Abstract
Purpose
To compare the clinical efficacy of percutaneous functional spinal unit cementoplasty (PFSUP) and posterior spinal fixation combined with vertebroplasty (PSF + VP) for the treatment of symptomatic chronic osteoporotic vertebral fractures (SCOVFs).
Method
Thirty-one patients with SCOVFs were included in this retrospective study and divided into PFSUP (n = 14) and PSF + VP (n = 17) groups. Visual analog scores (VAS) and Oswestry Disability Index (ODI) were recorded before and after surgery and at the last follow-up. Besides, the local kyphosis angle (LKA) and sagittal vertical axis (SVA) were measured. The operation duration, number of X-ray exposures, amount of blood loss, bed rest duration, hospitalization duration, and presence of complications were recorded.
Result
The VAS, ODI, LKA, and SVA after surgery and at the last follow-up were significantly improved in both groups compared to preoperative measurements. The PFSUP group experienced shorter operation duration (78.2 ± 13.1 vs. 124.7 ± 14.7, p < 0.001), less blood loss (31.1 ± 8.1 vs. 334.7 ± 70.9, p < 0.001), more X-ray exposures (92.1 ± 14.3 vs. 29.4 ± 5.5, p < 0.001), shorter bed rest duration (12.4 ± 3.8 vs. 43.4 ± 10.0, p < 0.001), shorter hospitalization (6.6 ± 2.4 vs. 10.9 ± 2.7, p < 0.001), lower complication rate (28.5% vs. 64.7%, p < 0.05), and higher cement leakage rate (42.9% vs. 5.8%, p < 0.05) than the PSF + VP group.
Conclusion
During the treatment of SCOVFs, the combination of PFSUP and PSF + VP can restore spinal stability, improve kyphosis, and relieve pain. PFSUP can reduce blood loss and complications, early mobilization, and shorten the hospital stay, but it is associated with a higher cement leakage rate and more radiation exposure.
Introduction
Osteoporotic vertebral compression fractures (OVCFs) are one of the most common complications of osteoporosis in elderly men and postmenopausal women. Most cases of OVCFs can be treated with bed rest, bracing, oral nonsteroidal anti-inflammatory drugs, or percutaneous vertebral augmentation, thereby achieving pain relief and fracture healing [Citation1]. Delayed diagnosis or inadequate treatment of OVCFs can lead to a lack of bone healing and result in conditions such as vertebral instability, intravertebral cleft formation, and even vertebral collapse. These complications are collectively known as symptomatic chronic osteoporotic vertebral fractures (SCOVFs). It is now understood that SCOVFs mostly occur at the thoracolumbar spine, characterized by complex clinical symptoms, including refractory lower back pain caused by fracture nonunion and vertebral instability, neurological impairment resulting from local kyphosis and/or fracture fragments protruding into the spinal canal. Furthermore, kyphosis can reduce thoracic and abdominal volumes, resulting in organ compression symptoms, such as pulmonary and digestive dysfunction [Citation2, Citation3]. Conservative treatment is ineffective for SCOVFs, which often exacerbates the condition and increases the risk of complications [Citation4, Citation5]. Current treatment options include percutaneous vertebral augmentation techniques such as percutaneous vertebroplasty (PVP) and percutaneous kyphoplasty (PKP), open surgery such as anterior vertebral resection with reconstruction, posterior osteotomy with long-segment internal fixation, and posterior internal fixation combined with PVP for fractured vertebrae [Citation3–7]. Open surgery can reconstruct spinal stability, improve kyphosis, and perform spinal canal decompression if necessary. However, open surgery is associated with major surgical trauma, excessive bleeding, and a high rate of complications, especially in elderly patients with severe osteoporosis and underlying medical conditions [Citation8]. Percutaneous vertebral augmentation can provide rapid analgesia and enhanced recovery, but it has a limited role in improving kyphosis and restoring spine stability. Moreover, it is associated with high rates of cement leakage, potentially leading to cement loosening or displacement into the spinal canal [Citation9]. Furthermore, patients with SCOVFs often experience chronic low back pain, disk space narrowing, and instability due to endplate disk complex (EDC) injury, which may persist even after vertebral augmentation [Citation10–13]. Moreover, there is no effective way to prevent cement leakage, as well as disk degeneration and adjacent vertebral fractures secondary to cement leakage into the disk space.
In 2015, Varga et al. [Citation14] reported using percutaneous cement discoplasty (PCD) for treating the disk vacuum sign associated with lumbar disk degeneration, yielding satisfactory clinical results. In recent years, PCD has been extensively used to treat degenerative spinal diseases, such as discogenic low back pain, degenerative scoliosis, and lumbar instability [Citation15, Citation16]. Furthermore, percutaneous discectomy combined with PCD has been applied to treat lumbar disk herniation with endplate osteochondritis [Citation17]. Importantly, PCD can restore the disk space height and relieve low back and radiating leg pain caused by mechanical instability. Although the bone cement used in PCD does not achieve fusion with the host bone, the implantation of cement may contribute to the stability of the intervertebral region, thereby promoting posterior bony structure fusion, resulting in long-term stability [Citation18].
Herein, we performed a comprehensive treatment approach called percutaneous functional spinal unit cementoplasty (PFSUP), which involved treating the fractured vertebral body, the injured endplate disk complex (EDC), and the adjacent vertebrae as a single unit using cementoplasty during a single procedure. This study aimed to evaluate the clinical outcomes of PFSUP and compare them with posterior spinal fixation with vertebroplasty (PSF + VP) in the treatment of SCOVFs.
Subjects and methods
Subjects
A total of 31 patients with SCOVFs who underwent surgical treatment in our hospital from July 2018 to March 2020 were analyzed retrospectively. Based on the surgical procedures performed, patients were divided into two groups: PFSUP (n = 14) and PSF + VP (n = 17). The inclusion criteria included: (1) patients aged >60 years old with a bone mineral density T-score of < −2.5 (measured by dual-energy X-ray absorptiometry); (2) patients with persistent low back pain and kyphotic deformity of more than 3 months; (3) The fractured vertebral body showed low signal intensity on T1-weighted imaging (WI), and intermediate, high or mixed intermediate signal intensities on T2WI, suggesting an old fracture that had not healed or a fresh fracture combined with an old fracture; (4) patients who had complete follow-up data for at least 24 months. Exclusion criteria included: (1) patients with non-osteoporotic vertebral fractures caused by tumor, inflammation, or trauma; (2) patients who had previous surgery at the affected vertebrae or the adjacent vertebrae; (3) patients who were followed up for less than 24 months or lost to follow-up.
Surgical techniques
All operations were performed under general anesthesia. Patients were placed in the prone position. Soft pillows were placed under the chest and bilateral iliac crests, with the abdomen hanging free and maintaining the thoracolumbar segment in a hyperextension position. Then the surgeon used both hands to apply pressure on the apex of the kyphosis to reduce the fracture. For patients in the PFSUP group, unilateral extra-pedicular PKP was performed in accordance with the procedure described by a previous study [Citation19]. Fluoroscopy was used to determine the location of the fractured vertebra and the entry point on the skin was marked according to the distance from the entry point to the midline calculated on the CT imaging. The junction between the base of the superior articular process and the transverse process was used as the entry point. Then the trocar was inserted into the vertebrae. The position of the trocar was confirmed using lateral and anteroposterior X-rays. Once the trocar reached the anterior 1/3 of the vertebral body laterally and exceeded the center of the anteroposteriorly, a balloon was placed in the compressed vertebral body and inflated to expand it. If cement leakage occurred into the disk space, bone cement injection continued until the vertebral body and disk space were adequately filled. If there was no intervertebral leakage or insufficient cement filling in the disk space, the trocar was retracted to the posterior edge of the vertebral body and tilted toward the injured disk, and cement was implanted into the disk space. Finally, PVP was performed in the adjacent vertebrae adjacent to the injured EDC. If both disks were injured (2 cases), cement was implanted into the disk space and vertebral bodies above and below the fractured vertebra. In this study, cement leakage into the disk space occurred in 8 patients, and satisfactory cement distribution in the disk space was achieved in 6 patients, obviating the need for additional PCD. For patients in the PSF + VP group, a posterior median incision was made, and the paravertebral muscles were dissected subperiosteally to expose the bilateral entry points for pedicle screw placement. A balloon was then inserted into the fractured vertebra via a transpedicular approach. The pressure in the balloon was gradually increased to restore the vertebral body height. Cement was then implanted into the fractured vertebra through a bilateral transpedicular approach under fluoroscopy. For the adjacent vertebrae above and below the fractured vertebra, cement was implanted into the anterior 1/3 of the vertebral body through a bilateral transpedicular approach, filling the anterior 2/3 of the pedicle screw path. Then the pedicle screws were inserted and fixed in place with pre-bent connecting rods. A drainage tube was placed after washing the wound with normal saline, and the incision was sutured in layers.
Postoperative management
In the PFSUP group, patient ambulation was observed after 6 h of surgery. In the PSF + VP group, the drainage tube was removed at 48 h or when the drainage volume decreased to less than 50 ml per 24 h after surgery. Then, patients were instructed to ambulate with a rigid lumbar brace. All patients in the two groups received anti-osteoporotic therapy and were instructed to perform lower back muscles.
Evaluation of clinical outcomes
The operation duration, number of X-ray exposures, amount of blood loss, occurrence of complications, duration of bed rest, and hospitalization duration in the two groups were recorded. The visual analog scale (VAS) was used to evaluate the degree of low back pain. Zero represented the absence of pain, while 10 points denoted maximal pain. The Oswestry Disability Index (ODI) was used to evaluate the activities of daily living. The VAS and ODI were recorded before, after surgery, and at the last follow-up in both groups.
Evaluation of the imaging findings
Before surgery, all patients underwent full-length flexion and extension X-rays of the spine, two-dimensional computed tomography (2DCT), and spine MRI. After the operation and at the last follow-up, all patients underwent full-length spine X-rays. The LKA was defined as the angle formed between two lines drawn parallel to the superior endplate of the vertebral body one level above the fractured vertebra and parallel to the inferior endplate of the vertebral body one level below the fractured vertebra on lateral X-ray. The sagittal vertical axis (SVA) was measured as the distance between a C7 vertical plumb line and the superior-posterior corner of the sacrum on a full-length lateral spine X-ray. 2DCT was performed after surgery to observe cement leakage, and the cement leakage rate was calculated. If low back pain recurred during the follow-up period, MRI was performed to confirm the occurrence of adjacent vertebral fracture, and the adjacent vertebral fracture rate at the last follow-up was recorded.
Statistical analysis
All statistical analyses were conducted using SPSS 25.0 software (SPSS, Chicago, Illinois, USA). Continuous variables were tested for normal distribution using the Shapiro-Wilk test. Data with normal distribution and homogeneity of variance were expressed as mean ± SD. Comparisons between groups were performed using two independent samples t-tests, followed by multiple comparisons using the least significant difference (LSD) t-test. Within-group comparisons were performed using the paired samples t-test. The differences in sex, cement leakage rate, and adjacent vertebral fracture rate between the two groups were compared using the Chi-squared test. A P-value < 0.05 was statistically significant.
Results
Clinical characteristics of patients
In the PFSUP group, there were 4 males and 10 females, with an average age of 75.50 years, BMI of 21.5, and T-Score of −2.99. Eight of these 14 patients had a history of trauma (including 3 with sprain injuries and 5 with simple falls). The mean duration of symptoms was 124.6 ± 31.1 days. The fractures occurred at T11 (n = 3), T12 (n = 5), L1 (n = 4), and L2 (n = 2). In the PSF + VP group, there were 5 males and 12 females, with an average age of 73.88 years, BMI of 20.9, and T-Score of −3.04. Among these 17 patients, 10 had a history of trauma (5 with sprain injury and 5 with simple falls). The mean duration of symptoms was 130.3 ± 26.1 days. The fractures occurred at T11 (n = 3), T12 (n = 5), L1 (n = 6), and L2 (n = 3). Patients in the PFSUP and PSF + VP groups were followed up for 30.6 ± 4.8 months and 33.9 ± 6.2 months, respectively. The clinical characteristics of patients in the two groups are shown in . The amount of blood loss was less in the PFSUP group than in the PSF + VP group (31.1 ± 8.1 ml vs. 334.7 ± 70.9 ml, p < 0.001). The PFSUP group was associated with a significantly shorter duration of bed rest than the PSF + VP group (12.4 ± 3.8 h vs. 43.4 ± 10.0 h, p < 0.001). Compared with the PSF + VP group, the PFSUP group experienced a significantly shorter hospitalization duration (6.6 ± 2.4 days vs. 10.88 ± 2.74 days, p < 0.001). However, the number of X-ray exposures was significantly higher in the PFSUP group compared with the PSF + VP group (92.1 ± 14.3 vs. 29.4 ± 5.5, p < 0.001, ).
Table 1. The clinical characteristics of patients in the two groups.
Table 2. The general results in two groups during their duration of hospital stay.
Clinical outcomes
Compared with preoperative scores, the VAS was significantly improved after surgery and at the last follow-up in both groups. The VAS scores after surgery were significantly better in the PFSUP group than in the PSF + VP group (p < 0.05); however, the VAS at the last follow-up was comparable between the two groups. Compared with preoperative scores, the ODI was significantly improved at 3 months after surgery and the last follow-up in both groups (p < 0.05). No significant difference was found in the ODI between the two groups at 3 months after surgery and the last follow-up ().
Table 3. The radiological results and clinical outcomes in two groups at admission, after operation and at the last follow up.
Imaging findings
No significant difference was found in LKA between the two groups before surgery (27.5 ± 5.0 vs. 25.7 ± 5.4, p = 0.268), after surgery (14.9 ± 3.3 vs. 15.7 ± 5.6, p = 0.624) and at the last follow-up (19.1 ± 2.5 vs. 20.5 ± 4.7, p = 0.305). Both groups achieved significant improvements in LKA after surgery (p < 0.05 for both groups), and there was no significant difference in angle correction between the two groups after surgery (12.6 ± 4.7 vs. 9.9 ± 3.8, p = 0.09). At the final follow-up, the loss of correction was 4.2°±2.7° in the PFSUP group and 4.8° ±2.3° in the PSF + VP group (p = 0.51), which resulted in a significant difference in LKA between measurements taken postoperatively and at the last follow-up (p < 0.05 for both groups). Compared with the preoperative measurements, the SVA was significantly improved after surgery and at the last follow-up in the two groups (p < 0.05 for both groups). However, the SVA was significantly increased at the last follow-up compared to the postoperative values in both groups (p < 0.05 for both groups), but the final SVA still showed significant improvement compared with preoperative values for both groups (p < 0.05 for both groups, ). The typical case in PFSUP group is shown in , and the other typical case in PSF + VP group is shown in .
Figure 1. Case 1. A 74-years old female patient complained refractory low back pain after lumbar sprain 90 days ago. A-B: X-ray at admission demonstrated L2 compression fracture with narrowed L1/2 disk space. C: Preoperative CT sagittal reconstruction show L2 collapse and osteophyte formation with upper endplate fracture. D: MRI at admission showed severe compressed and subacute fracture of L2, L1/2 disk space at T2 WI show disk injury with heterogeneous signal. E: Unilateral extra-pedicular PKP was performed at the injured vertebra, fluoroscopy show cement leak into the injured disk after adequate distribution in the L2. F-G, H: Postoperative X-ray and CT sagittal reconstruction show adequate cement distribution in the injured vertebra and disk, the cement bridge is generated between the vertebra and disk..
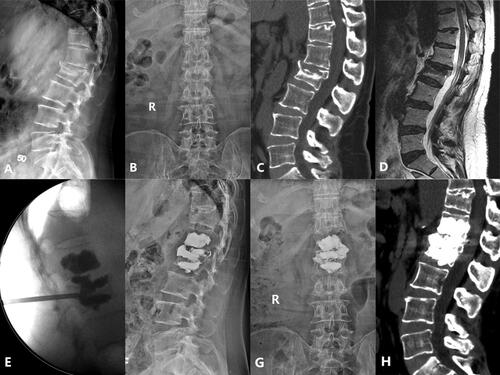
Figure 2. Case 1. A 74-years old female patient complained refractory low back pain after lumbar sprain 90 days ago. A: Preoperative full-length spine X-ray of the same patient show local kyphosis angle is 24.1°, the sagittal vertical axis(SVA) is -29.9 mm B: at the last follow up(35 months after operation), full-length spine X-ray show local kyphosis angle is 11.9°, the sagittal vertical axis(SVA) is 19.8 mm, the cement keeps in the original place.
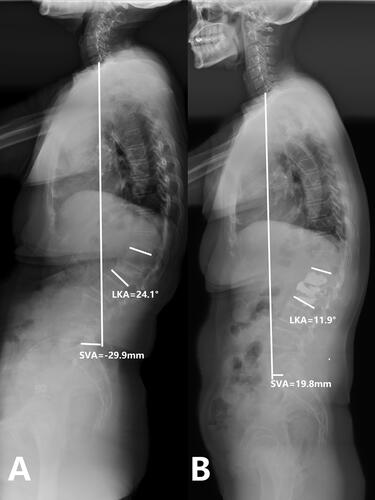
Figure 3. Case 2. PSV + VP. A 69-years old female patient complained refractory low back pain with functional dyspepsia which was persistent for about 6 months without any injury. A-B: X-ray at admission demonstrated L1 compression fracture with narrowed L1/2 disk space. C: Preoperative CT sagittal reconstruction show L1 collapse and intra-vertebral vacuum cleft with upper endplate fracture. D: MRI at admission showed severe compressed intra-vertebral vacuum cleft of L2, L1/2 disk space at T2 WI show disk injury with heterogeneous signal. E-F,G: Postoperative X-ray and CT sagittal reconstruction show adequate cement distribution in the injured vertebra, with bone cement reinforced screws fixation at the adjacent vertebrae.
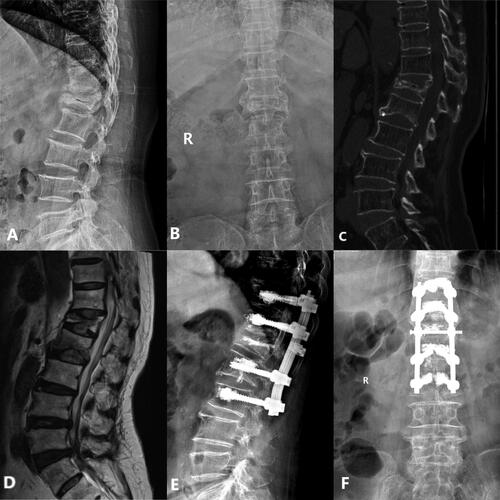
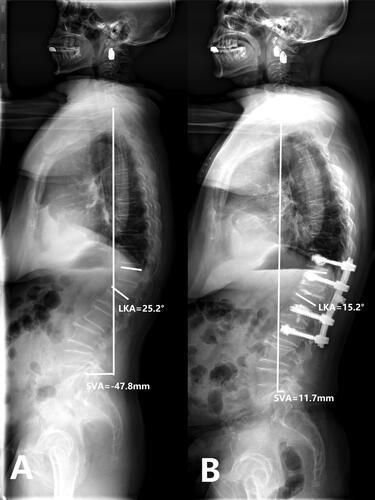
Figure 4. Case 2. PSV + VP. A 69-years old female patient complained refractory low back pain with functional dyspepsia which was persistent for about 6 months without any injury. A: Preoperative full-length spine X-ray of the same patient show local kyphosis angle is 25.2°, the sagittal vertical axis(SVA) is -47.8 mm. B: at the last follow up(33 months after operation), full-length spine X-ray show local kyphosis angle is 15.2°, the sagittal vertical axis(SVA) is 11.7 mm, the cement and internal fixation keeps in the original place.
Complications
In the PFSUP group, the cement leakage rate was 42.9% (6/14), and the total complication rate was 28.5% (4/14). In the PSF + VP group, the cement leakage rate was 5.8% (1/17), and the total complication rate was 64.7% (11/17). There were significant differences in the cement leakage rate and total complication rate between the two groups (p = 0.028 and p = 0.042, respectively, ).
Table 4. The complications in two groups at Peri-operative period and at the last follow up.
Discussion
In the present study, two procedures were used to treat SCOVF. We found that after two years of follow-up, PFSUP and PSF + VP could reduce LKA, restore SVA, and achieve pain relief and functional improvement. Compared with the PSF + VP group, the PFSUP group experienced less blood loss, lower complication rates, earlier ambulation, and shorter hospital duration, substantiating the advantages of this minimally invasive surgery, although it was associated with higher bone cement leakage rate and more X-ray exposure.
Three months after surgery, patients with SCOVF experienced complications such as fracture nonunion, instability, and kyphosis. Accordingly, conservative treatment alone often leads to poor results [Citation20]. A consensus has been reached on using percutaneous vertebral augmentation techniques such as PVP or PKP in patients without vertebral instability and kyphosis [Citation21, Citation22]. However, no standardized treatment option has been established for patients with kyphotic deformity and instability. Various approaches have been reported, including posterior long-segment fixation, PSF + VP, and anterior reconstruction surgery. However, due to advanced age, multiple underlying diseases, and severe osteoporosis, patients may not tolerate open procedures well, leading to high complication rates [Citation23]. Among open surgeries, PSF + VP is a relatively less invasive procedure, and many studies have compared the efficacy of PSF + VP versus posterior long-segment fixation or anterior reconstruction surgery. Our results showed that PSF + VP could achieve similar clinical outcomes to other procedures while reducing complications and promoting recovery [Citation24, Citation25].
In a study by Katsumi et al., after PSF + VP was used to treat 43 patients with old vertebral compression fractures and kyphosis, significant pain relief, kyphosis correction, and satisfactory bony fusion were observed after two years of follow-up [Citation24]. Ishikawa et al. [Citation25] compared the efficacy of PSF + VP and posterior long-segment fixation with fusion in treating osteoporotic vertebral collapse with neurological impairment and found that both groups achieved comparable outcomes in terms of relief of clinical symptoms and improving neurological function, but PSF + VP was associated with high rates of correction loss. In the present study, the PSF + VP group showed significant improvement in VAS and ODI and significant correction of LKA and sagittal alignment, consistent with the literature [Citation24, Citation25].
Since diverse symptoms and imaging findings may develop with the progression of SCOVFs, we only included patients with SCOVFs with intersegmental instability and moderate kyphosis (Cobb angle less than 30°) in the present study. PFSUP and PSF + VP are not indicated for treating patients with rigid kyphosis, severe spinal stenosis, or neurological impairment.
In addition to the intervertebral vacuum cleft, patients with SCOVFs exhibit the vacuum sign in the disk space, possibly due to endplate and disk complex injury or intersegmental instability caused by endplate collapse [Citation13, Citation26, Citation27]. Morishita et al. [Citation28] found that patients with the intervertebral vacuum sign were likelier to suffer low back pain. Nakajima et al. [Citation29] believed that the vacuum sign in the disk space adjacent to the fractured vertebra indicated intervertebral instability, thus necessitating the interbody fusion procedure.
Severe vertebral collapse, particularly in the thoracolumbar junction, can lead to localized kyphotic deformity and persistent, refractory low back pain. If left uncorrected, this deformity can result in ongoing disability even after fracture healing [Citation30]. It is widely believed that for treating SCOVF with intervertebral instability or kyphosis, vertebral augmentation techniques cannot restore intervertebral stability, with limited effect on kyphosis correction and loss of correction following vertebral augmentation [Citation13, Citation31]. At present, posterior long-segment internal fixation, PSF + VP, and anterior reconstruction surgery have been reported to treat SCOVFs combined with intervertebral instability or kyphosis. Although open surgical procedures can restore spinal stability and correct kyphosis well, they have disadvantages, including invasiveness and high complication rates [Citation32]. It has been reported that the internal fixation failure rate is high due to severe osteoporosis, even after enhancing the strength of fixations with multiple methods [Citation33]. In the present study, PCD was performed in patients with SCOVFs with intervertebral instability and kyphosis to restore intervertebral disk height and stability. To mitigate the risk of adjacent vertebral fractures associated with bone cement implantation into the intervertebral disk [Citation16], PVP was performed on the adjacent vertebral body during the same procedure to avoid adjacent vertebral fractures and increase local stability. After an average follow-up of 24 months, we found that PFSUP could restore the spine’s stability, effectively relieve pain, and promote early mobilization of patients. Additionally, PFSUP is a minimally invasive procedure with minimal surgical trauma and less intraoperative blood loss, which can greatly reduce the rate of complications. The short setting time of bone cement allows for immediate stability, enabling early mobilization and reducing complications associated with prolonged bed rest.
Vertebral augmentation techniques used to treat SCOVFs have been associated with a high rate of cement leakage, with some studies reporting rates up to 75% [Citation34, Citation35]. The main pathways for cement leakage are through the vertebral body cleft and endplate fractures. Intervertebral leakage is the most common [Citation36], followed by paravertebral and spinal canal leakage. However, cement leakage into the vertebral venous plexus is relatively rare and may be related to severe compression and avascular necrosis of the vertebral body. During the PFSUP procedure, injections should continue if cement leakage occurs into the disk space until satisfactory cement distribution is observed, allowing simultaneous completion of PCD cement augmentation for the fractured vertebra.
Neurological impairment may occur after cement leaks into the spinal canal, especially for SOVCF patients. Once nerve injury is present, cement removal may be required by open surgery. In this study, the bone cement leakage rate was 28.6% in the PFSUP group, lower than reported in the literature [Citation6, Citation10], attributed to the following reasons. First, we did not include patients who developed cement leakage into the disk space for calculating the cement leakage rate. Second, to promote cement leakage into the disk space, the trocar was directed intentionally toward the disk space during surgery, which may reduce the probability of cement leakage into the spinal canal, and around the vertebral body [Citation37].
In the present study, the PFSUP group experienced more X-ray exposure than the PSF + VP group (170 times vs. 40 times, p < 0.01) since during PFSUP, bone cement was implanted into at least two vertebral bodies and one disk space, which led to more radiation exposure. In the future, modified surgical methods and tools such as lateral opening trocar should be introduced to optimize the procedure and reduce radiation exposure.
Correction of LKA and restoration of sagittal alignment are important in maintaining long-term outcomes and reducing the re-fracture risk [Citation38, Citation39]. A good sagittal alignment can promote mobility, relieve chronic fatigue and pain, improve patients’ psychological well-being, and reduce symptoms associated with organ compression. This study adopted LKA and SVA to evaluate the sagittal alignment. After the PFSUP procedure, the injured vertebra, disk, and bone cement were fused, making it challenging to accurately measure the anterior vertebral height, which was not considered a radiological outcome in this study. In these two groups, LKA and SVA were significantly improved after surgery and at the last follow-up, whereas a significant loss of correction of LKA was observed at the last follow-up compared to the postoperative measurements, consistent with a previous study [Citation24, Citation40].
This study has several limitations. First, it is a retrospective case-control study with low evidence and small sample size. Randomized controlled trials with large sample sizes and high levels of evidence are needed to verify the safety and efficacy of this procedure. Second, a large amount of bone cement used in PFSUP may lead to adverse effects, such as monomer toxicity and allergic reaction. Since the procedure is performed under general anesthesia, it may not be possible to accurately determine these adverse reactions, emphasizing the need for further investigation. Third, long-term complications, such as bone cement fracture and displacement, could not be observed in this study due to the short follow-up duration. Fourth, anti-osteoporosis drugs may affect clinical outcomes and imaging findings. It should be borne in mind that in the present study, patients did not take the same anti-osteoporosis drugs, which may have impacted the reliability of our results to some extent. Fifth, the advantages of the technique PFSUP over PSF + VP are related to the minimally invasive approach, which introduces a clinical bias. Accordingly, further research is required to evaluate the merits of PFSUP compared to other minimally invasive procedures, such as PKP.
In conclusion, for treating SCOVFs with intersegmental instability and kyphosis, PFSUP and PSF + VP can restore spinal stability, improve kyphotic deformity, and relieve pain. Compared with PSF + VP, PFSUP can reduce blood loss and complications, promote early mobilization, and shorten hospitalization duration, but it is associated with a higher cement leakage rate and more radiation exposure.
Ethics approval
The study was approved by the ethical committee of Xuzhou Central Hospital. Xuzhou Clinical School of Xuzhou Medical University.
Authors’ contributions
YX and ZL equally participated in the data collection, statistical analysis, article writing. JF, YX participated in data collection. WD participated in design of the study, and revision of the manuscript. All authors read and approve the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Blattert TR, Schnake KJ, Gonschorek O, et al. Nonsurgical and surgical management of osteoporotic vertebral body fractures: recommendations of the spine section of the german society for orthopaedics and trauma (DGOU). Global Spine J. 2018;8(2 Suppl):1–10. doi:10.1177/2192568217745823.
- Ito Y, Hasegawa Y, Toda K, Nakahara S. Pathogenesis and diagnosis of delayed vertebral collapse resulting from osteoporotic spinal fracture. Spine J. 2002;2(2):101–106. doi:10.1016/s1529-9430(01)00165-6.
- Lim J, Choi SW, Youm JY, Kwon HJ, Kim SH, Koh HS. Posttraumatic delayed vertebral collapse: Kummell’s disease. J Korean Neurosurg Soc. 2018;61(1):1–9. doi:10.3340/jkns.2017.0505.010.
- Ma R, Chow R, Shen FH. Kummell’s disease: delayed post-traumatic osteonecrosis of the vertebral body. Eur Spine J. 2010;19(7):1065–1070. doi:10.1007/s00586-009-1205-4.
- Adamska O, Modzelewski K, Stolarczyk A, et al. Is Kummell’s disease a misdiagnosed and/or an underreported complication of osteoporotic vertebral compression fractures? A pattern of the condition and available treatment modalities. J Clin Med. 2021;10(12):2584. doi:10.3390/jcm10122584.
- Liu T, Gu GN, Zhan CG, et al. Comparison of percutaneous vertebroplasty and percutaneous vertebroplasty combined with pediculoplasty for Kümmell’s disease: a retrospective observational study. J Orthop Surg Res. 2023;18(1):471. doi:10.1186/s13018-023-03957-5.
- Lee SH, Kim ES, Eoh W. Cement augmented anterior reconstruction with short posterior instrumentation: a less invasive surgical option for Kummell’s disease with cord compression. J Clin Neurosci. 2011;18(4):509–514. doi:10.1016/j.jocn.2010.07.139.
- Sakai Y, Kaito T, Takenaka S, et al. Complications after spinal fixation surgery for osteoporotic vertebral collapse with neurological deficits: Japan Association of Spine Surgeons with ambition multicenter study. J Orthop Sci. 2019;24(6):985–990. doi:10.1016/j.jos.2019.08.015.
- Jeong YH, Lee CJ, Yeon JT, et al. Insufficient penetration of bone cement into the trabecular bone: a potential risk for delayed bone cement displacement after kyphoplasty? Reg Anesth Pain Med. 2016;41(5):616–618. doi:10.1097/AAP.0000000000000445.
- O’Brien JP, Sims JT, Evans AJ. Vertebroplasty in patients with severe vertebral compression fractures: a technical report. AJNR Am J Neuroradiol. 2000;21(8):1555–1558.
- Lafforgue P, Chagnaud C, Daumen-Legré V, et al. The intravertebral vacuum phenomenon ("vertebral osteonecrosis"). Migration of intradiscal gas in a fractured vertebral body? Spine (Phila Pa 1976). 1997;22(16):1885–1891. doi:10.1097/00007632-199708150-00015.
- Karasick D, Eason MA. Vertebral pneumatocyst mimicking susceptibility artifact on MR imaging. AJR Am J Roentgenol. 1998;170(1):221–221. doi:10.2214/ajr.170.1.9423641.
- McKiernan F, Jensen R, Faciszewski T. The dynamic mobility of vertebral compression fractures. J Bone Miner Res. 2003;18(1):24–29. doi:10.1359/jbmr.2003.18.1.24.
- Varga PP, Jakab G, Bors IB, et al. Experiences with PMMA cement as a stand-alone intervertebral spacer: percutaneous cement discoplasty in the case of vacuum phenomenon within lumbar intervertebral discs. Orthopade. 2015;44 Suppl 1(2):S1–S7. doi:10.1007/s00132-014-3060-1.
- Sola C, Camino Willhuber G, Kido G, et al. Percutaneous cement discoplasty for the treatment of advanced degenerative disk disease in elderly patients. Eur Spine J. 2021;30(8):2200–2208. doi:10.1007/s00586-018-5547-7.
- Camino-Willhuber G, Norotte G, Bronsard N, et al. Percutaneous cement discoplasty for degenerative low back pain with vacuum phenomenon: a multicentric study with a minimum of 2 years of follow-up. World Neurosurg. 2021;155(12):e210–e217. doi:10.1016/j.wneu.2021.08.042.
- Tian QH, Lu YY, Sun XQ, et al. Feasibility of percutaneous lumbar discectomy combined with percutaneous cementoplasty for symptomatic lumbar disc herniation with modic type I endplate changes. Pain Physician. 2017;20(4):E481–E488. PMID: 28535556.
- Sola C, Camino Willhuber G, Kido G, et al. Expert’s comment concerning grand rounds case entitled "percutaneous cement discoplasty for the treatment of advanced degenerative disk disease in elderly patients": (C. Sola, et al., Eur Spine J; 2018: doi:10.1007/s00586-018-5547-7). Eur Spine J. 2021;30(8):2209–2210. doi:10.1007/s00586-020-06568-4.
- Fabbriciani G, Pirro M, Floridi P, et al. Osteoanabolic therapy: a non-surgical option of treatment for Kümmell’s disease? Rheumatol Int. 2012;32(5):1371–1374. doi:10.1007/s00296-010-1408-3.
- Wiggins MC, Sehizadeh M, Pilgram TK, et al. Importance of intravertebral fracture clefts in vertebroplasty outcome. AJR Am J Roentgenol. 2007;188(3):634–640. doi:10.2214/AJR.06.0542.
- Wei H, Dong C, Zhu Y, et al. Analysis of two minimally invasive procedures for osteoporotic vertebral compression fractures with intravertebral cleft: a systematic review and meta-analysis. J Orthop Surg Res. 2020;15(1):401. doi:10.1186/s13018-020-01938-6.
- Morishita S, Yoshii T, Okawa A, et al. Comparison of perioperative complications between anterior fusion and posterior fusion for osteoporotic vertebral fractures in elderly patients: propensity score-matching analysis using nationwide inpatient database. Clin Spine Surg. 2020;33(10):E586–E592. doi:10.1097/BSD.0000000000000992.
- Katsumi K, Hirano T, Watanabe K, et al. Surgical treatment for osteoporotic thoracolumbar vertebral collapse using vertebroplasty with posterior spinal fusion: a prospective multicenter study. Int Orthop. 2016;40(11):2309–2315. doi:10.1007/s00264-016-3222-3.
- Ishikawa Y, Watanabe K, Katsumi K, et al. Short- versus long-segment posterior spinal fusion with vertebroplasty for osteoporotic vertebral collapse with neurological impairment in thoracolumbar spine: a multicenter study. BMC Musculoskelet Disord. 2020;21(1):513. doi:10.1186/s12891-020-03539-0.
- Li KC, Li AF, Hsieh CH, et al. Another option to treat Kümmell’s disease with cord compression. Eur Spine J. 2007;16(9):1479–1487. doi:10.1007/s00586-006-0094-z.
- Patil S, Rawall S, Singh D, et al. Surgical patterns in osteoporotic vertebral compression fractures. Eur Spine J. 2013;22(4):883–891. doi:10.1007/s00586-012-2508-4.
- Morishita K, Kasai Y, Uchida A. Clinical symptoms of patients with intervertebral vacuum phenomenon. Neurologist. 2008;14(1):37–39. doi:10.1097/NRL.0b013e3180dc9992.
- Nakajima H, Uchida K, Honjoh K, et al. Surgical treatment of low lumbar osteoporotic vertebral collapse: a single-institution experience. J Neurosurg Spine. 2016;24(1):39–47. doi:10.3171/2015.4.SPINE14847.
- Sudo H, Ito M, Kaneda K, et al. Anterior decompression and strut graft versus posterior decompression and pedicle screw fixation with vertebroplasty for osteoporotic thoracolumbar vertebral collapse with neurologic deficits. Spine J. 2013;13(12):1726–1732. doi:10.1016/j.spinee.2013.05.041.
- Sun G, Jin P, Li M, et al. Height restoration and wedge angle correction effects of percutaneous vertebroplasty: association with intraosseous clefts. Eur Radiol. 2011;21(12):2597–2603. doi:10.1007/s00330-011-2218-z.
- Li HK, Hao DJ, Yang JS, et al. Percutaneous kyphoplasty versus posterior spinal fixation with vertebroplasty for treatment of Kümmell disease: A case-control study with minimal 2-year follow-up. Medicine (Baltimore). 2017;96(51):e9287. doi:10.1097/MD.0000000000009287.
- Kanayama M, Ishida T, Hashimoto T, et al. Role of major spine surgery using Kaneda anterior instrumentation for osteoporotic vertebral collapse. J Spinal Disord Tech. 2010;23(1):53–56. doi:10.1097/BSD.0b013e318193e3a5.
- Ha KY, Lee JS, Kim KW, et al. Percutaneous vertebroplasty for vertebral compression fractures with and without intravertebral clefts. J Bone Joint Surg Br. 2006;88(5):629–633. doi:10.1302/0301-620X.88B5.17345.
- Martin DJ, Rad AE, Kallmes DF. Prevalence of extravertebral cement leakage after vertebroplasty: procedural documentation versus CT detection. Acta Radiol. 2012;53(5):569–572. doi:10.1258/ar.2012.120222.
- Tanigawa N, Kariya S, Komemushi A, et al. Cement leakage in percutaneous vertebroplasty for osteoporotic compression fractures with or without intravertebral clefts. AJR Am J Roentgenol. 2011;196(6):1415–1418. doi:10.2214/AJR.09.2774.
- Georgy BA. Feasibility, safety and cement leakage in vertebroplasty of osteoporotic and malignant compression fractures using ultra-viscous cement and hydraulic delivery system. Pain Physician. 2012;15(3):223–228. PMID: 22622906.
- Wei Y, Tian W, Zhang GL, et al. Thoracolumbar kyphosis is associated with compressive vertebral fracture in postmenopausal women. Osteoporos Int. 2017;28(6):1925–1929. doi:10.1007/s00198-017-3971-x.
- Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18(10):1897–1904.
- Glassman SD, Bridwell K, Dimar JR, et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976). 2005;30(18):2024–2029. doi:10.1097/01.brs.0000179086.30449.96.
- Suk SI, Kim JH, Lee SM, et al. Anterior-posterior surgery versus posterior closing wedge osteotomy in posttraumatic kyphosis with neurologic compromised osteoporotic fracture. Spine (Phila Pa 1976). 2003;28(18):2170–2175. doi:10.1097/01.BRS.0000090889.45158.5A.
