Abstract
Background
Central airway stenosis (CAS) in infants is characterized by dysphonia, dyspnea, cyanosis, repeated apnea, and infection. This case series study aimed to evaluate the safety and efficacy of holmium laser, cryoablation and budesonide inhalation in treating infants with severe CAS.
Methods
This retrospective study reviewed medical records data of 28 infants with severe CAS who underwent holmium laser treatment with cryoablation and/or balloon dilatation and budesonide inhalation therapy at Shanghai Children’s Medical Center between June 2014 and May 2020. Outcomes were defined as treatment success when the stenotic area was <25% for the normal age group with stable reopening diameter at one-year follow-up.
Results
Patients’ mean age was 12.8 ± 8.8 months and 17 (60%) were male. Sixteen cases had web-like stenosis and 12 had scar contracture stenosis. Among 16 patients with web-like stenosis, 8 (50%) underwent balloon dilation with cryotherapy and 8 (50%) underwent balloon dilation only; treatment success was achieved in 10 (62.5%) cases and after revised treatments in 5 (31.25%) cases. Among 12 patients with scar contracture stenosis, 6 (50%) underwent balloon dilation with cryotherapy, 4 (33.3%) underwent cryotherapy and 2 (16.7%) underwent balloon dilation only; treatment success was achieved in 3 (23.1%) cases and after 1-4 revised treatments in 8 (61.5%) cases. Symptoms of the 2 unsuccessful (7.1%) cases were relieved after tracheal stent insertion. Neither severe adverse events nor complications were observed during follow-up.
Conclusion
Holmium laser with cryoablation followed by budesonide inhalation therapy safely and effectively cleans stenotic tissues and maintains airway reopening. Balloon dilation after holmium laser is recommended for treating web-like stenosis.
Introduction
Airway stenosis is a narrowing of the airway that blocks the passage of air into the lungs. The central airway includes the trachea and the main-stem bronchi. Central airway stenosis (CAS) is characterized by reduced airflow and dyspnea due to the minimized diameter of the central airway [Citation1]. In infants, CAS may occur congenitally or as the result of chronic inflammation, infection or cancer. The Freitag grading system classifies the severity of CAS according to the degree of stenosis in the airway diameter (%), which is defined as the diameter of the stenosis site divided by the normal diameter x 100%: grade I, ≦25%; grade II, 26–50%, grade III, 51–75%; grade IV, 76–90% [Citation2]. Unlike adult CAS, pediatric CAS generally involves benign lesions. However, the progression is fast because of the immature airway in infants. The symptoms include dysphonia, obvious dyspnea, cyanosis, repeated apnea, and infection, which together can be life-threatening. This retrospective case series study aimed to evaluate the safety and efficacy of mini-invasive treatment consisting of holmium laser, cryoablation, and budesonide inhalation in 28 infants with severe CAS.
Methods
Patients
This retrospective case series study reviewed the medical records data of 28 infants with severe CAS who underwent holmium laser excision at Shanghai Children’s Medical Center between June 2014 and May 2020. Inclusion criteria were: 1) infants or toddlers aged about 1 to 3 years with a history of long-term wheezing, apnea, and repeated lung infection; 2) CAS confirmed with chest CT 3-dimentional reconstruction images and bronchoscopy. No exclusion criteria were applied. All CAS cases had mucosal wall stenosis, including 16 cases with web-like stenosis and 12 with scar-contracture stenosis, categorized by the cause of stenosis. The severity of stenosis was evaluated using the Freitag grading system [Citation2]. Web-like stenosis is caused by a membrane that occludes the airway, with a small hole in the center supplying partial airflow. It is often seen in the tracheal web or trauma web caused by injury from tracheal intubation.2 Scar contracture stenosis is defined as fibromuscular stenosis, or granulation and fibrous tissue proliferation caused by inflammation or trauma.
Ethics statement
The study protocol was approved by the institutional review board of Shanghai Children’s Hospital. Because all retrospective data were deidentified, informed consent of parents or guardians was waived.
Surgical instruments
Flexible scopes were introduced per oral with a laryngeal mask airway (LMA), although in two cases were introduced via tracheal intubation. Depending on each patient’s age and body weight, different Olympus fiber bronchoscopes were used (Olympus Corp., Tokyo, Japan), including the P-260 fiber bronchoscope (OD 4.8 mm, operating channel 2.2 mm), XP-260 fiber bronchoscope (OD 2.8 mm, operating channel 1.2 mm), P290 electronic bronchoscope (OD 4.0 mm, operating channel 2.0 mm), or XP290 electronic bronchoscope (OD 2.8 mm, operating channel 1.2 mm), to detect the location of the CAS, estimate the diameter and length of the stenotic site, and evaluate the condition of the distal airway. Holmium laser equipment (DHL-1-A, Dahua, Wuxi, China), balloon dilatation catheter and guidewire (Bard Peripheral Vascular Inc., Tempe, AZ, USA), CO2 cryotherapy device (Erbe GmbH, Tuebingen, Germany), and rigid bronchoscope (Carl Stoz SE & Co. KG, Tuttlingen, Germany) were used for delivering the treatment.
Surgical procedures
After the patient fasted for 6 h, midazolam (0.1 mg/kg), fentanyl (1 μg/kg), and propofol (2 mg/kg) were intravenously injected to induce anesthesia, while lidocaine was sprayed for local anesthesia. Four cases with Grade IV subglottic stenosis underwent tracheotomy before bronchoscopy, and oxygen was supplied through a tracheal cannula during surgery. In two cases with grade IV tracheal granulation stenosis, two with left main bronchial opening, and two with inner trachea grade III inflammatory stenosis, rigid bronchoscopes were placed in the main airway for intraoperative ventilation.
Holmium laser ablation
The single-pulse energy of holmium laser was dependent on the properties of stenosis, initiating from 0.8 J for web-like stenosis and 1-1.2 J for scar contracture stenosis. The pulse frequency was 10 Hz. The 0.55 mm silicon fiber probe of holmium laser was delivered through the working channel of the bronchoscope and extended out of the channel by 0.5-1 cm to achieve thermal ablation of the stenosis site. For web-like stenosis, ablation was performed in the center of the membrane and then 3-4 sites were selected at 12, 3, 6, and 9 o’clock for further ablation (). For scar contracture stenosis, ablation was performed on the proliferated granulation tissues and adhered scar tissues.
Balloon dilatation
After holmium laser ablation, the balloon catheter reached the stenosis site through the glottis. The balloon sizes used ranged from 6mmx30mm to 10mmx30mm depending on stenosis severity. The applied pressure was 7-9 ATM. Under bronchoscopic monitoring, the balloon was fully dilatated and stayed at the stenosis site for 45-60 s. The same procedure was repeated 3-4 times. The morphology of the stenosis site was evaluated with the bronchoscope again after dilatation.
CO2 cryotherapy
CO2 cryotherapy was applied around the airway walls. Left granulation tissues and inflammatory proliferated tissues on the bronchial wall were cleaned using CO2.
Bleeding at the stenosis site was treated with diluted norepinephrine (1:10000) sprayed through the bronchoscope. After patients returned to bed, budesonide 1 mg inhalation therapy was administered 3 times every 4 h except in infants with tracheotomy. Home-use budesonide 0.25 mg inhalation therapy was administered every 12 h until the next bronchoscope examination.
Follow-up bronchoscope examination was performed twice per month during the first three months after discharge. If stenosis re-occurred, mini-invasive surgery was performed under bronchoscope; otherwise, patients were examined once every three months postoperatively.
Outcomes evaluation
Successful outcomes were defined as follows: 1) Cure: success in initial airway reopening. No further CAS symptoms. Image and fiber bronchoscope examinations indicate a significant improvement in the severity of stenosis. The area of airway stenosis is smaller than 25% normal range based on patient’s age. The reopening diameter is stable. No further treatment for stenosis is required. No stenosis is observed at one-year follow-up. 2) Effective: success in initial airway reopening. Attenuated CAS symptoms but with unstable reopening diameter. After 1-4 reopening treatments, the area of airway stenosis remains smaller than 25% normal range based on patient’s age at one year after treatment and with stable reopening diameter.
Unsuccessful treatment was defined as occurrence of one of the following conditions: 1) No obvious relief of CAS symptoms; 2) image and bronchoscope examinations indicate no improvement in the severity of stenosis; 3) unstable reopening diameter with treatment interval shorter than 3 months and more than 4 reopening treatments; 4) final airway obstruction.
Results
The baseline demographic and clinical characteristics of included patients are listed in . Patients’ mean age was 12.8 ± 8.8 months and 60.7% were male. The 28 patients included 15 with subglottic stenosis and 13 with tracheal stenosis as categorized by stenosis site. Regarding severity, 19 (67.9%) were grade III and 9 (32.1%) were grade IV. Regarding the cause and type of stenosis, 16 had web-like stenosis (median age: 7 months) and 12 had scar contracture stenosis (median age: 15 months). Among the 16 cases with web-like stenosis, 13 had trauma web caused by airway injury after intubation, and 3 had congenital subglottic web. Among the 12 cases with scar contracture stenosis, 9 had scar proliferation after airway surgery, tracheostomy, or intubation; and 3 had scar contracture stenosis caused by inflammatory-proliferation after infection.
Table 1. Patients’ demographic and clinical characteristics.
All 16 cases with web-like stenosis underwent holmium laser followed by balloon dilatation, and 8 of them further received CO2 cryotherapy. Ten cases were cured and 5 were considered effective. No re-stenosis occurred and dyspnea disappeared during one-year follow-up (). All 12 cases with scar contracture stenosis underwent holmium laser, while 2 of these patients further received balloon dilation, 4 further received CO2 cryotherapy, and 6 further received balloon dilatation and CO2 cryotherapy. Three cases were cured, and 8 were considered effective. No re-stenosis occurred and dyspnea was improved during the one-year follow-up (). Two unsuccessful cases who received 4 treatments still had repeated re-stenosis at the initial stenosis site, and the symptoms were relieved after tracheal stent insertion.
Figure 2. The bronchoscope image of a 27-month-old boy with severe subglottic web-like stenosis. (A) Grade IV subglottic web-like stenosis. (B) Holmium laser ablation. (C) Balloon dilatation after airway expansion. (D) The morphology of subglottic airway after holmium laser ablation and CO2 cryotherapy.
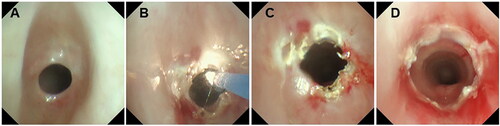
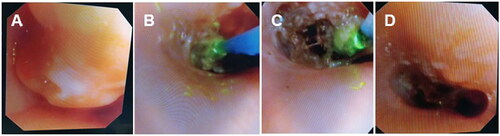
Figure 3. The bronchoscope image of a 15-month-old girl with severe pneumonia. Granulation and keloid proliferation at the site of tracheostomy. Severe subglottic scar contracture stenosis. (A) New granulation at the subglottic site of tracheostomy. (B-C) After expansion of airway airflow area, CO2 cryotherapy was applied to freeze the left granulation and keloid tissues for debridement. (D) Subglottic airway image after holmium laser ablation and CO2 cryotherapy.
Neither severe complications during treatment nor adverse effects were reported in any patient after treatment with holmium laser therapy. Only minor complications occurred. During holmium laser combined with balloon dilatation, all cases had little errhysis around the mucosa of the stenotic area. Three patients had more errhysis at the stenotic area after balloon dilatation, so 1:10000 norepinephrine was sprayed through the bronchoscope for hemostasis. In the present study, 20 cases with grade III tracheal CAS, 2 cases with grade IV left main bronchial CAS, and 2 cases with grade IV upper tracheal CAS had temporary oxygen desaturation and CO2 retention several times after general anesthesia and mini-invasive treatments (). Oxygen desaturation was relieved after treatment was paused and hyperbaric oxygen therapy was administered. Four cases with grade IV subglottic CAS underwent tracheotomy below the stenosis site before treatment and oxygen was supplied through a tracheal cannula during treatment, therefore hypoxia did not occur.
Discussion
In the present study, retrospective evaluation of the data of 28 infants with severe CAS demonstrated that holmium laser combined with cryoablation and/or balloon dilatation and budesonide inhalation therapy quickly cleaned stenotic tissues in web-like stenosis without re-stenosis after treatment in a majority of cases. Successful treatment in infants with scar contracture stenosis effectively improved clinical symptoms and maintained reopening. No severe complications or adverse effects were reported in any patient during or after treatment with holmium laser therapy. Here we share our experience with these cases.
Early age accompanied by congenital heart diseases and severe, repeated lung infection affect the respiratory tolerance of infants. Cases with severe airway stenosis tend to have respiratory failure, therefore it is necessary to relieve hypoxia symptoms in a timely manner. Two aspects are applied to delivering treatments for severe CAS in infants: one is reopening the initial airway stenosis by extending the airflow area and improving symptoms of hypoxia and CO2 retention as soon as possible; the other is maintaining airway control as long as possible and preventing re-stenosis. Although airway stenosis can be corrected with surgical resection of the stenosis site, surgical treatment is limited by several factors, including complications such as granulation tissues and airway stenosis after surgery, significantly different success rates in different medical centers, and high expense [Citation3–5].
Thermal ablation (such as laser, electrocoagulation, and argon knife) is the most common approach to treating airway web-like and scar contracture stenoses. Airway stenosis is opened rapidly by thermal ablation. However, animal study found that injuries caused by the argon knife and electrocoagulation treatments are most evident, leading to a high rate of tracheal granulation stenosis [Citation6]. Some researchers are opposed to excessive clearance of granulation in benign airway stenosis by thermal ablation [Citation7, Citation8]. During resection of proliferative web-like and scar tissues, the thermal effect also damages cartilages and mucosa in the airway. The best solution for severe CAS is balloon dilatation [Citation9]. Before dilatation, laser treatment can be used for treating web-like, granulation tissues, and harder scar lesions, while CO2 cryotherapy used in the late stage freezes left granulation tissues and attenuates edema in the airway mucosa. Cryoablation decreases the blood supply and consequently inhibits growth of newborn tissues, accelerates death of local tissues, and prevents the occurrence of re-stenosis [Citation3, Citation10].
Holmium laser has the following advantages: 1) It is solid-pulse laser with a 2100 mm wavelength, very close to water’s absorption peak of 1950 mm. It is hydrophilic, touch-triggered, and has minimal heat injury to surrounding tissues. The heat-injury zone is 0.5-1.0 mm so that it delicately carves diseased tissues and prevents re-stenosis [Citation11, Citation12]. 2) The silica fiber of holmium laser has various sizes: 200, 365, 550, and 1000 μm. The fiber is so thin that it can pass bronchoscopes with diameter 2.8 mm and channel 1.2 mm, which is especially suitable for infants. 3) The manipulation is performed under a bloodless visual field because injury caused by thermal solidification to tissues surrounding the incised site is less, and hemostasis is effective [Citation13]. 4) Holmium laser has weaker treatment power in the airway, which is controlled at 8-12w, while the temperature of tissues is maintained at 38-40 °C. This stimulates the mucosa in the local airway, promoting metabolism in local tissues and enhancing growth of the mucosa in normal tissues [Citation14]. As such, Holmium laser demonstrates the advantages of minimal injury and precise manipulation. Combining it with other mini-invasive treatments relieves severe airway stenosis, increases the airflow area, and improves prognosis.
The key point after treating initial severe CAS is to provide maintenance treatment to prevent re-stenosis. Among the 28 cases, all were successfully re-expanded after initial treatment, but 15 cases had re-stenosis during follow-up so they received 1-4 re-expansion treatments. Two cases still had re-stenosis after receiving 4 reopening treatments, so they were able to accept tracheal stent insertion afterwards. Other studies have reported that the success rate of bronchoscopy on benign stenosis is up to 87%, and the rate of re-stenosis is 40–70% [Citation15, Citation16]. Re-stenosis can be prevented by the following methods: 1) Using holmium laser as the main approach for thermal ablation. The duration of ablation at each site is less than 5 sec. A large treatment area is not suitable for loosing scar and granulation. Left tissues occluding the airway can be cleaned with CO2 cryotherapy and forceps. 2) Full cryotherapy at the stenosis site is necessary after treatment [Citation17]. 3) Infection in the local mucosa aggravates the injury levels of tissues and inflammation in airway, leading to proliferation of granulation tissue [Citation18, Citation19]. Aseptic surgical procedure decreases the risk of infection at the stenosis site during and after surgery. 4) Fibroblasts resulting in granulation proliferation reach peak growth at the lesion site on the 7th day, so the interval for reevaluation must be shorter than two weeks. If the initial dyspnea recurs after getting better, then immediate reevaluation should be performed. 5) Glucocorticoids have strong anti-inflammatory effects. A study reported that endoscopic balloon dilatation combined with local glucocorticoid prevented airway re-stenosis [Citation20]. Yokoi et al [Citation21] also reported that budesonide inhalation inhibited benign scar formation in airway. The present study also gave inhaled budesonide 0.25 mg twice daily for local anti-inflammatory treatment.
Limitations
The present study has several limitations, including its retrospective design, which limits the extent to which results can be generalized to other population and also does not rule out selection bias. This is also a single-center study with a small sample size, which also limits generalization of the results. Further prospective study with a large cohort in multiple centers is needed for reaching a sound conclusion about treatments for severe CAS in infants.
Conclusions
For severe CAS in infants, holmium laser ablation combined with cryotherapy and/or balloon dilatation followed by budesonide inhalation therapy safely and effectively cleans stenotic tissues and maintains airway reopening during one-year postoperative follow-up only with minor complications.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1–6. PMID: 15187010. doi:10.1164/rccm.200210-1181SO.
- Freitag L, Ernst A, Unger M, Kovitz K, Marquette CH. A proposed classification system of central airway stenosis. Eur Respir J. 2007;30(1):7–12. PMID: 17392320. doi:10.1183/09031936.00132804.
- Antón-Pacheco JL, Cano I, Comas J, et al. Management of congenital tracheal stenosis in infancy. Eur J Cardiothorac Surg. 2006;29(6):991–996. PMID: 16675228. doi:10.1016/j.ejcts.2005.12.061.
- Chiu PP, Kim PC. Prognostic factors in the surgical treatment of congenital tracheal stenosis: a multicenter analysis of the literature. J Pediatr Surg. 2006;41(1):221–225. discussion 221-225. PMID: 16410137. doi:10.1016/j.jpedsurg.2005.10.043.
- Wright CD. Treatment of congenital tracheal stenosis. Semin Thorac Cardiovasc Surg. 2009;21(3):274–277. PMID: 19942127. doi:10.1053/j.semtcvs.2009.06.002.
- Zhang J, Wang T, Wang J, et al. Effect of three interventional bronchoscopic methods on tracheal stenosis and the formation of granulation tissues in dogs. Chin Med J (Engl). 2010;123(5):621–627. PMID: 20367992.
- Bagheri R, Majidi M, Khadivi E, Attar AS, Tabari A. Outcome of surgical treatment for proximal long segment post intubation tracheal stenosis. J Cardiothorac Surg. 2013;8(1):35. PMID: 23452927. doi:10.1186/1749-8090-8-35.
- Yao Z. Updates on diagnosis and treatment of tracheal stenosis in infants. Int J Pediatrics. 2017;6:152–157.
- Lee KH, Ko GY, Song HY, Shim TS, Kim WS. Benign tracheobronchial stenoses: long-term clinical experience with balloon dilation. J Vasc Interv Radiol. 2002;13(9):909–914. PMID: 12354825. doi:10.1016/s1051-0443(07)61774-6.
- Collins NC. Is ice right? Does cryotherapy improve outcome for acute soft tissue injury? Emerg Med J. 2008;25(2):65–68. PMID: 18212134. doi:10.1136/emj.2007.051664.
- Fisher JC. The power density of a surgical laser beam: its meaning and measurement. Lasers Surg Med. 1983;2(4):301–315. PMID: 6865637. doi:10.1002/lsm.1900020403.
- Tooher R, Sutherland P, Costello A, Gilling P, Rees G, Maddern G. A systematic review of holmium laser prostatectomy for benign prostatic hyperplasia. J Urol. 2004;171(5):1773–1781. PMID: 15076275. doi:10.1097/01.ju.0000113494.03668.6d.
- Squiers JJ, Teeter WA, Hoopman JE, et al. Holmium: YAG laser bronchoscopy ablation of benign and malignant airway obstructions: an 8-year experience. Lasers Med Sci. 2014;29(4):1437–1443. PMID: 24584844. doi:10.1007/s10103-014-1536-1.
- Hayashi K, Hecht P, Thabit G, 3rd, et al. The biologic response to laser thermal modification in an in vivo sheep model. Clin Orthop Relat Res. 2000;373(373):265–276. PMID: 10810487. doi:10.1097/00003086-200004000-00033.
- Rahman NA, Fruchter O, Shitrit D, Fox BD, Kramer MR. Flexible bronchoscopic management of benign tracheal stenosis: long term follow-up of 115 patients. J Cardiothorac Surg. 2010;5(1):2. PMID: 20078894. doi:10.1186/1749-8090-5-2.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope. 2009;119(2):272–283. PMID: 19160408. doi:10.1002/lary.20056.
- Dalkowski A, Fimmel S, Beutler C, Zouboulis CC. Cryotherapy modifies synthetic activity and differentiation of keloidal fibroblasts in vitro. Exp Dermatol. 2003;12(5):673–681. PMID: 14705809. doi:10.1034/j.1600-0625.2003.00015.x.
- Ragoowansi R, Cornes PG, Moss AL, Glees JP. Treatment of keloids by surgical excision and immediate postoperative single-fraction radiotherapy. Plast Reconstr Surg. 2003;111(6):1853–1859. PMID: 12711944. doi:10.1097/01.PRS.0000056869.31142.DE.
- Squire R, Brodsky L, Rossman J. The role of infection in the pathogenesis of acquired tracheal stenosis. Laryngoscope. 1990;100(7):765–770. PMID: 2362536. doi:10.1288/00005537-199007000-00013.
- Edmondson NE, Bent J. 3rd. Serial intralesional steroid injection combined with balloon dilation as an alternative to open repair of subglottic stenosis. Int J Pediatr Otorhinolaryngol. 2010;74(9):1078–1081. PMID: 20708131. doi:10.1016/j.ijporl.2010.05.027.
- Yokoi A, Nakao M, Bitoh Y, Arai H, Oshima Y, Nishijima E. Treatment of postoperative tracheal granulation tissue with inhaled budesonide in congenital tracheal stenosis. J Pediatr Surg. 2014;49(2):293–295; discussion 295. discussion 295. PMID: 24528970. doi:10.1016/j.jpedsurg.2013.11.041.