Abstract
Objective
The inhibition of the Hippo pathway through targeting the Yes-associated protein (YAP) presents a novel and promising approach for treating tumors. However, the efficacy of YAP inhibitors in the context of breast cancer (BC) remains incompletely understood. Here, we aimed to investigate the involvement of YAP in BC's metabolic reprogramming and reveal the potential underlying mechanisms. To this end, we assessed the function of verteporfin (VP), a YAP-TEAD complex inhibitor, on the glycolytic activity of BC cells.
Methods
We evaluated the expression of YAP by utilizing immunohistochemistry (IHC) in BC patients who have undergone 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) prior to biopsy/surgery. We employed RNA immunoprecipitation (RIP) and fluorescent in situ hybridization (FISH) assays to assess the interaction between YAP mRNA and human antigen R (HuR) in BC cells. The biological importance of YAP in the metabolism and malignancy of BC was evaluated in vitro. Finally, the effect of VP on glycolysis was determined by using 18F-FDG uptake, glucose consumption, and lactate production assays.
Results
Our studies revealed that high expression of YAP was positively correlated with the maximum uptake value (SUVmax) determined by 18F-FDG PET/CT imaging in BC samples. Inhibition of YAP activity suppressed glycolysis in BC. The mechanism underlying this phenomenon could be the binding of YAP to HuR, which promotes glycolysis in BC cells. Treatment with VP effectively suppressed glycolysis induced by YAP overexpression in BC cells.
Conclusion
VP exhibited anti-glycolytic effect on BC cells, indicating its therapeutic value as an FDA-approved drug.
Introduction
Breast cancer (BC) is the one of most common cancer and the leading cause of cancer-related deaths in numerous countries, especially among women [Citation1, Citation2]. To enhance the prognosis and therapeutic outcomes of BC patients, it is imperative to identify molecular targets and accelerate the development of targeted drugs.
The Hippo-YAP pathway, initially discovered in Drosophila, regulates organ size and tissue homeostasis [Citation3], and is essential for glucose metabolism [Citation4]. YAP, the primary downstream effector of this pathway, acts as a signaling integrator that governs cellular proliferation and differentiation [Citation5]. Dysregulation of this pathway has been implicated in the progression of various tumors, including in BC [Citation6]. In the nuclei, YAP interact with transcriptional enhancer activation domains (TEAD) to promote cellular proliferation and inhibit apoptosis [Citation7, Citation8]. Increased YAP expression and its nuclear localization have been documented in BC cells [Citation6]. Compared to normal breast tissue, the mRNA levels of YAP were higher in BC. For BC samples, YAP were higher in luminal tumor than in triple-negative breast cancer (TNBC) [Citation9]. However, the therapeutic potential of hippo pathway inhibitors has not been rigorously tested in BC.
Verteporfin (VP), a drug approved by the FDA for macular degeneration, acts as a suppressor of the YAP-TEAD complex by interrupting YAP-TEAD interactions [Citation10], thereby inhibiting YAP-induced tumor development [Citation11]. Verteporfin treatment of BC cells (MCF7 and MDA-MB-231) sensitized them to tamoxifen and doxorubicin and significantly reduced tumor cell proliferation and survival rates [Citation9]. Previous results have also shown that YAP1-TEAD1 complex also regulates the carcinogenic phenotype of BC cells by modulating glycolysis [Citation12]. Nevertheless, the potential anti-glycolysis efficacy of VP remains largely unexplored in BC.
Cancer cells exhibit the accelerated Warburg effect, in which they rely on aerobic glycolysis rather than mitochondrial oxidative phosphorylation which is crucial for ATP production in normal, non-tumorigenic cells. This phenomenon results in increased glucose consumption and lactate production even in the presence of sufficient oxygen [Citation13], which provides a favorable environment for tumor growth, invasion, and metastasis, while inhibiting the anti-cancer immune response [Citation14]. Aerobic glycolysis is also observed in the development and progression of BC. Positron emission tomography/computed tomography (PET/CT) using [18F]-fluorodeoxyglucose (18F-FDG) is a widely used noninvasive imaging technique to monitor in vivo glucose metabolism, and the maximum uptake value (SUVmax) of 18F-FDG has a positive correlation with tumor progression [Citation15]. Thus, by measuring glucose metabolism through 18F-FDG PET/CT imaging, doctors can diagnose and stage tumor patients, and optimize treatment options [Citation16].
Here, we demonstrate that YAP is highly expressed in BC cells and positively correlated with SUVmax determined by 18F-FDG PET/CT imaging in BC patients, confirming the critical role of YAP in promoting the glycolysis of BC cells. Mechanically, we show that binding of Human antigen R (HuR) to YAP mRNA promotes YAP-mediated glycolytic activation. We further demonstrate that treatment with VP leads to a reduction in 18F-FDG uptake, glucose consumption, and lactate production, thus inhibiting the Warburg effect in BC cells. Our findings suggest that VP is a promising therapeutic agent for BC with enhanced glycolysis.
Materials and methods
Clinical samples
A retrospective analysis was conducted using data from 18 patients who were diagnosed with BC and underwent biopsy or surgery at our hospital between January 2018 and December 2020. All eligible patients with clinicopathologic data available had not undergone chemotherapy or radiation prior to receiving 18F-FDG PET/CT imaging. The SUVmax of the lesion was determined by manually placing the region of interest (ROI). The SUVmax values of the 18 patients were ranked, with the first 9 values classified as the FDG low group and the last 9 values as the FDG high group. Before our study, we obtained informed consent from all participants, and this study was approved by the ethics committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Cell culture and transfection
The BC cell lines MCF-7 (ER+, PR+, HER2-), SK-BR-3 (ER-, PR-, HER2+), BT-549 (ER-, PR-, HER2-) and MDA-MB-231 (ER-, PR-, HER2-) were used in this study. The cells were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified essential medium (DMEM), supplemented with 1% penicillin-streptomycin (Invitrogen) and 10% fetal bovine serum (Gibco) at 37 °C and 5% CO2. Transfection of the cells was performed using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions. The overexpression plasmid of YAP was synthesized by Dingke Co., Ltd. (Shenzhen, China). The siRNA sequences used in this study are provided below:
Sense of HuR, AGGACGUAGAAGACAUGUUCUTT,
Antisense of HuR, AGAACAUGUCUUCUACGUCCUTT;
Sense of YAP, AGAUACUUCUUAAAUCACATT,
Antisense of YAP, UGUGAUUUAAGAAGUAUCUTT.
RNA extraction, quantitative real-time PCR (qRT-PCR)
The procedures for RNA extraction and qRT-PCR are described elsewhere [Citation17, Citation18]. Primer sequences are listed below:
YAP: Forward ACCGTTTCCCAGACTACCTTG,
Reverse TTCTATGTTCATTCCATCTCCTTCC;
HuR: Forward TGTTCTCTCGGTTTGGGCGGAT,
Reverse TCTTCTGCCTCCGACCGTTTGT;
CTGF: Forward CTGCGAGGAGTGGGTGTG,
Reverse GCTCTAATCATAGTTGGGTCTGG.
RNA immunoprecipitation (RIP)
The Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, Bedford, MA) was utilized according to the manufacturer’s protocol to conduct RIP. Briefly, approximately 1 × 107 BC cells were collected, washed with PBS, then lysed with RIP lysis buffer. Magnetic beads were washed twice with RIP wash buffer and then incubated with 5 μg of the antibody for 2 h at 4 °C. The lysate was incubated overnight with the bead/antibody complex at 4 °C on a rotator. The abundance of HuR was determined by qRT-PCR analysis. HuR antibody (Abcam) and IgG antibody (Abcam) were used as positive and negative controls, respectively.
Fluorescent in situ hybridization (FISH)
The cells were fixed using a 4% paraformaldehyde solution, permeabilized with 0.3% Triton X-100, and prehybridized prior to labeling probes. The probes were then applied and incubated at 37 °C overnight in the dark. A FISH kit from RiboBio was utilized following the manufacturer’s instructions, and images were acquired using a confocal microscope from Leika. The HuR and YAP probes were synthesized by Sangon Biotech. The sequences of the probe are listed below:
HuR, 5’CY5-CUCUGAGCUCGGGCGAGCAUACGACACCUUA-3’CY5;
YAP, 5’FITC-UCUCCGAGUCCCCGCGGACGAGUCACGAUCUGAU-3’FITC.
Cellular proliferation, migration, and Western blotting assays
For the EdU (5-ethynyl-2′-deoxyuridine) assay, cells were incubated in an EdU-labeling reagent for 2 h. The proliferation activity was measured using ImageJ (National Institutes of Health, Bethesda, MD, USA), and images were acquired at 10× using a Zeiss microscope. The migration assay and Western blot were performed following previously published protocols [Citation17, Citation18]. Antibody for HuR was obtained from Abcam, and antibodies for β-actin and YAP were obtained from Cell Signaling Technology, respectively.
Seahorse assay
The Seahorse XF Glycolysis Rate Assay Kit (Agilent) and Seahorse XFe96 Analyzer (Agilent) were utilized to determine the extracellular acidification rate (ECAR) and oxygen consumption rate (OCR). Briefly, cells were seeded in a complete medium overnight into an XFe96 Cell Culture Microplate. After washing, the cells were incubated for 1 h in a 37 °C CO2-free incubator with XF RPMI medium containing HEPES, 10 mM glucose, 2 mM glutamine, and 1 mM pyruvate, before detection. Subsequently, cells were detected by adding 50 mM 2-deoxy-d-glucose and 0.5 μM rotenone and antimycin A (Rot/AA). The Seahorse Glycolytic Rate Assay report generator was used to provide the basal and compensatory glycolysis rates, which take into account the contribution of CO2 to extracellular acid produced by mitochondrial respiration. ECAR and OCR were measured to determine glycolysis and mitochondrial respiration, respectively.
18F-fluorodeoxyglucose (FDG) uptake assay
The cells were pre-cultured in a glucose-free culture medium for 6 h and subsequently incubated with 1 μCi/mL concentration of 18F-FDG in a glucose-free culture medium for 2 h. The cells were then washed with phosphate-buffered saline (PBS) and digested with 1 M sodium hydroxide. The radioactivity of 18F-FDG was quantified using a γ counter.
Glucose consumption and lactate production
The experiment involved plating 1 × 104 cells per well in a 24-well plate and culturing them overnight. Following this, the cells were washed with PBS twice and glucose consumption was determined using a glucose assay (Sigma). Additionally, lactate production was measured using a lactate assay kit (Njjcbio, China). To perform these measurements, the cell supernatant was collected and analyzed, and the absorbance of the cell medium was determined.
Immunohistochemical (IHC) assay
IHC staining of BC tissue was performed using previously described methods [Citation18]. The staining intensity of YAP was scored on a scale of 0–3, with 0 representing negative staining, 1 indicating weakly positive staining, 2 denoting moderately positive staining, and 3 representing strongly positive staining. A staining intensity score of less than 2 was defined as low expression, while a score of 2 or higher was considered to indicate high expression of YAP in the BC tissue.
Statistical analysis
Data were presented as mean ± standard deviation (SD). Differences between groups were assessed by t-test. Experiments were conducted in triplicate. A P-value was statistically significant at <0.05 (denoted by *p < 0.05; **p < 0.01, ***p < 0.001).
Results
The overexpression of YAP and glycolysis are associated in BC as measured by 18F-FDG PET/CT
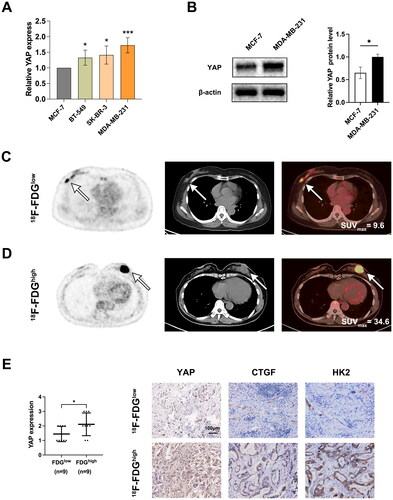
Initially, we measured the expression levels of YAP in BC cell lines. The findings revealed that MDA-MB-231 cells displayed elevated mRNA and protein levels of YAP (). To further evaluate the contribution of YAP to glycolysis in BC tissue samples, we analyzed 18F-FDG PET/CT images from BC patients. As illustrated in , specimens with strong YAP expression exhibited higher SUVmax. Additionally, BC patients with higher lesion SUVmax values had increased YAP expression compared to those with lower values (). In , BC patient with higher lesion SUVmax value was ER (-), PR (-), Her2 (3+), Ki67 (60%+), and BC patient with low SUVmax value was ER (90%), PR (95%), Her2 (1+), Ki67 (10%+). We also tested the protein levels of CTGF, a well-known downstream target of YAP [Citation19, Citation20], and HK2, a key enzyme involved in glucose metabolism in BC samples [Citation18]. In line with the expression pattern of YAP, the protein levels of CTGF and HK2 were both decreased in the samples from patients with low SUVmax compared with those from the high SUVmax group (). These results suggested that YAP may have a critical role in regulating glucose metabolism of BC cells.
Figure 1. 18F-FDG imaging shows that YAP overexpression is associated with glycolysis in BC. (A) qRT-PCT assay to measure YAP levels in BC cells. (B) The protein levels of YAP. (C, D) Typical 18F-FDG PET/CT imaging of patients with low (C) or high (D) SUVmax levels. (E) IHC assay assessment of YAP in patients with low and high SUVmax. Typical IHC images of YAP, CTGF, and HK2 in patients with low and high SUVmax. Data are mean ± SD for triplicate experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
YAP promotes BC cell glycolysis
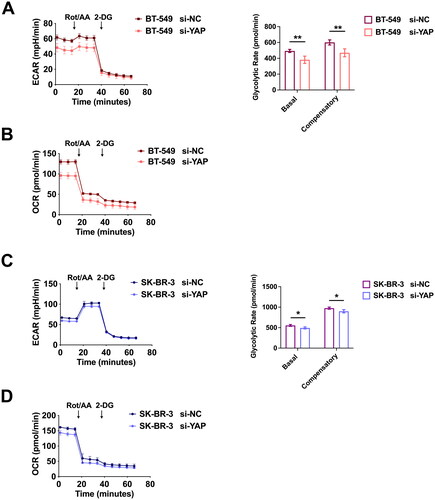
To demonstrate that YAP overexpression contributes to glycolysis, we conducted Seahorse experiments in BC cells and observed that YAP suppression significantly reduced ECAR levels (, left), as well as basal and compensatory glycolytic rates (, right), and OCR levels () in BT-549 cells. Similarly, YAP inhibition in SK-BR-3 cells reduced ECAR levels (, left), as well as basal and compensatory glycolytic rates (, right), and OCR levels ().
Figure 2. YAP promotes glycolysis in BC cells. (A) ECAR levels (left) and basal and compensatory glycolytic rates (right) in BT-549 cells transfected with si-YAP or control RNA. (B) the OCR levels of indicated BT-549 cells were measured. (C) ECAR levels (left) and basal and compensatory glycolytic rates (right) in SK-BR-3 cells transfected with si-YAP or control RNA. (D) OCR levels of indicated SK-BR-3 cells. Data are mean ± SD for triplicate experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
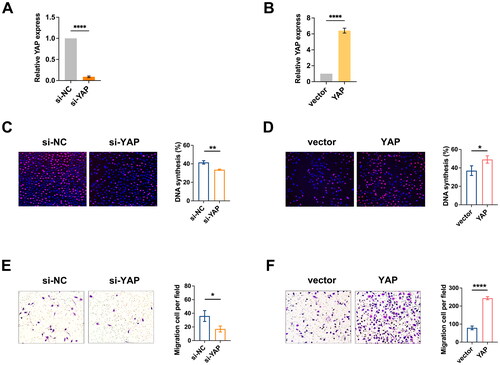
Subsequently, we conducted an EdU incorporation assay and Transwell assay in BC cells. The knockdown efficiency and overexpression efficiency of YAP mRNA in BT-549 cells were shown in , respectively. We indicated that suppression of YAP resulted in reduced proliferation of BC cells and inhibited cell migration (), respectively. In contrast, overexpression of YAP resulted in increased proliferation and migration of BC cells (), respectively. Taken together, these results confirm that YAP accumulation in BC cells stimulates glycolysis.
Figure 3. YAP promotes the growth of BC cells. (A) the knockdown efficiency of YAP in BT-549 cells. (B) the overexpression efficiency of YAP in BT-549 cells. (C-D) the EdU assay demonstrated that YAP knockdown inhibited BC cell growth (C), while YAP overexpression promoted BC cell growth (D), respectively. (E-F) Migration ability determined by Transwell assay demonstrated that YAP knockdown inhibited BC cell migration (E), while YAP overexpression promoted BC cell migration (F), respectively. Data are mean ± SD for triplicate experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Detection of binding of HuR to YAP in BC cells
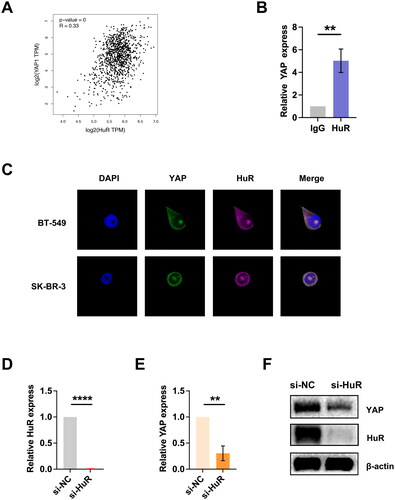
In the previous work, we demonstrated the essential role of HuR in the hypoxia-induced switch from oxidative phosphorylation to glycolysis [Citation18]. Additionally, HuR has been shown to regulate the YAP/TAZ pathway in malignant peripheral nerve sheath tumors (MPNST) cells, where it exerts important oncogenic functions [Citation21]. Using data from the GEPIA database, we analyzed the relationship between HuR and YAP expression in BC samples, which revealed a positive correlation between the two genes (). To investigate whether HuR directly binds YAP mRNA in BC cells, we performed RIP experiments. Consistently, the RIP assay demonstrated that YAP mRNA was significantly enriched by the HuR antibody (), suggesting HuR may regulate YAP expression in BC cells. Moreover, our FISH assay showed the co-localization of endogenous HuR and YAP mRNA in the cytoplasm of BC cell lines (). To further investigate the effect of HuR on YAP mRNA, we knocked down HuR in BC cells (the knockdown efficiency was shown in ) and observed a significant decrease in mRNA and protein levels of YAP in HuR-knockdown cells (). These findings provide strong evidence that HuR binds to and stabilizes YAP mRNA in BC cells, supporting the role of HuR in regulating YAP-mediated glycolysis in BC.
Figure 4. Detection of YAP binding to HuR in BC cells. (A) Analysis of the GEPIA database revealed a positive correlation between HuR and YAP in BC samples. (B) The RIP assay demonstrated that the HuR antibody significantly enriched YAP mRNA in BC cells. (C) The FISH assay verified the colocalization of endogenous HuR and YAP in cytoplasm. (D) Knockdown efficiency of HuR in BC cells. (E-F) Following HuR knockdown, mRNA (E) and protein (F) expression of YAP were downregulated. Data are mean ± SD for triplicate experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
VP inhibits YAP activity in BC cells
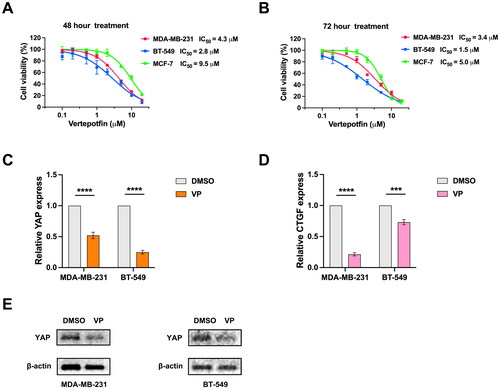
We then investigated whether pharmacological inhibition of YAP by VP would suppress glycolysis in BC cells. We determined the IC50 of VP for MDA-MB-231, BT-549 and MCF-7 cells using the BC cell line viability curve after 48 and 72 h of VP treatment (). As expected, we observed downregulation of YAP mRNA expression in BT-549 and MDA-MB-231 cell lines following treatment with VP (). Given that CTGF is a target gene of the YAP-TEAD1 transcription factor complex [Citation19, Citation20], we also evaluated the effects of VP treatment on the mRNA levels of CTGF and found it reduced following VP treatment in both BT-549 and MDA-MB-231 cells (). Additionally, we assessed the YAP protein levels in cell lines treated with VP and observed a dramatic decrease (). Our findings suggest that VP can inhibit YAP protein abundance and may subsequently reduce glycolysis in BC cells.
Figure 5. VP inhibits YAP activity in BC cells. (A) the cell line viability curve was used to determine the IC50 of VP for each cell line after 48 h of drug treatment. (B) the BC cell line viability curve was used to determine the IC50 of VP for each cell line after 72 h of drug treatment. (C) mRNA levels of YAP after VP treatment in the BT-549 and MDA-MB-231 cell lines. (D) mRNA levels of CTGF with VP treatment in the BT-549 and MDA-MB-231 cell lines. (E) YAP protein levels in cell lines treated with VP. Data are mean ± SD for triplicate experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Effect of VP therapy on glycolysis in BC cells
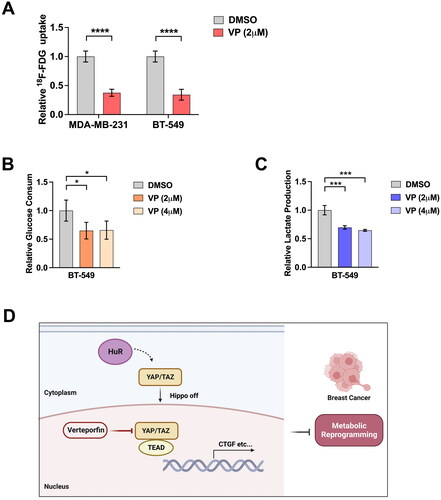
Finally, we explored the glycolytic regulation effects of VP in BC cells. We observed that VP treatment reduced 18F-FDG uptake rates in both BT-549 and MDA-MB-231 cells (). VP administration also reduced glucose consumption () and lactate production in BC cells (). Taken together, these results suggest that VP treatment inhibits YAP-induced glycolysis in BC cells ().
Figure 6. Effects of VP therapy on glycolysis in BC cells. (A) 18F-FDG uptake rates in BT-549 and MDA-MB-231 cell lines after VP. (B) Glucose consumption by BT-549 cells after indicated doses of VP. (C) Lactate production of BT-549 cells after indicated doses of VP. (D) Schematic diagram describing the role of YAP in glycolysis of BC. Data are mean ± SD for triplicate experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
The Hippo/YAP pathway is known to modulate biological function by regulating the expression of target genes, and YAP is a key transcription factor of this pathway [Citation22, Citation23]. YAP promotes cellular growth and proliferation, and its critical role has led to the development of small molecular inhibitors [Citation24]. In this study, we examined VP’s ability to suppress YAP overexpression-induced glycolysis in BC cells.
Inhibiting the interaction between YAP and TEAD provides a pharmacologically viable approach for blocking YAP oncoproteins [Citation11]. VP has been found to disturb the YAP-TEAD interaction and inhibit the expression of YAP target genes. In preclinical glioblastoma (GBM) models, VP has shown anti-invasive and antiproliferative effects, as well as associated survival benefits, indicating the therapeutic value of this drug for GBM treatment [Citation25]. Furthermore, VP could also be a treatment for the amplification and mutant of epidermal growth factor receptor GBM [Citation26]. Pharmacological inhibition of the mammalian target of rapamycin (mTOR) complexes 1/2 by torin1 and YAP by VP has been found to effectively block tumor growth in TNBC in vitro and in vivo, suggesting that the combination of these drugs could be used for treating TNBC [Citation27]. Previous studies have indicated that VP sensitizes BC cells to the effects of doxorubicin and tamoxifen, reducing tumor cell proliferation and survival [Citation9]. However, the impact of VP on controlling BC glycolysis is not well understood. Our results demonstrated that VP inhibits YAP activity and, as a result, inhibits YAP-induced glycolysis in BC cells.
The evaluation of glycolysis in vivo is currently best achieved by the use of 18F-FDG PET/CT. By 18F-FDG PET/CT scanning and IHC assay, our study confirms the association between YAP overexpression and up-regulated glycolysis in BC cells, thus highlighting the role of YAP in promoting glycolysis. HuR, also known as ELAVL1, is a protein that binds directly to target mRNAs for promoting their stability and expression. It is essential for hypoxia-induced glycolysis to switch from oxidative phosphorylation to glycolysis [Citation18]. HuR binds to a variety of RNA, mostly to U-rich extensions in the three prime untranslated regions (3′-UTR) [Citation21, Citation28], and increases the half-life of its target mRNAs [Citation28]. Elevated HuR expression was observed in BC cells [Citation29], which specifically stabilizes YAP levels. Collectively, our findings underscore the crucial regulatory role of YAP in BC metabolic reprogramming.
Our study has the following limitations. VP is a porphyrin derivative that acts as a photosensitizer and produces its primary biological activity through the short-lived singlet oxygen and reactive oxygen radicals [Citation30]. Although it has been reported that VP treatment can reduce YAP expression, it is worth noting that it can also induce oligomerization of several proteins related to major cellular processes, including autophagy and cytoskeleton maintenance [Citation10, Citation30]. In addition, VP has also been reported to have proteotoxic effects [Citation31]. Therefore, other downstream targets rather than the YAP-TEAD complex may also be involved in the inhibitory effect of VP on glucose metabolism.
In conclusion, our findings provide compelling evidence for the role of YAP in promoting glycolysis in BC cells, and demonstrate VP could be an effective inhibitor of YAP-induced glycolysis. These results suggest that targeting YAP activity by VP delivery may represent a promising therapeutic approach for BC treatment.
Disclosure statement
There are no conflicts of interest.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):1–9. doi:10.3322/caac.21492.
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi:10.1001/jama.2018.19323.
- Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–1133. doi:10.1016/j.cell.2007.07.019.
- Koo JH, Guan KL. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018;28(2):196–206. doi:10.1016/j.cmet.2018.07.010.
- Ibar C, Irvine KD. Integration of hippo-YAP signaling with metabolism. Dev Cell. 2020;54(2):256–267. doi:10.1016/j.devcel.2020.06.025.
- Yousefi H, Delavar MR, Piroozian F, et al. Hippo signaling pathway: a comprehensive gene expression profile analysis in breast cancer. Biomed Pharmacother. 2022;151:113144. doi:10.1016/j.biopha.2022.113144.
- Zhang Z, Lin Z, Zhou Z, et al. Structure-based design and synthesis of potent cyclic peptides inhibiting the YAP-TEAD protein-protein interaction. ACS Med Chem Lett. 2014;5(9):993–998. doi:10.1021/ml500160m.
- Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971. doi:10.1101/gad.1664408.
- Guimei M, Alrouh S, Saber-Ayad M, et al. Inhibition of yes-associated protein-1 (YAP1) enhances the response of invasive breast cancer cells to the standard therapy. Breast Cancer (Dove Med Press). 2020;12:189–199. doi:10.2147/bctt.S268926.
- Gibault F, Corvaisier M, Bailly F, et al. Non-photoinduced biological properties of verteporfin. Curr Med Chem. 2016;23(11):1171–1184. doi:10.2174/0929867323666160316125048.
- Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–1305. doi:10.1101/gad.192856.112.
- Lin C, Xu X. YAP1-TEAD1-Glut1 axis dictates the oncogenic phenotypes of breast cancer cells by modulating glycolysis. Biomed Pharmacother. 2017;95:789–794. doi:10.1016/j.biopha.2017.08.091.
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi:10.1126/science.123.3191.309.
- Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10(9):671–684. doi:10.1038/nrd3504.
- Ulaner GA. PET/CT for patients with breast cancer: where is the clinical impact? AJR Am J Roentgenol. 2019;213(2):254–265. doi:10.2214/ajr.19.21177.
- Groheux D, Martineau A, Teixeira L, et al. (18)FDG-PET/CT for predicting the outcome in ER+/HER2- breast cancer patients: comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis. Breast Cancer Res. 2017;19(1):3. doi:10.1186/s13058-016-0793-2.
- Jiang S, Zhang LF, Zhang HW, et al. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. Embo J. 2012;31(8):1985–1998. doi:10.1038/emboj.2012.45.
- Zhang LF, Lou JT, Lu MH, et al. Suppression of miR-199a maturation by HuR is crucial for hypoxia-induced glycolytic switch in hepatocellular carcinoma. Embo J. 2015;34(21):2671–2685. doi:10.15252/embj.201591803.
- Bum-Erdene K, Yeh IJ, Gonzalez-Gutierrez G, et al. Small-molecule cyanamide pan-TEAD·YAP1 covalent antagonists. J Med Chem. 2023;66(1):266–284. doi:10.1021/acs.jmedchem.2c01189.
- Si Y, Ji X, Cao X, et al. Src inhibits the hippo tumor suppressor pathway through tyrosine phosphorylation of Lats1. Cancer Res. 2017;77(18):4868–4880. doi:10.1158/0008-5472.Can-17-0391.
- Palomo-Irigoyen M, Pérez-Andrés E, Iruarrizaga-Lejarreta M, et al. HuR/ELAVL1 drives malignant peripheral nerve sheath tumor growth and metastasis. J Clin Invest. 2020;130(7):3848–3864. doi:10.1172/jci130379.
- Misra JR, Irvine KD. The hippo signaling network and its biological functions. Annu Rev Genet. 2018;52(1):65–87. doi:10.1146/annurev-genet-120417-031621.
- Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–828. doi:10.1016/j.cell.2015.10.044.
- Clara JA, Monge C, Yang Y, et al. Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat Rev Clin Oncol. 2020;17(4):204–232. doi:10.1038/s41571-019-0293-2.
- Barrette AM, Ronk H, Joshi T, et al. Anti-invasive efficacy and survival benefit of the YAP-TEAD inhibitor verteporfin in preclinical glioblastoma models. Neuro Oncol. 2022;24(5):694–707. doi:10.1093/neuonc/noab244.
- Vigneswaran K, Boyd NH, Oh SY, et al. YAP/TAZ transcriptional coactivators create therapeutic vulnerability to verteporfin in EGFR-mutant glioblastoma. Clin Cancer Res. 2021;27(5):1553–1569. doi:10.1158/1078-0432.Ccr-20-0018.
- Dai M, Yan G, Wang N, et al. In vivo genome-wide CRISPR screen reveals breast cancer vulnerabilities and synergistic mTOR/Hippo targeted combination therapy. Nat Commun. 2021;12(1):3055. doi:10.1038/s41467-021-23316-4.
- Abdelmohsen K, Panda AC, Munk R, et al. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol. 2017;14(3):361–369. doi:10.1080/15476286.2017.1279788.
- Heinonen M, Fagerholm R, Aaltonen K, et al. Prognostic role of HuR in hereditary breast cancer. Clin Cancer Res. 2007;13(23):6959–6963. doi:10.1158/1078-0432.Ccr-07-1432.
- Calses PC, Crawford JJ, Lill JR, et al. Hippo pathway in cancer: aberrant regulation and therapeutic opportunities. Trends Cancer. 2019;5(5):297–307. doi:10.1016/j.trecan.2019.04.001.
- Dey A, Varelas X, Guan KL. Targeting the hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov. 2020;19(7):480–494. doi:10.1038/s41573-020-0070-z.
