Abstract
Objective
Hilar cholangiocarcinoma (HCCA) is a highly aggressive biliary tract tumor. microRNAs (miRs) exert dual actions in various cancers. This paper seeks to expound on the functional mechanisms of miR-25-3p/dual specificity phosphatase 5 (DUSP5) in HCCA cell proliferation and migration.
Methods
HCCA-related data were downloaded from GEO database to screen out differentially-expressed genes. The potential target miR (miR-25-3p) and its expression in HCCA were analyzed on Starbase. The binding relation between miR-25-3p and DUSP5 was confirmed by dual-luciferase assay. Levels of miR-25-3p and DUSP5 in FRH-0201 cells and HIBEpics were determined by RT-qPCR and Western blot. miR-25-3p and DUSP5 levels were intervened with to explore their effects on FRH-0201 cells. The apoptosis, proliferation, migration, and invasion of FRH-0201 cells were evaluated by TUNEL, CCK8, scratch healing, and Transwell assays. Flow cytometry was conducted to assess FRH-0201 cell cycle. Levels of cell cycle-related proteins were determined by Western blot.
Results
DUSP5 was weakly-expressed and miR-25-3p was highly-expressed in HCCA samples and cells. miR-25-3p targeted DUSP5. miR-25-3p suppressed FRH-0201 cell apoptosis and increased cell proliferation, migration, and invasion. DUSP5 overexpression partially abrogated miR-25-3p overexpression-exerted effects on FRH-0201 cells. miR-25-3p stimulated G1/S phase transition of FRH-0201 cells by targeting DUSP5.
Conclusion
miR-25-3p regulated HCCA cell cycle and facilitated cell proliferation and migration by targeting DUSP5.
Introduction
Hilar cholangiocarcinoma (HCCA) is a highly aggressive malignant neoplasm of the proximal extrahepatic bile ducts and accounts for 50%-70% of all biliary tract tumors.Citation1 Despite its high proportion, HCCA is a rare condition and is thus difficult to investigate.Citation2 Recent years witness an increase in the incidence of HCCA and mortality of HCCA patients on a worldwide scale.Citation3 The major curative modalities for HCCA patients include hepatic and vascular resection and liver transplantation.Citation4 However, less than half of HCCA tumors are resectable and postoperative recurrence is still high, thus leading to poor prognosis.Citation5,Citation6 Under such circumstances, ablative therapies, intra-arterial therapies, chemotherapy, target therapies, immunotherapy, and radiotherapy have become available options for HCCA patients.Citation7 Even so, more efforts are urgently needed to seek for therapeutic strategies to achieve better overall survival, response rates, and tumor control in HCCA patients.
microRNAs (miRNAs) are small noncoding RNAs and negative regulators of gene expression.Citation8 By binding to a complementary sequence in the 3′-untranslated regions (3′-UTRs) of the mRNA, miRNAs are involved in the post-transcriptional regulation of target genes.Citation9 Several miRNAs have been ascertained to exert promotional or inhibitory effects on cancer progression.Citation10 The oncogenic miR-25-3p shows high expression levels in pancreatic ductal adenocarcinoma, and its high expression pattern correlates with the shorter survival time of patients.Citation11 The elevation of miR-25-3p expression also accelerates gastric cancer growth and invasion in vitro.Citation12 Additionally, miR-25-3p is implicated in the process of cholangiocarcinoma development and progression,Citation13 making miR-25-3p worthy of study.
Dual specificity phosphatase 5 (DUSP5), or VH1-like phosphatase-3 (VH3), is a member of the DUSP family.Citation14 Lately, DUSP5 has been recognized as a direct transcription target of p53 the tumor suppressor,Citation15 and DUSP5 itself has been confirmed as a cancer inhibitor.Citation16 DUSP5 not only presents a low expression pattern in ovarian cancer cells and colorectal cancer cells Citation17,Citation18 but participates in cell proliferation and migration through the nuclear-factor-kappa-B (NF-κB) and mitogen-activated protein kinase/extracellular signal-related protein kinase (MEK/ERK) pathways.Citation19,Citation20 Nonetheless, the role of DUSP5 in HCCA remains elusive. The interaction between miR-25-3p and DUSP5 in HCCA is also worth considering, given the regulatory action of miR-95 on DUSP5 expression in gastric cancer.Citation21 Therefore, based on the GEO database, the paper screened differentially expressed genes (DEGs) by bioinformatics analysis and provided the foundation for gene therapy. Specifically, we explored the effects of miR-25-3p/DUSP5 on cell proliferation, migration, invasion, and cycle distribution in HCCA and the related mechanism, in a hope of providing more insights into the effective treatment of HCCA.
Materials and methods
Bioinformatics analysis
HCCA-associated datasets GSE34166 and GSE68292 were obtained from GEO database (https://www.ncbi.nlm.nih.gov/gds/?term=). Data were processed using GEO2R and DEGs were screened out by the threshold of p < 0.05 and |log fold-change (FC)|>1.5. The volcano map of DEGs was generated by visual hierarchical clustering analysis. The selected DEGs were input into Venny mapping software 2.1 to get the intersection target genes. The expression pattern of miR-25-3p in HCCA was analyzed using Starbase (https://starbase.sysu.edu.cn/).
Cell culture
Human intrahepatic bile duct endothelial cells HIBEpics and human cholangiocarcinoma FRH-0201 cells (both from Otwo Biotech, Shenzhen, China) were cultured separately in RPMI 1640 medium comprising 1% penicillin-streptomycin and 10% fetal bovine serum (FBS), and Dulbecco’s modified Eagle’s medium (DMEM) consisting of 1% penicillin-streptomycin and 10% FBS (all from Yaji Biotechnology, Shanghai, China) at 37 °C in a humidified incubator with 5% CO2. Upon 85% cell confluence, cells were detached using 0.25% trypsin and passaged once every 3 days. The 3rd passage cells at the exponential phase were selected for subsequent experimentation.
Cell transfection and grouping
FRH-0201 cells at the exponential phase were transfected using Lipofectamine 3000 (L3000015, Invitrogen, Carlsbad, CA, USA) with miR-25-3p mimic (200 nM/well), miR-25-3p inhibitor (200 nM/well) and overexpression (oe) plasmid oe-DUSP5 (5 μg/well) Citation22 as well as their negative controls (NCs) (all from RiboBio, Guangzhou, China) in strict compliance with the specifications. Afterwards, cells were further cultured in a constant temperature incubator for 48 h before experimentation.
FRH-0201 cells were assigned into 9 groups: 1) control group (without any treatment), 2) miR-mimic group (transfected with miR-25-3p mimic), 3) mimic NC group (transfected with miR-25-3p mimic NC), 4) miR-inhi group (transfected with miR-25-3p inhibitor), 5) inhi-NC group (transfected with miR-25-3p inhibitor NC), 6) oe-DUSP5 group (transfected with oe-DUSP5), 7) oe-NC group (transfected with overexpression empty plasmid), 8) miR mimic + oe-DUSP5 group (simultaneously transfected with miR-25-3p mimic and oe-DUSP5), 9) miR mimic + oe-NC group (simultaneously transfected with miR-25-3p mimic and overexpression empty plasmid).
Dual-luciferase reporter gene assay
The binding sequences of miR-25-3p and DUSP5 were predicted on Starbase database (https://starbase.sysu.edu.cn). The DUSP5 wild-type (WT) and mutant (MT) luciferase reporter gene vectors (RiboBio, Guangzhou, China) were constructed and separately delivered into FRH-0201 cells together with miR-25-3p mimic or mimic NC. A Dual-luciferase Reporter Gene Assay System (Promega, Madison, WI, USA) was adopted to measure luciferase activity after 48 h of transfection.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Cells were harvested by centrifugation, followed by total RNA extraction from cells using TRIzol kits (R401-01, Vazyme, Nanjing, China). An ultraviolet spectrophotometer (A30221, Thermo Scientific, Rockford, IL, USA) was utilized to determine RNA concentration. After that, RNA was inversely transcribed into cDNA using the SuperScript III Reverse Transcription kit (18080-044, Invitrogen). PCR reaction system was added following the specifications of SYBR Green qPCR Master Mix (HY-K0501, MedChemExpress, Monmouth Junction, NJ, USA), followed by RT-qPCR on an ABI Prism 7300 system (ABI, Foster City, CA, USA) for 10 min at 95 °C, and 40 cycles of 90 °C for 15 s, 60 °C for 20 s, and 72 °C for 30 s. U6 and GAPDH were used as an internal reference. The 2-ΔΔCt method was adopted for data analysis. The primers used in RT-qPCR were synthesized by Sangon Biotech (Shanghai, China), and their sequences are listed in .
Table 1. Primer sequences in RT-qPCR.
Western blot
Cells were washed twice with pre-cold phosphate buffer saline and lysed with radioimmunoprecipitation assay buffer (Boster, Wuhan, China) to extract total protein. Protein concentration was determined using a bicinchoninic acid protein quantification kit (HonorGene, Changsha, China). The protein was denatured by boiling, followed by separation using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transfer to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) at a constant current. The membranes were first blocked in 5% nonfat milk powder for 90 min and subsequently incubated with diluted anti-DUSP5 (1:1000, ab255611, Abcam, Cambridge, MA, USA), anti-cyclin D1 (1:5000, ab134175, Abcam), anti-cyclin dependent kinase 4 (CDK4) (1:5000, ab108357, Abcam), and anti-CDK6 (1:5000, ab124821, Abcam) overnight at 4 °C. After 3 washes with Tris-buffered saline-Tween-20 (TBST), the membranes were incubated at room temperature for 1 h with horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G (1:10000, ab205718, Abcam). Finally, the membranes were washed thrice with TBST, incubated with enhanced chemiluminescence solution (AWB0005, Abiowell, Changsha, China) for 1 min, and imaged using a Gel Imaging System (Clinx Science Instruments, Shanghai, China), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal reference. Image J software (NIH, Bethesda, MD, USA) was employed for gray value analysis.
TUNEL staining for cell apoptosis
Cells were cultured for 24 h on cover slides, then fixed at room temperature in 4% paraformaldehyde solution for 30 min, and stained with fluorescein isothiocyanate following the specifications of TUNEL Apoptosis Detection kit (Yeasen Biotechnology, Shanghai, China). TUNEL-positive cells were observed under a fluorescence microscope (Leica, Solms, Germany).
Cell counting kit 8 (CCK8) assay
After 48 h of transfection, FRH-0201 cell proliferation was assessed using a CCK8 kit (HY-K0301, MedChemExpress). Cell suspension was seeded in 96-well plates (100 μL/well). Three duplicated wells were reserved for each group. Then, 10 μL CCK8 solution was added into each well for 24 h of culture. Optical density (OD) values at 450 nm were estimated.
Scratch healing assay for cell migration
Horizontal lines were scratched behind the 6-well plate. Approximately 5 × 105 cells were added in each well and cultured until 90%-95% confluency, and scratches were made perpendicular to the horizontal line using a pipette tip. The images of the scratch at 0 h were captured, and 3 visual fields were selected randomly per group. After 24 h of culture in a 37 °C incubator, cells were fixed in 4% paraformaldehyde for 20 min, stained for 5 min with 0.1% crystal violet, and observed under an inverted microscope (KS3000, Zeiss-Kontron, Germany).
Transwell assay for cell invasion
Serum-free DMEM (PM150312-HR, Yaji Biotechnology) and Matrigel (356234, Corning, Tewksbury, MA, USA) were added into pre-cold Transwell chamber, followed by the addition of 100 μL cell suspension (containing about 1 × 105 cells). The basolateral chamber was added with 700 μL DMEM containing 10% FBS. After 24 h of culture at 37 °C, the apical chamber was taken out, and cells were fixed for 20 min in 4% paraformaldehyde, stained for 5 min with 0.1% crystal violet, observed and counted under an inverted microscope (KS3000, Zeiss-Kontron).
Flow cytometry for cell cycle
FRH-0201 cells (2 × 105 cells/well) were seeded in 6-well plates and cultured in FBS-free DMEM. After starvation for 24 h for cell cycle synchronization, cells were cultured for 48 h in DMEM containing 10% FBS. Then, cells were collected by centrifugation and fixed at 20 °C for 24 h in 70% ethanol (Servicebio, Wuhan, China). Cell cycle was assessed using a Cell Cycle Assay kit (E-CK-A351, Elabscience, Wuhan, China). Cells in different phases of cell cycle were observed using a flow cytometer (Beckman Coulter, Fullerton, CA, USA).
Statistical analysis
Statistical analysis was processed using GraphPad 8.0 software. Cell experiment was repeated 3 times independently. Data were mean ± standard deviation unless otherwise stated. Pairwise comparisons were made using independent sample t test, and data in 3 or more different groups were compared using one-way analysis of variance (ANOVA), with Tukey’s test as post hoc test. The p < 0.05 was set as the threshold of statistical significance.
Results
Bioinformatics analysis of key genes in HCCA
A total of 10 samples, including 2 controls, 6 HCCA patients, and 2 patients with chronic pancreatic necrosis (excluded) were present in dataset GSE34166 from GEO database, and we identified 999 DEGs (444 upregulated genes and 555 downregulated genes) (). There were 12 samples, including 3 controls and 9 HCCA patients in GSE68292 dataset, and we identified 125 DEGs (84 upregulated genes and 41 downregulated genes) (). After intersection, totally 15 DEGs were found, including 12 upregulated genes and 3 downregulated genes (DUSP5, DUSP2, ARHGEF2) (). Given the earlier finding that miR-25-3p and cholangiocarcinoma are associated,Citation13 we analyzed miR-25-3p expression in HCCA and found that miR-25-3p was highly-expressed (, p < 0.01).
Figure 1. Bioinformatics analysis of key genes in HCCA. (A) Volcano map of DEGs in GSE34166 dataset; (B) Volcano map of DEGs in GSE68292 dataset; (C) Venn diagram of DEGs in both datasets, with red representing upregulated genes and blue downregulated genes; (D) High expression level of miR-25-3p in HCCA analyzed by Starbase.
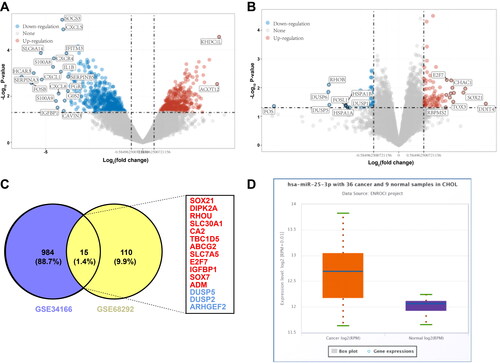
miR-25-3p targets DUSP5 in HCCA
Further, human intrahepatic bile duct endothelial cells HIBEpics and human cholangiocarcinoma FRH-0201 cells were cultured in vitro. RT-qPCR and Western blot revealed that miR-25-3p expression was incremented and DUSP5 mRNA and protein levels were decremented in FRH-0201 cells relative to HIBEpics (, p < 0.01). A potential binding site between DUSP5 and miR-25-3p was discovered on Starbase database (). Dual-luciferase reporter gene assay demonstrated that luciferase activity was weakened after transfection of miR-25-3p mimic and DUSP5-WT (p < 0.01), while no substantial change occurred after transfection of miR-25-3p mimic and DUSP5-MT (, p < 0.01), indicating a potential binding relation between DUSP5 and miR-25-3p. FRH-0201 cells were subsequently transfected with miR-25-3p mimic or inhibitor. RT-qPCR and Western blot delineated that miR-25-3p expression was elevated and DUSP5 mRNA and protein levels were reduced in the miR mimic group, while the opposite trend was observed in the miR-inhi group (, p < 0.01). These results illustrated that miR-25-3p downregulated DUSP5 in HCCA.
Figure 2. miR-25-3p targets DUSP5 in HCCA. (A) The binding site of DUSP5 and miR-25-3p predicted on Starbase database; (B) Interaction between DUSP5 and miR-25-3p analyzed by dual-luciferase reporter gene assay; (C) The levels of miR-25-3p and DUSP5 RNA measured by RT-qPCR; (D) DUSP5 protein level determined by Western blot; (E) miR-25-3p and DUSP5 RNA expression levels measured by RT-qPCR; (F) DUSP5 protein level after transfection determined by Western blot. Cell experiment was repeated 3 times. Data were mean ± standard deviation. Pairwise comparisons in figures B-D were made using independent sample t test, and comparisons in figures E/F were made using one-way ANOVA, followed by Tukey’s test. *p<0.05, **p < 0.01.
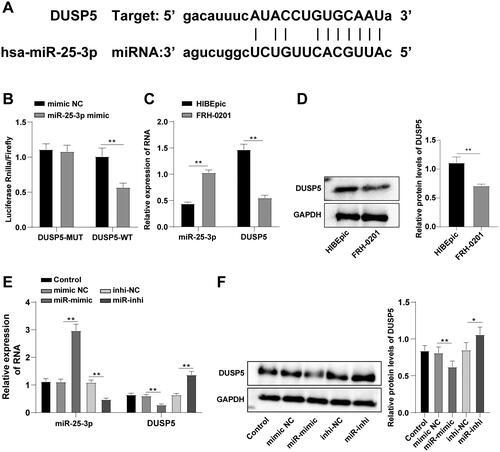
miR-25-3p potentiates HCCA cell proliferation and migration by targeting DUSP5
Then, we analyzed the effect of miR-25-3p on FRH-0201 cell behaviors. TUNEL staining showed decreased apoptosis after miR-25-3p overexpression and increased apoptosis after miR-25-3p inhibition (, p < 0.01). CCK8 assay unraveled enhanced proliferative ability in the miR-mimic group and reduced proliferative ability in the miR-inhi group (, p < 0.01). Scratch healing and Transwell assays exhibited that miR-25-3p overexpression increased cell migration and invasion, while miR-25-3p inhibition exerted the opposite effect on cell migration and invasion (, p < 0.01). Collectively, miR-25-3p stimulated HCCA cell proliferation, invasion, and migration and compromised apoptosis.
Figure 3. miR-25-3p potentiates HCCA cell proliferation and migration by targeting DUSP5. (A) Cell apoptosis assessed by TUNEL staining; (B) Cell proliferation assessed by CCK-8 assay; (C) Cell migration assessed by scratch healing assay; (D) Cell invasion assessed by Transwell assay. Cell experiment was repeated 3 times. Data were mean ± standard deviation. Comparisons among groups were made using one-way ANOVA, followed by Tukey’s test. **p < 0.01.
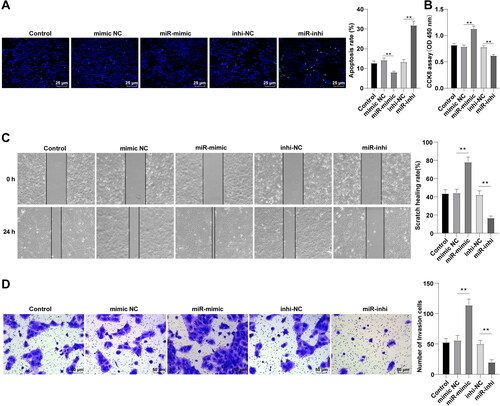
DUSP5 overexpression partially negates miR-25-3p-mediated promotional effects on HCCA cell proliferation and migration
We further analyzed the role of DUSP5 in FRH-0201 cell behaviors by introducing oe-DUSP5 plasmids and miR-25-3p mimic into FRH-0201 cells. Western blot manifested that DUSP5 protein level was raised in the miR-mimic + oe-DUSP5 group (, p < 0.01). As indicated in , the number of apoptotic cells was augmented in the miR-mimic + oe-DUSP5 group (p < 0.01). Relative to the miR-mimic + oe-NC group, the miR-mimic + oe-DUSP5 group witnessed decreased proliferation (, p < 0.01), migration, and invasion of FRH-0201 cells (, p < 0.01). It could thus be inferred that DUSP5 overexpression could partly nullify the promotional action of miR-25-3p on HCCA cell proliferation and migration.
Figure 4. DUSP5 overexpression partially negates miR-25-3p-mediated promotional effect on HCCA cell proliferation and migration. (A) DUSP5 protein level measured by Western blot; (B) Cell apoptosis assessed by TUNEL staining; (C) Cell proliferation assessed by CCK-8 assay; (D) Cell migration assessed by scratch healing assay; (E) Cell invasion assessed by Transwell assay. Cell experiment was repeated 3 times. Data were mean ± standard deviation. Comparisons among groups were made using one-way ANOVA, followed by Tukey’s test. *p<0.05, **p < 0.01.
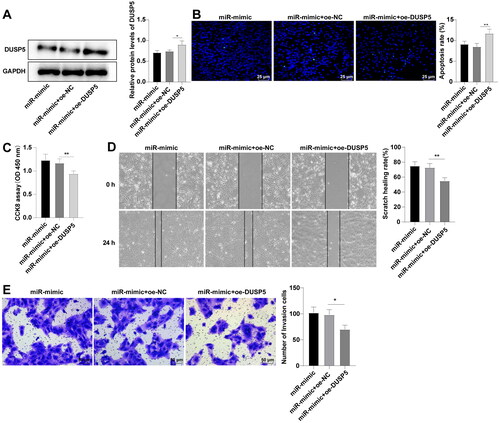
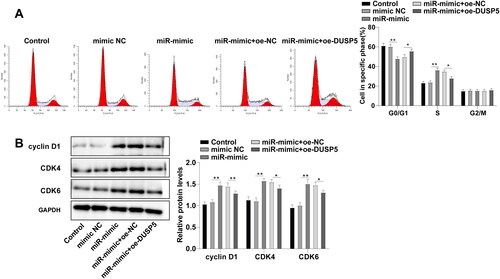
miR-25-3p facilitates G1/S phase transition of HCCA cells by targeting DUSP5
To clarify the functional mechanism of DUSP5 in FRH-0201 cell behaviors, we analyzed the effect of DUSP5 on FHR-0201 cell cycle. Flow cytometry demonstrated that the percentages of cells in G0/G1 phase were 61.37%, 60.37%, 47.13%, 49.84%, and 55.78%, and the percentages of cells in S phase were 23.45%, 23.95%, 36.44%, 35.05% and 28.13% in the control, mimic NC, miR-mimic, miR-mimic + oe-NC, and miR-mimic + oe-DUSP5 groups, respectively (, p < 0.05), confirming that miR-25-3p could arrest FRH-0201 cells in G0/G1 phase by targeting DUSP5. As indicated by Western blot, cell cycle-related proteins cyclin D1, CDK4, and CDK6 were upregulated in the miR-mimic group compared to those in the mimic NC group, while the miR-mimic + oe-DUSP5 group showed decreased levels relative to the miR-mimic + oe-NC group (, p < 0.05). Conjointly, miR-25-3p regulated G1/S phase transition of FRH-0201 cells by targeting DUSP5, thus promoting FRH-0201 cell proliferation.
Figure 5. miR-25-3p facilitates G1/S phase transition of HCCA cells by targeting DUSP5. (A) Cell cycle assessed by flow cytometry; (B) Levels of cell cycle-associated proteins determined by Western blot. Cell experiment was repeated 3 times. Data were mean ± standard deviation. Comparisons were analyzed using one-way ANOVA, followed by Tukey’s test. *p<0.05, **p < 0.01.
Discussion
HCCA, also known as Klatskin tumor, is an advanced malignancy with worse patient survival that often occurs in the elderly.Citation23 miRNAs participate in cholangiocarcinoma pathogenesis and can be used as diagnostic and prognostic biomarkers and therapeutic targets in cholangiocarcinoma.Citation13 The changes in expression or activity of DUSP enzymes are related to human cancer.Citation24 The current study revealed that miR-25-3p modulated G1/S phase transition of HCCA cells by targeting DUSP5, thus prompting HCCA cell proliferation, migration, and invasion.
Out of 999 DEGs from GSE34166 datasets and 125 DEGs from GSE68292 dataset, we managed to identify 15 intersection DEGs that might be associated with HCCA. Among these genes, DUSP5 is considered a direct transcriptional target of tumor suppressor p53.Citation25 Additionally, DUSP5 exhibits low expression patterns in Hodgkin’s lymphoma cells and other tumor cells from hematopoietic and non-hematopoietic malignancies relative to nonmalignant cells.Citation26 Thereby, we hypothesized the same mechanism of DUSP5 in HCCA and selected DUSP5 as the study subject. miR-25-3p is a member of the known oncogenic miR-25-93-106b cluster.Citation27 Starbase database reveals high expression of miR-25-3p in HCCA. Moreover, bioinformatics analysis showed high expression of miR-25-3p and low expression of DUSP5 in HCCA. The same expression patterns were observed in the FRH-0201 cells we cultured in vitro. Our results elucidated that miR-25-3p targeted DUSP5 in HCCA, evidenced by the decreased DUSP5 levels after miR-25-3p overexpression and increased levels of DUSP5 after miR-25-3p knockdown. Previous findings elicited that DUSP5 is the target gene of miR-95 and miR-203,Citation21,Citation28 the expression patterns and functions of which in HCCA remain elusive and require in-depth investigation in the future.
Cancer cells divide continuously and excessively, and cancer cell cycle control is critical for the maintenance of cell viability.Citation29 Cell cycle is composed of 4 different phases: G1, S, G2, and M phase.Citation30 The whole cell cycle mechanism is under the strict control of CDKs and cyclin.Citation31 Cyclin D1, in particular, can regulate the growth of cells in G1 phase by forming active kinase complexes with CDK4 and CDK6.Citation32,Citation33 Linc00858 affects colorectal cancer cell cycle progression by sponging miR-25-3p.Citation34 miR-25-3p upregulation, which is possibly induced by inflammatory mediators during autoimmune neuroinflammation, is linked to the downregulation of Pten and Klf4, genes known as regulators of cell cycle.Citation35 miR-25-3p facilitates the entry of osteosarcoma cells into the S phase and protects osteosarcoma cells from G0/G1 arrest.Citation36 Similarly, our results illustrated that the number of HCCA cells in G0/1 phase was decreased after miR-25-3p overexpression, and increased after DUSP5 overexpression. Furthermore, long noncoding RNA linc01503 mediates cell cycle progression in gastric cancer by epigenetically silencing DUSP5.Citation37 The elevated expression of long non-coding RNA ROR1-AS1 stimulates colon cancer cycle by downregulating DUSP5 in combination with EZH2.Citation38 The restoration of DUSP5 results in the arrest during the gastric cancer transition from G1 to S phase in the cell cycle.Citation39 Our results indicated that the levels of cyclin D1, CDK4, and CDK6 in HCCA cells were incremented after miR-25-3p overexpression and diminished after DUSP5 overexpression. To conclude, miR-25-3p regulated G1/S phase transition of HCCA cells by targeting DUSP5, and DUSP5 overexpression annulled miR-25-3p-facilitated G1/S phase transition of HCCA cells. Cyclins and CDKs enable cells to advance between different phases of the cell cycle, and thereby regulate cell growth and proliferation.Citation40 The enforced miR-25-3p expression promotes gastric cancer cell growth, invasion, and migration in vitro.Citation12 miR-25-3p targets PTEN to facilitate proliferation and inhibit apoptosis of esophageal cancer cells.Citation41 Likewise, our experiments revealed that overexpression of miR-25-3p decreased apoptosis, increased proliferation, migration, and invasion of HCCA cells, while inhibition of miR-25-3p produced the opposite effect. DUSP5 can inhibit proliferation, migration, and invasion of ovarian cancer cells.Citation18 Likewise, our results manifested that the enhanced proliferative, migratory, and invasive abilities of HCCA cells mediated by miR-25-3p were partially abolished by DUSP5 overexpression.
In a word, this study managed to find out that miR-25-3p regulates G1/S phase transition of HCCA cells and facilitates HCCA cell growth by targeting DUSP5. Notwithstanding, the present study has been limited by several flaws. First, the present study lacks an in vivo assay to validate the obtained results. What’s more, the concrete mechanism by which DUSP5 modulates G1/S phase transition of HCCA cells or other mechanisms of DUSP5 in affecting HCCA cell proliferation and migration remains to be further explained. In future research, the specific mechanism of DUSP5 in the G1/S phase transition of HCCA cells and the pathways involved shall be explored.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Soares KC, Jarnagin WR. The landmark series: hilar cholangiocarcinoma. Ann Surg Oncol. 2021;28(8):1–9. doi:10.1245/s10434-021-09871-6.
- Zaydfudim VM, Rosen CB, Nagorney DM. Hilar cholangiocarcinoma. Surg Oncol Clin N Am. 2014;23(2):247–263. doi:10.1016/j.soc.2013.10.005.
- Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–588. doi:10.1038/s41575-020-0310-z.
- Anderson B, Doyle MBM. Surgical considerations of hilar cholangiocarcinoma. Surg Oncol Clin N Am. 2019;28(4):601–617. doi:10.1016/j.soc.2019.06.003.
- Sapisochin G, Ivanics T, Subramanian V, Doyle M, Heimbach JK, Hong JC. Multidisciplinary treatment for hilar and intrahepatic cholangiocarcinoma: A review of the general principles. Int J Surg. 2020;82S:77–81. doi:10.1016/j.ijsu.2020.04.067.
- Soares KC, Kamel I, Cosgrove DP, Herman JM, Pawlik TM. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr. 2014;3(1):18–34. doi:10.3978/j.issn.2304-3881.2014.02.05.
- Inchingolo R, Acquafredda F, Ferraro V, et al. Non-surgical treatment of hilar cholangiocarcinoma. World J Gastrointest Oncol. 2021;13(11):1696–1708. doi:10.4251/wjgo.v13.i11.1696.
- Saliminejad K, KhorramKhorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–5465. doi:10.1002/jcp.27486.
- Burroughs AM, Ando Y. Identifying and characterizing functional 3’ nucleotide addition in the miRNA pathway. Methods. 2019;152:23–30. doi:10.1016/j.ymeth.2018.08.006.
- Puik JR, Meijer LL, Le Large TY, et al. miRNA profiling for diagnosis, prognosis and stratification of cancer treatment in cholangiocarcinoma. Pharmacogenomics. 2017;18(14):1343–1358. doi:10.2217/pgs-2017-0010.
- Zhang J, Bai R, Li M, et al. Excessive miR-25-3p maturation via N(6)-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10(1):1858. doi:10.1038/s41467-019-09712-x.
- Ning L, Zhang M, Zhu Q, Hao F, Shen W, Chen D. miR-25-3p inhibition impairs tumorigenesis and invasion in gastric cancer cells in vitro and in vivo. Bioengineered. 2020;11(1):81–90. doi:10.1080/21655979.2019.1710924.
- Shi T, Morishita A, Kobara H, Masaki T. The role of microRNAs in cholangiocarcinoma. IJMS. 2021;22(14):7627. doi:10.3390/ijms22147627.
- Patterson KI, Brummer T, O’Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418(3):475–489. doi:10.1042/bj20082234.
- Zhang H, Zheng H, Mu W, et al. DUSP16 ablation arrests the cell cycle and induces cellular senescence. FEBS J. 2015;282(23):4580–4594. doi:10.1111/febs.13518.
- Cai C, Chen JY, Han ZD, et al. Down-regulation of dual-specificity phosphatase 5 predicts poor prognosis of patients with prostate cancer. Int J Clin Exp Med. 2015;8(3):4186–4194. doi:.
- Ding J, Li J, Wang H, et al. Long noncoding RNA CRNDE promotes colorectal cancer cell proliferation via epigenetically silencing DUSP5/CDKN1A expression. Cell Death Dis. 2017;8(8):e2997. doi:10.1038/cddis.2017.328.
- Wang L, Hu J, Qiu D, et al. Dual-specificity phosphatase 5 suppresses ovarian cancer progression by inhibiting IL-33 signaling. Am J Transl Res. 2019;11(2):844–854. doi:.
- Bornigen D, Tyekucheva S, Wang X, et al. Computational reconstruction of NFkappaB pathway interaction mechanisms during prostate cancer. PLoS Comput Biol. 2016;12(4):e1004820. doi:10.1371/journal.pcbi.1004820.
- Nokin MJ, Bellier J, Durieux F, et al. Methylglyoxal, a glycolysis metabolite, triggers metastasis through MEK/ERK/SMAD1 pathway activation in breast cancer. Breast Cancer Res. 2019;21(1):11. doi:10.1186/s13058-018-1095-7.
- Du M, Zhuang Y, Tan P, Yu Z, Zhang X, Wang A. microRNA-95 knockdown inhibits epithelial-mesenchymal transition and cancer stem cell phenotype in gastric cancer cells through MAPK pathway by upregulating DUSP5. J Cell Physiol. 2020;235(2):944–956. doi:10.1002/jcp.29010.
- Zhao Z, Zheng J, Ye Y, Zhao K, Wang R, Wang R. MicroRNA253p regulates human nucleus pulposus cell proliferation and apoptosis in intervertebral disc degeneration by targeting Bim. Mol Med Rep. 2020;22(5):3621–3628. doi:10.3892/mmr.2020.11483.
- Zhang X, Liu H. Klatskin tumor: a population-based study of incidence and survival. Med Sci Monit. 2019;25:4503–4512. doi:10.12659/MSM.914987.
- Rios P, Nunes-Xavier CE, Tabernero L, Kohn M, Pulido R. Dual-specificity phosphatases as molecular targets for inhibition in human disease. Antioxid Redox Signal. 2014;20(14):2251–2273. doi:10.1089/ars.2013.5709.
- Ueda K, Arakawa H, Nakamura Y. Dual-specificity phosphatase 5 (DUSP5) as a direct transcriptional target of tumor suppressor p53. Oncogene. 2003;22(36):5586–5591. doi:10.1038/sj.onc.1206845.
- Staege MS, Muller K, Kewitz S, et al. Expression of dual-specificity phosphatase 5 pseudogene 1 (DUSP5P1) in tumor cells. PLoS One. 2014;9(2):e89577. doi:10.1371/journal.pone.0089577.
- Gruszka R, Zakrzewska M. The oncogenic relevance of miR-17-92 cluster and its paralogous miR-106b-25 and miR-106a-363 clusters in brain tumors. IJMS. 2018;19(3):879. doi:10.3390/ijms19030879.
- Altan Z, Sahin Y. miR-203 suppresses pancreatic cancer cell proliferation and migration by modulating DUSP5 expression. Mol Cell Probes. 2022;66:101866. doi:10.1016/j.mcp.2022.101866.
- Matthews HK, Bertoli C, de Bruin RAM. Cell cycle control in cancer. Nat Rev Mol Cell Biol. 2022;23(1):74–88. doi:10.1038/s41580-021-00404-3.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013.
- Dickson MA, Schwartz GK. Development of cell-cycle inhibitors for cancer therapy. Curr Oncol. 2009;16(2):36–43. doi:10.3747/co.v16i2.428.
- Paternot S, Colleoni B, Bisteau X, Roger PP. The CDK4/CDK6 inhibitor PD0332991 paradoxically stabilizes activated cyclin D3-CDK4/6 complexes. Cell Cycle. 2014;13(18):2879–2888. doi:10.4161/15384101.2014.946841.
- Dong P, Zhang C, Parker BT, You L, Mathey-Prevot B. Cyclin D/CDK4/6 activity controls G1 length in mammalian cells. PLoS One. 2018;13(1):e0185637. doi:10.1371/journal.pone.0185637.
- Zhan J, Tong J, Fu Q. Long non‑coding RNA LINC00858 promotes TP53‑wild‑type colorectal cancer progression by regulating the microRNA‑25‑3p/SMAD7 axis. Oncol Rep. 2020;43(4):1267–1277. doi:10.3892/or.2020.7506.
- Zare-Chahoki A, Ahmadi-Zeidabadi M, Azadarmaki S, Ghorbani S, Noorbakhsh F. Inflammation in an animal model of multiple sclerosis leads to microRNA-25-3p dysregulation associated with inhibition of Pten and Klf4. Iran J Allergy Asthma Immunol. 2021;20(3):314–325. doi:10.18502/ijaai.v20i3.6337.
- Rao HC, Wu ZK, Wei SD, et al. MiR-25-3p serves as an oncogenic microRNA by downregulating the expression of merlin in osteosarcoma. Cancer Manag Res. 2020;12:8989–9001. doi:10.2147/CMAR.S262245.
- Ma Z, Gao X, Shuai Y, et al. EGR1-mediated linc01503 promotes cell cycle progression and tumorigenesis in gastric cancer. Cell Prolif. 2021;54(1):e12922. doi:10.1111/cpr.12922.
- Wang XY, Jian X, Sun BQ, Ge XS, Huang FJ, Chen YQ. LncRNA ROR1-AS1 promotes colon cancer cell proliferation by suppressing the expression of DUSP5/CDKN1A. Eur Rev Med Pharmacol Sci. 2020;24(3):1116–1125. doi:10.26355/eurrev_202002_20162.
- Shin SH, Park SY, Kang GH. Down-regulation of dual-specificity phosphatase 5 in gastric cancer by promoter CpG island hypermethylation and its potential role in carcinogenesis. Am J Pathol. 2013;182(4):1275–1285. doi:10.1016/j.ajpath.2013.01.004.
- Giacoia EG, Miyake M, Lawton A, Goodison S, Rosser CJ. PAI-1 leads to G1-phase cell-cycle progression through cyclin D3/cdk4/6 upregulation. Mol Cancer Res. 2014;12(3):322–334. doi:10.1158/1541-7786.MCR-13-0543.
- Zhang L, Tong Z, Sun Z, Zhu G, Shen E, Huang Y. MiR-25-3p targets PTEN to regulate the migration, invasion, and apoptosis of esophageal cancer cells via the PI3K/AKT pathway. Biosci Rep. 2020;40(10):BSR20201901. doi:10.1042/BSR20201901.
