Abstract
Objective
The study aimed to compare the incidence of intraoperative endplate injury in patients who underwent Transforaminal interbody fusion (TLIF) and mini-open lumbar interbody fusion (LLIF) surgery. The independent risk factors related to endplate injury in LLIF procedure were analyzed.
Methods
A total of 199 patients who underwent LLIF (n = 106) or TLIF (n = 93) surgery from June 2019 to September 2021 were reviewed. The endplate injury was assessed by postoperative sagittal CT scan. A binary logistic analysis model were used to identify independent risk factors related to LLIF endplate injury based on univariate analysis.
Results
There was an obvious difference in the occurrence of intraoperative endplate injury between LLIF (42/106, 39.6%) and TLIF group (26/93, 28%), although it did not reach the significant level. L1 CT value (OR = 0.985, 95% CI = 0.972–0.998), cage position (OR = 3.881, 95% CI = 1.398–10.771) and height variance (OR = 1.263, 95% CI = 1.013–1.575) were independent risk factors for endplate injury in LLIF procedure. According to the cage settlement patterns, there 5 types of A to E. The severity of the facet joint degeneration was positively related to the occurrence of endplate injury.
Conclusions
The incidence of intraoperative endplate injury is higher in LLIF than in TLIF procedures. Low bone quantity, cage posterior position and larger height variance are risk factors to induce endplate injury in LLIF surgery. The facet joint degeneration may be related to severe endplate injuries and even fractures.
Introduction
Degenerative diseases of the lumbar spine arise from aging and degeneration of the lumbar spine, accompanied by pathological changes of lumbar spinal stenosis, intervertebral foraminal stenosis, and nerve root compression. It can lead to interstitial claudication and radiation pain and numbness in the lower extremity [Citation1]. Transforaminal interbody fusion (TLIF) is performed to attain direct decompression of nerve root by evacuating the hyperplastic articular eminence, ligamentum flavum, bony redundancy, prolapsed nucleus pulposus, and other compression-causing tissues. It has achieved satisfactory clinical outcomes [Citation2, Citation3] but can cause harm to the posterior ligaments, muscle tissue, and articular eminence joints [Citation4]. Indirect decompression of the compressed nerve tissue can be gained through a lateral approach with a larger cage implanted in the intervertebral space, which has acquired widespread interest [Citation5]. TLIF has the advantages of minimally invasive, strong anterior and middle column support, indirect spinal canal decompression, and restoration of coronal and sagittal spinal sequences. As a result, it has been widely applied in recent years for the surgical treatment of degenerative scoliosis, lumbar spinal stenosis, lumbar spondylolisthesis, and even partial lumbar spine trauma, infection and tumor [Citation6, Citation7].
Lateral lumbar interbody fusion (LLIF) is another common minimally invasive technique for interbody fusion. LLIF can cause some access-related complications, such as injury to the psoas major muscle, blood vessels, femoral nerve, and genitofemoral nerve [Citation8]. Notably, intraoperative endplate injury is the most common complication in LLIF surgery [Citation9, Citation10]. Intraoperative endplate injury is considered to be any violation (discontinuity or abrasion) of either or both adjacent vertebral endplates. Severe endplate injury may occur followed by immediate intraoperative cage settling, cage retropulsion or even vertebral fracture. Weight-bearing activities can also lead to the onset of late-onset subsidence for patients suffering from endplate injury without cage subsidence, especially for those who received stand-alone LLIF surgery without an internal fixation device. However exclude endplate injury without cage subsidence is rarely reported [Citation11, Citation12]. LLIF requires a larger cage for indirect decompression. During the insertion of a larger cage, the stress between the cage and endplate surface is more prominent, which is more likely to cause intraoperative endplate injury. However, in vitro biomechanical studies suggest that LLIF cage is more difficult to settle than TLIF cage [Citation13]. One recent study by Jin et al. has reported the occurrence of intraoperative endplate injury with the incidence of 20.4% for patients receiving minimally invasive surgery (MIS)-LLIF, which is related to gender, sagittal disk angle in the extended position, and endplate sclerosis [Citation14]. Therefore, it is necessary to examine the incidence of intraoperative endplate injury during LLIF, and its predisposing factors.
Although cage subsidence after lumbar fusion has been studied, few studies have provided direct comparative data of cage settling between LLIF and TLIF surgery, as well as the risk factors. In this study, patients with or without cage subsidence who underwent TLIF and mini-open LLIF surgery were recruited, the incidence of intraoperative endplate injury between the two groups was compared. Moreover, the independent risk factors related to endplate injury in LLIF procedures were analyzed, in order to help surgeons make decisions.
Materials and methods
Patient population
This study was approved by our Institutional Review Board and informed consent was waived due to the retrospective nature of this study. Patients who were treated with open TLIF with bilateral pedicle screw instrumentation and mini-open LLIF with or without bilateral pedicle screw instrumentation from June 2019 to September 2021 were recruited in the current study. All the patients included in this study experienced pain or palsy derived from degenerative lumbar diseases and were not relieved by conservative treatment. The inclusion criteria were as follows: (1) Degenerative lumbar spinal stenosis; (2) Lumbar spondylolisthesis; (3) Underwent initial LLIF and TLIF procedure, (4) Single-segment fusion; (5) Patients with complete data. The exclusion criteria were as follows: (1) Combined developmental spinal stenosis; (2). Surgical segment is L5S1; (3) More than II degree spondylolisthesis; (4) Severe osteoporosis; (5) Patients with scoliosis; (6) Patients with previous lumbar spine infection or fracture; (7) patients without complete data. All patients underwent LLIF and TLIF utilizing the Keystone system (SanYou, Inc., Shanghai, China) and the COUGAR system (Depuy Spine Inc., Raynham, MA, USA) respectively.
Surgical technique and equipment
TLIF and LLIF procedures were performed according to the methods reported before [Citation10]. Bilateral pedicle screw and rod were enforced right after TLIF and 3–5 days after the LLIF. Each procedure was performed by a fellowship-trained orthopedic spine surgeon with a minimum of 5 years of experience in performing LLIF and TLIF surgery.
Radiographic evaluation
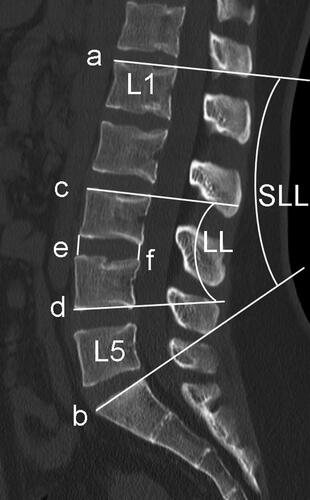
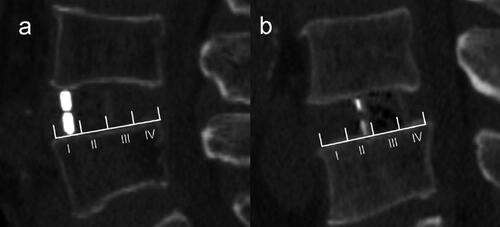
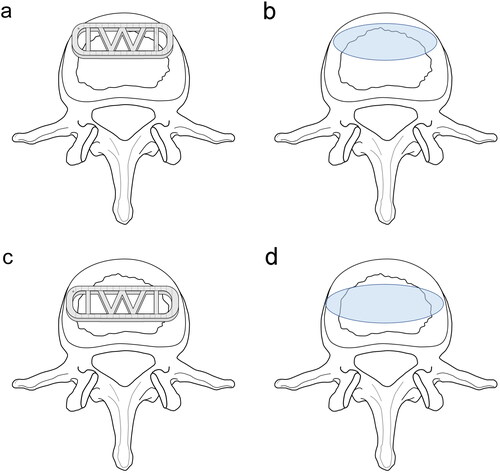
Pfimann Classification of the intervertebral disk was assessed by preoperative magnetic resonance imaging (MRI) [Citation15]. Patients underwent Computed tomography (CT) examination before TLIF and LLIF surgery, and the following parameters were collected, including lumbar lordosis (LL), segment lumbar lordosis (SLL), intervertebral space height (IH), lumbar CT value (L1 CT value) (). Then the CT examination was performed again on the 3rd postoperative day before the patient’s weight-bearing activities. Obvious intraoperative endplate injury can be identified by intraoperative C-arm fluoroscopy, but the accuracy is worse than that of CT examination, and C-arm fluoroscopy cannot be identified for minor endplate injury. Therefore, CT was used to evaluate end-plate injury on the 3rd postoperative day. Intervertebral space height (IH), cage position, endplate injury and patterns were identified by postoperative CT sagittal images. IH is the mean value of the anterior and posterior height of intervertebral space. L1 CT value is the mean HU value of 3 elliptic regions of interest placed on the transverse section of L1 vertebral body trabecular bone 20. Height variance represents the difference between cage height and intervertebral space height. The caudal endplate was equally divided into four zones (I-IV). According to whether the anterior-inferior edge of the cage falls on the zone I or not, the cage location is divided into 2 groups, including anterior located and posterior located (). Endplate injury was defined as discontinuity of the endplate. Radiographic assessment was independently performed by two spinal surgeons who were blinded to the study information. A third reviewer was available for adjudication in case of disagreement.
Statistical analysis
To compare baseline demographics and clinical data, an independent t-test was used for the parametric continuous variables, and the Mann-Whitney U test on non-parametric variables. Chi-square or Fisher’s exact tests were used for categorical variables. Variables with a significant difference in the univariate analyses were introduced into a binary logistic regression model for the exploration of risk factors related to endplate injury in LLIF surgery. P values less than 0.05 were considered statically significant.
Results
Characteristics and intraoperative endplate injury of patients who received LLIF or TLIF surgery
The study comprised 106 patients in the LLIF group and 93 patients in the TLIF group. The demographic data and occurrence rate of endplate injury between LLIF and TLIF groups were shown in . There were no significant statistical differences in basic patient profiles, including sex, age, BMI, diagnosis, L1 CT value, LL, SLL and disk Pfirrmann classification and surgical segment. In addition, the incidence of intraoperative endplate injury between LLIF group (64/106, 60.38%) and TLIF group (67/93, 72.06%) showed an obvious difference, although the difference did not reach a significant level (p = 0.083).
Table 1. Characteristics And intraoperative endplate injury of patients received LLIF or TLIF surgery.
Comparison of influencing factors related to endplate injury during LLIF surgery
A comparison of influencing factors related to endplate injury during LLIF surgery was listed in . Univariate analysis revealed that more females, older age, lower L1 CT value, cage posterior position, larger height variance, and lower IH were found in the group with endplate injury during LLIF procedures, which showed a significant difference (p < 0.05).
Table 2. Univariate Analysis for endplate injury in LLIF group.
Then clinical variables with a significant difference were introduced into the binary logistic regression model, including sex, age, L1 CT value, cage position and height variance. Height variance and IH are related factors, and the former was chosen instead of the latter in the model because height variance represented the theoretical height of disk space that can be distracted. The model revealed that lower L1 CT value (OR: 0.985; 95%CI: 0.972–0.998; p < 0.05), larger height variance (OR: 1.263; 95%CI: 0.972–0.998; p < 0.05), and cage posterior located (OR: 3.881; 95%CI: 1.398–10.771; p < 0.001) were independent risk factors for the occurrence of intraoperative endplate injury ().
Table 3. Binary logistic regression analysis for endplate injury in LLIF group.
The patterns of intraoperative endplate injury during LLIF procedures
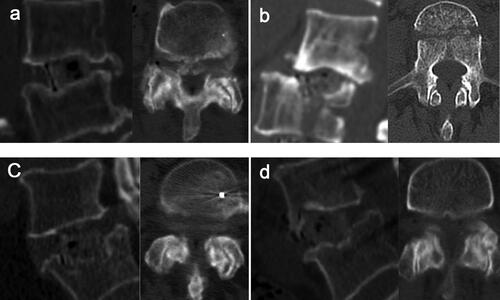
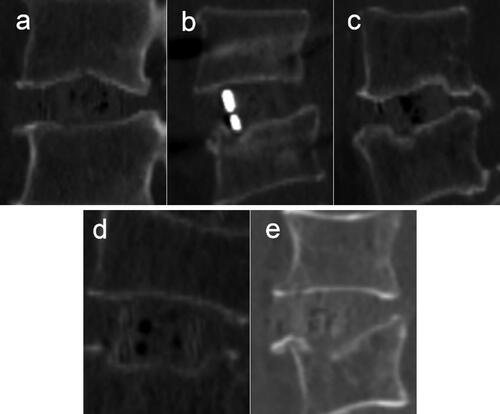
The patterns of intraoperative endplate injury during LLIF procedures were further analyzed and summarized according to the shape of endplate damage. There are 5 patterns according to the levels of cage subsidence and settling, five patterns were identified. The five patterns were as follows: Type A: Endplate abrasion, without cage settling; Type B: one edge of the cage settles into the vertebral body; Type C: two diagonal edges of the cage settle into the vertebral bodies; Type D: The upper or lower surface of the cage settles into the vertebral body; Type E: Fracture of the superior or inferior vertebral body, the fracture line is distributed along the coronal plane (). The severity of endplate damage of types 1 to 5 is increasing.
Figure 3. The patterns of intraoperative endplate injury during LLIF procedures. (a) Endplate abrasion, without cage settling. (b) one edge of the cage settles into the vertebral body. (c) two diagonal edges of the cage settle into the vertebral bodies. (d) the upper or lower surface of the cage settles into the vertebral body. (e) Fracture of the superior or inferior vertebral body, the fracture line is distributed along the coronal plane.
Discussion
Intraoperative endplate injury and cage subsidence were distinguished in the current study. LLIF procedure was determined to be more prone to intraoperative endplate injury than TLIF surgery, because of the placement of a large fusion device, a concept that is prone to intraoperative endplate injury [Citation16]. In TLIF procedure, the cage is implanted with an interlaminar spreader for pre-distraction, which reduces stress on the contact surface between the cage and the endplate [Citation17]. In some ways, the LLIF cage is “knocked” into space, whereas the TLIF cage is inserted. An expandable interbody spacer could be implanted gently and significantly reduce the incidence of late-onset subsidence during LLIF procedure [Citation18, Citation19]. However, further studies are needed to assess the intraoperative endplate injury, indirect decompression effects and clinical outcomes of the expandable cage, especially for stand-alone LLIF.
Association of bone quality with intraoperative endplate injury
Osteoporosis has long been recognized as a risk factor for intraoperative endplate injury [Citation20] and late-onset cage subsidence [Citation21]. Consistent with the previous evidence, a lower hounsfield units (HUs) value was determined to be more prone to injury based on the present findings [Citation22, Citation23]. Reduced bone quality significantly facilitates intraoperative endplate injury [Citation24]. Modic changes and concomitant endplate sclerosis are potential predictors for preventing cage subsidence [Citation25, Citation26]. Endplate stiffness is more important for intraoperative endplate injury and late on-set subsidence than bone quantity of the vertebral body. Therefore, the especially peripheral part should be strong enough to tolerate the altered load transfer following cage insertion in a finite element study [Citation27].
Association of cage characteristics with intraoperative endplate injury
Cage characteristics also play a role in these complications. Most researchers believe that low cage width is a crucial risk factor for subsidence, because it cannot cover the bilateral periphery of the endplate better [Citation28, Citation29]. However, a retrospective clinical study has concluded that no significant correlation between fusion length and sedimentation [Citation30], which is inconsistent with our findings. Currently, the titanium cage and polyetheretherketone (PEEK) cage are the two most common cages in clinical practice [Citation31]. In a systematic review by Kersten who compared a PEEK cage with a bone graft, titanium cage, and carbon fiber cage, no difference was found between PEEK and titanium cage [Citation31]. In the current study, PEEK cage was applied. The position of the cage device during LLIF surgery is still controversial, but anterior placement may reduce stress between the cage and endplate [Citation32, Citation33]. In our study, the height of the front wall of the cage is larger than the back wall and the lordosis is 6 degrees. We believe there is stress distribution nonuniformity when the cage is implanted into disk space. The stress between the endplate and anterior-superior or anterior-inferior edge could be the highest. To protect patients from subsidence after MIS LIF, the surgeon needs to take care with the caudal endplate during cage insertion. If a caudal bilateral (Type 2) endplate breach is detected, supplemental posterior fixation to arrest progression and facilitate fusion is recommended [Citation30]. In the present study, endplate damage is significantly reduced when the front edge of the cage is located on the anterior part of the epiphyseal ring (). Shiga divided the caudal endplate into 5 regions in the sagittal CT films, and suggested the center of the cage falling on zone II would achieve better lordosis correction and the fewest endplate injuries [Citation34]. In fact, when the cage center is located on zone II, the front edge of the cage falls on the anterior part of the epiphyseal ring, which is consistent with our results. In addition, the cage height has been determined to be a positive factor for intraoperative endplate injury, and a cage height of no more than 11 mm is suggested [Citation12, Citation28]. In our study, height variance and cage height were identified to be two strongly correlated variables and the former plays a key role in intraoperative endplate injury. In the process of determining the appropriate height of the cage, cage molds were determined from small size to big size step by step, and damage to the endplate is uncertain. Thus the magnitude of the preoperative disk space should be considered except for the surgeon’s touch during the cage trial stage. A height difference of less than 5 mm between LLIF cage and disk space could reduce the risk of endplate injury.
Association of degeneration of facet joints with intraoperative endplate injury
Moreover, the effect of degeneration and tropism of the facet joint on indirect decompression was noticed. Although several authors mentioned degeneration and tropism of the facet joint did not correlate with indirect decompression [Citation35, Citation36], in our study, severe endplate injury and even vertebral fractures occurred in patients with serious joint degeneration (). It was concluded that, as part of the three-joint complex, degeneration of facet joints could potentially prevent the intervertebral distraction in the axial direction. After the degeneration of the facet joints, the bilateral facet joints are stiff and even hyperostosis, resulting in adhesion and even fusion between the facet joints. Due to the decreased ability of adhesion, fusion and expansion of the facet joints between the vertebral bodies, the stress on the vertebral endplate increases significantly after the implantation of the fusion cage, resulting in endplate injury. It is known that endplate injury is closely related to the decrease of intervertebral space height and cage subsidence or displacement during follow-up, and some cases may require reoperation [Citation37]. Therefore, it is necessary to strengthen the prevention of endplate injury. Once it occurs, timely and effective treatment and close follow-up should be taken.
Limitations
There are limitations to this study. First, this is a retrospective, single-center study and the study sample size is insufficient, and the clinical findings should be verified in a multicenter study with a larger sample size. Second, the number of surgical segments and the width of the cage cannot be assessed because those with scoliosis and multi-segment fusion were excluded, and only the 18 mm wide cage was available in this study. Finally, because this is a retrospective study, and most patients did not follow up in time to acquire functional scores and imaging examinations. The impact of intraoperative endplate injury on bony fusion, clinical outcome and the progressive cage subsidence after the operation are still unclear because of a short follow-up time, in particular, the impact of endplate injury without cage settling on standalone patients requires further study.
Conclusion
The incidence of intraoperative endplate injury is higher in LLIF than in TLIF procedures, possibly because the different stress distribution caused by the indirect decompression mechanism when the cage is implanted. Low bone quality, and big height variance are risk factors for intraoperative end-plate injury in LLIF surgery and the front edge of the cage falls on the anterior part of the epiphyseal ring will reduce the incidence of intraoperative endplate injury. Indirect decompression of neural structures is not recommended when the facet joint has degenerated to the point of a “locked” joint. The cage length and height should be determined based on the vertebral body and the height of the disk space.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Burgstaller JM, Steurer J, Gravestock I, et al. Long-term results after surgical or nonsurgical treatment in patients with degenerative lumbar spinal stenosis: A prospective multicenter study. Spine (Phila Pa 1976). 2020;45(15):1–8. doi:10.1097/BRS.0000000000003457.
- Fleege C, Rickert M, Rauschmann M. [The PLIF and TLIF techniques. Indication, technique, advantages, and disadvantages]. Orthopade. 2015;44(2):114–123. doi:10.1007/s00132-014-3065-9.
- Uysal M, Ozalay M, Derincek A, Kochai A, Turker M. Effect of plif and tlif on sagittal spinopelvic balance of patients with degenerative spondylolisthesis. Acta Orthop Traumatol Turc. 2018;52(4):272–276. doi:10.1016/j.aott.2018.03.001.
- Asil K, Yaldiz C. Retrospective comparison of radiological and clinical outcomes of plif and tlif techniques in patients who underwent lumbar spinal posterior stabilization. Medicine (Baltimore). 2016;95(17):e3235. doi:10.1097/MD.0000000000003235.
- Limthongkul W, Tanasansomboon T, Yingsakmongkol W, Tanaviriyachai T, Radcliff K, Singhatanadgige W. Indirect decompression effect to central canal and ligamentum flavum after extreme lateral lumbar interbody fusion and oblique lumbar interbody fusion. Spine (Phila Pa 1976). 2020;45(17):E1077–E1084. doi:10.1097/BRS.0000000000003521.
- Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including plif, tlif, mi-tlif, olif/atp, llif and alif. J Spine Surg. 2015;1(1):2–18. doi:10.3978/j.issn.2414-469X.2015.10.05.
- Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (xlif): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435–443. doi:10.1016/j.spinee.2005.08.012.
- Woods KR, Billys JB, Hynes RA. Technical description of oblique lateral interbody fusion at l1-l5 (olif25) and at l5-s1 (olif51) and evaluation of complication and fusion rates. Spine J. 2017;17(4):545–553. doi:10.1016/j.spinee.2016.10.026.
- Sharma AK, Kepler CK, Girardi FP, Cammisa FP, Huang RC, Sama AA. Lateral lumbar interbody fusion: Clinical and radiographic outcomes at 1 year: A preliminary report. J Spinal Disord Tech. 2011;24(4):242–250. doi:10.1097/BSD.0b013e3181ecf995.
- Tohmeh AG, Khorsand D, Watson B, Zielinski X. Radiographical and clinical evaluation of extreme lateral interbody fusion: Effects of cage size and instrumentation type with a minimum of 1-year follow-up. Spine (Phila Pa 1976). 2014;39(26):E1582–1591. doi:10.1097/BRS.0000000000000645.
- Kim WJ, Lee JW, Kim SM, et al. Precautions for combined anterior and posterior long-level fusion for adult spinal deformity: Perioperative surgical complications related to the anterior procedure (oblique lumbar interbody fusion). Asian Spine J. 2019;13(5):823–831. doi:10.31616/asj.2018.0304.
- Satake K, Kanemura T, Yamaguchi H, Segi N, Ouchida J. Predisposing factors for intraoperative endplate injury of extreme lateral interbody fusion. Asian Spine J. 2016;10(5):907–914. doi:10.4184/asj.2016.10.5.907.
- Atarod M. An evaluation of occupant dynamics during moderate-to-high speed side impacts. Proc Inst Mech Eng H. 2021;235(5):546–565. doi:10.1177/0954411921994937.
- Kim YH, Ha KY, Kim KT, et al. Risk factors for intraoperative endplate injury during minimally-invasive lateral lumbar interbody fusion. Sci Rep. 2021;11(1):20149. doi:10.1038/s41598-021-99751-6.
- Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26(17):1873–1878. doi:10.1097/00007632-200109010-00011.
- Gabel BC, Hoshide R, Taylor W. An algorithm to predict success of indirect decompression using the extreme lateral lumbar interbody fusion procedure. Cureus. 2015;7(9):e317. doi:10.7759/cureus.317.
- Gum JL, Reddy D, Glassman S. Transforaminal lumbar interbody fusion (tlif). JBJS Essent Surg Tech. 2016;6(2):e22. doi:10.2106/JBJS.ST.15.00003.
- Frisch RF, Luna IY, Brooks DM, Joshua G, O’Brien JR. Clinical and radiographic analysis of expandable versus static lateral lumbar interbody fusion devices with two-year follow-up. J Spine Surg. 2018;4(1):62–71. doi:10.21037/jss.2018.03.16.
- Li YM, Frisch RF, Huang Z, et al. Comparative effectiveness of expandable versus static interbody spacers via mis llif: A 2-year radiographic and clinical outcomes study. Global Spine J. 2020;10(8):998–1005. doi:10.1177/2192568219886278.
- Okano I, Jones C, Salzmann SN, et al. Endplate volumetric bone mineral density measured by quantitative computed tomography as a novel predictive measure of severe cage subsidence after standalone lateral lumbar fusion. Eur Spine J. 2020b;29(5):1131–1140. doi:10.1007/s00586-020-06348-0.
- Agarwal N, Faramand A, Alan N, et al. Lateral lumbar interbody fusion in the elderly: A 10-year experience. J Neurosurg Spine. 2018;29(5):525–529. doi:10.3171/2018.3.SPINE171147.
- Hiyama A, Sakai D, Katoh H, Nomura S, Sato M, Watanabe M. Comparative study of cage subsidence in single-level lateral lumbar interbody fusion. J Clin Med. 2022;11(5):1374. doi:10.3390/jcm11051374.
- Xi Z, Mummaneni PV, Wang M, et al. The association between lower hounsfield units on computed tomography and cage subsidence after lateral lumbar interbody fusion. Neurosurg Focus. 2020;49(2):E8. doi:10.3171/2020.5.FOCUS20169.
- Jones C, Okano I, Salzmann SN, et al. Endplate volumetric bone mineral density is a predictor for cage subsidence following lateral lumbar interbody fusion: A risk factor analysis. Spine J. 2021;21(10):1729–1737. doi:10.1016/j.spinee.2021.02.021.
- Liu J, Ding W, Yang D, et al. Modic changes (MCS) associated with endplate sclerosis can prevent cage subsidence in oblique lumbar interbody fusion (OLIF) stand-alone. World Neurosurg. 2020;138:e160–e168. doi:10.1016/j.wneu.2020.02.047.
- Okano I, Jones C, Rentenberger C, et al. The association between endplate changes and risk for early severe cage subsidence among standalone lateral lumbar interbody fusion patients. Spine (Phila Pa 1976). 2020a;45(23):E1580–E1587. doi:10.1097/BRS.0000000000003668.
- Polikeit A, Ferguson SJ, Nolte LP, Orr TE. The importance of the endplate for interbody cages in the lumbar spine. Eur Spine J. 2003;12(6):556–561. doi:10.1007/s00586-003-0556-5.
- Le TV, Baaj AA, Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976). 2012;37(14):1268–1273. doi:10.1097/BRS.0b013e3182458b2f.
- Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine. 2013;19(1):110–118. doi:10.3171/2013.4.SPINE12319.
- Malham GM, Parker RM, Blecher CM, Seex KA. Assessment and classification of subsidence after lateral interbody fusion using serial computed tomography. J Neurosurg Spine. 2015;23(5):589–597. doi:10.3171/2015.1.SPINE14566.
- Li ZJ, Wang Y, Xu GJ, Tian P. Is peek cage better than titanium cage in anterior cervical discectomy and fusion surgery? A meta-analysis. BMC Musculoskelet Disord. 2016;17(1):379. doi:10.1186/s12891-016-1234-1.
- Park SJ, Lee CS, Chung SS, Kang SS, Park HJ, Kim SH. The ideal cage position for achieving both indirect neural decompression and segmental angle restoration in lateral lumbar interbody fusion (llif). Clin Spine Surg. 2017;30(6):E784–E790. doi:10.1097/BSD.0000000000000406.
- Hiyama A, Katoh H, Sakai D, Sato M, Tanaka M, Watanabe M. Cluster analysis to predict factors associated with sufficient indirect decompression immediately after single-level lateral lumbar interbody fusion. J Clin Neurosci. 2021;83:112–118. doi:10.1016/j.jocn.2020.11.014.
- Shiga Y, Orita S, Inage K, et al. Evaluation of the location of intervertebral cages during oblique lateral interbody fusion surgery to achieve sagittal correction. Spine Surg Relat Res. 2017;1(4):197–202. doi:10.22603/ssrr.1.2017-0001.
- Park D, Mummaneni PV, Mehra R, et al. Predictors of the need for laminectomy after indirect decompression via initial anterior or lateral lumbar interbody fusion. J Neurosurg Spine. 2020;24:1–7. doi:10.3171/2019.11.SPINE19314.
- Navarro-Ramirez R, Lang G, Moriguchi Y, et al. Are locked facets a contraindication for extreme lateral interbody fusion? World Neurosurg. 2017;100:607–618. doi:10.1016/j.wneu.2016.11.059.
- Xie H, Ouyang Z, Zhang H. Radiographic analysis of pedicle screw retractor-assisted transforaminal lumbar interbody fusion for single-segment spondylolisthesis in adults: A retrospective study and technical note. Orthop Surg. 2022;14(9):2219–2229. doi:10.1111/os.13441.