Abstract
This paper highlights the pervasive misconception concerning 1994 findings from Hatch et al. about ozone (O3) tissue dose in humans versus rats. That study exposed humans to 0.4 ppm and rats to 2 ppm 18O-labeled O3 and found comparable incorporation of 18O into bronchoalveolar lavage constituents. However, during O3 exposure humans were exercising, which increased their ventilation rate five-fold, while rats were at rest. This resulted in similar O3 tissue doses between the two species, and predominantly explained the comparable 18O incorporation at five-fold different concentrations. The five-times higher exercising human inhalation rate offset the five-times lower concentration, producing the same human dose expected at rest at 2 ppm (i.e. 0.4 ppm × 4686 L/2 hour ≈ 2 ppm × 998 L/2 hour). In 2013, Hatch et al. showed that resting humans and resting rats experienced fairly comparable 18O incorporation at the same O3 exposure concentration and activity state into BALF cells. Despite these findings, we show here that in the peer-reviewed literature a substantial proportion of researchers continue to perpetuate the misunderstanding that human lung tissue doses of O3 are simply 3–5 times greater than rat doses at the same O3 concentration, due to interspecies differences, and not considering activity state. It is important to correct this misconception to ensure an appropriate understanding of the implications of O3 studies by the scientific community and policy experts making regulatory decisions (e.g. the US Environmental Protection Agency’s National Ambient Air Quality Standards for O3).
Introduction
Ozone (O3) is a photochemical air pollutant that arises secondarily from a series of complex reactions in the troposphere between nitrogen oxides, volatile organic compounds, and the ultraviolet spectrum of sunlight (Costa, Citation2008a). Historically, toxicological literature has shown that relatively high levels of O3 (>1 parts per million) contribute to laboratory animal morbidity and mortality (Stokinger, Citation1957), and in humans lower levels can cause diminished lung function (Schelegle, Citation2001) and may exacerbate asthma (). O3 is one of six “criteria” air pollutants for which regulatory standards have been set by the US Environmental Protection Agency (USEPA) through the National Ambient Air Quality Standards (NAAQS) program. The O3 NAAQS level is currently set at 0.070 parts per million (ppm) for an annual fourth-highest daily eight-hour maximum, averaged over three years. Historical values for the O3 NAAQS have been 0.12 ppm for a one-hour maximum averaging time, and 0.08, 0.075, and currently 0.070 ppm for an eight-hour maximum averaging time. Typically, the justifications for these NAAQS have been an extrapolation from available controlled O3 exposures of exercising human subjects, with support from human epidemiology and laboratory animal toxicity studies (USEPA, Citation2013). These animal toxicological studies utilize relatively high exposure levels of O3 (0.2–3 ppm) to demonstrate a range of acute and chronic inhalation toxicities.
Figure 1. O3 concentration-response from animal studies – the dose makes the poison. O3 concentration (in ppb) versus severity of response Depuydt et al., Citation1999; Dye et al., Citation1999; Mittler et al., Citation1956; Scheel et al., Citation1959; Schelegle et al., Citation2001; Stokinger, Citation1957.
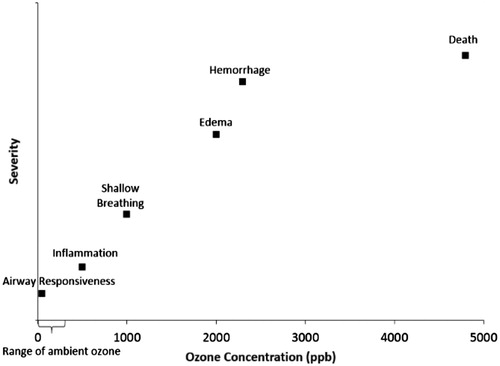
By contrast, according to the latest O3 Integrated Science Assessment (ISA; USEPA, Citation2013), from 2007 to 2009 the median eight-hour daily maximum concentration across all US sites was 0.040 ppm, which is 5–75 times lower than the animal toxicity study concentrations mentioned above. While the ISA (USEPA, Citation2013) generally considers pollutant concentrations within one or two orders of magnitude of ambient conditions to be relevant, experimental animal exposure concentrations appreciably higher than those experienced by humans are not environmentally relevant or particularly useful in identifying health effects likely to occur in humans exposed under ambient conditions. This is because the mechanisms that cause toxicity at high concentrations may be entirely different than those operating at lower concentrations. This is the concept of dose-dependent transitions in mechanisms of toxicity (Slikker et al., Citation2004). For this reason, a correct understanding of the relevance of experimental animal O3 exposure concentrations to humans exposed to O3 environmentally (i.e. cross-species extrapolation) is crucial.
In speaking with O3 researchers about their work, it became apparent that there is a common misconception about what O3 concentrations produce equal lung tissue doses in rats and humans. Some researchers seem to erroneously believe that simply due to interspecies differences, rats must be exposed to O3 concentrations 3–5 times higher than humans to produce the same dose to the lungs. The misconception is that human lung tissue doses of O3 are 3–5 times greater than rat lung tissue doses at the same O3 concentration due to interspecies differences in dosimetry. Furthermore, in speaking with O3 researchers, it became apparent that the basis of this misconception (and its perpetuation) lies in a narrow interpretation of the findings of Hatch et al. (Citation1994).
Briefly, Hatch et al. (Citation1994) exposed eight male human volunteers (18–35 years of age) to 0.4 ppm 18O-labeled O3 (18O3) for two hours with intermittent 15-minute periods of heavy treadmill exercise. Additionally, two dose groups of resting male rats (n = 19 and 20) were exposed to 0.4 and 2 ppm 18O-labeled O3 for two hours, respectively. Tissue dose of O3 was assessed using microgram per gram (μg/g) 18O incorporation into bronchoalveolar lavage fluid (BALF) constituents from rats and humans collected 0–1 hour after exposure. BALF is a well-established specimen that can safely be collected, and has been used for dose–response studies (e.g. release of inflammatory cells into the BALF). Hatch et al. considered the BALF cells and surfactant to have an alveolar origin, based on histological evidence, so in the following text we refer to this as the alveolar region (Hatch et al., Citation1994, Citation2013; Pinkerton et al., Citation2015). In general, BALF found in distal airspaces (i.e. alveolar region) in the lower part of the lung collected from healthy, never-smoker individuals has a characteristic profile of cellular and acellular constituents. Likewise, it is common for acutely and chronically diseased individuals (e.g. acute interstitial pneumonia, diffuse alveolar damage, sarcoidosis) to have a characteristic profile of cellular and acellular constituents found in their BALF as well (Meyer & Raghu, Citation2011). Hatch et al. (Citation1994) found that exercising humans had 4–5 times higher 18O concentrations in their BALF constituents than did resting rats at the same O3 concentration of 0.4 ppm, with the implication being that O3 toxicity in resting rats underestimates dose in exercising humans. Citing Hatch et al. (Citation1994), Barreno et al. (Citation2013) correctly reported that the dose of O3 delivered to the lower lungs is the product of O3 concentration, exposure time, and minute ventilation (VE); thus, exercising during exposure increases the VE of subjects and subsequently, the total dose of O3 delivered to the lungs. However, many other researchers do not caveat nor document the importance of the difference in activity state when citing Hatch et al. (Citation1994), but rather simply imply that rats, across the board, must be exposed to a 3–5 times higher O3 concentration than humans to achieve an equal dose, due to interspecies differences in dosimetry. This is in contrast to Hatch et al. (Citation1994), who suggested that exercise may be more important than species differences in determining alveolar O3 dose. Consistent with this conclusion, the 4–5 times higher alveolar O3 dose observed in exercising humans compared to resting rats in Hatch et al. (Citation1994) is most simply explained primarily by the approximately five-fold increase in ventilation rate in the human subjects induced by exercise. Hatch et al. (Citation2013) repeated this experiment with humans at rest, and found comparable incorporation of 18O into BALF cells between rats and humans exposed to 0.4 ppm 18O3. They found twice as much incorporation of 18O into the BALF surfactant in humans compared to rats, but the implications of this for lung cell dose is uncertain, and the authors, as well as ourselves, chose to use the BALF cell (not surfactant) 18O incorporation as the measure of alveolar tissue dose. Hatch et al. (Citation2013) noted that the BALF cell 18O incorporation was five-times higher in the exercising human subjects, compared to those at rest. They were exercising intermittently for two hours and breathed a total of 4686 L (i.e. 3876 L for 60 total minutes of intermittent exercising + 810 L for 60 total minutes of intermittent rest between exercise periods = 4686 L total for the two-hour intermittent exercise scenario; note the addition error in Table 3 of Hatch et al., Citation2013) compared to only 998 L total for two hours at rest, resulting in a 4.7-fold difference and almost perfectly explaining the difference in alveolar tissue 18O incorporation.
In this paper, we investigated the prevalence of the misconception that rats must simply be exposed to a concentration of O3 3–5 times higher than humans to achieve a comparable tissue dose, through a search of the scientific, peer-reviewed literature. We present here the finding that this misconception is quite prevalent among those who cite the work of Hatch et al. (Citation1994).
Methods
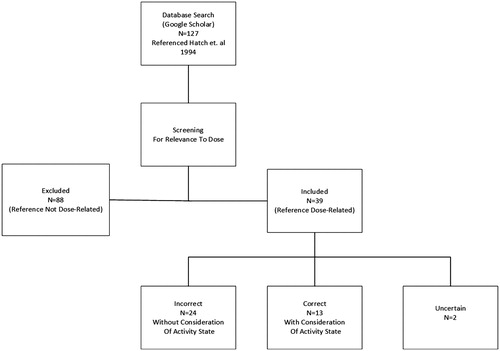
To assess the pervasiveness of the misunderstanding that rats must be exposed to a concentration of O3 3–5 times higher than a human in order to achieve a similar alveolar tissue dose, we first searched for the Hatch et al. (Citation1994) manuscript in Google Scholar (17 May 2016) and used the “cited by” function to identify all of the published papers that cited the Hatch et al. (Citation1994) manuscript. These papers comprised a broad spectrum of studies (i.e. in vitro to in vivo), which were further screened to exclude those articles that did not specifically address the comparability of/differences in rat versus human tissue doses (e.g. referenced Hatch et al. for methods only) (). Then the papers were categorized as “Correct”, “Incorrect” or “Uncertain” () based on their use/interpretation of the findings of Hatch et al. (Citation1994) regarding rat versus human alveolar doses. “Correct” articles were those wherein study text reflected a proper understanding of the influence of activity state in discussing species differences in alveolar dose at given exposure concentrations (e.g. studies that had an appreciation for increases in ventilation rate and corresponding alveolar dose due to exercise, and/or acknowledged the importance of considering rest versus exercise when comparing doses across species, either explicitly or simply through appropriately caveated text). For example, the following sentence is properly caveated as to activity state (although “effects” should arguably more appropriately be “doses”), and so is deemed to reflect a “correct” understanding of Hatch et al. (Citation1994), “The effect of O3 exposures in resting rats underestimate those observed in exercising human” (Pryor et al., Citation1996). “Incorrect” articles were ones wherein study text perpetuated an iteration of the misunderstanding that the human alveolar dose, across the board, is simply 3–5 times greater than rat tissue doses at the same O3 concentration (e.g. Miller et al., Citation2016). The authors ascribed this to some difference (e.g. interspecies, sensitivity, age, cardiac condition) in dosimetry, did not explicitly or implicitly consider activity state as a factor (e.g. Stiegel et al., Citation2016, ), and/or assumed comparability/equivalency of rodent exposure at rest to exercising human exposure at the same concentration. For some articles, it was not clear as to whether there was a proper appreciation of the influence of activity state on alveolar dose in different species. Thus, these studies were deemed “uncertain”. For example, in discussing their rodent study exposure concentration, Elder et al. (Citation2000) indicate, “The exposure concentration of O3 (1 ppm) is high, but is similar to an exposure to 0.25 ppm in an exercising human (Hatch et al., Citation1994)”. Given that rodents in the Elder et al. study were not made to exercise, for purposes of the current study it was assumed that this sentence is implicitly discussing resting rodent versus exercising human dose, although this was not explicitly stated by the authors (i.e. 1 ppm not caveated by “at rest”). The prevalence of the three categories was then calculated as the percent of published papers citing Hatch et al. (Citation1994) regarding rat versus human alveolar dose with either a “correct”, “incorrect” or “uncertain” interpretation (). We assessed all included studies based on these criteria, regardless of whether the subject of the study’s investigation was rats or other mammals, or was even an in vitro study. That is because we were assessing the prevalence and perpetuation of the stated misconception in the literature, not how often this misconception was used to explicitly choose O3 concentrations in a rat study.
Table 1. Dose-related quotes from papers referencing Hatch et al. (Citation1994).
Results
In total, 127 articles were identified that referenced the Hatch et al. (Citation1994) study. Of these, 88 articles referenced Hatch et al. but did not address interspecies differences in alveolar dose specifically. For example, this includes referencing for methods, experimental design, and/or the analysis/incorporation of 18O into the BALF and its constituents, and so were unrelated to the question at hand and were excluded from further analysis. Thirteen of the remaining 39 articles (33%) properly referenced Hatch et al. (Citation1994) in regards to the importance of activity state/ventilation rate (i.e. exercising humans versus resting rats) in estimating relevant alveolar doses (). Twenty-four articles (62%) that referenced Hatch et al. (Citation1994) perpetuated the misconception that human alveolar doses of O3 should simply be expected to be 3–5 times greater than rat doses at equal exposure concentrations (). In addition, one article, Oakes et al. (Citation2013), went as far as to say that a rat O3 exposure concentration of 2 ppm was “comparable” to a human exposure concentration of 0.2 ppm in susceptible human subjects. It is not clear how the authors reached this conclusion (i.e. a 10-fold higher rat exposure concentration is comparable), but they cited Hatch et al. (Citation1994). The two remaining articles (5%) were deemed not clear in the researchers’ citation/interpretation of Hatch et al. (Citation1994) and were placed in the “uncertain” category (). Overall, the available articles included both in vivo (i.e. human, rats, mice, monkeys) and in vitro assays, and covered a range of dates from 1998 to 2016.
Our review found that most authors who cited Hatch et al. (Citation1994) for their findings of 18O incorporation in rats versus humans used it to inappropriately explain or justify an O3 animal exposure scenario based on the misconception (i.e. interspecies differences in O3 dose, with humans experiencing higher doses than rats). As the studies did not use a different metric of tissue dose and merely used concentration as a surrogate, results for/discussion of other dose metrics is not relevant for the limited purpose of this paper.
Discussion
We found that a misunderstanding/misapplication of the findings of Hatch et al. (Citation1994) is pervasive, with researchers not properly understanding and/or appropriately considering that the original Hatch et al. (Citation1994) study exposed humans and rats under very different activity states (i.e. resting versus exercising), which explains the differences in measured alveolar dose. This misconception occurred in 62% of the studies identified (24 of 39 studies; ), published up to present day. Thus, these studies continue to perpetuate the misconception that rats receive a 3–5 times lower alveolar dose than humans at the same O3 concentration simply due to interspecies differences in dosimetry.
Although Hatch et al. (Citation2013) specifically demonstrated that resting human subjects achieve a BALF cell 18O3 dose (5.6 ± 1.7 μg/g dry weight) somewhat (i.e. 25%) lower than that of resting rats (7.5 ± 1.6 μg/g dry weight) at the same exposure concentration (and much lower than exercising humans), with two-fold higher 18O incorporation into human BALF surfactant (Table 4 of Hatch et al., Citation2013), the paper apparently accomplished little in terms of correcting the common misunderstanding based on the previous paper (Hatch et al., Citation1994), as evidenced by results of the current study. In our findings, more than half of the papers did not account for the critical consideration that Hatch et al. (Citation1994) results from resting rats are being compared to those from exercising humans. Given the fact that exercising humans breathed approximately five times more air than those at rest (i.e. 3876 L for 60 total minutes of intermittent exercising + 810 L for 60 total minutes of intermittent rest between exercise periods = 4686 L total for the two-hour intermittent exercise scenario/998 L total for the two-hour resting scenario = 4.7-fold difference; note the addition error in Table 3 of Hatch et al., Citation2013) as well as other considerations (see below), it is not particularly surprising that exercising humans accumulated approximately five times more 18O in BALF cells than resting rats. Ultimately, this results in the incorrect conclusion that high, environmentally-irrelevant O3 exposure concentration (e.g. >500 ppb) rat study results are directly relevant to environmentally-exposed humans, without accounting for human activity and simply ignoring the fact that exposure concentration, duration and exercise state are the primary determinants of the alveolar dose of O3 in humans and rats, which are more important than potential interspecies differences in dosimetry.
In addition to the results of the Hatch et al. (Citation2013) study itself, other considerations support that the human equivalent concentration (HEC)/effective dose corresponding to a rat study exposure concentration should not be five times lower at comparable activity levels. These considerations, which suggest that the rat may be an appropriate if not conservative model for humans (i.e. the HEC is not lower than the rat concentration), include:
Interspecies differences in alveolar dose;
USEPA interspecies dosimetric adjustment procedures; and
Modeled predictions of O3 absorption in respiratory tract regions of interest (i.e. alveolar region).
These topics and their implications for interspecies (rat versus human) dosimetric differences are briefly discussed below.
Interspecies differences in lung dose
Rats breathe more air per unit body weight (BW) than humans, which is a consideration judged by Hatch et al. (Citation2013) to be relevant and appropriate for discussion. An example of this is provided in Appendix 2 of Hatch et al. (Citation2013), which uses an allometric equation to calculate that the rat ventilation rate per unit BW is 2.82 times higher than that for humans. Based on this consideration, the authors acknowledge that rats should receive a higher alveolar dose and that this fact is contrary to the notion that rats may underestimate human O3 dose. By corollary, it is also contrary to the idea that a five-fold higher exposure concentration is needed for rats to receive the same alveolar dose of 18O as humans. In fact, because rats should receive a higher alveolar dose, despite their discussion of Hatch et al. (Citation1994) results (based on a comparison that does not consider the increased ventilation rate produced by exercise), the study authors conclude that, "The finding that human resting BALF 18O dose approximates that of the resting rat BALF 18O dose is unexpected". In other words, if any interspecies differences in dose would be expected, based on the knowledge that rats breathe more air per unit BW than humans, the study authors would expect the rat to overestimate human dose. Thus, in addition to the results of the Hatch et al. (Citation2013) study itself, which should be given the greatest weight, this consideration judged to be relevant by Hatch et al. themselves suggests that the HEC corresponding to a rat study O3 exposure concentration should not be lower at the same level of physical activity, much less five times lower. If anything, it suggests that humans should receive a lower dose.
Standard USEPA interspecies dosimetric adjustment procedures
This section discusses standard USEPA dosimetric adjustment method considerations to demonstrate general expectations about the interspecies extrapolation of dose. O3 is a Category 2 gas based on its physical/chemical and toxicokinetic characteristics and their influence on sites of toxicity. More specifically, O3 is a Category 2 gas because it has the ability to exert both portal of entry (POE) and systemic effects. For Category 2 gases, USEPA suggests different interspecies dosimetric adjustment procedures depending on where the specific adverse effect being evaluated occurred (i.e. POE versus systemic). That is, when a POE effect produced by a Category 2 gas is being evaluated, the default dosimetric adjustments for a Category 1 gas (which tend to be highly reactive and undergo rapid, irreversible reactions in the respiratory tract) are recommended, while the dosimetric adjustments for a Category 3 gas are recommended when a Category 2 gas has produced a systemic effect (pp. 4–17, USEPA, Citation1994). Accordingly, when a POE effect is induced in the pulmonary region, whether by a Category 1 or Category 2 gas, the dosimetric adjustments for a Category 1 gas are used. Thus, when O3 exerts POE effects, the interspecies dosimetric adjustment procedure used is that for a Category 1 gas.
In general, for pulmonary region effects (from the respiratory bronchioles to the alveoli) caused by either a Category 1 or 2 gas, the HEC is calculated as the animal (e.g. rat) exposure concentration multiplied by the regional gas dose ratio for the pulmonary region (RGDRPU). The RGDRPU accounts for animal-to-human differences in deposition and absorption within the pulmonary region. USEPA (Citation2009) provides a rat-to-human RGDRPU of 3.5. This indicates that not only is the HEC generally not five times lower than the rat exposure concentration for pulmonary effects, it is generally expected to be approximately 3.5 times higher. Thus, for example, the HEC that would correspond to a rat exposure concentration of 400 ppb for pulmonary effects would be approximately 1400 ppb using this standard procedure (i.e. HEC = animal exposure concentration × RGDRPU = 400 ppb × 3.5 = 1400 ppb). Simply being aware of typical rat-to-human RGDRPU values should lead a researcher to question and critically evaluate the basis for any expectation of a five-fold lower HEC, which would correspond to an RGDRPU of 0.2 (e.g. HEC = animal exposure concentration × RGDRPU = 400 ppb ×0.2 = 80 ppb, which would account for a five-fold higher dose in humans compared to rats). Thus, consideration of standard USEPA interspecies dosimetric adjustment procedures for POE effects suggests that the HEC corresponding to a rat study exposure concentration should be higher than the rat concentration, not lower, much less five times lower.
Modeled predictions of O3 absorption
Furthermore, while the above discussion on interspecies dosimetric adjustment procedures is general in nature, O3 absorption has been modeled in both humans and rats. Tables 3–5 of USEPA (Citation2012) provides such modeling results with the aim of simulating how interspecies anatomical and physiological differences influence the transport of O3 throughout the airways and alveoli. Briefly, contrary to the notion that rats may underestimate human dose by five-fold at the same exposure concentration, the modeling results showed that total absorbed amount of O3 (g/kg BW) was found to be 30% higher in rats. More specifically and importantly, the amount of O3 absorbed per surface area and unit time (g/cm2/min) in the alveolar region was reported to be slightly higher (11%) in the rat, although still comparable to that in humans. Similar to other considerations (e.g. empirical data from Hatch et al., Citation2013), this information suggests that despite previous erroneous notions about the rat underestimating human alveolar O3 dose, the rat may be an appropriate if not conservative model for human alveolar dose. That is, the relevant considerations and information discussed above do not support that the HEC corresponding to a rat study exposure concentration should be lower than the rat concentration, much less five times lower.
Conclusions
In conclusion, our experience and research indicate that:
It is common for researchers to assert that the HEC/effective dose for O3 is 3–5 times lower than the rat exposure concentration (i.e. that rats must simply be exposed to O3 concentrations 3–5 times higher than humans to produce the same dose to the lower lung);
As a result, this misconception has essentially become “common knowledge” among O3 researchers, even though it is erroneous;
This erroneous assertion appears to have originated and persisted due to an inappropriate/incongruous comparison of study results for exercising humans versus resting rats (Hatch et al., Citation1994), which is often insufficiently caveated when cited or in some instances has not been made clear; and
The misconception is now prevalent in the scientific literature.
This frequently accepted but erroneous notion is entirely inconsistent with expectations about potential interspecies differences in O3 dose at the same air concentration, as well as with currently available data. These expectations are based on considerations of interspecies differences in alveolar dose, USEPA dosimetric adjustment procedures, and modeled predictions of O3 absorption. More importantly, the erroneous notion on the part of 62% of the research papers citing Hatch et al. (Citation1994) in regard to the HEC for a given rat O3 exposure concentration being five-fold lower is contrary to the Hatch et al. (Citation2013) demonstration of comparable 18O incorporation into alveolar BALF cells in resting humans and rats. Inopportunely, it appears that the comparison of mismatched exposure scenario results (exercising humans versus resting rats) from Hatch et al. (Citation1994) has also resulted in many researchers incorrectly concluding that high, environmentally-irrelevant O3 exposure concentration (e.g. >500 ppb) rat study results are relevant to environmentally-exposed humans, without considering physical activity. Thus, based on our review this misunderstanding is prevalent in the scientific literature and regrettably will be difficult to correct. Having said that, it is important for the scientists and policy makers responsible for making regulatory decisions (e.g. setting the O3 NAAQS) to have a more refined and accurate understanding of the applicability of doses used in O3 studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Barreno RX, Richards JB, Schneider DJ, et al. (2013). Endogenous osteopontin promotes ozone-induced neutrophil recruitment to the lungs and airway hyperresponsiveness to methacholine. An J Physiol Lung Cell Mol Physiol 305:L118–29.
- Boussouar A, Araneda S, Hamelin C, et al. (2009). Prenatal ozone exposure abolishes stress activation of Fos and tyrosine hydroxylase in the nucleus tractus solitarius of adult rat. Neurosci Lett 452:75–8.
- Cho HY, Gladwell W, Yamamoto M, Kleeberger SR. (2013). Exacerbated airway toxicity of environmental oxidant ozone in mice deficient in Nrf2. Oxid Med Cell Long 2013:14.
- Cho HY, Morgan DL, Bauer AK, Kleeberger SR. (2007). Signal transduction pathways of tumor necrosis factor-mediated lung induced by ozone in mice. Am J Respir Crit Care Med 175:829–39.
- Costa DL. (2008a). Chapter 28: air pollution in perspective. In: Klaassen CD (ed) The basic science of poisons Casarett & Doull's. 7th ed. New York: McGraw Hill, 1119–56.
- Costa DL. (2008b). Alternative test methods in inhalation toxicology: challenges and opportunities. Exp Toxicol Pathol 60:105–9.
- Crémillieux Y, Servais S, Berthezène Y, et al. (2008). Effects of ozone exposure in rat lungs investigated with hyperpolarized 3 He MRI. J Magn Reson Imaging 27:771–6.
- Depuydt P, Joos GF, Pauwels RA. (1999). Ambient ozone concentrations induce airway hyperresponsiveness in some rat strains. Eur Respir J 14:125–31.
- Dye JA, Ledbetter AD, Schladweiler MC, et al. (2015). Whole body plethysmography reveals differential ventilatory responses to ozone in rat models of cardiovascular disease. Inhal Toxicol 27:14–25.
- Dye JA, Madden MC, Richards JH, et al. (1999). Ozone effects on airway responsiveness, lung injury, and inflammation. Comparative rat strain and in vivo/in vitro investigations. Inhal Toxicol 11:1015–40.
- Elder ACP, Gelein R, Finkelstein JN, et al. (2000). Pulmonary inflammatory response to inhaled ultrafine particle is modified by age, ozone exposure, and bacterial toxin. Inhal Toxicol 12:227–46.
- Gackière F, Saliba L, Baude A, et al. (2011). Ozone inhalation activates stress-responsive regions of the CNS. J Neurochem 117:961–72.
- González-Guevara E, Martínez-Lazcano JC, Custodio V, et al. (2014). Exposure to ozone induces a systemic inflammatory response: possible source of the neurological alterations induced by this gas. Inhal Toxicol 26:485–91.
- Haque R, Umstead TM, Freeman WM, et al. (2009). The impact of surfactant protein-A on ozone-induced changes in the mouse bronchoalveolar lavage proteome. Proteome Sci 7:12.
- Hatch GE, McKee J, Brown J, et al. (2013). Biomarkers of dose and effect of inhaled ozone in resting versus exercising human subjects: comparison with resting rats. Biomark Insights 8:53–67.
- Hatch GE, Slade R, Harris LP, et al. (1994). Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med 150:676–83.
- Huffman LJ, Beighley CM, Frazer DG, et al. (2006). Increased susceptibility of the lungs of hyperthyroid rats to oxidant injury: specificity of effects. Toxicology 225:119–27.
- Huffman LJ, Judy DJ, Brumbaugh K, et al. (2001). Hyperthyroidism increases the risk of ozone-induced lung toxicity in rats. Toxicol Appl Pharmacol 173:18–26.
- Kleeberger SR, Ohtsuka Y, Zhang LY, Longphre M. (2001). Airway responses to chronic ozone exposure are partially mediated through mast cells. J Appl Physiol 90:713–23.
- Kumarathasan P, Vincent R, Goegan P, et al. (2002). Alteration in aromatic hydroxylation and lipid oxidation status in the lungs of rats exposed to ozone. Toxicol Mech Methods 12:195.
- Martinez-Lazcano JC, Gonzalex-Guevara E, Rubio MDC, et al. (2013). The effects of ozone exposure and associated injury mechanisms on the central nervous system. Rev Neurosci 24:337–52.
- Meyer KC, Raghu G. (2011). Bronchoalveolar lavage for the evaluation of interstitial lung disease: is it clinically useful? Eur Respir J 38:761–9.
- Mikerov AN, Phelps DS, Gan X, et al. (2014). Effect of ozone exposure and infection on bronchoalveolar lavage: sex differences in response patterns. Toxicol Lett 230:333–44.
- Miller DB, Karoly ED, Jones JC, et al. (2015). Inhaled ozone (O3)-induces changes in serum metabolomic and liver transcriptomic profiles in rats. Toxicol Appl Pharmacol 286:65–79.
- Miller DB, Snow SJ, Schladweiler MC, et al. (2016). Acute ozone-induced pulmonary and systemic metabolic effects are diminished in adrenalectomized rats. Toxicol Sci 150:312–22.
- Mittler S, Hedrick D, King M, Gaynor A. (1956). Toxicity of ozone: I. Acute toxicity. Industrial medicine and surgery, July 1956, 301–6.
- Nadadur SS, Costa DL, Slade R, et al. (2005). Acute ozone-induced differential gene expression profiles in rat lung. Environ Health Perspect 113:1717–22.
- Oakes JL, O’Connor BP, Warg LA, et al. (2013). Ozone enhances pulmonary innate immune response to a toll-like receptor-2 agonist. Am J Respir Cell Mol Biol 48:27–34.
- Ong CB, Kumagai K, Brooks PT, et al. (2015). Ozone-induced type 2 immunity in nasal airways development and lymphoid cell dependence in mice. Am J Respir Cell Mol Biol 54:331–40.
- Perpu RSP, Garcia C, Dostal D, Sethi R. (2010). Enhanced death signaling in ozone-exposed ischemic-reperfused hearts. Mol Cell Biochem 336:55–64.
- Pinkerton KE, Gehr P, Crapo JD. (2015). Architecture and cellular composition of the air-blood barrier. In: Parent RA (ed.) Comparative biology of the normal lung. Vol. 2. Boca Raton, FL: CRC Press, 121–8.
- Pryor WA, Bermudez E, Cueto R, Squadrito GL. (1996). Detection of aldehydes in bronchoalveolar lavage of rats exposed to ozone. Fundam Appl Toxicol 34:148–56.
- Scheel LD, Dobrogorski OJ, Mountain JT, et al. (1959). Physiologic, biochemical, immunologic and pathologic changes following ozone exposure. J Appl Physiol 14:67–80.
- Schelegle ES, Alfaro MF, Putney L, et al. (2001). Effect of C-fiber-mediated, ozone-induced rapid shallow breathing on airway epithelial injury in rats. J Appl Physiol 91:1611–18.
- Shore SA, Williams ES, Chen L, et al. (2011). Impact of aging on pulmonary responses to acute ozone exposure in mice: role of TNFR1. Inhal Toxicol 23:878–88.
- Slikker W, Andersen ME, Bogdanffy MS, et al. (2004). Dose-dependent transitions in mechanisms of toxicity. Toxicol Appl Pharmacol 201:203–25.
- Snow SJ, Gordon CJ, Bass VL, et al. (2016). Age-related differences in pulmonary effects of acute and subchronic episodic ozone exposures in Brown Norway rats. Inhal Toxicol 28:313–23.
- Soulage C, Perrin D, Cottet-Emard JM, et al. (2004). Central and peripheral changes in catecholamine biosynthesis and turnover in rats after a short period of ozone exposure. Neurochem Int 45:979–86.
- Stiegel MA, Pleil JD, Sobus JR, Madden MC. (2016). Inflammatory cytokines and white blood cell counts response to environmental levels of diesel exhaust and Ozone inhalation. PLoS One 11:e0152458. DOI: 10.1371/journal.pone.0152458
- Stokinger HE. (1957). Evaluation of the hazards of ozone and oxides of nitrogen; factors modifying toxicity. AMA Arch Ind Health 15:181–90.
- Thomson EP, Goegan P, Kumarathasan, Vincent R. (2004). Air pollutants increase gene expression of the vasoconstrictor endothelin-1 in the lungs. Biochim Biophys Acta 1689:75–82.
- Tsujino I, Kawakami Y, Kaneko A. (2005). Comparative simulation of gas transport in airway models of rat, dog, and human. Inhal Toxicol 17:475–85.
- USEPA (United States Environmental Protection Agency). (1987). Update to the health assessment document and addendum for dichloromethane (methylene chloride): pharmacokinetics, mechanism of action and epidemiology. Review draft (EPA/600/8-87/030A). Washington, DC: Office of Health and Environmental Assessment.
- USEPA (United States Environmental Protection Agency). (1992). EPA request for comments on draft report on cross-species scaling factor for cancer assessment [57 FR 24152, 5 June 1992].
- USEPA (United States Environmental Protection Agency). (1994). Methods for derivation of inhalation reference concentrations and application of inhalation dosimetry (EPA/600/8-90/066F). Research Triangle Park (NC).
- USEPA (United States Environmental Protection Agency). (2009). Status report: advances in inhalation dosimetry of gases and vapors with portal of entry effects in the upper respiratory tract (EPA/600/R-09/072). Research Triangle Park (NC).
- USEPA (United States Environmental Protection Agency). (2012). Advances in inhalation gas dosimetry for derivation of a reference concentration (RfC) and use in risk assessment (EPA/600/R-12/044). Washington (DC).
- USEPA. (2013). Integrated science assessment for ozone and related photochemical oxidants (Final). National Center for Environmental Assessment (NCEA) (EPA/600/R-06/076F, February, 1251).
- van Bree L, Dormans JAMA, Boere AJF, Rombout PJA. (2001). Time study on development and repair of lung injury following ozone exposure in rats. Inhal Toxicol 13:703–17.
- Vella RE, Pillon NJ, Zarrouki B, et al. (2015). Ozone exposure triggers insulin resistance through muscle c-Jun N-terminal kinase activation. Diabetes 64:1011–24.
- Wagner JG, Jiang Q, Harkema JR, et al. (2007). Ozone enhancement of lower airway allergic inflammation is prevented by γ-tocopherol. Radic Biol Med 43:1176–88.
- Ward WO, Ledbetter AD, Schladweiler MC, Kodavanti UP. (2015). Lung transcriptional profiling: insights into the mechanisms of ozone-induced pulmonary injury in Wistar Kyoto rats. Inhal Toxicol 27: 80–92.
- Yang Q, Ge MQ, Kokalari B, et al. (2015). Group 2 innate lymphoid cells mediate ozone-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 137:571–8.
- Zhao Q, Simpson LG, Driscol KE, Leikauf GD. (1998). Chemokin regulation of ozone-induced neutrophil and monocyte inflammation. Am J Physiol: Lung Cell Mol Physiol 274:L39–L46.
- Zhong J, Allen K, Rao X, et al. (2016). Repeated ozone exposure exacerbates insulin resistance and activates innate immune response in genetically susceptible mice. Inhal Toxicol 28:383–92.