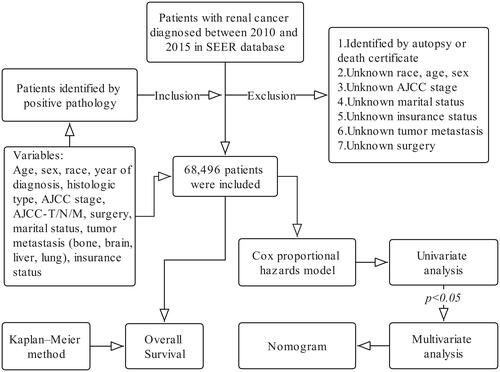
Histologically diagnosed renal cancer patients between 2010 and 2015 were included in this study by using SEER*Stat software [(version 8.3.9.2); National Cancer Institute, Bethesda, MD, USA]. The study variables were patient race, gender, age, histological type, AJCC stage, AJCC–T/N/M stage, surgery to the primary site, distant metastasis, vital status and survival time.
The primary endpoint was OS. Survival time from diagnosis of renal cancer to death from any disease was defined as OS. The inclusion and exclusion criteria are shown in .
Patients were divided into five subgroups based on their age at diagnosis:<50 years, 51–60 years, 61–70 years, 71–80 years, and >80 years. The histologic types of renal cancer were divided into renal cell carcinoma (RCC), spindle cell carcinoma (Spcc), renal endocrine carcinoma (Edc), squamous cell carcinoma (Sqcc), nephroblastoma (Npb) and other types of renal epithelial tumors (Oet) according to the ICD-0–3 morphological classification criteria.
Statistical analysis
Using the US population per 100,000 person-years as the denominator, SEER*Stat software (version 8.3.9.2) was used to calculate the incidence of renal cancer, and univariate and multivariate Cox regression analysis was used to analyze the variables that affected OS of patients with renal cancer. A Kaplan–Meier method with a log-rank test was used to calculate OS rates. In addition, according to the multivariate Cox regression analysis results, the 1–, 3–, and 5-year prognostic nomograms were constructed. A calibration curve was constructed to evaluate the accuracy of predicting the 1-, 3-, and 5-year survival rates. All statistical analyses were performed using R version 4.1.1. A p value of <0.05 was considered statistically significant.
Results
Incidence of renal cancer
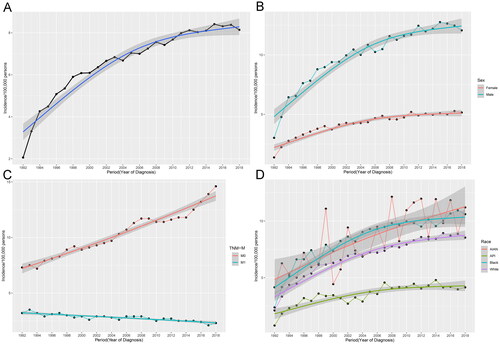
We calculated the annual percentage changes (APC) of renal cancer patients from 1992 to 2018 using the weighted least squares method. As shown in , the incidence rate based on age at diagnosis from 1992 to 2018 was 2.06, 3.31, 4.26, 4.49, 5.08, 5.35, 5.9, 6.08, 6.09, 6.36, 6.66, 6.84, 6.68, 7.04, 7, 7.25, 7.56, 7.42, 7.68, 7.98, 8.12, 8.03, 8.1, 8.41, 8.31, 8.38 and 8.14/100,000 persons. The incidence of renal cancer has shown an increasing trend from 1992 to 2018. Since 2012, its incidence has reached a plateau with a stable incidence rate of 8/100,000 persons. We grouped them according to whether there was distant metastasis and found that the incidence of renal cancer without distant metastasis was increasing year by year. In contrast the incidence of renal cancer with distant metastasis was decreasing yearly (). In addition, we analyzed the incidence of renal cancer by gender and ethnicity. We found that male patients had a significantly higher incidence than female patients (), and blacks had a higher incidence than other ethnic groups. However, the incidence of Asian or Pacific Islanders (API) was lower than that of other races (). Overall, the incidence trend was more consistent with the overall incidence in male, black, and white patients.
Survival analysis
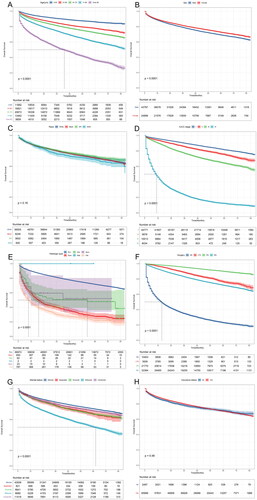
In total, 68,496 patients were enrolled in the study between 2010 and 2015. (). Based on the clinical data of the included population, we further analyzed the relationship between various clinical variables and OS in renal cancer patients using the Kaplan–Meier method. As shown in , elder patients at diagnosis were significantly associated with poorer OS. Compared with female renal cancer patients, OS was significantly lower in male patients (). The 5-year survival rate was greater than 70%, but there was no significant difference in the OS of patients of different races, (). When the disease progresses to AJCC-IV stage, the 5-year survival rate is less than 15% (). shows that Sqcc and Oet have the worst OS among the different histological types. Patients have the worst OS without surgery (). In the population of different marital status, the survival probability of widowed patients was significantly lower (). The prognosis is significantly poorer when a patient develops distant metastases from the primary tumor, such as bone, lung, liver, and brain metastases.
Figure 3. Correlation of different clinical variables with OS. (A) Age, (B) Sex, (C) Race, (D) AJCC-Stage, (E) Histological type, (F) Surgery, (G) Marital status, and (H) Insurance status (AIAN: American Indian/Alaska Native; API: Asian or Pacific Islander; RCC: renal cell carcinoma; Spcc: spindle cell carcinoma; Edc: renal endocrine tumor; Sqcc: squamous cell carcinoma; Oet: other types of renal epithelial tumors; Npb: nephroblastoma; NS: no surgery; LTE: local tumor excision; PN: partial nephrectomy; RN: radical nephrectomy).
We further performed survival analysis in ascending subgroups based on sex and race. In terms of sex, the effects of race, marital status, AJCC stage, and surgery on prognosis were analyzed. As shown in the Supplementary Figure 2, male patients generally shortened survival time regardless of race, marital status, and surgery. In terms of race, we did the same analysis, At <51 years, black patients had a lower probability of survival, and at 51–60 years, American Indian/Alaska Native had a lower survival probability (Supplementary Figure 1A). Among patients with AJCC–III/IV, black patients have a lower probability of survival than other races (Supplementary Figure 1C). There appears to be little difference in the prognosis of renal cancer patients by ethnicity regarding marital status (Supplementary Figure 1B) and surgery (Supplementary Figure 1D).
In addition, we analyzed the prognosis after different surgery for different histological types of renal cancer (Supplementary Figure 3). Survival time was highest in patients with Rcc who underwent partial nephrectomy. However, except Npb, no surgery, and radical nephrectomy patients had significantly poorer OS in different histologic types.
Univariate and multivariate cox regression model construction
As shown in , clinical variables such as race, gender, age, insurance status, marital status, histological type, AJCC stage, AJCC–T/N/M stage, surgery at the primary site, and metastatic disease were included in the univariate Cox regression. As a result of analysis, gender, age, marital status, histological type, AJCC stage, AJCC–T/N/M stage, surgery, and metastatic disease were significantly correlated with the OS of renal cancer patients. The above relevant variables with p value <0.05 were included for multivariate Cox regression analysis. The results showed that gender, age, marital status, histological type, AJCC stage, AJCC–T/N/M stage, surgery, and metastatic disease were independent prognostic factors in renal cancer patients.
Table 2. Univariate and multivariate Cox regression analyses of risk factors for renal cancer patients.
Prognostic nomogram
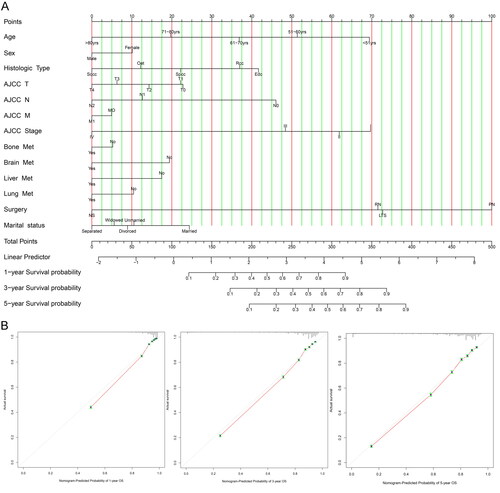
Based on the results of multivariate Cox regression analysis, a nomogram was constructed to predict the prognosis of renal cancer patients at 1, 3, and 5 years (). The total predicted score was obtained by adding the scores of each variable.
Figure 4. (A) Predicted 1-, 3-, and 5-year prognosis nomogram. (B) Calibration curves (AIAN: American Indian/Alaska Native; API: Asian or Pacific Islander; RCC: renal cell carcinoma; Spcc: spindle cell carcinoma; Edc: renal endocrine tumor; Sqcc: squamous cell carcinoma; Oet: other types of renal epithelial tumors; NS: no surgery; LTE: local tumor excision; PN: partial nephrectomy; RN: radical nephrectomy) (since there were only 2 Npb patients, they were not included).
Calibration curve
Validating prognostic nomogram for predicting 1-, 3-, and 5-year OS accuracy in renal cancer patients by constructing calibration curves. As shown in , the predicted survival rate was in good agreement with the observed survival rate, and the Concordance index (C-index) was 0.82, which also confirms the accuracy of the nomogram.
Discussion
Renal cancer is one of the most common and challenging malignant tumors found in clinics [Citation1]. With the progress of the global economy and society, the possibility of early detection of renal cancer has significantly increased [Citation12]. However. problems such as missed diagnosis and late tumor detection still exist, resulting in a low 5-year survival rate for patients with advanced renal cancer [Citation13, Citation14]. Therefore, it is essential to investigate and identify prognostic factors in renal cancer patients.
We found that renal cancer has been increasing worldwide from 1992 to 2011. Some studies believed that was related to the increased use of imaging screening [Citation15–17]. However, the incidence of renal cancer has gradually stabilized since 2012, reaching a relatively stable level. Previous studies have suggested that this may be due to the gradual stabilization of the incidence of localized RCC [Citation18–20]. Our study suggests that a combination of the rising incidence of localized renal cancer and the declining incidence of advanced metastatic renal cancer may lead to plateau formation. In addition, the incidence of male patients is higher than that of women, while the incidence in women is only half that of men. The finding is consistent with previous research conclusions, which may be caused by different metabolic patterns of chromosome genes, sex hormones, and harmful habits such as smoking [Citation21–23].
In addition, we included 68,658 patients with renal cancer and analyzed the relationship between clinical variables and OS of patients in this study. We found that advanced age, male patients, widowed patients, Sqcc, high AJCC Stage, radical nephrectomy, and patients who did not undergo surgery had significantly lower survival rates. We further analyzed the effect of different surgical methods on the prognosis of patients with different histological types of renal cancer. Patients with Rcc who underwent partial nephrectomy (PN) had the highest survival probability. Patients without surgery or with RN generally had a lower survival probability Probably because most of the patients were in the advanced stage when surgery was performed or when the opportunity for surgery was lost. It is consistent with previous research [Citation24, Citation25]. We also analyzed survival in ascending subgroups based on sex and race. In Rcc patients of most races, different marital statuses, and different surgery, the survival time of male patients is generally shortened. There are also differences in prognosis among different races at different stages and ages. We performed univariate and multivariate COX regression analyses on the clinical variables of the included patients, suggesting that, except for ethnicity differences and insurance status, all the remaining factors had a significant impact on the OS of patients with renal cancer. Therefore, we concluded that besides the oncological characteristics of renal cancer patients will affect the prognosis of renal cancer patients, socioeconomic factors and environmental factors cannot be ignored to affect the prognosis of renal cancer patients [Citation26].
Finally, we constructed a nomogram and incorporated multiple clinical variables to predict the 1-, 3-, and 5-year OS of patients diagnosed with renal cancer. The C-index was 0.82, indicating that the nomogram had good accuracy in predicting OS.
However, our study also has some limitations. Firstly, the included population is mainly from18 regions of the United States, and the scope is relatively limited. It is hoped that populations from more regions can be included in the future to reduce bias. Secondly, our analysis is based on patients diagnosed between 2010 and 2015. The treatment mode for advanced tumors is relatively simple. However, the rapid progress of immunotherapy and targeted therapy may affect the OS of patients [Citation27]. Therefore, it is necessary to continue to follow up and update the impact of emerging treatment methods and drugs on the survival of renal cancer patients. Finally, although the SEER database has made great contributions to clinical research, it also has limitations, including the lack of tumor-specific survival data and limited data on comprehensive treatment, which makes it difficult for us to obtain appropriate data to comprehensively analyze the prognosis of renal cancer patients. Hopefully, this problem can be solved with the continuous improvement of data in the SEER database.
Conclusion
Overall, our study showed a gradual and stable trend in the incidence of renal cancer. In addition, we further found that age, gender, marital status, histological type, AJCC stage, metastatic disease, and surgery were independent parameters of prognosis for patients with renal cancer; therefore, socioeconomic factors, as well as environmental factors should be considered in predicting the prognosis of patients with renal cancer. Finally, based on the results of multivariate COX regression analysis, we constructed a nomogram for predicting 1, 3, and 5 years and used a Calibration curve for validation. This predictive tool can help clinicians achieve personalized predictions of patient outcomes and provide guidance for clinical follow-up and monitoring programs.
Authors’ contributions
(I) Conception and design: All authors. (II) Administrative support: JY Zhang, and CG Ge. (III) Provision of study materials or patients: SD Wang, and BS Zheng. (IV) Collection and assembly of data: All authors. (V) Data analysis and interpretation: All authors. (VI) Manuscript writing: All authors. (VII) Final approval of manuscript: All authors.
Ethical statement
Not applicable.
Table 1. Baseline characteristics of included patients.
| Abbreviations | ||
| SEER | = | The Surveillance, Epidemiology, and End Results |
| OS | = | Overall survival |
| APC | = | Annual percentage change |
| C-index | = | Concordance index |
| AIAN | = | American Indian/Alaska Native |
| API | = | Asian or Pacific Islander |
| RCC | = | renal cell carcinoma |
| Spcc | = | Spindle cell carcinoma |
| Edc | = | Renal endocrine tumor |
| Sqcc | = | Squamous cell carcinoma |
| Npb | = | Nephroblastoma |
| Oet | = | Other types of renal epithelial tumors |
| NS | = | No surgery |
| LTE | = | Local tumor excision |
| PN | = | Partial nephrectomy |
| RN | = | Radical nephrectomy |
Supplemental Material
Download PDF (3 MB)Acknowledgements
Thanks to all the authors for all their contributions to this study.
Disclosure statement
None.
Additional information
Funding
References
- Siegel R, Miller K, Fuchs H, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):1–9. doi:10.3322/caac.21654.
- Sung H, Ferlay J, Siegel R, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660.
- Haifler M, Neheman A, Zisman A. Has stage migration in renal cancer run its course? A SEER database analysis. Clin Genitourin Cancer. 2020;18(4):e368–e373. doi:10.1016/j.clgc.2020.01.005.
- Gandaglia G, Ravi P, Abdollah F, et al. Contemporary incidence and mortality rates of kidney cancer in the United States. Can Urol Assoc J. 2014;8(7–8):247–252. doi:10.5489/cuaj.1760.
- Patel AR, Prasad SM, Shih YC, Eggener SE. The association of the human development index with global kidney cancer incidence and mortality. J Urol. 2012;187(6):1978–1983. doi:10.1016/j.juro.2012.01.121.
- Chen W, Hill H, Christie A, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539(7627):112–117. doi:10.1038/nature19796.
- Burke HB. Outcome prediction and the future of the TNM staging system. J Natl Cancer Inst. 2004;96(19):1408–1409. doi:10.1093/jnci/djh293.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4.
- Park YH, Lee SJ, Cho EY, et al. Clinical relevance of TNM staging system according to breast cancer subtypes. Ann Oncol. 2019;30(12):2011. doi:10.1093/annonc/mdz223.
- Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi:10.1200/jco.2007.12.9791.
- Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: surveillance, epidemiology, and end results (SEER) database. JAMA Surg. 2018;153(6):588–589. doi:10.1001/jamasurg.2018.0501.
- Turajlic S, Swanton C, Boshoff C. Kidney cancer: the next decade. J Exp Med. 2018;215(10):2477–2479. doi:10.1084/jem.20181617.
- Bradley AJ, Maskell GF, Mannava A, Pollard A, Welsh T. Routes to diagnosis and missed opportunities in the detection of renal cancer. Clin Radiol. 2021;76(2):129–134. doi:10.1016/j.crad.2020.11.005.
- Zhou Y, Abel GA, Hamilton W, Singh H, Walter FM, Lyratzopoulos G. Imaging activity possibly signalling missed diagnostic opportunities in bladder and kidney cancer: a longitudinal data-linkage study using primary care electronic health records. Cancer Epidemiol. 2020;66:101703. doi:10.1016/j.canep.2020.101703.
- Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74–84. doi:10.1016/j.eururo.2018.08.036.
- Al-Bayati O, Hasan A, Pruthi D, Kaushik D, Liss MA. Systematic review of modifiable risk factors for kidney cancer. Urol Oncol. 2019;37(6):359–371. doi:10.1016/j.urolonc.2018.12.008.
- Abou Elkassem AM, Lo SS, Gunn AJ, et al. Role of imaging in renal cell carcinoma: a multidisciplinary perspective. Radiographics. 2021;41(5):1387–1407. doi:10.1148/rg.2021200202.
- Patel HD, Gupta M, Joice GA, et al. Clinical stage migration and survival for renal cell carcinoma in the United States. Eur Urol Oncol. 2019;2(4):343–348. doi:10.1016/j.euo.2018.08.023.
- Takagi T, Kondo T, Tanabe K. Stage migration of renal cell carcinoma at a single Japanese university hospital: 24-year study. Int J Urol. 2014;21(4):429–430. doi:10.1111/iju.12300.
- Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7(5):245–257. doi:10.1038/nrurol.2010.46.
- Lughezzani G, Paciotti M, Fasulo V, Casale P, Saita A. Gender-specific risk factors for renal cell carcinoma: a systematic review. Curr Opin Urol. 2019;29(3):272–278. doi:10.1097/mou.0000000000000603.
- Mancini M, Righetto M, Baggio G. Gender-related approach to kidney cancer management: moving forward. IJMS. 2020;21(9):3378. doi:10.3390/ijms21093378.
- Peired AJ, Campi R, Angelotti ML, et al. Sex and gender differences in kidney cancer. Clin Exp Evid Cancers (Basel). 2021;13(18):4588. doi:10.3390/cancers13184588.
- Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A, Autorino R. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol. 2017;71(4):606–617. doi:10.1016/j.eururo.2016.08.060.
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59(4):543–552. doi:10.1016/j.eururo.2010.12.013.
- Barata PC, Rini BI. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin. 2017;67(6):507–524. doi:10.3322/caac.21411.
- Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–3337. doi:10.1172/jci83871.