Abstract
Background
The prognostic significance of neural invasion (NI) in gastric cancer (GC) has not been established. This study is to investigate the characteristic and prognostic value of NI in GC.
Methods
592 patients who had undergone gastrectomy for GC were retrospectively analyzed. NI was defined when cancer cells infiltrated into the perineurium or neural fascicles by hematoxylin and eosin staining of surgical specimens. NI and the other clinical factors were analyzed.
Results
NI was detected in 270 of the 592 patients. NI was associated with tumor size, site, depth of invasion, lymph node metastasis, TNM stage, D dissection, tumor differentiation, Lauren classification, and blood vessel invasion. NI was associated with the overall survival. Multivariate analysis indicated that NI was not an independent prognostic factor for total patients, while NI independently predicted prognosis for age < 60 and lymph node metastasis negative patients by subgroup analysis. Concomitant existence of NI with tumor size ≥3cm, TNM stage III, or diffused Lauren classification independently predicted prognosis.
Conclusions
The frequency of NI is high in GC patients and increases with disease progression. NI is related to poor survival in GC patients who underwent curative gastrectomy and provides independent prognostic value for young and lymph node metastasis negative patients.
Introduction
Gastric cancer (GC) is the fifth most common cancer and the third most common cause of cancer death worldwideCitation1. The majority of gastric cancer patients are diagnosed at advanced stage and have poor prognoses despite surgery or other treatmentsCitation2,Citation3. The clinical-pathological characteristics such as histology, Tumor node metastasis (TNM) stage and lymphovascular infiltration have been identified as important factors to determine the prognosis of gastric cancerCitation4,Citation5. However, some biological behaviors of gastric cancer cannot be fully understood by the above factors. So, it is important to find new clinical-pathological characteristics that contribute to gastric cancer development.
Neural invasion (NI) is a pathologic process characterized by tumor invasion of nerve, and it is defined as the presence of cancer cells along the sides of nerves and/or inside the epineurial, perineurial and endoneurial spaces of the neuronal sheathCitation6. NI is a common pathologic feature in many malignances, including head and neck cancerCitation7, pancreatic cancerCitation8, colorectal cancerCitation9, prostate cancerCitation10 and GCCitation11. A great deal of evidence revealed NI as a high malignant process, which seriously worsens the prognosis in several types of cancer.
Although NI has been proven as an important prognostic factor in GC in previous studies, the prognostic value and clinical feature of NI in GC still remain debated. The aim of the study is to uncover the role of NI in gastric cancer.
Materials and methods
Patients
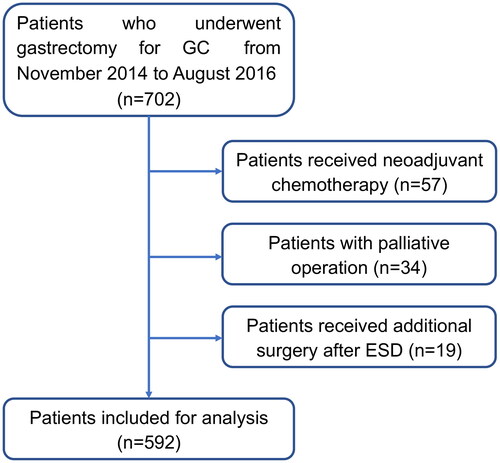
In this study, a total of 702 patients who underwent gastrectomy for gastric cancer from November 2014 to August 2016 in the First Affiliated Hospital of Nanjing Medical University were included. As shown in , patients who received palliative operation, neoadjuvant chemotherapy, or additional surgery after ESD was excluded. After exclusion, 592 patients were enrolled for analysis. All patients enrolled in the study had histologically confirmed adenocarcinoma of the stomach and underwent gastrectomy with proper lymph node dissection according to the Japanese Gastric Cancer Treatment Guideline (Version 4). In brief, for GC with cTIN0M0, D1-plus (D1+) lymphadenectomy was performed, while for GC with cN (+) or more than cT2 grade, D2 dissection should be performed. For total gastrectomy, D1+ lymphadenectomy were stations from 1 to 7, 8a, 9 and 11p, D2 dissection was consist of D1+ stations and stations 10, 11d, and 12a, splenectomy was not routinely performed. For distal gastrectomy, D1+ lymphadenectomy included stations 1, 3, 4 sb, 4d, 5, 6, 7, 8a and 9; D2 included D1-plus together with stations 11p, and 12a. In our institution, pStage I GC patients were not routinely recommended for adjuvant chemotherapy. For pStage II GC patients, adjuvant chemotherapy with S-1 is the standard treatment, whereas adjuvant chemotherapy with a doublet regimen (CapOX or SOX) was preferred for pStage III patients. This study was performed according to the ethics code of the World Medical Association (Declaration of Helsinki) and was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
Histopathological evaluation
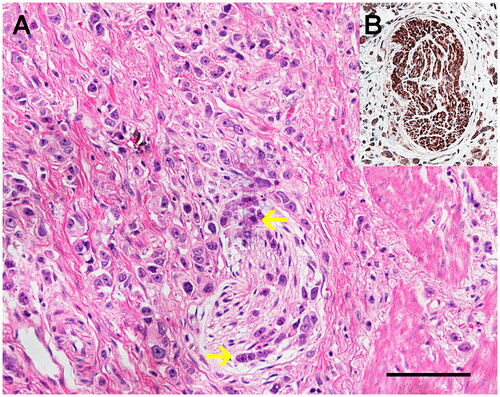
Surgical specimens were fixed in 10% formalin after being resected and embedded in paraffin. Paraffin-embedded blocks were then cut into 5 μm-thick sections for hematoxylin and eosin staining and specific neuronal marker (PGP9.5) staining. PGP9.5 staining was performed to label nerves within GC and NI was assessed as positive if the cancer cells presented along the sides of nerves and/or inside the epineurial, perineurial and endoneurial spaces of the neuronal sheath (). TNM stages were classified according to the 7th edition of the American Joint Committee on Cancer Staging Manual. Besides, the histologic type of gastric carcinoma was grouped according to the histological classification for gastric carcinoma by the World Health Organization (WHO).
Figure 2. (A) The representative microphotographs of neural invasion (NI) in gastric cancer assessed by HE staining (magnification: ×200, scale bar: 10 μm). NI was assessed as positive if the cancer cells presented along the sides of nerves and/or inside the epineurial, perineurial and endoneurial spaces of the neuronal sheath. (B) The nerve fiber was confirmed with the immunohistochemistry of a specific neuronal marker (PGP9.5).
Statistical analysis
Clinical information including gender, age, tumor size, tumor site, invasion depth, lymph node metastasis, TNM stage, tumor differentiation, Lauren classification, blood vessel invasion, NI, the Eastern Cooperative Oncology Group Performance Status (ECOG PS), BMI, and survival data were collected. The association between NI positivity and clinical characterizations was analyzed by the chi-squared test and Fisher’s exact test. The Kaplan-Meier method was employed to calculate the survival distribution and curves, and the log-rank test was used to evaluate the differences of survival curves. Overall survival (OS) was defined as the interval from diagnosis to the death of the patients or loss in the follow-up. Univariate analyses and multivariate analyses with the Cox regression method were performed to evaluate the prognostic significance of NI and the other clinicopathological factors. Multivariate p values were used to describe the independence of these factors. The 95% confidence (95%) was used to quantify the relationship between survival time and each independent factor. All p values were two-sided in tests, and p values less than 0.05 were considered to be statistically significant. Analysis was performed by the statistical package SPSS (SPSS, Chicago, IL, USA).
Results
A total of 592 patients who had undergone radical gastrectomy for gastric cancer were retrospectively analyzed; 438 patients were male and 154 were female. The median age was 62 years, ranging from 27 to 90 years. A total of 305 (51.52%) patients underwent total gastrectomy, 279 (47.13%) underwent distal gastrectomy, 8 (1.35%) underwent proximal gastrectomy. 25 (4.22%) patients received a combined procedure, and more in detail, 10 (1.69%) patients received a combined splenectomy, 6 (1.01%) underwent a combined cholecystectomy, 4 (0.68%) patients received a combined distal pancreatectomy with splenectomy, 2 (0.34%) underwent a combined appendicectomy, 1 (0.17%) received a resection of hepatic cysts, 1 (0.17%) underwent a partial transverse colectomy, and 1 (0.17%) underwent a left nephrectomy.
NI was detected as positive in 270 of the 592 patients (45.61%). The associations between NI and other clinicopathological factors are shown in . NI positivity was closely associated with tumor size, tumor site, depth of invasion (pT grade), lymph node metastasis (pN stage), TNM stage, tumor differentiation, Lauren classification, and blood vessel invasion. On the other side, NI positivity demonstrated no correlation with patients’ gender, age, ECOG scores, or BMI. In brief, the NI-positive patients showed a further tumor progression than the NI-negative cases, such as larger tumor size, more advanced in TNM stage, and more frequent lymph node metastasis and blood vessel invasion. Besides, NI was more likely to occurred in patients with poorly differentiated or diffuse Lauren classification. In addition, the higher the tumor location in the stomach, the more frequent NI detection in GC patients.
Table 1. Association of neural invasion (NI) status and other clinical-pathological characteristics.
In the early gastric cancer (pT1) subgroup, 6/148 (4.05%) patients showed positive NI. Interestingly, all 6 of them were in grade T1b and there were no NI positive patients in T1a patients.
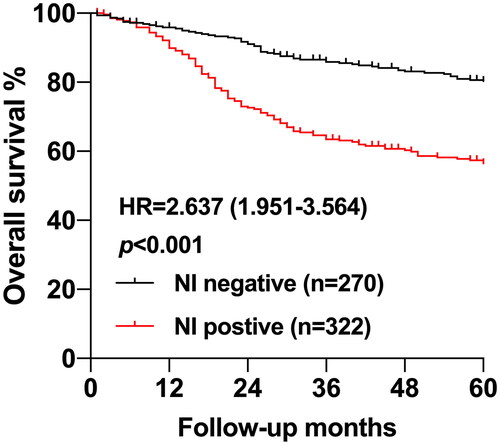
The median time of follow-up was 65 months, and the 5-year follow-up rate was 90.37%. 5-year OS of the total patients was 69.2%. 5-year OS of patients who had no NI was 80.0%, whereas that of patients with NI was 56.5%, demonstrated a significant difference in two groups (p < 0.001) (). The positivity of NI was closely associated with OS of patients who underwent radical gastrectomy in the univariate analysis (HR = 2.600, 95% CI:1.904-3.550, p < 0.001). Except for NI, age (HR = 1.973, 95% CI:1.398-2.783, p < 0.001), tumor size (HR = 3.192, 95% CI:2.175-4.685, p < 0.001), invasion depth (pT grade) (HR = 5.479, 95% CI:3.048-9.850, p < 0.001), lymph node metastasis (pN stage) (HR = 4.008, 95% CI:2.743-5.857, p < 0.001), TNM stage (HR = 4.099, 95% CI:2.919-5.758, p < 0.001), D dissection (HR = 6.994, 95% CI:4.823-10.14, p < 0.001), tumor differentiation (HR = 2.854, 95% CI:1.791-4.548, p < 0.001), Lauren classification (HR = 1.466, 95% CI:1.079-1.996, p = 0.015), blood vessel invasion (HR = 2.609, 95% CI:1.936-3.517, p < 0.001) and ECOG PS (HR = 1.663, 95% CI: 1.227-2.255, p < 0.001) also showed significant correlation with patients’ OS, while gender, tumor site, and BMI did not ().
Table 2. Univariate and Multivariate Cox regression analysis of overall survival in 592 GC patients, *p < 0.05, ** p < 0.01, ***p < 0.001.
Furthermore, we performed a multivariate analysis with the Cox regression method in order to further evaluate the prognostic significance of NI and other clinicopathological factors. However, multivariate analysis indicated that the positivity of NI was not an independent prognostic factor, while age (HR = 1.842, 95% CI: 1.303-2.604, p < 0.001), tumor size (HR = 1.612, 95% CI: 1.068-2.434, p = 0.023), invasion depth (HR = 2.330, 95% CI: 1.225-4.430, p = 0.009), lymph node metastasis (HR = 2.415, 95% CI: 1.587-3.675, p < 0.001), blood vessel invasion (HR = 1.430, 95% CI: 1.038-1.970, p = 0.029) were independent prognostic factors in GC patients ().
To further investigate the prognostic value of NI, we performed the stratification analysis according to different clinical-pathological variable. We divided these patients into different subgroups based on the gender (male group and female group), age (≥60 years group and <60 years group), tumor size (≥3cm group and <3cm group), tumor site (proximal group and non-proximal group), invasion depth (early (pT1) group and advanced (pT2-4) group), lymph node metastasis (positive group and negative group), TNM stage (stage III group and stage I/II group), tumor differentiation (poor-signet group and well-moderate group), Lauren classification (diffuse group, mixed group, and intestinal group), blood vessel invasion (positive group and negative group), and BMI (≥23 group and <23 group). Then, overall survivals were analyzed in these subgroups. The results were shown in and Figure S1. No significant differences of overall survival between NI positive and negative patients were overserved in tumor size < 3cm subgroup, early stage (pT1) subgroup, TNM stage I/II subgroup, and TNM stage III subgroup, while patients with NI showed a worse prognosis in the rest subgroup.
Table 3. Univariate survival analysis according to NI status, stratification for different clinical-pathological variable, *p < 0.05, **p < 0.01, ***p < 0.001.
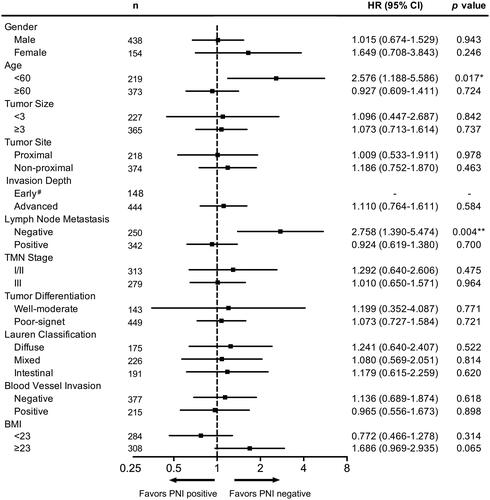
We also performed multivariate analysis with Cox regression method in these subgroups, it showed that NI was an independent prognostic factor in age < 60 subgroup (HR = 2.576, 95% CI: 1.188-5.586, p = 0.017) and lymph node metastasis negative subgroup (HR = 2.758, 95% CI: 1.390-5.474, p = 0.004) (). These data indicated NI as an important prognostic factor in these patients, which might require more aggressive treatment.
Figure 4. Multivariate Cox regression survival analysis according to NI status, stratification for different clinical-pathological variable, * p < 0.05, ** p < 0.01, *** p < 0.001. # the proportion of NI positive patients in early stage subgroup was too small for analysis.
Mounting evidence has demonstrated that the association of clinical factors might provide more information than either factor alone. It was reported that the concomitant existence of NI and lymphovascular invasion might serve as an independent prognostic factor for DFS and OS in GC patients. Therefore, we performed multivariate analysis with Cox regression according to the combination of NI status with other clinical-pathological variables. The results showed that the concomitant existence of tumor size ≥3cm with NI(HR = 1.517, 95% CI:1.083-2.127, p = 0.015), TNM stage III with NI (HR = 1.528, 95% CI:1.090-2.143, p = 0.014), and diffuse Lauren classification with NI (HR = 1.614, 95% CI:1.156-2.253, p = 0.005) independently predicted prognosis in GC patients ().
Table 4. Multivariate Cox regression survival analysis according to the combination of NI status with other clinical-pathological variables, *p < 0.05, **p < 0.01, ***p < 0.001. NI: Neural invasion, LNM: Lymph node metastasis, BVI: Blood vessel invasion. #The sample size of early GC (pT1) & NI + subgroup was not enough for analysis.
Discussion
As an important pathological feature, NI is frequently found in several cancers, ranging from 6.8% to 75.6% in oncologic patients. The incidence of NI is approximately 20% in colorectal cancerCitation12 and is reported much higher in biliary tract carcinoma (85% to 88%)Citation13,Citation14 and pancreatic cancer (50% to 80%)Citation8,Citation15. As for gastric cancer, the median rate of NI positivity was 40.9% (6.8–75.6%) according to a meta-analysis from 24 studies containing 30, 590 GC patients who underwent curative gastrectomyCitation16. In our study, NI was detected as positive in 270 of the 592 patients, which accounts for 45.61% in GC.
NI was reported to be associated with aggressive behavior, tumor recurrence, neoplastic pain, and poor survival in multiple tumors. In the study, we found NI positivity was associated with tumor size, tumor stage, lymph node metastasis, and blood vessel invasion, which were important features of advanced gastric cancer. Furthermore, we also detected the elevated incidences of NI in the poor-signet differentiated and diffused type GC which had more aggressive behavior.
The incidence of NI positivity in patients at pathological T1 and T2 stage is 12.03%, while increases to 68.66% with T3 and T4 lesions. Notably, 6/148 (4.05%) of the patients with T1 lesions presented with NI. T1 stage in GC can be further divided into T1a (mucosal GC) and T1b (submucosal GC). We found that all of these 6 patients were in T1b stage and no NI was detected in T1a stage patients. This phenomenon might be related to the different nerve distribution in gastric layer and need further investigation. More importantly, GC with T1 lesion is usually treated by Endoscopic mucosal resection (EMR) and Endoscopic submucosal dissection (ESD). Our data that identified the existence of NI in GC with T1 lesion indicated a potential non-curative risk of EMR or ESD, as NI represents a crucial route for local spread and potential recurrence.
Previous studies had demonstrated the features in epidemiological, clinical, and molecular aspects between Lauren diffuse histotype and intestinal histotype. In the study, elevated incidences of NI were detected in the diffused type gastric cancer patients, which is usually presented in younger patients with a lower male-female ratio.
Nagakawa et al. reported that the high ratio of NI in and biliary tract and pancreatic cancers might be associated with the high autonomic innervation in these organsCitation13, which might also explain the high NI incidence in GC. Interestingly, we also found that NI positivity correlated with the tumor site of GC. The NI incidence of GC located in upper 1/3 (proximal cancer) was higher than the other parts, which was similar to the previous reportsCitation17. This is possibly caused by the higher nerve density around the cardia, which support the notion of the correlation between organ innervation an NI. However, the underlying mechanism needs to be investigated in further studies.
Based on previous studies and our data, NI was highly related to the lymph node metastasis. NI positivity was detected only in 24.00% of patients without lymph node metastasis vs 61.40% of lymph node metastasis positive cases. Nevertheless, the perineural space is more likely to be considered as an independent route for tumor spread as it differs from lymphatic vessels in anatomy and ultrastructureCitation18. Therefore, these 24.00% patients with N0 stage and positive NI further indicated NI as an independent pathological feature rather than the subsequent consequence of lymph node metastasis.
NI has been identified as a critical prognostic factor in several cancer including pancreatic cancerCitation19, colorectal cancerCitation20,Citation21 and cholangiocarcinomaCitation22. Apart from these cancers, NI is also related to the poor prognosis and a high risk of recurrence in GC. In this study, by univariate survival analysis, we found that the 5-year overall survival in NI negative patients was 80.00%, while it reduced to 56.5% in NI positive cases. Therefore, considering the significant prognostic value of NI in GC, increasing researchers has recommend to incorporate NI status into GC TNM staging system for amelioration patients’ stratificationCitation23.
However, it remains a debate whether NI could serve as an independent factor for GC prognosis. Tanaka et al. reported that NI indicated a poor prognosis in gastric cancer patientsCitation24. Tianhang et al. and Bilici et al. found that NI was an independent prognostic factor for overall survival in patients with gastric cancer who underwent gastrectomyCitation11,Citation25. Franco et al. detected that NI did not result as an independent prognostic factor in gastric cancer, but NI emerged as an independent prognostic factor in the subgroup of patients with intestinal histotypeCitation17. Lee et al. showed that NI incidence was higher in the Signet Ring Cell Gastric Carcinoma (SRC) patients than in the non-SRC patients and SRC was an independent prognostic factor for the patients who were positive for NICitation26.
In our study, at multivariate analysis, NI positivity was not an independent prognostic factor in total GC patients, which may limit its application. In spite of this, Lorenzo et al. demonstrated the independent prognostic value of NI in GC patients with intestinal typeCitation17. Therefore, stratification analysis was performed according to different clinical-pathological variable. NI emerged as an independent prognostic factor in young (age < 60) and lymph node metastasis negative (pN0) patients. These results indicated that more aggressive treatment may be needed for these patients with NI. In addition, it was also reported that perineural invasion was independently associated with the early recurrence of GC patients after curative resectionCitation27. More importantly, based on the National Comprehensive Cancer Network (NCCN) Guidelines, options for pT2N0M0 GC patients after D2 lymph node dissection include surveillance or adjuvant chemotherapy. Patients with poorly differentiated or high-grade cancer, lymphovascular invasion, neural invasion or aged <50 years are candidates for adjuvant chemotherapy. Therefore, our results might contribute for recognizing the high-risk GC patients with age < 60 and negative lymph node metastasis who need more aggressive treatment.
It is widely accepted that the combined application of clinical factors might provide more information than either factor alone. Such markers have been applied for the diagnostic and prognosis in GC patients, including neutrophil-to-lymphocyte ratio (NLR)Citation28,Citation29, platelet-to-lymphocyte ratio (PLR)Citation30, and C-reactive protein/albumin ratio (CAR)Citation31. Moreover, it was reported that the concomitant existence of NI and lymphovascular invasion might serve as an independent prognostic factor for DFS and OS in GC patients. Therefore, we performed multivariate analysis with Cox regression according to the combination of NI status with other clinical-pathological variables. The results showed that the concomitant existence of tumor size ≥3cm with NI, TNM stage III with NI, and diffuse Lauren classification with NI independently predicted prognosis in GC patients. These combined markers could be used to predict the prognosis of GC patients who underwent curative gastrectomy more accurately and needs the further validation.
This study has some limitations. First, although NI has a significant prognostic value in GC, this feature can only be evaluated postoperatively. Therefore, this study was a retrospective study conducted in a single center which weakened the reliability of the data because of the nature of its collection. Second, the sample size was not large enough to distinguish the differences between different groups in subgroup analysis. Third, this study only included GC patients who underwent curative gastrectomy and did not involve IV stage GC, limiting our knowledge of the full spectrum of GC.
In summary, the retrospective analysis of a large series of GC patients who underwent curative gastrectomy confirmed the strong association between NI status and tumor size, tumor site, depth of invasion (T grade), lymph node metastasis, TNM stage, tumor differentiation, Lauren classification, and blood vessel invasion. Survival analysis revealed that NI predicted poor survival in GC patients, while it was not an independent prognostic factor according to Cox regression. Stratification analysis identified NI status as an independent prognostic factor in age < 60 subgroup and lymph node metastasis negative subgroup. Besides, the concomitant existence of tumor size ≥3cm with NI, TNM stage III with NI, and diffuse Lauren classification with NI independently predicted prognosis in GC patients. These new finding provided important information for detecting patients at high risk for poor prognosis after curative resection and for planning follow-up and treatment after surgery in GC patients.
Ethics statement
All procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients.
Author contributions
LW, YX, TJ, and YL were involved in data interpretation and statistical analysis. YS, JL, FL, WW, and DZ were involved in the design of the study. The post-polishing and post-typesetting work are also done by YS and JL. HX, LY, and ZX were involved in the preparation of the manuscript. All authors reviewed and approved the final manuscript.
Supplemental Material
Download TIFF Image (2.1 MB)Acknowledgements
We would like to thank the patients who gave their consent to having their data presented in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):1–8. doi:10.3322/caac.21660.
- Marrelli D, Pedrazzani C, Morgagni P, , et al. Changing clinical and pathological features of gastric cancer over time. Br J Surg. 2011;98(9):1273–1283. doi:10.1002/bjs.7528.
- Luebeck EG, Curtius K, Jeon J, Hazelton WD. Impact of tumor progression on cancer incidence curves. Cancer Res. 2013;73(3):1086–1096. doi:10.1158/0008-5472.Can-12-2198.
- Verlato G, Marrelli D, Accordini S, et al. Short-term and long-term risk factors in gastric cancer. World J Gastroenterol. 2015;21(21):6434–6443. doi:10.3748/wjg.v21.i21.6434.
- Marrelli D, Morgagni P, de Manzoni G, Marchet A, Baiocchi GL, et al. External validation of a score predictive of recurrence after radical surgery for non-cardia gastric cancer: Results of a follow-up study. J Am Coll Surg. 2015;221(2):280–290. doi:10.1016/j.jamcollsurg.2015.03.042.
- Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115(15):3379–3391. doi:10.1002/cncr.24396.
- Schmitd LB, Scanlon CS, D’Silva NJ. Perineural invasion in head and neck cancer. J Dent Res. 2018;97(7):742–750. doi:10.1177/0022034518756297.
- Demir IE, Ceyhan G O, Liebl F, D’Haese JG, Maak M, et al. Neural invasion in pancreatic cancer: the past, present and future. Cancers (Basel). 2010;2(3):1513–1527. doi:10.3390/cancers2031513.
- Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, , et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27(31):5131–5137. doi:10.1200/jco.2009.22.4949.
- Kang M, Oh JJ, Lee S, Hong SK, Lee SE, et al. Perineural invasion and lymphovascular invasion are associated with increased risk of biochemical recurrence in patients undergoing radical prostatectomy. Ann Surg Oncol. 2016;23(8):2699–2706. doi:10.1245/s10434-016-5153-z.
- Bilici A, Seker M, Ustaalioglu BB, Kefeli U, Yildirim E, et al. Prognostic significance of perineural invasion in patients with gastric cancer who underwent curative resection. Ann Surg Oncol. 2010;17(8):2037–2044. doi:10.1245/s10434-010-1027-y.
- Peng J, Sheng W, Huang D, Venook AP, Xu Y, et al. Perineural invasion in pT3N0 rectal cancer: the incidence and its prognostic effect. Cancer. 2011;117(7):1415–1421. doi:10.1002/cncr.25620.
- Nagakawa T, Mori K, Nakano T, Kadoya M, Kobayashi H, et al. Perineural invasion of carcinoma of the pancreas and biliary tract. Br J Surg. 1993;80(5):619–621. doi:10.1002/bjs.1800800526.
- Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. JAMA Surg. 2014;149(6):565–574. doi:10.1001/jamasurg.2013.5137.
- Lenz J, Karasek P, Jarkovsky J, Muckova K, Dite P, et al. Clinicopathological correlations of nestin expression in surgically resectable pancreatic cancer including an analysis of perineural invasion. J Gastrointestin Liver Dis. 2011;20(4):389–396.
- Deng J, You Q, Gao Y, Yu Q, Zhao P, et al. Prognostic value of perineural invasion in gastric cancer: a systematic review and meta-analysis. PLoS One. 2014;9(2):e88907. doi:10.1371/journal.pone.0088907.
- De Franco L, Marrelli D, Voglino C, Vindigni C, Ferrara F, et al. Prognostic value of perineural invasion in resected gastric cancer patients according to lauren histotype. Pathol Oncol Res. 2018;24(2):393–400. doi:10.1007/s12253-017-0257-8.
- Takahashi T, Ishikura H, Motohara T, Okushiba S, Dohke M, et al. Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol. 1997;65(3):164–170. doi:10.1002/(sici)1096-9098(199707)65:3 < 164::aid-jso4 > 3.0.co;2-4.
- Crippa S, Pergolini I, Javed AA, Honselmann KC, Weiss MJ, et al. Implications of perineural invasion on disease recurrence and survival after pancreatectomy for pancreatic head ductal adenocarcinoma. Ann Surg. 2022;276(2):378–385. doi:10.1097/sla.0000000000004464.
- Alotaibi AM, Lee JL, Kim J, Lim SB, Yu CS, et al. Prognostic and oncologic significance of perineural invasion in sporadic colorectal cancer. Ann Surg Oncol. 2017;24(6):1626–1634. doi:10.1245/s10434-016-5748-4.
- Al-Sukhni E, Attwood K, Gabriel EM, LeVea CM, Kanehira K, et al. Lymphovascular and perineural invasion are associated with poor prognostic features and outcomes in colorectal cancer: A retrospective cohort study. Int J Surg. 2017;37:42–49. doi:10.1016/j.ijsu.2016.08.528.
- Zhang Z, Zhou Y, Hu K, Wang D, Wang Z, et al. Perineural invasion as a prognostic factor for intrahepatic cholangiocarcinoma after curative resection and a potential indication for postoperative chemotherapy: a retrospective cohort study. BMC Cancer. 2020;20(1):270. doi:10.1186/s12885-020-06781-w.
- Aurello P, Berardi G, Tierno SM, Rampioni Vinciguerra GL, Socciarelli F, et al. Influence of perineural invasion in predicting overall survival and disease-free survival in patients With locally advanced gastric cancer. Am J Surg. 2017;213(4):748–753. doi:10.1016/j.amjsurg.2016.05.022.
- Tanaka A, Yoshikawa H, Okuno K, Koh K, Watatani M, et al. The importance of neural invasion (NI) as a prognostic factor in diffuse invasive gastric cancer. Surg Today. 1997;27(8):692–695. doi:10.1007/bf02384978.
- Tianhang L, Guoen F, Jianwei B, Liye M. The effect of perineural invasion on overall survival in patients with gastric carcinoma. J Gastrointest Surg. 2008;12(7):1263–1267. doi:10.1007/s11605-008-0529-4.
- Lee D, Son SY, Kim YB, Han SU, Hur H. Neural invasion is a significant contributor to peritoneal recurrence in signet ring cell gastric carcinoma. Ann Surg Oncol. 2018;25(5):1167–1175. doi:10.1245/s10434-018-6371-3.
- Chen L, Lin J, Chen LZ, Chen Y, Wang XJ, et al. Perineural invasion and postoperative complications are independent predictors of early recurrence and survival following curative resection of gastric cancer. Cancer Manag Res. 2020;12:7601–7610. doi:10.2147/cmar.S264582.
- Fan X, Wang D, Zhang W, Liu J, Liu C, et al. Inflammatory markers predict survival in patients with advanced gastric and colorectal cancers receiving anti-PD-1 therapy. Front Cell Dev Biol. 2021;9:638312. doi:10.3389/fcell.2021.638312.
- Shimozaki K, Nakayama I, Takahari D, Kamiimabeppu D, Osumi H, et al. A novel clinical prognostic index for patients with advanced gastric cancer: possible contribution to the continuum of care. ESMO Open. 2021;6(5):100234. doi:10.1016/j.esmoop.2021.100234.
- Zhang X, Li JH, Zhang Q, Li QQ, Zhang KP, et al. Relationship between prognostic nutritional index and mortality in overweight or obese patients with cancer: A multicenter observational study. J Inflamm Res. 2021;14:3921–3932. doi:10.2147/jir.S321724.
- Namikawa T, Shimizu S, Yokota K, Tanioka N, Munekage M, et al. Neutrophil-to-lymphocyte ratio and C-reactive protein-to-albumin ratio as prognostic factors for unresectable advanced or recurrent gastric cancer. Langenbecks Arch Surg. 2022;407(2):609–621. doi:10.1007/s00423-021-02356-w.