Abstract
Background: Pancreatic carcinoma (PC) is a global health threat with a high death rate. miRNAs are implicated in tumor initiation and progression. This study explored the expression of miR-425-5p in PC patients and its correlation with tumor immune microenvironment (TIME).
Method: miR-425-5p expression in cancer tissues and adjacent non-tumor tissues of PC patients was examined by RT-qPCR. The levels of immune cells and cytokines were measured by flow cytometry and ELISA. The correlation of miR-425-5p with TNM stage and TIME was assessed by Spearman method. The death of PC patients was recorded through 36-month follow-ups. The prognosis of patients was assessed by Kaplan-Meier curves.
Results: miR-425-5p expression was upregulated in PC tissues and elevated with increasing TNM stage. miR-425-5p expression was positively correlated with TNM stage. The PC tissues had decreased levels of CD3+, CD4+, CD8+, and natural killer (NK) cells, CD4+/CD8+ ratio, IL-2, and INF-γ, but increased levels of Tregs, IL-4, IL-10, and TGF-β. miR-425-5p level in cancer tissues was positively correlated with Tregs/IL-10/TGF-β, but negatively related to CD3+/CD4+/CD8+/NK cells and IL-2/INF-γ. Moreover, high miR-425-5p expression predicted a poor prognosis in PC patients.
Conclusion: miR-425-5p is upregulated in PC patients and is prominently associated with the TIME, and high miR-425-5p predicts a poor prognosis in PC patients.
Introduction
Pancreatic carcinoma (PC) is construed as a dominant cause of cancer-associated death worldwide, and the global burden of PC has more than doubled during the last 25 years [Citation1]. According to the tumor location, PC can be classified into pancreatic head cancer (PHC) and pancreatic body/tail cancer (PBTC) [Citation2]. Due to the poor prognosis, the quantity of deaths in PC (466,000) is almost close to the number of new cases (496,000) [Citation3]. PC is normally difficult to diagnose at an early stage because of its asymptomatic nature, and patients normally do not exhibit symptoms until PC has metastasized or progressed into an advanced stage where the tumor cannot be surgically removed; however, early diagnosis, as well as surgical removal of PC, can elevate the survival rate to 39%, while late detection can reduce the survival rate to 3% [Citation4]. Despite advancements in cancer therapy, PC still represents one of the most lethal malignancies with an estimated 5-year overall survival of 11% [Citation5]. Indeed, the poor 5-year survival is partly attributed to late diagnosis and limited treatment options, as well as biophysical and molecular properties of tumors that result in resistance [Citation6]. Therefore, it is of high priority to further investigate the mechanism underlying PC pathogenesis and explore novel and efficient biomarkers to provide new insights into early diagnosis and treatment.
microRNAs (miRNAs) are broadly acknowledged as small non-coding RNAs with roughly 22 nucleotides in length, which confer an essential role in the post-transcriptional modulation of gene expression either by suppression mRNA translation or by facilitating mRNA degradation [Citation7, Citation8]. miRNAs are powerful mediators of a plethora of cellular activities, such as cell differentiation, growth, development, and apoptosis [Citation9]. Intrinsically, numerous miRNAs primarily function as oncogenes or tumor suppressors, and the dysregulation of miRNAs is exceedingly related to tumor initiation, metastasis, and progression [Citation10]. Through much-valuable literature review, it is interesting to note that miR-425-5p is capable of serving as an oncogene to promote neoplasm occurrence, metastasis, and development in numerous cancer types [Citation11–13]. Likewise, miR-425-5p is highly expressed in tumor tissues from PC patients, and it expression is intensively associated with differentiation degree, clinical stage, and lymph node metastasis [Citation14]. These findings made it plausible to postulate miR-425-5p as a potent biomarker for PC.
The interactions between immune cells, tumor cells, and cytokines present in the tumor immune microenvironment (TIME), which are classified into pro-tumor or anti-tumor, primarily determine the tendency of anti-tumor immunity, and despite the elimination of tumors by immune system via the cancer immune cycle, tumors seem to ultimately evade immune surveillance by forming an immunosuppressive microenvironment [Citation15]. An existing study has uncovered that malignant progression, resistance to therapy, and poor prognosis of PC are strongly associated with the immunosuppressive property of tumor microenvironment (TME) [Citation16]. Among the immune cells in the TME, T lymphocytes (especially CD4+ and CD8+) interact with tumor cells to maintain the suppressive TIME, further impacting the occurrence, development, and metastasis of tumors [Citation17–19]. The CD8 T cell priming is essentially directed as a corroboration work between innate immune cells such as natural killer (NK) cells with the CD4+ T cells in adoptive immunity [Citation20]. T regulatory lymphocytes (Tregs) can repress abnormal and excessive immune responses to nonself- or self-antigens to sustain immune homeostasis, which is also implicated in tumor development by inhibiting anti-tumor immunity [Citation21]. Therefore, it is of paramount significance to investigate the TIME in PC and the composition and inhibitory characteristics of TIME to provide new ideas for immunotherapy of PC.
Prior research has highlighted recent signs of progress in understanding the complicated interplay between miRNA dysregulation and TME [Citation22]. However, reports regarding the association of miR-425-5p with immune microenvironment characteristics in PC are limited. To this end, this general study regarding PC intended to investigate the expression level of miR-425-5p in PC patients and its correlation with TIME characteristics.
Materials and Methods
Ethics Statement
This study was approved by the Academic Ethics Committee of Suining Central Hospital. All enrolled subjects were informed of the study purpose and signed the informed consent.
Study Subjects
Totally 96 pairs of PC tissues and adjacent non-tumor tissues 5 cm away from the tumor were obtained from 96 PC patients who underwent surgery in Suining Central Hospital from June 2017 to June 2019, and then rapidly frozen in liquid nitrogen at −80 °C for subsequent usage. There were 14 patients who could not be followed up or withdrew halfway, so 82 patients were finally included. None of them received preoperative radiotherapy or chemotherapy. There were 52 males and 30 females with a mean age of 60.43 ± 7.16 years. There were 56 cases of PHC and 26 cases of PBTC. According to the tumor node metastasis (TNM) staging criteria established by the Union for International Cancer Control, 15 patients were in stage I, 32 patients were in stage II, and 35 patients were in stage III.
Inclusion and Exclusion Criteria
Inclusion criteria included: (1) diagnosed with PC by pathological examination; (2) no preoperative radiotherapy, chemotherapy, targeted therapy, and immunotherapy; (3) with complete clinical data and surgical specimen tissues; (4) with completed follow-up records. Exclusion criteria included: (1) combined with other primary malignant tumors; (2) in a special physical state, such as surgery, trauma, pregnancy, and lactation; (3) complicated with other infections and inflammatory diseases.
Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
RT-qPCR was adopted to measure miR-425-5p expression in the tissues. The extraction of total RNA from samples was conducted using TRIzol® reagent (Thermo Fisher Scientific, Waltham, MA, USA). RNA was subsequently reverse-transcribed into cDNA using reverse transcription kits (Takara, Kusatsu, Shiga, Japan). qPCR reactions were carried out utilizing SYBR Premix Ex Taq (Takara). Thermal cycling conditions were: 95 °C for 30 s, and then 40 cycles of 95 °C for 5 s and 60 °C for 30 s. RT-qPCR was performed on an ABI 7500 system (ABI, Carlsbad, CA, USA). Relative expression level of miR-425-5p after normalization to the internal control U6 was calculated using the 2-ΔΔCt method. Primer sequences are exhibited in .
Table 1. Primer sequences.
Detection of Immune Cells by Flow Cytometry
The tumors were weighted and sliced into small fragments, followed by digestion in 10 mL digestion solution (phosphate-buffered saline supplemented with type I collagenase [200 U/mL], hyaluronidase, and DNase I [100 µg/mL]) at 37 °C for 60 min. The single-cell suspension was acquired by the grinding of digested tissues and their filtration using a 70-µm cell strainer (BD Biosciences, San Jose, CA, USA). The cell suspension (100 µg/mL) was stained for 30 min using the following antibodies: CD3-phycoerythrin (PE)-CY5 (ab157300, Abcam, Cambridge, UK), CD4-fluorescein isothiocyanate (FITC; ab59474, Abcam), CD56-FITC (ab234250, Abcam), CD8-PE (ab28017, Abcam), CD25-PE (61-0251-82, Thermo Fisher Scientific), and forkhead box protein 3 (FoxP3)-PE-CY5 (15-4777-42, Thermo Fisher Scientific) at 4 °C. Flow cytometry was performed on a FACS Canto II flow cytometer (BD Biosciences) and data were analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of interleukin (IL)-2, IL-4, IL-10, interferon (INF)-γ, and transforming growth factor (TGF)-β in the tissues from patients were determined using corresponding IL-2 (E-EL-H0099c, Elabscience Biotechnology, Wuhan, Hubei, China), IL-4 (E-EL-H0101c, Elabscience Biotechnology), IL-10 (E-EL-H6154, Elabscience Biotechnology), INF-γ (PI511, Beyotime, Shanghai, China), and TGF-β (PT880, Beyotime) ELISA kits. The absorbance values of samples were detected on a microplate reader (Bio-Rad 680, Bio-Rad, Hercules, CA, USA), and the concentration of each cytokine in the tissues was calculated based on the formula.
Patient Follow-Up
After discharge, the patients were followed up by telephone and outpatient reexamination for 36 months. The survival information of all patients was collected and analyzed, and the death of PC patients was recorded. If the patient was not followed up, he/she was considered a loss case and was no longer recorded.
Data Analysis
Statistical analysis and mapping of data were performed using SPSS 22.0 (IBM Corp. Armonk, NY, USA) and GraphPad Prism 8.01 (GraphPad Software Inc., San Diego, CA, USA). The normal distribution was examined by Shapiro-Wilk test. Data were depicted as mean ± standard deviation (SD). Comparisons between two groups were processed by the t-test. Spearman method was performed to analyze the correlation between miR-425-5p with TNM stage and TIME. Kaplan-Meier curve was plotted to analyze the outcomes, and log-rank test was employed to analyze survival differences. p < 0.05 was considered statistically significant.
Results
miR-425-5p Was Upregulated in PC
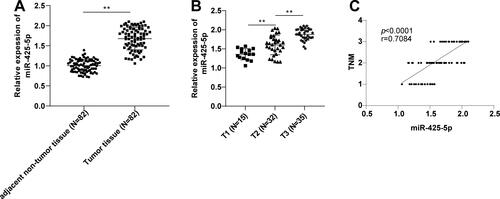
The difference in miR-425-5p expression levels between cancer tissues and adjacent non-tumor tissues of PC patients was compared. RT-qPCR revealed that miR-425-5p level in PC tissues was higher than that in adjacent non-tumor tissues (p < 0.01) () and increased with the TNM stage (p < 0.01) (). Spearman analysis revealed that miR-425-5p expression in cancer tissues of PC patients was positively correlated with TNM stage (p < 0.0001, r = 0.7084) (). Overall, miR-425-5p was highly expressed in PC tissues and showed a positive correlation with TNM stage.
Figure 1. miR-425-5p was upregulated in PC. (A-B) miR-425-5p expression levels in patient tissues were determined by RT-qPCR. (C) Correlation between miR-425-5p expression in cancer tissues of PC patients and TNM stage was assessed by Spearman correlation analysis. Data were exhibited as mean ± SD and comparisons between two groups were performed using the t-test. **p < 0.01.
Changes in Immune Cell Levels in PC
Evidence suggests that malignant progression and poor prognosis of PC are strongly associated with immunosuppression in the TME [Citation16]. Therefore, we used flow cytometry to detect the changes in immune cell levels in the tissues of PC patients. Compared with those in adjacent non-tumor tissues, the levels of CD3+, CD4+, CD8+, NK cells, and the ratio of CD4+/CD8+ were remarkably decreased and Tregs were prominently increased in PC tissues (all p < 0.001) (), which indicated a decrease in immune function of PC patients.
Table 2. Changes of immune cell levels in PC (mean ± SD).
Changes in Cytokine Levels in PC
ELISA showed that PC tissues had lower levels of immune factors IL-2 and INF-γ, but higher levels of IL-4, IL-10, and TGF-β than adjacent non-tumor tissues (all p < 0.01) ().
Table 3. Changes of immune factor levels in PC (mean ± SD).
Correlation between miR-425-5p and TIME in PC
We further analyzed the correlation of miR-425-5p with immune cells and immune factors in the TIME using Spearman analysis. The expression level of miR-425-5p in PC tissues was positively related to Treg, IL-10, and TGF-β levels (p < 0.05), inversely correlated with the levels of CD3+, CD4+, CD8+, and NK cells, as well as IL-2 and INF-γ (p < 0.05), but not correlated with IL-4 level (p > 0.05) ().
Table 4. Correlation analysis between miR-425-5p and immune microenvironment in PC patients.
Correlation between miR-425-5p and Prognosis in PC Patients
To investigate the prognostic value of miR-425-5p in PC patients, PC patients were deliberately separated into the low miR-425-5p expression group (N = 41) and high miR-425-5p expression group (N = 41) according to the median level of miR-425-5p. The relationship between miR-425-5p expression and clinical characteristics of PC patients was also analyzed, which revealed that there were no statistically significant differences in sex, age, and location of disease onset between the high and low miR-425-5p expression groups (p > 0.05) (), but there were significant differences in terms of lymph node metastasis status, TNM stage, and carbohydrate antigen 199 (CA199) level (p < 0.05) (). Further comparative analysis uncovered that miR-425-5p expression was elevated with increasing TNM stage. The low miR-425-5p expression group mainly consisted of patients with TNM stage I and II PC, and the high miR-425-5p expression group mainly consisted of patients with TNM stage II and III PC (). In addition, PC patients in the two groups were followed up to analyze the postoperative survival. The results unveiled that patients in the low miR-425-5p expression group had higher survival rates than those in the high miR-425-5p expression group (p < 0.05) (), indicating that high miR-425-5p expression predicted poor postoperative prognosis in PC patients.
Figure 2. Relationship between miR-425-5p and prognosis in PC patients. Survival curves were plotted using the regular follow-up data. Log-rank was used to test differences in Kaplan-Meier curves.
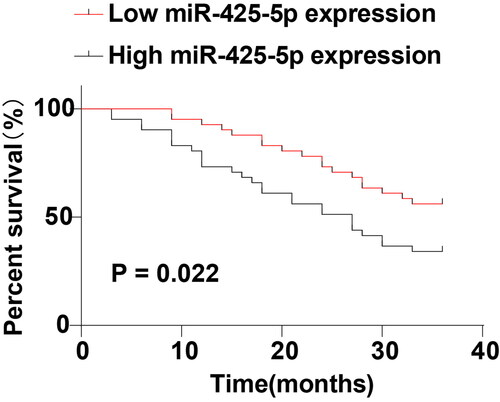
Table 5. Correlation of miR-425-5p expression with clinical characteristics of PC patients.
Table 6. Correlation of miR-425-5p expression with TNM stage.
Discussion
PC accounts for roughly 1.8% of all cancers, resulting in 4.6% of all global cancer-related deaths and 3.9% of global cancer disability-adjusted life [Citation23]. PC symptoms are atypical and the tumor progresses very rapidly, lacking sensitive early diagnostic markers [Citation24]. A majority of pancreatic tumors respond poorly to therapeutic approaches owing to the highly desmoplastic and immunosuppressive TME [Citation25]. Particularly, the TIME mainly comprises distinct immune cells in tumor islets and is extremely linked with anti-tumor immunological status in the TME [Citation26]. Equally importantly, numerous miRNAs take a crucial role in cancer progression, partake in the tumor immune process, and act in conjunction with miRNA sponges, which can serve as effective biomarkers for cancer diagnosis or prognosis prediction [Citation27]. Through much-valuable literature review, we learned that the adjacent non-tumor tissues in oral squamous cell carcinoma, lung adenocarcinoma, and colorectal cancer are all obtained at a distance of 5 cm from the tumor [Citation28–30]. Currently, adjacent non-tumor tissues are generally defined as the tissues 5 cm away from the tumor, with the purpose to ensure that the obtained tissues are as normal as possible. Therefore, in addition to tumor tissues, we collected the adjacent non-tumor tissues 5 cm away from the tumor in this study and explored miR-425-5p expression in PC patients. Our findings evinced the high expression of miR-425-5p in PC tissues and its evident correlation with TIME.
Accumulating evidence suggests that miR-425-5p is highly expressed in various cancers and can contribute to tumorigenesis and tumor development through regulation of different pathways [Citation13, Citation31]. Conversely, knockdown of miR-425-5p impedes tumor cell proliferation and migration as well as triggers apoptosis in cancers, such as hepatocellular carcinoma [Citation32] and cervical cancer [Citation33]. Accordingly, we noted that miR-425-5p was upregulated in PC tissues and elevated with TNM stage. Previous research has elicited increased miR-425-5p expression in PDAC cell lines, acting as a promising diagnostic marker for PDAC [Citation34]. Convincing evidence has documented the functionality of miRNAs as novel biomarkers, effective prognostic indicators, and promising therapeutic targets in PC [Citation35]. In other cancers including gastric cancer [Citation36] and lung cancer [Citation37], miR-425-5p overexpression is tightly related to advanced TNM stages and is considered an effective marker of poor outcome in several cancers [Citation36, Citation37]. In addition, PC patients with high miR-425-5p expression were more likely to develop lymph node metastasis and progress to advanced TNM stages, with more cases showing higher CA199 level. As well-known, CA199 is considered the best validated biomarker and an indicator of aberrant glycosylation in PC, which functions as a biomarker, predictor, and promoter in PC [Citation38]. Previous scholars have also uncovered the correlation of miR-425-5p expression with lymph node metastasis, differentiation degree, and clinical stage [Citation14]. Taken together, the above evidence and findings underscored the involvement of miR-425-5p in PC development.
However, the prognostic role of miR-425-5p in PC has not been elucidated yet. Therefore, we performed the Kaplan-Meier curve to analyze the prognostic value of miR-425-5p level in PC patients after surgery, firstly, to provide more diagnostic biomarkers for clinical prognostic assessment of PC, and secondly, to provide new ideas and directions for exploring immunotherapy of PC. Our results innovatively elicited that PC patients with low miR-425-5p expression had higher survival rates after surgery. Many studies have reported the assessment of poor prognosis by detecting miRNA expression levels in resected tumor tissues. For instance, disease-free survival and overall survival are shorter in gastric cancer patients with high miR-6792-3p expression in tissues, suggesting that high miR-6792-3p 3p is indicative of poor prognosis in gastric cancer [Citation39]. A study has assessed the prognostic value of miR-32 expression in non-small cell lung cancer by Kaplan-Meier method and found that patients with low miR-32 expression exhibit a shorter overall survival than those with high miR-32 expression [Citation40]. Therefore, it is of importance to assess poor prognosis following surgery by determining miR-425-5p levels in resected tumor tissues. Altogether, high miR-425-5p expression predicted poor postoperative prognosis in PC patients.
TME comprises a complicated immune cell environment, including NK cells, macrophages, and dendritic cells involved in innate immune responses, and T lymphocytes and Tregs involved in adaptive immune responses [Citation41, Citation42]. The immunological constituents within tumors are exceedingly linked with tumor progression, metastasis, and recurrence [Citation43]. NK cells can recognize and directly kill tumor cells or shape various anti-tumor immune responses, which are crucial immune components in manipulating tumor development, but the frequency of NK cell infiltration is extremely low in PDAC tumors (<0.5%) [Citation44]. Upon activation, CD4+ T cells are capable of differentiating into CD4+ helper T cells and secreting IL-2 to further activate CD8+ cytotoxic lymphocytes (CTLs) that are essential in killing tumor cells, but CTLs become exhausted and dysfunctional resulting from immunosuppression as well as immune-related tolerance in the TIME during the rapid tumor progression [Citation45]. Tregs can effectively infiltrate pancreatic malignancies in a very early stage of oncogenesis and contribute to establishing an immunosuppressed TIME, and Treg accumulation in blood or tissue is extensively associated with shortened survival in PDAC [Citation46]. Th1 factors (INF-γ and IL-2) are linked with anti-tumor functions such as cytophagic and cytotoxic enhancement, while Th2 factors (IL-10, IL-4, and TGF-β) exert a suppressive action in the TIME to facilitate tumor metastasis and recurrence [Citation47]. As indicated, PC tissues presented diminished CD3+, CD4+, CD8+, NK cells, and the CD4+/CD8+ ratio, increased Tregs, decreased IL-2 and INF-γ, and raised IL-4, IL-10, and TGF-β levels. It is noteworthy that PC is predominantly featured by a highly immunosuppressive TME and evasion of immune surveillance [Citation48].
miRNAs act primarily in different cellular compartments of the TME to modulate the function of fibroblasts and immune cells and/or affect the function of other cell types through cell-to-cell communication [Citation49]. Indeed, elevated miR-425-5p levels may be tightly related to the progress of immunosuppressive microenvironment in thymoma [Citation50]. In PC tissues, miR-425-5p shows a positive association with IL-23, which is a vital inflammatory factor in the TME and can accelerate tumor angiogenesis and repress CD8+ cell infiltration [Citation14]. Accordingly, our findings uncovered that miR-425-5p expression was positively correlated with Tregs, IL-10, and TGF-β levels, but inversely linked with the levels of CD3+, CD4+, CD8+, NK cells, IL-2, and INF-γ in PC tissues.
To summarize, this current study elucidated that miR-425-5p was highly expressed in PC tissues and was prominently correlated with TIME. However, there are several inevitable limitations. The sample size included is limited. The follow-up time for prognostic assessment is relatively short. In future studies, we shall conduct a multi-center prospective study and expand the sample size to increase confidence in the results. Moreover, the precise mechanism of miR-425-5p in PC warrants in-depth investigation.
Authors’ contributions
SJ is the guarantors of integrity of the entire study and contributed to the study concepts, study design, definition of intellectual content; DHK contributed to the literature research, clinical studies, data analysis; JLT contributed to the data acquisition, statistical analysis, manuscript editing, manuscript review.
Ethics approval and consent to participate
This study was approved by the Academic Ethics Committee of Suining Central Hospital. All enrolled subjects were informed of the study purpose and signed the informed consent. All procedures were strictly implemented according to the Declaration of Helsinki.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18(7):1–7. doi:10.1038/s41575-021-00457-x.
- Sun K, Mylavarapu C, Crenshaw A, et al. Pancreatic head vs pancreatic body/tail cancer: are they different? World J Gastrointest Oncol. 2022;14(3):716–723. doi:10.4251/wjgo.v14.i3.716.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660.
- Girish BP, Dariya B, Mannarapu M, Nagaraju GP, Raju GSR. Targeting the tumor microenvironment of pancreatic ductal adenocarcinoma using nano-phytomedicines. Semin Cancer Biol. 2022;86(Pt 2):1155–1162. doi:10.1016/j.semcancer.2021.06.014.
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708.
- Gromisch C, Qadan M, Machado MA, Liu K, Colson Y, Grinstaff MW. Pancreatic adenocarcinoma: unconventional approaches for an unconventional disease. Cancer Res. 2020;80(16):3179–3192. doi:10.1158/0008-5472.CAN-19-2731.
- Chen L, Heikkinen L, Wang C, Yang Y, Sun H, Wong G. Trends in the development of miRNA bioinformatics tools. Brief Bioinform. 2019;20(5):1836–1852. doi:10.1093/bib/bby054.
- Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M. Deciphering miRNAs’ action through miRNA editing. IJMS. 2019;20(24):6249. doi:10.3390/ijms20246249.
- Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–5465. doi:10.1002/jcp.27486.
- Ali Syeda Z, Langden SSS, Munkhzul C, Lee M, Song SJ. Regulatory mechanism of microRNA expression in cancer. IJMS. 2020;21(5):1723. doi:10.3390/ijms21051723.
- Yan YF, Gong FM, Wang BS, Zheng W. MiR-425-5p promotes tumor progression via modulation of CYLD in gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21:2130–2136.
- Liu D, Zhang H, Cui M, Chen C, Feng Y. Hsa-miR-425-5p promotes tumor growth and metastasis by activating the CTNND1-mediated beta-catenin pathway and EMT in colorectal cancer. Cell Cycle. 2020;19(15):1917–1927. doi:10.1080/15384101.2020.1783058.
- Zhou JS, Yang ZS, Cheng SY, Yu JH, Huang CJ, Feng Q. miRNA-425-5p enhances lung cancer growth via the PTEN/PI3K/AKT signaling axis. BMC Pulm Med. 2020;20(1):223. doi:10.1186/s12890-020-01261-0.
- Lu Y, Wu X, Wang J. Correlation of miR-425-5p and IL-23 with pancreatic cancer. Oncol Lett. 2019;17(5):4595–4599. doi:10.3892/ol.2019.10099.
- Lv B, Wang Y, Ma D, et al. Immunotherapy: reshape the tumor immune microenvironment. Front Immunol. 2022;13:844142. doi:10.3389/fimmu.2022.844142.
- Ren B, Cui M, Yang G, et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17(1):108. doi:10.1186/s12943-018-0858-1.
- Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi:10.1038/nrclinonc.2017.101.
- Dangaj D, Bruand M, Grimm AJ, et al. Cooperation between constitutive and inducible chemokines enables t cell engraftment and immune attack in solid tumors. Cancer Cell. 2019;35(6):885–900 e810. doi:10.1016/j.ccell.2019.05.004.
- Ostios-Garcia L, Villamayor J, Garcia-Lorenzo E, Vinal D, Feliu J. Understanding the immune response and the current landscape of immunotherapy in pancreatic cancer. World J Gastroenterol. 2021;27(40):6775–6793. doi:10.3748/wjg.v27.i40.6775.
- Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234(6):8509–8521. doi:10.1002/jcp.27782.
- Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080–2089. doi:10.1111/cas.14069.
- Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK. miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov. 2016;6(3):235–246. doi:10.1158/2159-8290.CD-15-0893.
- Lippi G, Mattiuzzi C. The global burden of pancreatic cancer. Arch Med Sci. 2020;16(4):820–824. doi:10.5114/aoms.2020.94845.
- Ariston Gabriel AN, Wang F, Jiao Q, et al. The involvement of exosomes in the diagnosis and treatment of pancreatic cancer. Mol Cancer. 2020;19(1):132. doi:10.1186/s12943-020-01245-y.
- Liu L, Kshirsagar PG, Gautam SK, et al. Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies. Theranostics. 2022;12(3):1030–1060. doi:10.7150/thno.64805.
- Mao X, Xu J, Wang W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20(1):131. doi:10.1186/s12943-021-01428-1.
- He B, Zhao Z, Cai Q, et al. miRNA-based biomarkers, therapies, and resistance in cancer. Int J Biol Sci. 2020;16(14):2628–2647. doi:10.7150/ijbs.47203.
- Pushalkar S, Ji X, Li Y, et al. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012;12:144. doi:10.1186/1471-2180-12-144.
- Feng Y, Tong X, Zhang B, Mao G, Huang H, Ma H. Effect of FAM196B in human lung adenocarcinoma. J Cancer. 2018;9(14):2451–2459. doi:10.7150/jca.24907.
- Pan R, Zhou C, Dai J, et al. Endothelial PAS domain protein 1 gene hypomethylation is associated with colorectal cancer in Han Chinese. Exp Ther Med. 2018;16(6):4983–4990. doi:10.3892/etm.2018.6856.
- Zhu Y, Dou H, Liu Y, et al. Breast cancer exosome-derived miR-425-5p induces cancer-associated fibroblast-like properties in human mammary fibroblasts by TGFbeta1/ROS signaling pathway. Oxid Med Cell Longev. 2022;2022:5266627. doi:10.1155/2022/5266627.
- Wu H, Shang J, Zhan W, Liu J, Ning H, Chen N. miR‑425‑5p promotes cell proliferation, migration and invasion by directly targeting FOXD3 in hepatocellular carcinoma cells. Mol Med Rep. 2019;20(2):1883–1892. doi:10.3892/mmr.2019.10427.
- Zhang Y, Yang Y, Liu R, Meng Y, Tian G, Cao Q. Downregulation of microRNA-425-5p suppresses cervical cancer tumorigenesis by targeting AIFM1. Exp Ther Med. 2019;17(5):4032–4038. doi:10.3892/etm.2019.7408.
- Makler A, Narayanan R, Asghar W. An exosomal miRNA biomarker for the detection of pancreatic ductal adenocarcinoma. Biosensors (Basel). 2022;12(10):831. doi:10.3390/bios12100831.
- Daoud AZ, Mulholland EJ, Cole G, McCarthy HO. MicroRNAs in pancreatic cancer: biomarkers, prognostic, and therapeutic modulators. BMC Cancer. 2019;19(1):1130. doi:10.1186/s12885-019-6284-y.
- Zhang Z, Wen M, Guo J, et al. Clinical value of miR-425-5p detection and its association with cell proliferation and apoptosis of gastric cancer. Pathol Res Pract. 2017;213(8):929–937. doi:10.1016/j.prp.2017.05.009.
- Fu Y, Li Y, Wang X, Li F, Lu Y. Overexpression of miR-425-5p is associated with poor prognosis and tumor progression in non-small cell lung cancer. Cancer Biomark. 2020;27(2):147–156. doi:10.3233/CBM-190782.
- Luo G, Jin K, Deng S, et al. Roles of CA19-9 in pancreatic cancer: biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188409. doi:10.1016/j.bbcan.2020.188409.
- Luo Z, Rong Z, Zhang J, et al. Circular RNA circCCDC9 acts as a miR-6792-3p sponge to suppress the progression of gastric cancer through regulating CAV1 expression. Mol Cancer. 2020;19(1):86. doi:10.1186/s12943-020-01203-8.
- Bai Y, Wang YL, Yao WJ, et al. Expression of miR-32 in human non-small cell lung cancer and its correlation with tumor progression and patient survival. Int J Clin Exp Pathol. 2015;8:824–829.
- Ni X, Chen C, Cui G, Ding W, Liu J. Crosstalk of RNA adenosine modification-related subtypes, establishment of a prognostic model, and immune infiltration characteristics in ovarian cancer. Front Immunol. 2022;13:932876. doi:10.3389/fimmu.2022.932876.
- Chen F, Li J, Wang H, Ba Q. Anti-tumor effects of Chinese medicine compounds by regulating immune cells in microenvironment. Front Oncol. 2021;11:746917. doi:10.3389/fonc.2021.746917.
- Fu T, Dai LJ, Wu SY, et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J Hematol Oncol. 2021;14(1):98. doi:10.1186/s13045-021-01103-4.
- Huber M, Brehm CU, Gress TM, et al. The immune microenvironment in pancreatic cancer. IJMS. 2020;21(19):7307. doi:10.3390/ijms21197307.
- Fu X, Sun G, Tu S, et al. Hsa_circ_0046523 mediates an immunosuppressive tumor microenvironment by regulating miR-148a-3p/PD-L1 axis in pancreatic cancer. Front Oncol. 2022;12:877376. doi:10.3389/fonc.2022.877376.
- Gartrell RD, Enzler T, Kim PS, et al. Neoadjuvant chemoradiation alters the immune microenvironment in pancreatic ductal adenocarcinoma. Oncoimmunology. 2022;11(1):2066767. doi:10.1080/2162402X.2022.2066767.
- Guo Q, Li J, Lin H. Effect and molecular mechanisms of traditional Chinese medicine on regulating tumor immunosuppressive microenvironment. Biomed Res Int. 2015;2015:261620. doi:10.1155/2015/261620.
- Ding G, Shen T, Yan C, Zhang M, Wu Z, Cao L. IFN-gamma down-regulates the PD-1 expression and assist nivolumab in PD-1-blockade effect on CD8+ T-lymphocytes in pancreatic cancer. BMC Cancer. 2019;19(1):1053. doi:10.1186/s12885-019-6145-8.
- Sempere LF, Powell K, Rana J, Brock AA, Schmittgen TD. Role of non-coding RNAs in tumor progression and metastasis in pancreatic cancer. Cancer Metastasis Rev. 2021;40(3):761–776. doi:10.1007/s10555-021-09995-x.
- Wang B, Xiao H, Yang X, et al. A novel immune-related microRNA signature for prognosis of thymoma. Aging (Albany NY). 2022;14(11):4739–4754. doi:10.18632/aging.204108.
