Abstract
Background
Tendon-exposed wounds are complex injuries with challenging reconstructions and no unified treatment mode. Furthermore, insufficient tissue volume and blood circulation disorders affect healing, which increases pain for the patient and affects their families and caretakers.
Review
As modern medicine advances, considerable progress has been made in understanding and treating tendon-exposed wounds, and current research encompasses both macro-and micro-studies. Additionally, new treatment methods have emerged alongside the classic surgical methods, such as new dressing therapies, vacuum sealing drainage combination therapy, platelet-rich plasma therapy, and live-cell bioengineering.
Conclusions
This review summarizes the latest treatment methods for tendon-exposed wounds to provide ideas and improve their treatment.
Background
In recent years, tendon-exposure trauma has become an increasingly common clinical problem owing to lifestyle changes and increased incidences of traffic accidents, mechanical injuries, burns, and diabetes (thus diabetic foot), making it a popular clinical research topic.Citation1 Tendon explant repair is a complex biological process with a challenging treatment and extensive recovery time, which psychologically and economically burdens patients and their families.Citation2 Globally, between 0.45 and 3.33% of the population has a history of leg ulcers,Citation3 and an estimated £8.3 billion was spent in the United Kingdom in 2018 to assess and treat 3.8 million people with wounds, which is a considerable drain on healthcare resources.Citation4 Tendons lie directly beneath the soft tissue; thus, areas with less soft tissue coverage, such as the back of the hands and feet, are highly susceptible to tendon outgrowth wounds following skin defect or necrosis. Since tendons are fibrous connective tissues with a relatively poor blood supply, they are mainly nourished by synovial fluid from the synovial tendon sheaths rather than by vascular perfusion. Consequently, once a large skin defect has formed, self-healing of the tendon is difficult, and it is prone to infection, often requiring clinical intervention.Citation5,Citation6
Modern medicine offers various approaches for treating exposed-tendon wounds, and as the global population ages, medical practitioners are placing more emphasis on this area of research, which has expanded beyond the macroscopic level into the microscopic level. Therefore, this article reviews the latest advances in treating exposed-tendon wounds.
Review
Physiology of Wound Healing
Physiologically, wound healing is roughly divided into four dynamic processes: platelet aggregation, inflammation, cell proliferation, and remodeling. Each segment is continuous and overlapping,Citation7 and complex interactions between various cell types, biomolecules, and the extracellular matrix (ECM) work together to produce inflammatory responses, synthesize granulation tissue, and restore the epithelium ().Citation8,Citation9
Figure 1. Pathophysiological mechanisms in the pathogenesis of tendon exposed wounds. The mechanisms include bacterial infection, poor vascularization, insufficient growth factors and lack of repair cells.
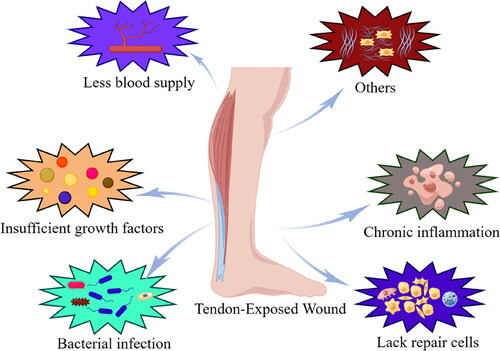
Common wounds usually undergo timely progression from the inflammatory phase to the end of the remodeling phase, but each healing stage is delayed or stagnated for tendon-exposed wounds, delaying overall wound healing and making self-healing difficult.Citation10 Tendon exposed wounds are chronic wounds and the treatment can be very tricky.Citation11,Citation12 Adequate debridement and establishment a healthy granular bed is a critical step to healing.Citation13 And it is a prerequisite for numerous specific clinical interventions. In fact, the healing process of tendon exposed wounds has its own unique characteristics comparison with dermal wound repair as follows: firstly, because of the lack of blood flow to the tendon itself, the possibility of tendon necrosis after prolonged exposure is a concern. At the same time, the difficulty of local granulation tissue growth in the tendon wound leads to a higher rate of necrosis in the skin graft or flaps compared to the dermal wound.Citation14 Secondly, tendon exposed wounds are difficult owing to the poor local blood supply, a lack of relevant GFs, and wound infection. Meanwhile tendons have a relatively poor blood supply since they are composed of fibrous connective tissue, which can be exacerbated by trauma and basal blood supply disorders; thus, granulation tissue formation is ineffective. Moreover, surgical procedures to repair the trauma, such as skin grafting, local flaps, or free skin grafts, put the tissue cells in a perpetual state of ischemia and hypoxia, so they cannot obtain nutrients or accumulate metabolic products. Consequently, skin grafting and flap surgeries have a high failure rate.Citation5,Citation6 Thirdly, lacking quantities and reduced activities of relevant GFs affect wound healing. Numerous GFs promote cell proliferation and vascular regeneration, accelerate tissue epithelialization, and regulate fibroblasts, accelerating wound healing. However, in tendon-exposed wounds, low concentrations of GFs severely hinder wound-healing processes.Citation15 Finally, wound infection slows the healing process. The skin is the body’s most important barrier against the outside world, and breaks in the skin leave the wound highly susceptible to bacterial infection, severely hindering the healing process by releasing various toxins and enzymatic substances. If the infection is severe and cannot be contained via the immune system or antibiotic treatment, long-term persistent damage to the wound will keep it in the inflammatory phase, prolonging or stalling healing.Citation16–18
General Treatment of Tendon Outgrowth Wounds
Exposed tendon wounds can be treated according to the TIME principles: tissue debridement, infection control, moisture balance and wound margins.Citation19
Debridement
Debridement is now considered a core component of wound care.Citation20 According to Williams et al. studies, aggressive debridement significantly improves the likelihood of chronic wound healing.Citation21 Debridement is the removal of dead cells, leaving only viable tissue. In fact, all layers need to be carefully explored without a tourniquet when treating the wound in order to observe the tissue blood supply. A 2–3 mm excision of the skin around the tissue, as well as all damaged and contaminated soft tissues, is generally considered.Citation22,Citation23 When tendon component resection is required, the need for additional surgical treatment should be determined by whether the extent of tendon resection has an impact on motor function. We consider tendon suturing or other treatment only when the extent of resection is too large and affects the motion of the affected limb. Finally, the wound should be flushed with plenty of saline and pulsed irrigation is recommended to further reduce the risk of infection. For optimal healing, the first debridement operation should be performed within 6–12 h after the injury. Finally, soft tissue coverage should be planned in the form of a phase I procedure (e.g., suture, skin graft, or flap) or a phase II procedure (e.g., vacuum-assisted suture [VAC], PRP, or a new dressing), depending on the intraoperative wound condition, should be considered. If the wound is severely contaminated or infected, consider a subsequent secondary debridement operation or repeat debridement every 48–72 h until the wound is clean.Citation13,Citation15
Antibiotic Therapy
In addition to surgical debridement, the wound should be treated prophylactically with intravenous antibiotics. Numerous reports have noted a significant reduction in wound infection rates in patients who received antibiotic prophylaxis compared to the treatment group that did not receive antibiotic prophylaxis, and it is believed that an important factor in reducing infection rates is the early use of antibiotics.Citation24–26 Despite the importance of antibiotic therapy in wounds, there are still no specific guidelines. In most cases, we consider antibiotic therapy starting with cephalosporins followed by sensitive antibiotics after culture results are obtained.
Moisture Balance
Related studies point out that moist wounds heal faster and have less risk of infection. If the wound appears dry, a dressing will need to be added to retain moisture.Citation27Wound edges need to be kept flat. If the edges are rolled up, they need to be properly excised to promote epithelialization.Citation28
Flap Transplantation
The skin flap method is a classic surgical technique for repairing skin and soft tissue defects caused by congenital or acquired trauma.Citation29,Citation30 Autologous flap transplantation has strong anti-infective properties, good histocompatibility, high softness, and key roles in functional reconstruction. However, the primary features of tendon-exposed wounds are soft tissue loss and tendon exposure. Thus, these wounds have local fibrous connective tissue areas with relatively poor blood supply to the wound base, which is not suitable for simple closure or separate skin transplantation. Even if these defects are minor, the reconstruction survival rate is low,Citation31 likely because immediate debridement of tendon-exposed wounds does not provide a suitable wound bed for successful flap transplantation. Successful flap transplantation requires the formation of a healthy granulation tissue bed at the base of the wound. However, wound healing additives, such as GF-rich dressings,Citation32,Citation33 matrix protein substrates,Citation34 hyperbaric oxygen,Citation35 skin substitutes,Citation36 and wound healing techniques (e.g., negative pressure drainage), can promote granulation tissue formation on the tendon’s surface.Citation37,Citation38
There are many types of flaps, and appropriate flaps and surgical methods should be selected for repairing wounds of different parts and depths. According to Marchesi’s summary of flap grafting for Achilles tendon exposed wounds, we can get inspiration: when the tendon exposed wound diameter (<2 cm) is Small defects, local rotational flaps or V–Y advancement flaps can be considered to complete the surgery quickly; when the wound diameter (2–4 cm) is moderate defects, it is necessary to use propeller perforator flaps or bipedicled flaps to minor donor site morbidity and ensure the blood supply of the flap; when the wound diameter (4–8 cm) is large defects, in the absence of contraindications, it is mostly necessary to treat with microsurgical assistance to ensure the normal blood supply of the flap; finally, when the wound diameter (>8 cm) is very large defects, the use of a latissimus dorsi flap may be considered.Citation13 For instance, tendon adhesion in the later stage limits skin grafting for full-thickness skin defects with tendon exposure. However, Carty et al. reported that applying a fasciocutaneous flap in a local defect wound of an exposed hand tendon was a good choice for preventing postoperative tendon adhesion.Citation39,Citation40 Straub et al. also used platelet-rich fibrin (PRF) as a lubricating layer to protect skin grafts from tendon movement.Citation32 Moreover, the postoperative survival of the flap depends on the abundance of oxygenated blood at the flap’s distal edge. Therefore, when the wound exceeds a certain size, it is difficult to achieve satisfactory results by simply covering it with a single large flap, especially for tendon-exposed wounds. For such problems, endovascular interventional therapy has long been used in flap transplantation, with obvious advantages and a wide application.Citation31 For example, Li et al. reported the successful application of a chimeric perforator flap pedicled with the descending branch of the lateral circumflex femoral artery and the lateral femoral muscle flap to repair a large area of deep wounds in the ankle, noting that this was an effective and relatively safe method.Citation41 Similarly, Martin et al. reported the successful repair of an open wound with an exposed right forearm tendon using a flap by anastomosis of the radial artery.Citation42
In addition, the skin flap is generally accompanied by subcutaneous soft tissue, and there are apparent scars and other problems after healing. Therefore, the autologous skin flap may not meet the patients’ long-term functional and esthetic needs when it comes to hand and foot tendon exposure wounds. However, one study suggests that autologous dermal transplantation using the vacuum-assisted closure (VAC) system is an effective alternative for small, shallow wounds with surrounding tissue damage that cannot form a local flap.Citation43,Citation44 They reported that the technique is simple, fast, and inexpensive and successfully repaired soft tissue defects of the Calf, foot, and toe. Similarly, in recent years, perifascial areolar tissue (PAT) has been commonly used in the wound surrounding the damaged tissue in tendon-exposed wounds.Citation42 PAT refers to very thin, loose connective tissue on the deep fascia rich in blood supply due to the many vascular plexuses, and it has great advantages for healing wounds exposed to rigid structures, such as tendons.Citation45 The autologous flap is advantageous due to the rich blood supply, wear resistance, and lack of immune rejection. However, there are also well-known limitations, such as an insufficient flap donor area, immense surgical trauma, and postoperative appearance.Citation46
Adjunctive Treatment of Tendon Outgrowth Wounds
With advances in modern medicine, various techniques and materials have emerged to adjunctive treat exposed-tendon wounds, improve flap survival rate. In addition to the classic combined vacuum sealing drainage (VSD) therapy,Citation47 Platelet-rich plasma (PRP) therapy,Citation48 new dressings,Citation49 and cell therapyCitation50 have emerged. The following sections review the latest adjunctive treatment advances for tendon explants to provide ideas and treatment assistance.
Artificial Skin Combined With VSD
Developments in tissue engineering technologies have resulted in artificial dermal substitutes that effectively repair tendon-exposed wounds.Citation51–55 Applying an artificial dermis to soft tissue defect wounds overcomes the dependence of traditional flaps on autologous soft tissue, solves the problem of an insufficient supply of autologous skin, and is richly available. Moreover, the surgical method is simple and easy, the surgical trauma is minimal, and the postoperative limb function and appearance are greatly improved.Citation48,Citation52 However, the artificial dermis alone may not quickly achieve sufficient granulation for tendon-exposed wounds, making the wound very susceptible to bacterial infection.Citation56
Many recent studies have demonstrated that negative pressure VSD can timely remove wound exudates, inhibit bacterial colonization, reduce tissue edema, increase local blood perfusion, and promote cell proliferation and the formation of protein synthesis factors.Citation44,Citation57,Citation58 Therefore, it is common to use an artificial dermis combined with VSD to reconstruct tendon-exposed wounds.Citation36,Citation46,Citation58–60 For example, Zhu et al. used negative pressure VSD combined with an artificial dermis to successfully treat 36 cases of tendon and other structurally exposed wounds, shortening the patient’s hospitalization time with satisfactory curative effects.Citation60 Similar alternative VAC methods on the artificial dermis can also successfully repair soft tissue coverage of complex wounds in the foot and ankle.Citation58 However, successful tissue engineering requires a trinity of scaffolds, cells, and a rich environment.Citation43 Artificial dermis grafts have the scaffold function of vascular reconstruction and collagen formation, which allows fibroblast-like cell, macrophage, and lymphocyte migration and ingrowth and promotes capillary ingrowth.Citation51,Citation55 At the same time, VSD provides a certain degree of mechanical force for wound healing, resulting in better attachment of the artificial dermis’ collagen layer to the wound. Sufficient attachment accelerates the process of fibroblast crawling and vascular ingrowth in the collagen layer of the artificial dermis, promoting dermis formation and vascularization of the wound, which creates a suitable wound bed for autologous grafts. Lee et al. provide an example of this; they used dermal substitutes and negative pressure wound therapy to treat hardware-exposed soft tissue defects.Citation36 Another report suggested that simple skin transplantation with many flaps had high tendon adhesion rates and noted that artificial skin had a considerable advantage for tendon sliding after reconstructing tendon-exposed wounds.Citation53
Although an artificial dermis combined with VSD has advantages for repairing tendon-exposed wounds, there are also apparent deficiencies. As a blood-deficient tissue, the tendon must form a sufficient wound bed before secondary autologous skin transplantation is possible, and it can take two to four weeks to form sufficient granulation tissue.Citation51,Citation53 Thus, the total treatment time is relatively long. Additionally, the artificial dermis is a tissue engineering product that is relatively expensive. These disadvantages may be a psychological and economic burden for some patients.Citation47,Citation52
Platelet-Rich Plasma (PRP) and Other Blood-Related Products
PRP is an autologous serum obtained by centrifugation and separation of whole blood in vitro with a platelet concentration higher than that of standard plasma.Citation61 Hematologists first introduced this concept in 1970 for the main purpose of treating thrombocytopenia.Citation62 However, studies have shown that PRP is rich in GFs, chemokines, and cytokines, so many researchers have a strong interest in the potential of PRP in regenerative medicine.Citation63 PRP has been widely used in dermatology, plastic surgery, sports medicine, and oral and maxillofacial surgeries, amongst other disciplines.Citation64 Many studies have demonstrated the beneficial effects and encouraging patient outcomes of PRP therapy in clinical practice ().Citation63,Citation65,Citation66
Figure 2. Mechanisms of PRP for tendon exposure wounds. PRP promotes the secretion of growth factors of PDGF, egf other growth factors. At the same time, it inhibits the inflammatory response and reduces the production of local ROS. Finally, these cytokines act on MSCs, macrophages fibroblasts, etc., and ultimately promote wound healing by means of inflammatory responses, proliferation and remodeling.
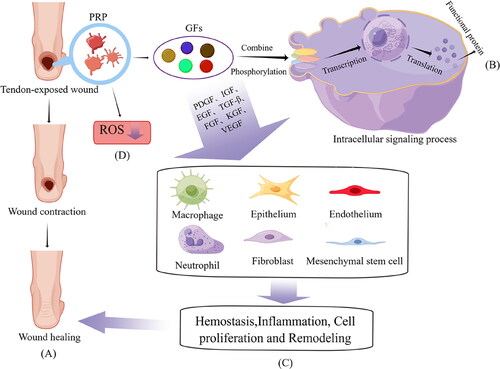
Moreover, PRP has certain advantages for refractory wound healing (e.g., tendon-exposed wounds); for example, they promote faster wound healing.Citation64,Citation67,Citation68 In addition, platelet alpha-particles are rich in GFs, such as PDGF, insulin-like growth factor (IGF) 1, vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), epidermal GF, and TGF-β1.Citation69 These are hypothesized to stimulate cell proliferation and migration, angiogenesis, granulation tissue formation, and ECM production, thus involving the entire wound healing process.Citation70 However, the tendon belongs to fibrous connective tissue and has no abundant blood supply, leading to a relatively low GF level. Furthermore, effective healing becomes difficult once a skin defect occurs above the tendon. Therefore, applying PRP in these and other fields can induce supraphysiological levels of GF to theoretically stimulate the regression of chronic pathological processes.
As previously mentioned, platelets are the first cells to respond in wound healing, initiating wound reconstruction. Platelets activate once a clot has formed, releasing GFs from the alpha-particles to stimulate healing.Citation62,Citation71 Like many cytokines, GFs bind ligands to related cell surface receptors, and then the membrane surface receptors change to transmit signals to the cell. These changes promote the phosphorylation and activation of specific protein kinases in cells, eventually leading to the translocation of phosphorylated kinases to the nucleus. This phosphorylated transcription factor enables the transcription and translation of wound healing-related target genes that ultimately perform coding functions.Citation61,Citation72
The primary roles of various GFs differ, but in general, the goal is to coordinate wound healing. For example, PDGF initiates callus formation through fibroblast and mesenchymal stem cell (MSC) proliferation and wound chemotaxis.Citation73 PDGF is also important in promoting endothelial cell proliferation in granulation tissue and angiogenesis.Citation74 VEGF, corresponding to its name, strongly stimulates vascular endothelial cell proliferation, thereby participating in neovascularization.Citation75 TGF-β stimulates MSC proliferation and differentiation and contributes to ECM formation through collagen production; it also helps mediate angiogenesis.Citation76–78 enhances endothelial and fibroblast migration and mitosis, stimulates angiogenesis and collagen formation, and has an important effect on tissue repair.Citation79 Finally, IGF stimulates cell growth and proliferation to promote wound healing.Citation80
Although numerous studies have shown that GFs in PRP are beneficial for reconstructing refractory wounds with tendon exposure,Citation64,Citation69,Citation81 other components, such as pro-inflammatory cytokines, have a negative impact.Citation82 For example, IL-1β plays an important role in cartilage damage and osteoarthritis and has been used to induce osteoarthritis in animal models.Citation83 Similarly, other inflammatory factors, such as TNF-α, induce cartilage damage and inhibit cartilage progenitor cell migration when it interacts with IL-1β, eventually leading to delayed cartilage healing. Furthermore, these inflammatory factors play a role in wound pain. Therefore, applying PRP to the joints may accelerate cartilage decomposition due to the pro-inflammatory reaction, ultimately inducing articular cartilage injury nonunion or aggravating existing arthritis.Citation70
In recent years, the effects of PRP on tendon-exposed wound healing have been verified in humans.Citation84,Citation85 For instance, Cervelli et al. used plasma and hyaluronic acid-binding gel in Achilles tendon-exposed wounds, finding that wound severity significantly decreased compared to the control group, almost all patients achieved wound closure, and no infections were recorded during the treatment.Citation81 Additionally, our group have performed a clinical study that included 12 patients (average age: 42 years) with tendon exposure due to soft tissue defects of various causes.Citation48 After fully debriding the patient’s wound, venous blood was extracted to obtain PRP by centrifugation. Then, the PRP was sprayed on the wound to form an autologous platelet gel, and healing was assessed based on the granulation tissue’s appearance and the wound’s size reduction. Overall, wound healing was observed in all patients, pain and serous discharge decreased within one week of treatment, and no side effects were observed throughout the follow-up period. Compared with other autologous cell therapies, the main advantage of using platelets as a regenerative agent in wound healing is that they can be extracted directly from the patient’s venous blood. Thus, PRP is readily available, has a high degree of safety, and has no possibility of immune rejection or blood-borne disease transmission. Moreover, it is simple to obtain and administer, does not require advanced preparation facilities, and can reduce postoperative pain and swelling.
In recent years, other blood-derived products have also been used to cover skin wounds,Citation86–88 for example, leukocyte- and PRF (L-PRF), which is a second-generation platelet concentrate for local use rich in fibrin, platelets, and white blood cells. It can also be obtained quickly and at a low cost without requiring activators and anticoagulants. Reports also indicate that L-PRF positively affects chronic ulcer wounds, and in vitro studies demonstrated that L-PRF exerted a very strong positive effect on the proliferation of all tested cell lines, especially fibroblasts and keratinocytes.Citation87,Citation89 Therefore, PRP and other blood products are generally advantageous for wound healing.
New Dressings
Wound dressings influence and accelerate the healing process,Citation49 and the types of wound dressings have advanced in recent years. Traditional dressings such as mainly gauze, cotton pads and bandages, are still widely used in clinical practice due to their low manufacturing costs and simplicity. However, these dressings have considerable disadvantages, such as poor wound moisturization, poor hemostasis, and a tendency to adhere to exudate, resulting in pain and new soft tissue defects during dressing changes. Therefore, modern dressings may be a more appropriate option.
Compared to conventional dressings, modern dressings encourage a moist environment for wound healing and have better biocompatibility and degradability, significantly affecting wound healing. Modern dressings commonly used in clinical practice include hydrogels, foams, cell-free matrices, and honey. For example, absorbable gelatin sponges combined with polyurethane membranes for refractory wounds with bone or tendon exposure is a novel and cost-effective wound reconstruction method.Citation90
A wide variety of novel dressings have been reported. To date, the literature has reported that honey, silver ionomer, and cell-free matrix dressings are most applicable for refractory wounds, which include tendon exposures. Firstly, honey has long been used as a wound dressing worldwide. Honey contains many bioactive components that promote wound healing through four stages: hemostasis, inflammation, proliferation, and remodeling.Citation91,Citation92 For example, Ahmed et al. recently reported a positive wound healing effect and confirmed the antibacterial and anti-inflammatory effects of honey,Citation93 reporting that the anti-inflammatory effect reduced edema and abnormal wound secretions and thus reduced the time to establish a clean wound bed. In addition, the bioactive substances in honey stimulate cell proliferation, collagen synthesis, and vascular and granulation tissue formation, all of which accelerate wound epithelialization and, thus, wound contraction, which facilitates wound healing and reduces the healing time. Honey also has antibacterial properties; its high viscosity protects against infection and reduces the wound’s bioburden.Citation94 Some have even noted pain relief and debris removal from wounds after applying honey.
Secondly, Loh et al. demonstrated the clinical efficacy of silver ionomer as a wound dressing for tendon-exposed lower limb wounds,Citation95 which provided a wet environment for the tissue defect, offering effective protection of the exposed tendon while absorbing wound exudate and promoting granulation tissue growth, resulting in safe and effective wound healing. Silver also has known antibacterial effects; silver ions in the dressing are oxidized upon contact with the wound, forming active silver ions. These ions interfere with bacterial function by chemically reducing or inactivating the biological activity of enzymes, inhibiting bacterial division, or directly killing the bacteria on the wound’s surface.Citation96–98
Finally, cell-free matrix dressings have been recently reported and well-received.Citation99–101 For example, Melandri et al. reported the excellent performance of a new human-derived cell-free dermal matrix for treating tendon-exposed distal lower limb wounds, suggesting this is a promising alternative for covering tendon-exposure wounds.Citation102 However, the function of these dressings is similar to that of other dressing types, mainly to promote granulation tissue formation and wound epithelialization and provide a moist environment for the wound. Therefore, new dressings offer several options for treating tendon-exposure wounds.
Live-Cell Bioengineering
Some products contain live cells, such as live adipose stem cells, fibroblasts, and dermis in combination with epidermal cells. Applying one or several types of live cells is an important point of differentiation between this method and the above-mentioned reconstruction methods. Hefton et al. and Leigh et al. were the first to describe the application of cultured epidermal sheets for treating skin defects,Citation103,Citation104 reporting that they healed or improved most soft tissue defect wounds and reduced the length of the patient’s hospital stay. Similarly, Jaime et al. concluded that frozen human allogeneic epidermal cultures were safe and effective for treating patients with tendon-exposure wounds, and they might apply to any lower limb skin defect.Citation105 However, in their study, all wounds were treated with a combination of VAC therapy and deepidermalized implants; as a result, they formed granulation tissue within a certain period postoperatively. Consequently, the exposed structures were effectively and adequately covered, and none of the patients’ wounds were infected. Therefore, sufficient granulation tissue and a clean wound surface created a suitable wound bed for the additional reconstructive treatments.Citation106 Nonetheless, tissue cultures are usually kept in a sterile environment and can directly cover the soft tissue defect after thawing; therefore, this method is simple, convenient, and free from donor complications.
Viable cryopreserved human placental membranes (vCHPM) are another effective wound coverage method.Citation107,Citation108 vCHPM contains natural components of the tissue that remain intact, including a structural matrix, GFs, and a natural mixture of endogenous live cells, including MSCs. The collagen-rich ECM also provides a structural scaffold for cell migration, proliferation, and differentiation, which is required for tissue repair, and the accompanying GFs and various cell types form a suitable basal bed for abnormal wounds. Suzuki et al. reported positive effects of vCHPM for managing complex wounds characterized by hardware, such as exposed tendons ().Citation109
Figure 3. Treatment of tendon exposed wounds. Treatment options for exposed tendon wounds include: flap grafting, artificial skin combined with VSD, platelet rich plasma and other blood-related products, new dressings, and so on.
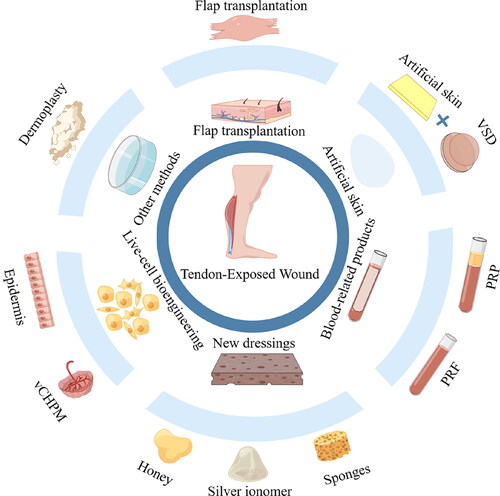
In summary, these approaches make it apparent that non-surgical treatment options promoting outpatient-based anatomical and functional tissue replacement without donor site complications are necessary to maximize the patient’s chances of recovery and mobility.
Conclusions
Exposed-tendon wounds are commonly found on the dorsal surface of the hand and foot, where impaired blood flow, a relative lack of GFs, and infection make wound reconstruction difficult, considerably affecting the patient’s quality of life. Consequently, healing such wounds is one of the most important challenges in medicine. This short review briefly outlines the treatment advances for tendon-exposed wounds, including flap grafts, VSD combined with an artificial dermis, PRP and other blood-related products, novel dressings, and live-cell bioengineering, as well as describes the biological process of general wound recovery. Although the evidence for these new therapies is limited, they all have encouraging clinical data supporting their use as new therapies.
Authors’ contributions
ZD and ZSL wrote the manuscript. GC prepared the reference, data. All authors reviewed the manuscript and approved the final version.
Ethical approval
This article is a review and should not be reviewed by the ethics Committee.
Informed consent
Informed written consent was obtained from the patient to publish their personal or clinical details information.
| Abbreviations | ||
| ECM | = | Extracellular matrix |
| FGF | = | Fibroblast growth factor |
| GF | = | Growth factor |
| IGF | = | Insulin-like growth factor |
| IL | = | Interleukin |
| L-PRF | = | Leukocyte- and platelet-rich fibrin |
| MSC | = | Mesenchymal stem cell |
| PAT | = | Perifascial areolar tissue |
| PDGF | = | Platelet-derived growth factor |
| PRF | = | Platelet-rich fibrin |
| PRP | = | Platelet-rich plasma |
| TGF-β | = | Transforming growth factor-beta |
| TNF-α | = | Tumor necrosis factor-alpha |
| VAC | = | Vacuum-assisted closure |
| vCHPM | = | viable Cryopreserved human placental membranes |
| VEGF | = | Vascular endothelial growth factor |
Acknowledgments
Not Applicable
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data used or analyzed during this study are included in this published article.
Additional information
Funding
References
- Yao J, Zeng Y, Yang J, et al. Repairing tendon-exposed wounds by combing the Masquelet technique with dermoplasty. Front Surg. 2022;9:1. doi:10.3389/fsurg.2022.995316.
- Li MX, Ma J, Zheng ZJ, Niu LB, Yang L. Clinical effect of bi-layered artificialdermis and autologous skin graft in repairing bone and/or tendon exposed wounds. Zhonghua Shao Shang Za Zhi. 2020;36(3):179–11. doi:10.3760/cma.j.cn501120-20191119-00437.
- Wen Q, Liu D, Wang X, et al. A systematic review of ozone therapy for treating chronically refractory wounds and ulcers. Int Wound J. 2022;19(4):853–870. doi:10.1111/iwj.13687.
- Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ Open. 2020;10(12):e045253. doi:10.1136/bmjopen-2020-045253.
- Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212(3):211–228. doi:10.1111/j.1469-7580.2008.00864x.
- Zhou L, Wei J, Liu L, Tao S, Dong Z. Composite sural neurocutaneous flap with gastrocnemius tendon for repairing defects of Achilles tendon and overlying soft tissue. J Orthop Surg (Hong Kong). 2020;28(3):2309499020971863. 2309499020971863. doi:10.1177/2309499020971863.
- Perez-Favila A, Martinez-Fierro ML, Rodriguez-Lazalde JG, et al. Current therapeutic strategies in diabetic foot ulcers. Medicina (Kaunas). 2019;55(11):714. doi:10.3390/medicina55110714.
- Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. doi:10.1098/rsob.200223.
- Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. doi:10.1007/s12325-017-0478-y.
- Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370–378. doi:10.1111/bjd.13954.
- Shoham Y, Kogan L, Weiss J, et al. Wound ‘dechronification’ with negatively-charged polystyrene microspheres: a double-blind RCT. J Wound Care. 2013;22(3):144–155. 148, 150-2 passim. doi:10.12968/jowc.2013.22.3.144.
- Silverstein G. Dermal regeneration template in the surgical management of diabetic foot ulcers: a series of five cases. J Foot Ankle Surg. 2006;45(1):28–33. doi:10.1053/j.jfas.2005.10.005.
- Marchesi A, Parodi PC, Brioschi M, et al. Soft-tissue defects of the Achilles tendon region: Management and reconstructive ladder. Review of the literature. Injury. 2016;47 Suppl 4(Suppl 4):S147–S153. doi:10.1016/j.injury.2016.07.053.
- Helgeson MD, Potter BK, Evans KN, Shawen SB. Bioartificial dermal substitute: a preliminary report on its use for the management of complex combat-related soft tissue wounds. J Orthop Trauma. 2007;21(6):394–399. doi:10.1097/BOT.0b013e318070c028.
- Liu P, Liu Y, Ke CN, et al. Therapeutic effect of autologous concentrated growth factor on lower-extremity chronic refractory wounds: A case report. World J Clin Cases. 2021;9(18):4797–4802. doi:10.12998/wjcc.v9.i18.4797.
- Goldberg SR, Diegelmann RF. What makes wounds chronic. Surg Clin North Am. 2020;100(4):681–693. doi:10.1016/j.suc.2020.05.001.
- Lee YK, Lee M. Treatment of infected Achilles tendinitis and overlying soft tissue defect using an anterolateral thigh free flap in an elderly patient: a case report. Medicine (Baltimore). 2018;97(35):e11995. doi:10.1097/MD.0000000000011995.
- George P, DeJesus RA. Venous flow-through flap reconstruction following severe finger wound infection: case report. J Reconstr Microsurg. 2009;25(4):267–269. doi:10.1055/s-0029-1215525.
- Leaper DJ, Schultz G, Carville K, et al. Extending the TIME concept: what have we learned in the past 10 years? Int Wound J. 2012;9 Suppl 2(suppl 2):1–19. doi:10.1111/j.1742-481X.2012.01097.x.
- Dayya D, O’Neill OJ, Huedo-Medina TB, Habib N, Moore J, Iyer K. Debridement of diabetic foot ulcers. Adv Wound Care (New Rochelle). 2022;11(12):666–686. doi:10.1089/wound.2021.0016.
- Williams D, Enoch S, Miller D, et al. Effect of sharp debridement using curette on recalcitrant nonhealing venous leg ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen. 2005;13(2):131–137. doi:10.1111/j.1067-1927.2005.130203.x.
- Nowak M, Mehrholz D, Barańska-Rybak W, Nowicki RJ. Wound debridement products and techniques: clinical examples and literature review. Postepy Dermatol Alergol. 2022;39(3):479–490. doi:10.5114/ada.2022.117572.
- Ziegler B, Fischer S, Pieper D, Mathes T, Kneser U, Hirche C. Evidence and trends in burn wound debridement: an evidence map. Plast Surg (Oakv). 2020;28(4):232–242. doi:10.1177/2292550320928553.
- Gosselin RA, Roberts I, Gillispie WJ. Antibiotics for preventing infection in open limb fractures. Cochrane Database Sys Rev. 2004;2004:CD003764.
- Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop Relat Res. 1989;243(243):36–40. doi:10.1097/00003086-198906000-00006.
- Yarrow J, Rahman S, Marsden N, Pallister I, Hemington-Gorse S. Management of open lower limb injuries in South West England and Wales. Ann R Coll Surg Engl. 2015;97(1):35–39. doi:10.1308/003588414X14055925058472.
- Field FK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167(1A):2S–6S.
- Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle). 2014;3(7):445–464. doi:10.1089/wound.2013.0473.
- Kang GC, Por YC, Tan BK. In vivo tissue engineering over wounds with exposed bone and tendon: autologous dermal grafting and vacuum-assisted closure. Ann Plast Surg. 2010;65(1):70–73. doi:10.1097/SAP.0b013e3181a72f77.
- Attinger CE, Ducic I, Hess CL, Basil A, Abbruzzesse M, Cooper P. Outcome of skin graft versus flap surgery in the salvage of the exposed achilles tendon in diabetics versus nondiabetics. Plast Reconstr Surg. 2006;117(7):2460–2467. doi:10.1097/01.prs.0000219345.73727.f5.
- Lin CT, Chen SG, Chen TM, Dai NT, Chang SC. Bipedicled flap for the reconstruction of soft tissue defects of the Achilles tendon. Ann Plast Surg. 2015;74(4):484–487. doi:10.1097/SAP.0b013e3182a1e508.
- Straub A, Brands R, Borgmann A, et al. Free skin grafting to reconstruct donor sites after radial forearm flap harvesting: a prospective study with Platelet-Rich Fibrin (PRF). J Clin Med. 2022;11(12):3506. doi:10.3390/jcm11123506.
- Miyanaga T, Haseda Y, Daizo H, et al. A perifascial areolar tissue graft with topical administration of basic fibroblast growth factor for treatment of complex wounds with exposed tendons and/or bones. J Foot Ankle Surg. 2018;57(1):104–110. doi:10.1053/j.jfas.2017.08.026.
- Heckmann A, Radtke C, Rennekampff HO, et al. Einzeitige Defektdeckung von denudiertem Knochen und freiliegenden Sehnen mittels MATRIDERM® und Spalthaut. Möglichkeiten und Grenzen [One-stage defect closure of deperiosted bone and exposed tendons with MATRIDERM® and skin transplantation]. Unfallchirurg. 2012;115(12):1092–1098. German. doi:10.1007/s00113-011-2003-0.
- Kang N, Hai Y, Liang F, Gao CJ, Liu XH. Preconditioned hyperbaric oxygenation protects skin flap grafts in rats against ischemia/reperfusion injury. Mol Med Rep. 2014;9(6):2124–2130. doi:10.3892/mmr.2014.2064.
- Lee SK, An YS, Choy WS. Management of hardware-exposed soft tissue defects using dermal substitutes and negative pressure wound therapy. Ann Plast Surg. 2023;90(3):242–247. doi:10.1097/SAP.0000000000003440.
- Andrews BT, Smith RB, Chang KE, Scharpf J, Goldstein DP, Funk GF. Management of the radial forearm free flap donor site with the vacuum-assisted closure (VAC) system. Laryngoscope. 2006;116(10):1918–1922. doi:10.1097/01.mlg.0000235935.07261.98.
- Mundinger GS, Stalder MW, Lee J, et al. Autologous heterogeneous skin construct closes traumatic lower extremity wounds in pediatric patients: a retrospective case series. Int J Low Extrem Wounds. 2023;22(1):103–112. doi:10.1177/1534734621992284.
- Carty MJ, Taghinia A, Upton J. Fascial flap reconstruction of the hand: a single surgeon’s 30-year experience. Plast Reconstr Surg. 2010;125(3):953–962. doi:10.1097/PRS.0b013e3181cc964c.
- Sobanko JF, Fischer J, Etzkorn JR, et al. Local fasciocutaneous sliding flaps for soft-tissue defects of the dorsum of the hand. JAMA Dermatol. 2014;150(11):1187–1191. doi:10.1001/jamadermatol.2014.954.
- Li RG, Zeng CJ, Yuan S, et al. Reconstruction of large area of deep wound in the foot and ankle with chimeric anterolateral thigh perforator flap. Orthop Surg. 2021;13(5):1609–1617. doi:10.1111/os.13046.
- Martin JP, Chambers JA, Long JN. Use of radial artery perforator flap from burn-injured tissues. J Burn Care Res. 2008;29(6):1009–1011. doi:10.1097/BCR.0b013e31818ba0e5.
- Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg. 2006;117(7 Suppl):121S–126S. doi:10.1097/01.prs.0000225450.12593.12.
- Repta R, Ford R, Hoberman L, Rechner B. The use of negative-pressure therapy and skin grafting in the treatment of soft-tissue defects over the Achilles tendon. Ann Plast Surg. 2005;55(4):367–370. doi:10.1097/01.sap.0000181342.25065.60.
- Hayashi A, Komoto M, Tanaka R, et al. The availability of perifascial areolar tissue graft for deep cutaneous ulcer coverage. J Plast Reconstr Aesthet Surg. 2015;68(12):1743–1749. doi:10.1016/j.bjps.2015.08.008.
- Lv Z, Wang Q, Jia R, Ding W, Shen Y. Pelnac® artificial dermis assisted by VSD for treatment of complex wound with bone/tendon exposed at the foot and ankle, a prospective study. J Invest Surg. 2020;33(7):636–641. doi:10.1080/08941939.2018.1536177.
- Muangman P, Engrav LH, Heimbach DM, et al. Complex wound management utilizing an artificial dermal matrix. Ann Plast Surg. 2006;57(2):199–202. doi:10.1097/01.sap.0000218636.61803.d6.
- Deng Z, Long ZS, Gong FP, Chen G. The efficacy and safety of platelet-rich plasma in the tendon-exposed wounds: a preliminary study. J Orthop Surg Res. 2022;17(1):497. doi:10.1186/s13018-022-03401-0.
- Shi C, Wang C, Liu H, et al. Selection of appropriate wound dressing for various wounds. Front Bioeng Biotechnol. 2020;8:182. doi:10.3389/fbioe.2020.00182.
- Johnson EL, Tassis EK, Michael GM, Whittinghill SG. Viable placental allograft as a biological dressing in the clinical management of full-thickness thermal occupational burns: Two case reports. Medicine (Baltimore). 2017;96(49):e9045. doi:10.1097/MD.0000000000009045.
- Lee LF, Porch JV, Spenler W, Garner WL. Integra in lower extremity reconstruction after burn injury. Plast Reconstr Surg. 2008;121(4):1256–1262. doi:10.1097/01.prs.0000304237.54236.66.
- Lv Z, Yu L, Wang Q, Jia R, Ding W, Shen Y. The use of dermal regeneration template for treatment of complex wound with bone/tendon exposed at the forearm and hand, a prospective cohort study. Medicine (Baltimore). 2019;98(44):e17726. doi:10.1097/MD.0000000000017726.
- Shores JT, Hiersche M, Gabriel A, Gupta S. Tendon coverage using an artificial skin substitute. J Plast Reconstr Aesthet Surg. 2012;65(11):1544–1550. doi:10.1016/j.bjps.2012.05.021.
- Wang HJ, Wei LG, Wang CH, et al. A new form of artificial skin to promote permanent wound coverage: a preliminary report. Ann Plast Surg. 2017;78(3 Suppl 2):S148–S152. doi:10.1097/SAP.0000000000001021.
- Yeong EK, Yu YC, Chan ZH, Roan TL. Is artificial dermis an effective tool in the treatment of tendon-exposed wounds? J Burn Care Res. 2013;34(1):161–167. doi:10.1097/BCR.0b013e3.182685f0a.
- Hsu KF, Chiu YL, Chiao HY, et al. Negative-pressure wound therapy combined with artificial dermis (Terudermis) followed by split-thickness skin graft might be an effective treatment option for wounds exposing tendon and bone: a retrospective observation study. Medicine (Baltimore). 2021;100(14):e25395. doi:10.1097/MD.0000000000025395.
- Hutchison RL, Craw JR. Use of acellular dermal regeneration template combined with NPWT to treat complicated extremity wounds in children. J Wound Care. 2013;22(12):708–712. doi:10.12968/jowc.2013.22.12.708.
- Pu LL. An alternative approach for soft-tissue coverage of a complex wound in the foot and ankle with vacuum-assisted closure over artificial dermis and subsequent skin graft. J Plast Reconstr Aesthet Surg. 2009;62(12):e682-4–e684. doi:10.1016/j.bjps.2008.08.032.
- Eo S, Kim Y, Cho S. Vacuum-assisted closure improves the incorporation of artificial dermis in soft tissue defects: Terudermis(®) and Pelnac(®). Int Wound J. 2011;8(3):261–267. doi:10.1111/j.1742-481X.2011.00780.x.
- Zhu B, Cao D, Xie J, Li J, Chen Z, Bao Q. Clinical experience of the use of Integra in combination with negative pressure wound therapy: an alternative method for the management of wounds with exposed bone or tendon. J Plast Surg Hand Surg. 2021;55(1):1–5. doi:10.1080/2000656X.2020.1781140.
- Collins T, Alexander D, Barkatali B. Platelet-rich plasma: a narrative review. EFORT Open Rev. 2021;6(4):225–235. doi:10.1302/2058-5241.6.200017.
- Verma R, Kumar S, Garg P, Verma YK. Platelet-rich plasma: a comparative and economical therapy for wound healing and tissue regeneration. Cell Tissue Bank. 2023;24(2):285–306. doi:10.1007/s10561-022-10039-z.
- Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21(20):7794. doi:10.3390/ijms21207794.
- Tian J, Cheng LH, Cui X, et al. Application of standardized platelet-rich plasma in elderly patients with complex wounds. Wound Repair Regen. 2019;27(3):268–276. doi:10.1111/wrr.12702.
- Le A, Enweze L, DeBaun MR, Dragoo JL. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11(4):624–634. doi:10.1007/s12178-018-9527-7.
- Grambart ST. Sports medicine and platelet-rich plasma: nonsurgical therapy. Clin Podiatr Med Surg. 2015;32(1):99–107. PMID: 25440421. doi:10.1016/j.cpm.2014.09.006.
- Liu X, Li X, Wei W, et al. Local autologous platelet rich plasma injection combined with platelet rich fibrin filling as the main treatment for refractory wounds: a case series. Front Surg. 2022;9:1003691. doi:10.3389/fsurg.2022.1003691.
- Meznerics FA, Fehérvári P, Dembrovszky F, et al. Platelet-rich plasma in chronic wound management: a systematic review and meta-analysis of randomized clinical trials. J Clin Med. 2022;11(24):7532. doi:10.3390/jcm11247532.
- Grigore TV, Cozma C. Platelet-rich plasma as a site-targeted approach in wound healing: a molecular perspective. Discoveries (Craiova). 2018;6(4):e87. doi:10.15190/d.2018.8.
- Xiong G, Lingampalli N, Koltsov J, et al. Men and women differ in the biochemical composition of platelet-rich plasma. Am J Sports Med. 2018;46(2):409–419. doi:10.1177/0363546517740845.
- Akbarzadeh S, McKenzie MB, Rahman MM, Cleland H. Allogeneic platelet-rich plasma: is it safe and effective for wound repair? Eur Surg Res. 2021;62(1):1–9. doi:10.1159/000514223.
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9(1):49–61. doi:10.1016/s1359-6101(97)00036-1.
- Rasubala L, Yoshikawa H, Nagata K, Iijima T, Ohishi M. Platelet-derived growth factor and bone morphogenetic protein in the healing of mandibular fractures in rats. Br J Oral Maxillofac Surg. 2003;41(3):173–178. doi:10.1016/s0266-4356(03)00075-5.
- Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125(4):917–928. doi:10.1083/jcb.125.4.917.
- Bauer SM, Bauer RJ, Velazquez OC. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovascular Surg. 2005;39(4):293–306. doi:10.1177/153857440503900401.
- Joyce ME, Roberts AB, Sporn MB, Bolander ME. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110(6):2195–2207. doi:10.1083/jcb.110.6.2195.
- Ng F, Boucher S, Koh S, et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112(2):295–307. doi:10.1182/blood-2007-07-103697.
- Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174–187. doi:10.1051/ject/200638174.
- Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93–103.
- Fabi S, Sundaram H. The potential of topical and injectable growth factors and cytokines for skin rejuvenation. Facial Plast Surg. 2014;30(2):157–171. doi:10.1055/s-0034-1372423.
- Cervelli V, Lucarini L, Spallone D, Brinci L, de Angelis B. Use of platelet rich plasma and hyaluronic acid on exposed tendons of the foot and ankle. J Wound Care. 2010;19(5):186, 188–90. doi:10.12968/jowc.2010.19.5.48045.
- Rikkers M, Dijkstra K, Terhaard BF, et al. Platelet-rich plasma does not inhibit inflammation or promote regeneration in human osteoarthritic chondrocytes in vitro despite increased proliferation. Cartilage. 2021;13(2_suppl):991S–1003S. doi:10.1177/1947603520961162.
- Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res. 2008;26(12):1643–1648. doi:10.1002/jor.20683.
- De Angelis B, Lucarini L, Orlandi F, et al. Regenerative surgery of the complications with Morton’s neuroma surgery: use of platelet rich plasma and hyaluronic acid. Int Wound J. 2013;10(4):372–376. doi:10.1111/j.1742-481X.2012.00992.x.
- Massara M, Pucci G, Stilo G, et al. The role of cord blood platelet gel in the management of a diabetic foot with tendon exposure. Regen Med. 2021;16(12):1051–1056. doi:10.2217/rme-2021-0044.
- Zhang H, Wang S, Lei C, Li G, Wang B. Experimental study of negative pressure wound therapy combined with platelet-rich fibrin for bone-exposed wounds. Regen Med. 2022;17(1):23–35. doi:10.2217/rme-2021-0043.
- Pinto NR, Ubilla M, Zamora Y, Del Rio V, Dohan Ehrenfest DM, Quirynen M. Leucocyte- and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: a prospective cohort study. Platelets. 2018;29(5):468–475. doi:10.1080/09537104.2017.1327654.
- Zhang S, Cao D, Xie J, Li H, Chen Z, Bao Q. Platelet-rich fibrin as an alternative adjunct to tendon-exposed wound healing: a randomized controlled clinical trial. Burns. 2019;45(5):1152–1157. doi:10.1016/j.burns.2019.01.007.
- Wang Y, Wang X, Zhao Y, Zhao Y, Ruan S, Cao H. Effect of leukocyte-platelet fibrin-rich wound reconstruction followed by full-thickness skin grafting in the treatment of diabetic foot Wagner grade 4 ulcer gangrene (toe area). Platelets. 2023;34(1):2131752. doi:10.1080/09537104.2022.2131752.
- Yu P, Hong N, Chen M, Zou X. Novel application of absorbable gelatine sponge combined with polyurethane film for dermal reconstruction of wounds with bone or tendon exposure. Int Wound J. 2023;20(1):18–27. doi:10.1111/iwj.13832.
- Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev. 2015;2015(3):CD005083. doi:10.1002/14651858.CD005083.pub4.
- Biglari B, Moghaddam A, Santos K, et al. Multicentre prospective observational study on professional wound care using honey (Medihoney™). Int Wound J. 2013;10(3):252–259. doi:10.1111/j.1742-481X.2012.00970.x.
- Ahmed AA, Eltregy S, Kandil MI. Honey dressing: a missed way for orthopaedic wound care. Int Orthop. 2022;46(11):2483–2491. doi:10.1007/s00264-022-05540-9.
- Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules. 2018;23(9):2392. doi:10.3390/molecules23092392.
- Loh ML, Goh BKL, Kong Y, et al. Combination therapy of oxidised regenerated cellulose/collagen/silver dressings with negative pressure wound therapy for coverage of exposed critical structures in complex lower-extremity wounds. Int Wound J. 2020;17(5):1356–1365. doi:10.1111/iwj.13406.
- Stanirowski PJ, Wnuk A, Cendrowski K, Sawicki W. Growth factors, silver dressings and negative pressure wound therapy in the management of hard-to-heal postoperative wounds in obstetrics and gynecology: a review. Arch Gynecol Obstet. 2015;292(4):757–775. doi:10.1007/s00404-015-3709-y.
- Xu D, Chu T, Tao G. Clinical study on the efficacy of silver ion dressing combined with prontosan gel dressing in the treatment of diabetic foot ulcers and the effect on serum inflammatory factors. Evid Based Complement Alternat Med. 2021;2021:2938625. doi:10.1155/2021/2938625.
- Wang R, Guo Y, Li B, Zheng J, Tang Z, Shu M. Application effect of silver-containing dressings in the repair of chronic refractory wounds. Evid Based Complement Alternat Med. 2022;2022:3616923. doi:10.1155/2022/3616923.
- Melville JC, Bennetts NA, Tijerina L, Shum JV. The use of acellular urinary bladder matrix as coverage for fasciocutaneous free flap donor sites: an alternative to traditional grafting procedures. J Oral Maxillofac Surg. 2017;75(10):2254–2260. doi:10.1016/j.joms.2017.03.011.
- Cazzell S, Moyer PM, Samsell B, Dorsch K, McLean J, Moore MA. A prospective, multicenter, single-arm clinical trial for treatment of complex diabetic foot ulcers with deep exposure using acellular dermal matrix. Adv Skin Wound Care. 2019;32(9):409–415. doi:10.1097/01.ASW.0000569132.38449.c0.
- Lullove E. Acellular fetal bovine dermal matrix in the treatment of nonhealing wounds in patients with complex comorbidities. J Am Podiatr Med Assoc. 2012;102(3):233–239. doi:10.7547/1020233.
- Melandri D, Marongiu F, Carboni A, et al. A new human-derived acellular dermal matrix for 1-stage coverage of exposed tendons in the foot. Int J Low Extrem Wounds. 2020;19(1):78–85. doi:10.1177/1534734619884422.
- Hefton JM, Caldwell D, Biozes DG, Balin AK, Carter DM. Grafting of skin ulcerswith cultured autologous epidermal cells. J Am Acad Dermatol. 1986;14(3):399–405. doi:10.1016/s0190-9622(86)70048-0.
- Leigh IM, Purkis PE, Navsaria HA, Phillips TJ. Treatment of chronicvenous ulcers with sheets of cultured allogenic keratinocytes. Br J Dermatol. 1987;117(5):591–597. doi:10.1111/j.1365-2133.1987.tb07491.x.
- Bolívar-Flores YJ, Kuri-Harcuch W. Frozen allogeneic human epidermal cultured sheets for the cure of complicated leg ulcers. Dermatol Surg. 1999;25(8):610–617. doi:10.1046/j.1524-4725.1999.99022.x.
- Brandi C, Grimaldi L, Nisi G, et al. Treatment with vacuum-assisted closure and cryo-preserved homologous de-epidermalised dermis of complex traumas to the lower limbs with loss of substance, and bones and tendons exposure. J Plast Reconstr Aesthet Surg. 2008;61(12):1507–1511. doi:10.1016/j.bjps.2007.09.036.
- Caputo WJ, Vaquero C, Monterosa A, et al. A retrospective study of cryopreserved umbilical cord as an adjunctive therapy to promote the healing of chronic, complex foot ulcers with underlying osteomyelitis. Wound Repair Regen. 2016;24(5):885–893. doi:10.1111/wrr.12456.
- Frykberg RG, Gibbons GW, Walters JL, Wukich DK, Milstein FC. A prospective, multicentre, open-label, single-arm clinical trial for treatment of chronic complex diabetic foot wounds with exposed tendon and/or bone: positive clinical outcomes of viable cryopreserved human placental membrane. Int Wound J. 2017;14(3):569–577. doi:10.1111/iwj.12649.
- Suzuki K, Michael G, Tamire Y. Viable intact cryopreserved human placental membrane for a non-surgical approach to closure in complex wounds. J Wound Care. 2016;25(Sup10):S25–S31. doi:10.12968/jowc.2016.25.Sup10.S25.
