Abstract
Two recent intratracheal instillation toxicology studies in rats clearly show that a naturally occurring quartz, with occluded crystal surfaces (quartz isolate), produced significantly less inflammatory response than a crushed reference quartz (DQ12). Respirable-size quartz isolate was isolated from bentonite parent rock, without crushing or the use of chemicals, to ensure that the surface properties of the quartz particles were unaltered. The isolation technique utilized gentle mechanical dispersion followed by sedimentation in an aqueous medium. Extensive mineralogical and chemical characterizations were undertaken to define the physicochemical properties of the test materials. The characterizations showed significant, measurable physicochemical differences between the two quartz types. These differences may help to explain the difference in toxicological response associated with these materials. The evaluation methods and resulting data that characterized the chemical and physical properties of the instillation test materials are discussed. The data presented show that such characterizations are essential if meaningful correlations are to be made between test materials and their toxicological profiles.
In recent years, investigators have determined that the human toxicity of quartz varies depending on the circumstances of exposure (CitationCastranova et al., 1996b; CitationInternational Agency for Research on Cancer, 1997). Mechanistic studies by CitationDonaldson and Borm (1998) emphasized that the biological toxicity of quartz is related to its surface activity and that this toxicity may vary dramatically depending on the origin of the quartz. CitationFubini (1998) also found that the biological toxicity of quartz is reduced by substances affecting its surface activity. CitationVallyathan et al. (1991) noted that while the toxicity of crushed quartz declines with time after crushing, crushed quartz that has been kept in a clean, dry environment can retain significant measurable toxicity for years. CitationVallyathan et al. (1995) showed that inhalation of respirable-size, “freshly fractured” (crushed within days) quartz leads to enhanced lung injury which may be the result of free radicals on the freshly fractured surfaces. CitationHamilton (1999) suggested a function that could quantify the toxicity related to surface activity of crystalline silica. This function, expressed in surface activity units, takes into account mass, surface area, silica polytype, origin, and time-dependent activity decay coefficients. Most naturally occurring quartz found in the environment has been in contact with a variety of contaminants, such as aluminosilicates, carbon compounds, and metal ions, for long periods of time. These environmental contaminants can occlude the surface of the quartz particles with a thin layer of material that can alter the particle surface characteristics. Quartz particles with these occluded surfaces can have a level of biological activity that is very different from that of quartz particles with unoccluded surfaces, or the surfaces of particles of freshly crushed quartz.
The most direct way to compare the surface differences of crushed quartz and quartz with occluded surfaces, and to relate these differences to their toxicological properties, is to dose laboratory animals with the different quartz species and compare the toxicological effects. A major challenge to this comparison is the ability to obtain a sufficient quantity of respirable-size, occluded quartz particles without changing the particles' surfaces. A method was developed that produced sufficient quantities of material for characterization and toxicological testing.
The Fraunhofer Institute of Technology and Experimental Medicine conducted two rat intratracheal instillation toxicology studies to compare the biological effects of respirable-size, crushed quartz and a naturally occurring quartz with occluded surfaces. The results of these studies are reported in Creutzenberg et al. (Citation2003, Citation2008). In the first study, conducted in 2001, the animals were evaluated for 28 days post dosing. In the second study, conducted in 2004, the post-dosing observation period was 90 days. Both studies reported significant, persistent inflammation with the crushed quartz, while the natural quartz with occluded surfaces produced a much lower degree of inflammation that was nonpersistent. The physicochemical properties of the instillation test materials used in both studies were characterized in order to better understand the nature of their toxicity.
MATERIALS AND METHODS
Test Materials
Any toxicological study must be based on a thorough understanding of the chemical and physical properties of the test material. Any characterization of the test material should also include an understanding of the history of the material and how that may have affected its properties. The materials used in these studies were crushed quartz, quartz isolated from sodium bentonite, montmorillonite clay isolated from sodium bentonite, and titanium dioxide. All test materials were composed of respirable-size particles.
Reference Quartz
DQ12 quartz obtained from Bergbauforschung, Essen, Germany, was used as the reference quartz. DQ12 quartz has well-documented biological activity, and has served as a frequent positive control in toxicological studies. This material is a purified sand obtained from the Dörentrup kaolin deposit in Germany (CitationRobok, 1973; CitationClouter et al., 2001). The sand was crushed approximately 30 years ago and then air centrifuged in 1985 to separate the bulk material into fine, midsize, and coarse fractions. The fine fraction, used in the 28-day study, was designated as “Reference Quartz 2001.” The mid-size fraction, used in the 90-day study, was designated as “Reference Quartz 2004.”
Quartz Isolate
“Quartz Isolate” as used in this article refers to the naturally occurring, respirable-size, occluded quartz separated from sodium bentonite, without the use of crushing or chemical additives. Obtaining a sufficient quantity of natural, respirable-size quartz with occluded surfaces, without altering the surface characteristics of the particles, requires great care. Many possible sources for these particles were examined. Of these, crude sodium bentonite was found to be the only material from which the quartz particles could be readily isolated without the need to dry, crush, or chemically treat the material. Crude sodium bentonite, from the 110-to 112-million-year-old Newcastle formation in Wyoming, was used to obtain the occluded quartz particles used in these studies.
Sodium bentonite is composed principally of the clay mineral montmorillonite, which occurs in water-dispersible particles that are typically < 0.5 μ m in size. A low-shear, aqueous dispersion technique was developed that, over time, allows the quartz particles in the bentonite to become separated from the clay particles. This technique makes it unnecessary to dry, crush, or chemically disperse the crude clay to liberate the quartz particles from the clay matrix, and thereby minimizes the potential for altering the natural occluded crystal surfaces of the quartz grains. The naturally occurring, respirable-size quartz particles in the bentonite were separated from the aqueous clay dispersion and concentrated by centrifugation. (A more detailed description of the separation procedure is available from the authors upon request. Initial separation and concentration of the Quartz Isolate material was done by Southern Clay Products, Gonzales, TX.). The resulting quartz concentrates were purified by repeated suspension in deionized water and sedimentation, using Stokes's Law to calculate sedimentation time. Following this process, the < 0.5 μ m montmorillonite particles were first removed, followed by the > 5.0 μ m particles. This process allowed the final particle size distribution of the quartz concentrate to be brought into the 1 to 5 μ m range. Each resulting quartz concentrate was maintained as an aqueous slurry to eliminate possible effects that drying might have on the surface properties of the particles. The quartz concentrates used in the 28-day and 90-day studies were designated as “Quartz Isolate 2001” and “Quartz Isolate 2004,” respectively.
This isolation technique may be applicable to isolating quartz particles from other sedimentary source rocks as well.
Titanium Dioxide
The titanium dioxide used was Bayertitan T, lot 85/13928, from Bayer AG, Krefeld, Germany. This material was produced by flame hydrolysis of TiCl4 and is the rutile polymorph.
Positive Control
Both toxicological studies were designed to dose the animals, in the control and test groups, with the same mass of test material and the same dose of quartz. The quartz content in the two reference quartzes was greater than that in the quartz isolates. In order to achieve the proper dosing parameters, titanium dioxide was added to dilute the reference quartzes, to produce positive control mixtures that contained the same quartz content as their respective quartz isolate. The resulting positive control mixtures were designated Positive Control 2001, for the 28-day study, and Positive Control 2004, for the 90-day study.
Clay Isolate
The montmorillonite clay separated from the sodium bentonite used to obtain Quartz Isolate 2001 was designated as the clay isolate. This material, which contained less than 0.04% quartz, was obtained using the aqueous dispersion and centrifugation techniques described for the quartz isolates.
Chemical Composition
The chemical composition of the test materials was determined using the standard lithium metaborate/tetraborate fusion method for inductively coupled plasma (ICP) determination of the major and trace element concentrations (CitationJohnson et al., 1981). (Testing was conducted by Activation Laboratories Ltd., Ancaster, Ontario, Canada.) The lithium metaborate/tetraborate method ensures that the entire sample is dissolved, enabling major oxides and high field strength elements to be put into solution. The results of chemical analysis of the quartz isolates were used to confirm their mineralogy as determined by x-ray diffraction analysis.
Mineralogical Composition
An understanding of the mineralogy of the reference quartz and quartz isolate samples is important for determining the purity and crystallinity of the quartz. In the case of the quartz isolates it was also important to determine the presence of other mineral components that might affect the surface properties and toxicity of these materials. X-ray diffraction (XRD) analysis was used for determination and quantification of the mineralogical composition of test materials. The quantification of quartz employed the standard NIOSH method 7500, adapted for bulk samples (CitationNational Institutes of Occupational Safety and Health, 2003). The latest quartz standard, NIST Standard Reference Material (SRM) 1878a, which is currently used for α-quartz determinations, was used as the calibration standard (CitationNational Institute of Standards and Technology, 2005). Quantification of the trace minerals analcime and calcite used standard XRD procedures (CitationKlug et al., 1974). Quantification of the swelling clay mineral, montmorillonite, poses special problems due to its variable composition. Following standard practice, the chemical contributions of the nonmontmorillonite mineral phases were deducted from the total chemical analysis. The remaining chemical values were used to generate a chemical formula matching that of the montmorillonite unit cell structure, following the method of CitationRoss and Hendricks (1945). The amount of montmorillonite could then be estimated from the resulting chemical formula. Any excess SiO2 was identified as undefined silica.
XRD analysis also allows determination of the domain size, which is an important characteristic of quartz. A domain is defined as a volume within a crystalline material that has a coherent diffracting alignment. A single crystal quartz particle can have a multitude of domains. Domain size is determined by measuring the diffraction line broadening in an XRD pattern, and applying the Warren–Averbach equation (CitationWarren & Averbach, 1953). CitationMurata and Norman (1976) described a semi-empirical treatment of line broadening they termed the “crystallinity index.” The crystallinity index appears to be largely a function of domain size (up to about 1 μ m diameter) but may also be affected by crystal lattice distortions induced by mechanical stress.
Knowledge of the domain size or its surrogate, crystallinity index, is critical in the quantification of crystalline silica by x-ray diffraction. Using x-ray diffraction, the intensity of a quartz sample is compared with that of a standard reference material to determine the quartz content of the sample. Quartz that has a significantly smaller domain size than that of the standard reference material, as a result of either the natural crystallization process that formed it (e.g., quartz isolates) or postformation stress-induced trauma, such as that produced by intense grinding (e.g., reference quartz), can yield a diffraction intensity per unit mass that may be as little as 50% of that produced by large domain, untraumatized quartz. As a result of this effect, the quartz content of materials containing quartz with a low crystallinity index may be significantly underreported when using a high-crystallinity-index standard reference material, such as NIST SRM 1878a. When this happens most analysts attribute the missing silica to an amorphous phase. In these circumstances, there is no distinct, physically separable, amorphous phase, and reporting amorphous silica as an identified phase is incorrect. Rather, the missing material should be included in the quartz content or simply listed as undefined and not assigned to a specific phase. The mineralogical composition of the quartz materials used in this study, and reported in , was obtained using SRM 1878a as the calibration standard. When compared with the chemical analysis (), the quartz values do not account for all of the silica present in these materials. Because no physically separable, identifiable, amorphous silica phases were found, the excess silica has been identified here as “undefined silica.”
TABLE 2 Physical properties of instillation materials for the 2001 and 2004 Fraunhofer rat studies
TABLE 1 Chemistry of instillation materials used in the 2001 and 2004 Fraunhofer rat studies
Thermal Analysis
Thermal measurements, such as differential scanning calorimetry (DSC), can be used to obtain information about certain mineral characteristics that cannot be obtained by other methods. DSC measures thermal reactions, either endothermic or exothermic, during heating of the sample from room to high temperatures. DSC testing was conducted using a Netzsch simultaneous thermal analyzer (STA) 409 CD equipped with platinum crucibles for differential scanning calorimetry. Additionally, high-temperature XRD analysis was conducted using a Rigaku DMAX2200 in theta–theta configuration with HT Direct Heating Pt/Rh carriers and an HT 1600C furnace attachment (thermal XRD analyzer).
Particle Size Distribution and Surface Characteristics
Scanning electron microscope (SEM) examination of mineral particles allows direct determination of the shape of particles in a sample, as well as the statistical particle size distribution of the sample. Samples of test materials were carbon coated using a Denton Vacuum DV-502A vacuum evaporator and then viewed in a Hitachi S-4200 field emission scanning electron microscope (SEM), operating at 15 kV, with a 7-mm working distance. The long and short particle dimensions were measured from digital images for 1000 particles of each test material using ImagePro 4.5 image analysis software. The diameter and mass distributions for each material were then determined. The mass distribution data was then averaged for each test material to obtain the mass mean geometric diameter (MMGD) of that material.
Surface Area
Surface-area measurements become problematic when dealing with samples containing expandable clays. The conventional Brunauer–Emmett–Teller (BET) technique, which is typically used to determine surface area for most minerals, involves gas adsorption after heating the sample in vacuo. However, minerals such as montmorillonite will expand in water and reveal a much larger surface area. Because tissues of living organisms are aqueous systems, the BET method does not provide the proper environment in which to measure the surface area of expandable minerals used for in vivo toxicological testing. The ethylene glycol monoethyl ether (EGME) technique has been shown to be useful where expandable minerals are involved (CitationBower et al., 1959; CitationCarter et al., 1965). This method assumes that EGME is absorbed as a single monolayer on all exposed surfaces, both internal and external. For swelling clays, like montmorillonite, this results in much larger surface area measurements than those obtained with the BET method.
Electron Spin Resonance
The ability of quartz to generate free radicals appears to be an important aspect of its potential biological toxicity (CitationCastranova et al., 1996a; CitationChen et al., 1998). Electron spin resonance spectroscopy (ESR) enables identification and quantification of unpaired electrons in paramagnetic compounds that may act as free radicals. Because most ESR work on quartz has been conducted on pure quartz, this study sought to determine whether ESR would also be predictive of biological activity in mixed-mineral systems, such as the quartz isolates. ESR measurements were obtained using a Bruker EMX spectrometer at a frequency of 9.80 GHz; power 63.5 mW; receiver gain 50,000; time constant 0.04 s; modulation amplitude 1.0 gauss; scan time 41 s; and magnetic field 3487 ± 100 G. The Quartz Isolate 2004, Reference Quartz 2004, and an additional quartz sample, Min-U-Sil 5 (a very fine, crushed, single crystal quartz), were first analyzed in aqueous slurry form using the DMPO/hydrogen peroxide spin adduct method described by CitationCastranova et al. (1996b) to compare their abilities to generate relatively long-lived OH• free radicals. These analyses were conducted both with and without deferoxamine, an iron chelator that has been shown to eliminate OH• radicals generated by quartz (CitationCastranova et al., 1996b; CitationChen et al., 1998). The samples were then also analyzed as dry powders to determine their silicon source free radicals. Each dry sample was then dry crushed in a Fritsch IEC ball mill for 10 min and then immediately reanalyzed.
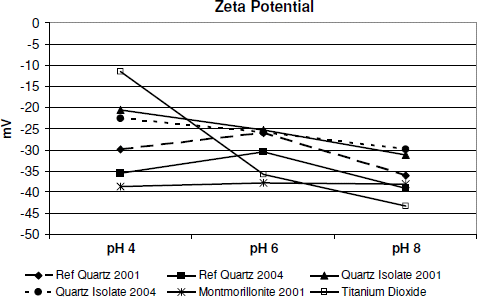
Zeta Potential Measurements
Zeta potential measures the electrical potential of colloidal particles in an aqueous suspension and can provide useful information about the boundary between colloidal particles and the medium in which they are suspended. Zeta potential values are affected by the pH of the solution and so are typically measured at several pH levels. Three 0.1% suspensions of each test material were prepared by adding the material to deionized water and processing for 3 min in a Waring blender at 14,500 rpm. The pH of the three suspensions for each material was then adjusted to 4, 6, and 8, respectively, using HCl or NaOH. Each suspension was then injected into a test cell and tested with a Malvern Zetasizer Nano ZS using the standard ZTS program.
Examination of Recovered Particles
Techniques that were developed to study the fate of inorganic and organic fibers in the lung have also been found to be useful in detecting changes to quartz particles after residence in the lung (CitationHesterburg et al., 1996a, Citation1996b). Plasma ashing is considered the best method for isolating particles from lung tissue without introducing artifacts. In this technique, samples are not heated above 40°C so delicate inorganic structures remain unaltered. The residue from plasma ashing is typically composed of mineral phases and a mixture of anhydrous oxides and salts from the inorganic constituents of the tissue.
A preliminary validation study was conducted with samples of the quartz isolate and reference quartz, mixed with lung tissues harvested from untreated rats. This study shows no effects of the plasma ashing procedures on the morphological properties of the particles.
At the conclusion of the life portion of the 90-day study whole lungs were harvested from the test animals and cartilaginous airway tissue was then removed by dissection. The lungs were then freeze-dried. Standard preservation methods, using formalin and phosphate buffers, were avoided so as not to alter the particle surfaces. Lung samples included lungs from untreated animals (air control) and saline-instilled control animals, Quartz Isolate 2004-instilled samples, and Positive Control 2004-instilled samples. In each case, a 100-mg lung sample was ashed in an oxygen plasma using an LTA-504 low-temperature oxygen plasma asher (LFE Plasma Systems, Clinton, MA) for 16 h at a chamber pressure of 1 mm mercury. The resulting ash was dispersed in 100 ml hot water. An aliquot of the suspended ash was then filtered onto a 0.2-μ m pore diameter Nuclepore polycarbonate membrane filter. The filtered ash was then mounted on an SEM stub, carbon coated in a vacuum evaporator, and placed in an SEM for imaging. SEM photomicrographs were examined to determine whether there were any differences between pre-and post-instillation quartz particle surface morphology and particle size. Semi-quantitative analysis of 10 particles from each lung of each of six animals was carried out for seven major oxides, using the energy-dispersive spectroscopy (EDS) function of the microscope. The same EDS analyses were also performed on 10 particles from each of the stock samples of Quartz Isolate 2001 and Quartz Isolate 2004.
RESULTS AND DISCUSSION
The 28-day and 90-day studies show significant toxicological differences between the reference quartz, composed of crushed DQ12, and quartz isolates, with occluded surfaces. These differences are paralleled by striking differences in the physicochemical nature of the respective quartz particles.
summarizes the chemical analysis of the test materials used in the 28-day and 90-day studies. The chemistry of each quartz isolate was used to confirm its mineralogy as determined by x-ray diffraction analysis. These data show that the chemical compositions of the two quartz isolates are very similar, as are the chemical compositions of the two reference quartz samples. The data also shows that the chemical compositions of the quartz isolates differ significantly from those of the reference quartz samples. Some of these differences are due to the presence of residual montmorillonite and other trace minerals associated with the quartz isolates.
The physical properties of the test materials used in these studies are summarized in . SEM analysis shows the MMGD of the Quartz Isolate 2001 to be 3.69 μ m, which is nearly 3 times larger than the 1.31 μ m MMGD of the reference quartz used in the 28-day study. Particle size is known to play a significant role in the toxicity of crystalline silica. Due to the large difference in particle size between the test materials used in the 28-day study, it was not possible to determine whether the results of the study were due to differences in the toxicity of the quartz species, or related to their particle size. To eliminate this problem, the quartz test materials used in the 90-day study were selected to have similar particle sizes. The MMGD of Quartz Isolate 2004 was 3.58 μ m, which compared well with the MMGD of 3.01 μ m for the reference quartz. The Bayertitan T titanium dioxide, used to create the positive control material, had an MMGD of 0.81 μ m.
Recent research has shown that sample surface area, sample particle size, and sample particle number, rather than sample weight (mass), are the proper metrics in toxicological studies of particulate materials (CitationOberdörster et al., 2005; CitationStoeger et al, 2006; CitationWittmaack, 2007). Although surface area may provide a useful metric when dealing with pure test substances, the presence of materials having very large surface areas, such as montmorillonite in the quartz isolates, can overwhelm the contribution of other materials, making data interpretation problematic. Further, use of the standard BET surface area measurement does not reflect the actual surface area of water-expandable minerals, such as montmorillonite, when they are used for in vivo toxicological studies. As previously discussed, the surface area values from the EGME method are considered to be a more accurate measure of surface area when dealing with expandable-mineral-containing materials, such as the quartz isolates used in these studies. The surface area data, presented in , show the dramatic difference between the results obtained using these two methods. These data also show the significant difference in surface area between the reference quartz and quartz isolate test materials.
XRD analysis of Reference Quartz 2001 and Reference Quartz 2004 showed these materials to be mineralogically identical, with both materials containing 87% quartz. Neither kaolin nor rubber contamination, from the grinding process, was detected in either material as previously reported (CitationRobok, 1973). Though not absolutely identical, Quartz Isolate 2001 and Quartz Isolate 2004 have very similar mineralogical compositions containing 62% and 60% quartz, respectively.
Chemical analysis of the two reference quartzes showed that they were both composed of 99.3% silica. The lack of correlation of these results with the XRD mineralogical results indicates either that the crystallinity of both reference quartz samples is significantly less than that of the NIST SRM 1878a reference standard or that they may contain 13% undefined silica. In consideration of the method used to prepare these samples, the authors feel it is likely that the undefined silica, quantified by XRD, may actually be composed of quartz particles whose crystalline structure has been altered by the crushing process. These particles yield XRD patterns that differ in crucial details from the pattern of the NIST SRM 1878a standard quartz. In either case, this indicates that NIST SRM 1878a, although commonly used as a calibration standard for toxicological studies, may not be the appropriate standard to use for all quartz studies.
XRD analysis also revealed that the domain size and crystallinity index values of Quartz Isolate 2001 and 2004 are 0.085 μm/1.6 and 0.076 μ m/1.6, respectively. The domain size and crystallinity index values of Reference Quartz 2001 and 2004 are 0.16 μ m/6.0 and 0.19 μ m/5.0, respectively. These results show a significant, fundamental difference in the characteristics of the crystal structure between the quartz isolate and reference quartz materials.
A unique characteristic of quartz is that it undergoes a reversible, crystallographic phase transition from α quartz to β quartz at 573°C. This transition can be detected using DSC methods. A DSC scan of Reference Quartz 2004 clearly showed an α quartz to β quartz transition at 573°C. This transition was confirmed by high temperature XRD analysis. In contrast, DSC analysis of Quartz Isolate 2004 did not reveal any indication of an α quartz to β quartz phase transition even when the sample was heated to 775°C. However, when 13% Reference Quartz 2004 was mixed with 87% Quartz Isolate 2004 the resulting DSC plot did show the reversible α ↔ β transition at 573°C. As a result, a high-temperature XRD study was conducted to investigate this unusual thermal behavior of the quartz isolates. When a sample of Quartz Isolate 2004 was heated to well above the α ↔ β phase transition temperature, the resulting XRD pattern showed that the quartz was in the β form. This confirmed that the phase transition did indeed take place, even though it was undetected in the DSC study. This same behavior has previously been observed in polycrystalline quartz from soils (CitationDrees et al., 1989). The lack of an α ↔ β transition endotherm in this type of quartz may be attributed either to a very high degree of crystal lattice distortion, resulting from impurities, or to the presence of many very small domains, all with slightly different transition temperatures.
These data reveal that the nature of the internal crystal organization of the reference quartz is significantly different from that of the quartz isolates. The domain size of the quartz isolates is quite small, showing that these crystals have numerous disruptions that affect the crystalline continuity within a single particle. In contrast, the reference quartz has a much larger domain size indicating that there are significantly fewer disruptions within the crystal structure of each particle. The thermal data also show that, in contrast to the particles of reference quartz, the quartz isolate particles are not acting as single crystals but, rather, as assemblages of numerous smaller crystals.
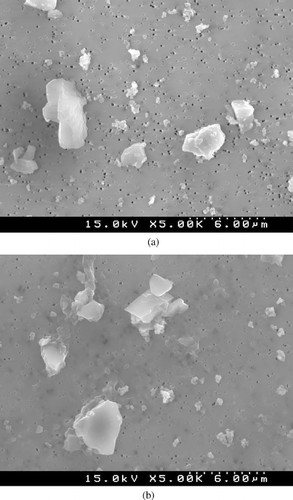
SEM photomicrographs show that the reference quartz is composed of highly angular, crushed fragments of larger quartz crystals (). In contrast, SEM photomicrographs of the quartz isolate show it to be composed of agglomerates of unbroken quartz intergrown with montmorillonite and undefined silica. Additionally, the quartz isolate particles appear to have filmy edges that may be an expression of thin layers of montmorillonite that coat the particles ().
The results of ESR testing showed that Quartz Isolate 2004, Reference Quartz 2004, and Min-U-Sil 5 all produced ESR spectra that indicate they possess the ability to generate Si (Si• and SiO•) and long-lived OH• free radicals. In wet preparations, the OH• radical peak heights in these ESR spectra show that the relative ability to generate free radicals was in the order of Reference Quartz 2004 = Min-U-Sil 5 > Quartz Isolate 2004. The Si radicals peak heights show an order of Quartz Isolate 2004 > Reference Quartz 2004 = Min-U-Sil 5 (). The addition of deferoxamine greatly reduced or eliminated the induced free radical signal from all the samples tested. Many earlier studies relate the intensity of the ESR signals for OH•, Si•, and SiO• directly to biological activity (CitationFubini et al., 1990; CitationCastranova et al., 1996a). The results of this study indicate that this relationship appears to be valid only when dealing with pure quartz, however. While the quartz isolate in the present study generates a significant ESR signal, the results of the toxicological studies show that it is significantly less biologically active than the reference quartz samples (CitationCreutzenberg, 2008). The explanation for this apparent discrepancy may lie in the presence of Fe2 + and Fe3 + ions in the residual montmorillonite associated with the quartz isolates, which may create radicals through a Fenton reaction. Apparently, these radicals are not as biologically active as the radicals generated on the fractured surfaces of the reference quartz.
TABLE 3 Electron spin resonance of Quartz Isolate 2004 and Reference Quartz 2004
CitationCastranova (1997), CitationFubini (1998), and others have shown that there is a relative decline of the ESR signal and biological toxicity of crushed quartz with time. After the initial dry ESR analyses, the Quartz Isolate 2004, Reference Quartz 2004, and Min-U-Sil 5 samples were each crushed for 10 min in a Fritsch IEC centrifugal ball mill. The resulting crushed samples were immediately reexamined by ESR. The ESR peak height for all three quartz samples increased slightly. This experiment shows that the strength of the ESR signal may not be indicative of relative biological toxicity. Further, when dealing with materials in which quartz particles are intimately associated with other minerals, such as the quartz isolates, the ESR data could be misleading and may not be a predictor of biological activity.
Some researchers feel that the electrical potential of the surface of respirable-size particles, as represented by their zeta potential, may be an indicator of their toxicological potential. The results of zeta potential measurements of the test materials in the present studies are presented in . It is apparent that the patterns of the zeta potential plots of the quartz isolate samples are different from those of the other test materials. Depending on pH, the zeta potential of Reference Quartz 2001 and 2004 is from 3 to 57% higher than that of Quartz Isolates 2001 and 2004 respectively. This indicates that the reference quartzes have a much more electrically reactive surface than do the quartz isolates.
SEM photomicrographs of quartz isolate and reference quartz before instillation (stock material) and after recovery from rat lungs from the 90-day study are shown in , a and b, and , a and b, respectively. These figures show that the surface morphology of both quartz materials was unchanged, either by 90 days of residence in the rat lungs or by the plasma ashing technique used to recover the particles.
FIG. 4 (a) SEM photomicrograph of quartz isolate before instillation in rat lung (5000×). (b) SEM photomicrograph of quartz isolate recovered from rat lung after 90 days by low-temperature plasma ashing (5000×).
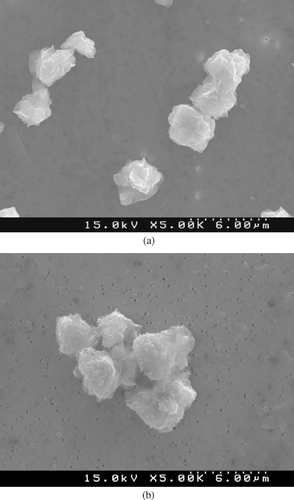
FIG. 5 (a) SEM photomicrograph of reference quartz before instillation in rat lung (5000×). (b) SEM photomicrograph of reference quartz recovered from rat lung after 90 days by low-temperature plasma ashing (5000×).
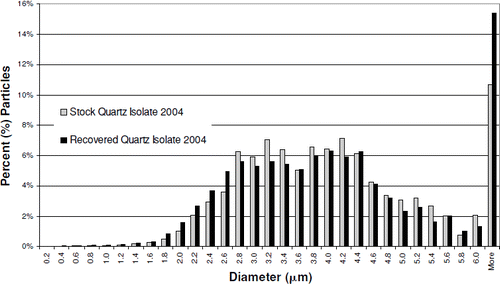
SEM images of the Quartz Isolate 2004 and Positive Control 2004 samples were also evaluated to determine particle size distribution, prior to instillation and following recovery from rat lungs. Particles recovered from the Quartz Isolate 2004-exposed lungs appear to show an increase in the number of fine particles compared to the stock material (). The median diameter of the recovered Quartz Isolate 2004, based on particle number, is 2.3 μ m, compared to 2.6 μ m for the stock material. The difference between these two materials is most pronounced in particles with diameters between 3.8 and 2.8 μ m, and less than 1.4 μ m. Increased lung inflammation is known to contribute to an increase in the amount of inorganic fine particulate residue present after low-temperature plasma ashing. The presence of this residue, associated with lung injury, may account for at least a portion of the difference in particle size distribution between the stock and recovered Quartz Isolate 2004. There was no difference in the mass median diameter between the recovered Quartz Isolate 2004 and the stock Quartz Isolate 2004 (). The mass of the finer particles in the recovered material was balanced by the occurrence of a few larger more massive particles.
FIG. 6 Recovered Quartz Isolate 2004 particle diameter distribution vs. stock material. Median diameter: stock particles, 2.6 μ m; recovered particles, 2.3 μ m.
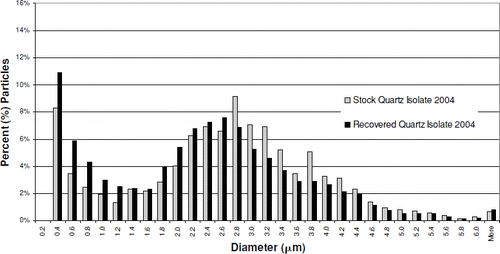
FIG. 7 Recovered Quartz Isolate 2004 particle mass distribution vs. stock material. Median diameter: stock particles, 3.8 μ m; recovered particles, 3.8 μ m.
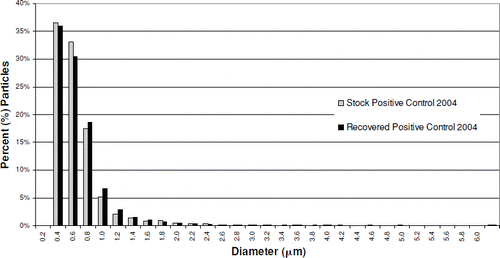
The particle size distribution of both the positive control stock material and the recovered positive control material, based on particle number, is dominated by the particles of titanium dioxide used as the diluent in their preparation (). The median diameter of both positive control materials, and the titanium dioxide, is 0.5 μ m. The mass median diameters of the positive control stock and recovered materials were 3.1 and 3.4 μ m, respectively. The smaller mass median diameter of the stock material may reflect the affects of the ultrasonic processing, which was necessary to break up aggregates of titanium dioxide in that material, prior to measurement, rather than being indicative of any changes occurring in the lung. It does not appear that there was any meaningful change in the particle size distribution of the positive control material after 90 days in rat lungs.
FIG. 8 Recovered Positive Control 2004 particle diameter distribution vs. stock material. Median diameter: stock particles, 0.5 μ m; recovered particles, 0.5 μ m.
The results of the EDS analysis of the Quartz Isolate 2004, recovered from rat lungs, and from the Quartz Isolate 2004 stock are summarized in . Also included in this table are wet chemical analysis results of the stock Quartz Isolate 2004. The analyses are normalized to 100% for the seven oxides reported. The EDS results for the stock Quartz Isolate 2004 compares well with the wet chemical analysis data. There is no significant difference in the chemistry of the recovered quartz isolate particles and the stock material. The lack of change in the aluminum oxide and magnesium oxide values is evidence that no clay is being lost from the particles during their 90-day residence time in the animal lung.
FIG. 9 Recovered Positive Control 2004 particle mass distribution vs. stock material. Median diameter: stock particles, 3.1 μ m; recovered particles, 3.4 μ m.
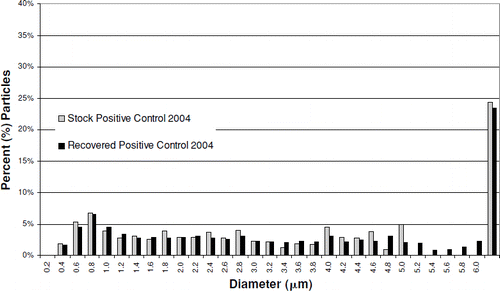
TABLE 4 EDS chemical analysis of stock Quartz Isolate 2004 and Quartz Isolate 2004 recovered from rat lungs after 90 days by plasma ashing
The toxicological studies showed a noticeable initial inflammation with the reference quartz, quartz isolate, and clay isolate test materials. In the 28-day study the initial inflammatory response to the clay isolate was equal to that of the reference quartz but decreased significantly by day 3 postdosing. In both the 28-day and 90-day studies the inflammation from the reference quartz samples persisted and increased over time. In contrast, the inflammation from the quartz isolates declined significantly over the course of the studies, though not as rapidly or to the degree found with the clay isolate. The presence of montmorillonite in the quartz isolates and the similarity of the inflammatory response profile between the quartz isolates and the clay isolate suggest that montmorillonite plays a role in the quartz isolate's inflammatory response. The amount of inflammation, as well as the progression of the inflammation over time, attributable to either the montmorillonite or quartz in the quartz isolate has yet to be determined.
SUMMARY AND CONCLUSIONS
Physicochemical and mineralogical characterization tests conducted on the quartz samples used in the 28-day and 90-day rat intratracheal instillation toxicology studies show that there are significant differences between the chemical, physical, and mineralogical properties of the crushed reference quartz and the quartz isolates with occluded surfaces. These differences are particularly striking when viewed in the context of the results of the toxicological studies, which clearly showed that the quartz isolates produced a significantly lower degree of inflammatory response than the reference quartz at the termination of the studies. The physicochemical and mineralogical differences of the test materials used in these studies provide useful information that helps to elucidate their toxicological differences.
In the published literature on quartz toxicology the quartz materials that have been used have not always been properly or thoroughly characterized. The results presented here clearly show that there can be significant differences between quartz species. The importance of a comprehensive characterization of any test substance, especially those that contain quartz, cannot be overstated. Without proper and thorough physicochemical and mineralogical characterization of mineral test materials used in toxicology studies, it may be impossible to draw meaningful conclusions from resulting toxicological data.
This study was supported by the Sorptive Minerals Institute, Washington, DC. The authors thank the following individuals and organizations for their help in developing data for this article: Dr. Marc Herpfer, Oil-Dri Corporation, Chicago, who directed surface area measurement using BET and EGME methods; Dr. Vincent Castranova and Dr. Steven Leonard, NIOSH, Morgantown, WV, who conducted the electron spin resonance studies; Dr. Don Eisenhour, AMCOL International, Arlington Heights, IL, who obtained the zeta potential test data; Gary Tomaino, Mineral Technologies, Inc., Easton, PA, who conducted the differential scanning calorimetry and high-temperature x-ray diffraction analysis; Dr. Haydn Murray, Indiana University, Bloomington, IN, who allowed use of his laboratory for developing the separation techniques; and Dr. M. I. Knudson, Southern Clay Products, Gonzales, TX, who provided the initial quartz concentrates used in both rat studies. The authors also thank Dr. John Domanski for his very helpful comments and suggestions.
REFERENCES
- C. A. Bower, and J. O. Goertzen. (1959). Surface area of soils and clays by an equilibrium ethylene glycol method. Soil Sci. 87:289–292.
- D. L. Carter, M. D. Heilman, and C. L. Gonzalez. (1965). Ethylene glycol monoethyl ether for determining surface area of silicate minerals. Soil Sci. 100:356–360.
- V. Castranova, W. H. Pailes, N. S. Dalal, P. R. Miles, L. Bowman, V. Vallyathan, and D. Pack. (1996a). Enhanced pulmonary response to the inhalation of freshly fractured silica as compared with aged dust exposure. Appl. Occup. Environ. Hyg. 11:937–941.
- Silica and silica-induced lung diseases. V. Castranova, V. Vallyathan, and W. E. Wallace. CRC Press, Boca RatonFL, (1996b) .
- V. Castranova, V. Vallyathan, D. M. Ramsey, J .L. McLaurin, D. Pack, S. Leonard, M. W. Barger, J. Y. C. Ma, N. S. Dalal, and A. Teass. (1997). Augmentation of pulmonary reactions to quartz inhalation by trace amounts of iron-containing particles. Environ. Health Perspect. 105 (Suppl. 5):1315–1324.
- F. Chen, Y. Lu, L. M. Demers, Y. Rojanasakul, X. Shi, V. Vallyathan, and V. Castranova. (1998). Role of hydroxyl radical in silica-induced NF-kappa B activation in macrophages. Ann. Clin. Lab. Sci. 28:1–13.
- A. Clouter, D. Brown, D. Höhr, P. Borm, and K. Donaldson. (2001). Inflammatory effects of respirable quartz collected in workplaces versus standard DQ12 quartz: Particle surface correlates. Toxicol. Sci. 63:90–98.
- O. Creutzenberg, G. Oberdörster, S. G. Ampian, W. F. Moll, R. D. Hamilton, and H. Muhle. (2003). Inflammatory effects of quartz samples after intratracheal instillation in rats: Meeting abstract. Toxicol. Sci. 72 (2 Suppl):45.
- O. Creutzenberg, T. Hansen, H. Ernst, H. Muhle, G. Oberdörster, and R. Hamilton. (2008). Toxicity of a quartz with occluded surfaces in a 90-day intratracheal instillation study in rats. Inhal. Toxicol. in press
- K. Donaldson, and P. J. A. Borm. (1998). The quartz hazard, a variable entity. Ann. Occup. Hyg. 42:287–294.
- L. R. Drees, L. P. Wilding, N. E. Smeck, and A. L. Senkayi. Silica in soils: Quartz and disordered silica polymorphsMinerals in soil environments 2nd, J. B. Dixon, and S. B. Weed. Soil Science Society of America, MadisonWI, (1989) 913–974.
- B. Fubini. (1998). Surface chemistry and quartz hazard. Ann. Occup. Hyg. 42:521–530.
- B. Fubini, E. Giamello, M. Volante, and V. Bolis. (1990). Chemical functionalities at the silica surface determining its reactivity when inhaled. Formation and reactivity of surface radicals. Toxicol. Ind. Health 6:571–598.
- R. D. Hamilton. (1999). Surface activity, an improved model for the characterization of crystalline silica dose 7th Internationals Symposium on Particle Toxicology, Maastricht, the Netherlands, October 13–15, 1999, abstr. t82
- T. W. Hesterburg, G. A. Hart, J. Chavalier, W. C. Miiller, R. D. Hamilton, J. Bauer, and P. Thevenaz. (1996a). Lung biopersistence and in vitro dissolution rate predict the pathogenic potential of synthetic vitreous fibers. Inhal. Toxicol. 12 (suppl 3):91–97.
- T. W. Hesterburg, W. C. Miiller, R. P. Musselman, O. Kamstrup, R. D. Hamilton, and P. Thevenaz. (1996b). Biopersistence of man-made vitreous fibers and crocidolite asbestos in the rat lung following inhalation. Toxicol. Sci. 29:267–279.
- International Agency for Research on Cancer. (1997). Silica, some silicates, coal dust, and para-aramid fibrils. IARC Monogr. Eval. Carcinogen. Risk Chem. Hum. 68:41–242.
- W. M. Johnson, and J. A. Maxwell. Rock and mineral analysis 2nd. John Wiley & Sons, New York, (1981) 109–111.
- H. P. Klug, and L. E. Alexander. X-ray diffraction procedures: For polycrystalline and amorphous materials 2nd,. Wiley-Interscience, New York, (1974) .
- K. J. Murata, and M. B. NormanII. (1976). An index of crystallinity for quartz. Am. J. Sci. 276:1120–1130.
- National Institutes of Occupational Safety and Health. (2003). Silica, crystalline, by XRD: Method 7500, Issue 4 http://www.cdc.gov/niosh/nmam/pdfs/7500.pdf
- National Institute of Standards and Technology. (2005). Certificate of analysis, Standard Reference Material 1878a, respirable alpha quartz Available at: https://srmors.nist.gov/view_cert.cfm?srm=1878A
- G. Oberdörster, E. Oberdörster, and J. Oberdörster. (2005). Nanotoxicology: An emerging discipline evolving from studies of ultrafine particle. Environ. Health Perspect. 113:823–839.
- K. Robok. (1973). Standard quartz DQ12 < 5 μ m for experimental pneumoconiosis research projects in the Federal Republic of Germany. Ann. Occup. Hyg. 16:63–66.
- C. S. Ross, and S. B. Hendricks. Minerals of the montmorillonite group, their origin, and relation to soils and clays.. U.S. Department of the Interior, Geological Survey, WashingtonDC, (1945) Professional Paper 205-B.
- T. Stoeger, C. Reinhard, S. Takenaka, A. Schroeppel, E. Karg, B. Ritter, J. Heyder, and H. Schulz. (2006). Instillation of six different ultrafine carbon particles indicates a surface area threshold dose for acute lung inflammation in mice. Environ. Health Perspect. 114:328–333.
- V. J. Vallyathan, H. Kang, K. van Dyke, N. S. Dalal, and V. Castranova. (1991). Response of alveolar macrophages to in vitro exposure to freshly fractured versus aged silica dust: the ability of Prosil 28, an organosilane material, to coat silica and reduce its biological reactivity. J. Toxicol. Environ. Health 33:303–315.
- V. Vallyathan, V. Castranova, D. Pack, S. Leonard, J. Shumaker, A. F. Hubbs, D. A. Shoemaker, D. M. Ramsey, J. R. Pretty, and J. L. McLaurin. (1995). Freshly fractured quartz inhalation leads to enhanced lung injury and inflammation. Potential role of free radicals. Am. J. Respir. Crit. Care 152:1003–1009.
- B. E. Warren, and B. L. Averbach. (1953). The effect of cold-work on X-ray patterns. J. Appl. Phys. 21:595–599.
- K. Wittmaack. (2007). In search of the most relevant parameter for quantifying lung inflammatory response to nanoparticle exposure: Particle number, surface area or what?. Environ. Health Perspect. 115:187–194.