 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Epidemiological studies indicate association between elevated air pollution and adverse health effects. Several mechanisms have been suggested, including translocation of inhaled ultrafine carbon (UFC) particles into the bloodstream. Previous studies in healthy subjects have shown no significant pulmonary translocation of UFC-particles. This study aimed to assess if UFC-particles translocate from damaged alveolar compartment in subjects suffering from chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF).
Methods
Eleven COPD and nine IPF subjects were exposed to a 100 nm UFC-particle-aerosol labeled with Indium-111. Activity in the body was followed up for 10 days using gamma camera planar-imaging as well as in blood and urine samples.
Results
The pulmonary central to periphery activity ratio was significantly higher for COPD as compared to IPF subjects at exposure, 1.8 and 1.4, respectively and remained constant throughout the test period. Ten days after exposure, the estimated median pulmonary translocation of UFC particles was 22.8 and 25.8% for COPD and IPF, respectively. Bound activity was present in blood throughout the test period, peaking at 24-h postinhalation with a median concentration of 5.6 and 8.9 Bq/ml for the COPD and IPF, respectively. Median bound activity excreted in urine (% of inhaled) after 10 days was 1.4% in COPD and 0.7% in IPF. Activity accumulation in liver and spleen could not be demonstrated.
Conclusions
Our results suggest that UFC particles leak through the damaged alveolar barrier to the bloodstream in COPD and IPF patients probably distributing in a wide spectrum of whole-body tissues.
Introduction
Epidemiological research has demonstrated an association between exposures to polluted ambient air and adverse health effects. Increased death rates have been reported after long-term exposure to high levels of air pollution (Rosenlund et al. Citation2006; Brook et al. Citation2010, Citation2018; Atkinson et al. Citation2014; Mills et al. Citation2015; Beelen et al. Citation2014; ESC Heart Failure Association, Citation2015). Long-term effects include development of atherosclerosis (Brook et al. Citation2010; Mills et al. Citation2007), cardiovascular disease or stroke (World Health Organization 2016). Furthermore, several studies (Brook et al. Citation2010; Atkinson et al. Citation2014; Mills et al. Citation2015) have reported that even a few days of exposure to acutely elevated air pollution levels can cause adverse health effects and even death. It is unclear whether these pathological events are initiated locally in the lung by inducing systemic inflammation, or if ultra-fine (UF) inhaled particles are translocated to the bloodstream, causing inflammation or activation of coagulation processes (Brook et al. Citation2010). In addition, there are several occupational groups that are exposed to airborne particles daily in their profession, such as firefighters, welders or in industrial production, making it necessary to clarify the imposed risk of airborne particles to be able to take appropriate health precautions.
In previous projects, our research group provided with a new method to study translocation of UF carbon particles by labeling UF carbon particles (generated in a modified Technegas® device) with Indium-111 (111In-UFC) (Klepczyńska-Nyström et al. Citation2012; Sanchez-Crespo et al. Citation2011). 111In-UFC aerosol has two major advantages in comparison with Technegas aerosol, that is, aerosol labeled with Technetium 99 m (Tc99m), their radioactive half-life is longer (2.8 days compared to 6 hours for Tc99m), and their chemical stability is considerably better (the radioactive label is more strongly bound to the carbon particle (Sanchez-Crespo et al. Citation2011)). With this new method, we could show that the translocation of 111In-UFC particles deposited in the lungs was small (4.3%) in healthy subjects seven days after exposure (Klepczyńska-Nyström et al. Citation2012). However, it is reasonable to believe that there is a difference between healthy subjects and those with injured alveolar air-blood barriers, as for example in chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF). Hence, the aim of this study was to investigate if patients suffering from COPD and IPF showed higher degree of pulmonary translocation of UF particles as compared to previously reported values in healthy individuals (Wiebert et al. Citation2006; Klepczyńska-Nyström et al. Citation2012; Miller et al. Citation2017).
Materials and methods
This study was approved by the regional ethics committee (Dnr. 2015/178) and the hospital radiation protection committee of the Karolinska University Hospital. All subjects participating in this study gave written informed consent.
Subjects
During 2015, 25 patients with COPD diagnosis, responded to an advertisement in a local newspaper and were invited to an initial information meeting. All participants underwent pulmonary function testing (using spirometry). The subjects meeting the criteria of lung function consistent with moderate to severe COPD according to GOLD (Singh et al. Citation2019), that is, airway obstruction with FEV1 < 50% predicted (Hedenstrom et al., Citation1985, Citation1986), were included in the study. A total of 11 subjects met this criterion and additionally underwent a diffusing capacity (DLCO) measurement.
Thirty randomly selected patients diagnosed with IPF at Karolinska University Hospital according to international guidelines (American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society Citation2018), were invited to the study. Nine patients chose to participate and underwent the same pulmonary function testing as for the COPD group.
In this study, the subject’s degree of alveolo-capillary permeability to water soluble substances was characterized by gamma camera measurement of the clearance of Technetium-99m-diethylenetriaminepentaacetic acid (99mTc-DTPA) aerosol to the systemic circulation (Wollmer and Evander Citation1994). The 99mTc-DTPA aerosol was prepared using a commercial kit (TechneScan DTPA; Mallinckrodt Medical, Kansas City, MO, USA) and inhaled using a SmartVent™ aerosol generating system (Diagnostic Imaging Limited, Welford, Great Britain). According to the manufacturer, the mass median aerodynamic diameter (MMAD) of the delivered droplets was 1.3 µm. Approximately 100 MBq 99mTc-DTPA were administered to each subject. 99mTc-DTPA was administered under normal tidal breathing using a mouthpiece and nose-clip and the subject lying in supine position in the gamma camera couch. Directly after administration, the pulmonary retention of 99mTc-DTPA was imaged for 45 minutes, at a sampling interval of 60 seconds. A two-headed gamma camera (Symbia T16, Siemens Healthcare, Erlangen, Germany) equipped with low energy high resolution parallel-hole collimators was used. A region of interest (ROI) covering both lungs was drawn and the time activity curve (TAC) corresponding to the pulmonary clearance of 99mTc-DTPA during the imaging period was then obtained. The TAC was then decay corrected and fitted to a mono-exponential equation from which the pulmonary clearance half-life of 99mTc-DTPA was obtained.
Indium-labeled UF particles generation and subject exposure
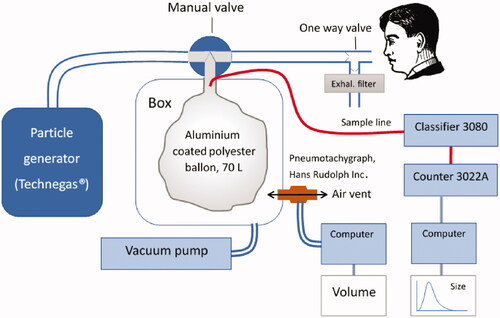
The methodology followed in this work has been previously described in detail (Sanchez-Crespo et al. Citation2011). A vial with 370 Megabecquerels (MBq) of 111In-hydrocloric acid was obtained from Amersham (Braunschweig, Germany). The hydrochloric acid was removed by evaporation. The operation was repeated by adding distilled water until neutral pH was achieved. Thereafter, 99% ethanol was added to the remaining Indium-111 as liquid carrier. About 0.14 ml of the resulting solution was placed in the graphite crucible of a first generation Technegas generator (Cyclomedica, Kingsgrove, Australia) modified to allow shorter burning times and lower burning temperature and run with air instead of argon, to cause the oxidation of the Indium-111 into In2O3, which is insoluble in water. The generator was then operated with 1 s burning time to generate 111In-UFC with around 100 nm count median diameter (CMD). As previously described 111In-UFC aerosol tends to agglomerate into larger particles after generation (Sanchez-Crespo et al. Citation2011), to minimize this process, the aerosol was diluted in a 70 L flexible and aluminum coated bag of polyester film (Mylar, Mono Content Group AB, Sweden), half filled with clean air. Aerosol particle sizes and concentrations in the bag were measured directly before and after patient exposure using a Scanning Mobility Particle Sizer system (SMPS; TSI, Incorporated Particle Instruments, Shoreview, MN, USA) consisting of an Electrostatic Classifier 3080 (equipped with a Differential Mobility Analyzer 3081) and a Condensation Particle Counter 3022 A. A schematic presentation of the particle generation and exposure setup is shown in . While wearing a nose clip subjects were instructed to spontaneously breath from the mouthpiece until an activity of approximately 5 MBq was reached as measured with a radiation-protection proportional counter LB 123 UMo (Berthold Technologies GmbH & Co Bad Wildbad, Germany) against their back. A pneumotachograph (Hans Rudolph Inc. USA) was used to continuously monitor the breathing pattern during exposure to ensure normal tidal breathing and measuring the total volume of inhaled 111In-UFC aerosol.
Figure 1. Particle generation and exposure setup. The Indium was manually loaded in the modified Technegas generator. After generation, the vacuum pump was started sucking the aerosol into the Mylar balloon. After checking particle sizes via the sampling line, the manual valve was turned, and inhalations started and quantified by the pneumotachograph.
To determine the percentage of free indium-111 activity at exposure, a sample of the inhaled aerosol was collected from the exposure bag on a 0.2 µm pore size Teflon filter (Pall Laboratory, Port Washington, NY, USA). The activity in the filter was then measured in a 3-inch sodium iodine well detector (Wizard Gamma Counter, PerkinElmer Inc., Waltham, MA, USA) with decay and background corrections. Thereafter, the filter was placed inside of a dialysis membrane tube (Spectrum Laboratories, New Brunswick, NJ, USA) together with 10 ml of 0.9% sodium chloride solution. The membrane tube was then submerged into 200 ml of 0.9% sodium chloride equilibration buffer. The activity concentration of free Indium-111 in the buffer was then determined after 24 hours of equilibration, by measuring the activity from a 20 ml buffer sample in the well counter. The percentage of inhaled free Indium-111 was then calculated as decay and background corrected total buffer activity in percent of reference filter activity. This process was subsequently repeated throughout the entire follow-up period for each subject to obtain an estimate of the chemical stability of the 111In-UFC labeling.
For each patient, the pulmonary aerosol deposition fraction (DF) was determined according to:
(1)
(1)
where AExh is the total exhaled activity collected in a Teflon filter with 0.2 µm pore size, VInh is the total volume of inhaled aerosol (measured with a pneumotachograph) and ABag is the activity concentration in the bag from sampling 1 liter of the aerosol through a Teflon filter with 0.2 µm pore size using a calibrated precision syringe (Harvard Apparatus, USA). All filter activities were measured in a dose calibrator (Capintec, INC, NJ, USA).
Pulmonary clearance of ultrafine particles, quantification and biodistribution
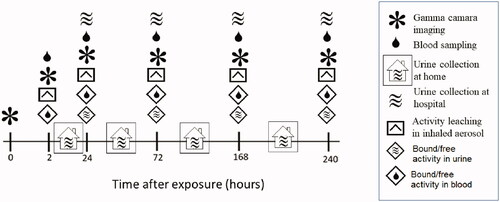
The complete timeline for activity tracking is shown in . A two-headed gamma camera (Symbia T16, Siemens Healthcare, Erlangen, Germany) equipped with medium energy plane parallel-hole collimators, was used to image the deposition and kinetics of 111In-UFC aerosol activity distribution. Gamma camera image acquisition was performed with the subjects lying in the supine position. A 10 minutes anterior and posterior planar images over thorax and abdomen were simultaneously performed directly after aerosol exposure, as well as at 2, 24, 72, 168- and 240-hour postexposure () using a 256 × 256 image matrix size. A 10 minutes background image was also taken at every visit and subsequently removed from the anterior and posterior images by simple pixelwise subtraction. The background corrected anterior and posterior images were then corrected for radioactivity decay with reference to exposure time. Finally, the geometric mean image (GM) at a given time ‘t’ post aerosol inhalation was then pixel-wise calculated according to:
(2)
(2)
Figure 2. Follow-up protocol after inhalation of an aerosol of ultrafine graphite particles labeled with Indium-111. Activity over the thorax and abdomen were measured in subjects with a large field of view gamma camera. In vitro leaching tests were performed on a filtered sample of the original inhaled aerosol using a dialysis membrane diffusion technique and a sodium iodine well chamber. Blood samples were drawn at every hospital visit and the activity concentration measured in a sodium iodine well counter. Total urine excretion between hospital visits were stored in separate containers and the total excreted activity measure in combination with every visit to the hospital from day 2. Total activity excreted through urine was measured in a sodium iodine well counter.
Rectangular ROIs covering each lung were drawn on the GM (t = 0). These ROIs were then automatically subdivided in three equal parts, the apical, middle and basal. The middle ROI was subsequently divided in two halves where the central region (C) was defined as the subregion closest to the Hilum at each lung and the periphery regions (P) the remaining half plus the upper and lower thirds of the lungs. The C and P-ROIs were subsequently used for quantifying the UFC pulmonary retention throughout the entire test period of 10 days. TAC curves corresponding to the total lung as well as in the C and the P ROIs, were then obtained relative to the total activity deposition after exposure and the corresponding Central-to-Peripheral ratios (C/P), normalized to the difference in size between regions, calculated. The temporal variation of C/P ratios provides with a surrogate for the effect of mucociliar transport from the central airways to the total lung UFC clearance (Brown et al. Citation2002).
Translocated activity from the lungs was investigated in blood and urine samples as well as in the abdomen using gamma camera imaging. About 5 ml of peripheral venous blood was drawn at every subject visit to the hospital and total urine production in between visits were saved by the subjects in a container and subsequently collected at each visit to the hospital (). Activity concentrations in blood and urine were determined using a 3-inch sodium iodine well detector, with background and decay correction from aerosol exposure date. The total activity excreted through the urine in-between subject visits to the hospital was then calculated as the measured activity concentration in urine times the total collected urine volume and expressed as percentage of the initial activity deposited in the lungs. The amount of free activity in blood and urine samples was determined using the dialysis membrane diffusion technique (Sanchez-Crespo et al. Citation2011). Dialysis tubes filled with samples of blood or urine were submerged in an equilibration buffer consisting of a 200 ml, 0.9% sodium chloride solution. The total activity in the equilibration buffers was then measured after 24 hours of equilibration using a sodium–iodine well detector. Free activity concentration in the sample was then calculated as decay and background corrected buffer activity divided by the sample volume. The total amount of free activity in urine in-between subject visits to the hospital was then calculated as the measured free-activity concentration times the total volume of collected urine and expressed as percentage of the initial activity deposited in the lungs.
The image derived pulmonary TAC is measured relative to the activity in the lungs after administration. Hence, it represents the differential activity retention between aerosol administration and each of the measured time points ‘t’. An estimate of the cumulative pulmonary clearance of UFC particles (Cl(t)), can then be obtained by correcting the TAC for cumulative particle leaching (F(t)) according to:
(3)
(3)
In this study, the cumulative free activity measured in the urine was used as a surrogate for F(t). The F(t) was then calculated by sequentially adding the decay corrected total excreted free activity in urine from aerosol administration to each of the sampling time points and expressed as percentage of inhaled activity.
Statistical analysis
The Mann–Whitney U-test was used to test for statistical differences between patient groups with regards to pulmonary UFC deposition and clearance and pulmonary function data. Median and interquartile range (IQR) were used as descriptive statistics. To statistically analyze the Cl(t) between subjects and between groups, the values were interpolated to match the same sampling time-points as depicted in .
Results
Subjects
shows no statistically significant differences between groups regarding patient age, height and weight. COPD patients showed significatively stronger smoking habits than the IPF group (p = 0.0008, ). All subjects had respiratory symptoms as well as impaired lung function characteristic of the pathophysiology of their condition (). Specifically, the COPD subjects in this cohort are significantly more obstructive than the IPF subjects with FEV1 well below 50% of predicted (p = 0.0001, ). However, no statistically significant difference in diffusion capacity was found between groups (). DTPA clearance was significantly faster in the IPF group as compared to the COPD (p = 0.015, ) and healthy individuals (Brådvik et al. Citation2002).
Table 1. Patient clinical characteristics and pulmonary function data.
UFC aerosol properties at exposure
shows that the UFC particle characteristics at exposure, with regards to aerosol count median aerodynamic diameter and labeling efficiency was not statistically different between groups. The aerosol generation was stable during the entire project with a pooled median (IQR) diameter of 99.2 nm (89.9 − 115.4 nm) and with less than 0.2% of free activity at exposure (Median (IQR) = 0.15% (0.03 − 0.53%) for the pooled groups). Ten days after generation, the cumulative amount of free activity in saline (median (IQR)) was as low as 1.6% (1.2 − 3.5%) and 1.7% (1.2 − 1.8%) for the COPD () and the IPF patients (), respectively, demonstrating a very good chemical stability. shows that the deposition fraction, the number of deposited particles relative to the total inhaled, was higher in the COPD group as compared to IPF patients, but statistical significance was not reached (p = 0.07). also reveals that the UFC particles deposition in the central airways was significantly higher in COPD patients as compared to IPF, median C/P0 1.8 and 1.4 respectively (p = 0.008), indicating different UFC deposition pattern.
Table 2. 111In- UFC aerosol characteristics at exposure.
Table 3. Measured time activity distributions for COPD patients.
Table 4. Measured time activity distributions for IPF patients.
Measured activity biodistribution and estimated pulmonary clearance of UFC particle
After aerosol inhalation the C/P ratio remained constant during the entire follow-up period of 10 days for both patient groups ( and ). Further, and show an overall rapid decline of the pulmonary retention of activity in the lungs which was not statistically different between patient groups.
and reveal small amounts of free activity excreted in the urine for both groups of patients throughout the entire follow-up period. Ten days after inhalation, this amount accounted for less than 2% of the total inhaled activity in both groups which is comparable to the measured free activity in saline from a sample of the administered aerosol ( and ).
shows the calculated UFC particle clearance from the lungs after free activity leaching correction (EquationEquation (3)(3)
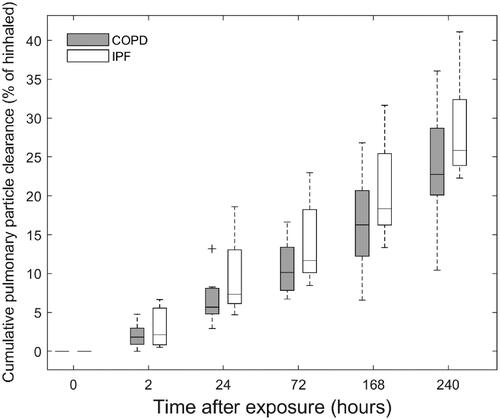
(3) ) and interpolation to the same sampling-time points. Compared to previously reported values in healthy individuals (Klepczyńska-Nyström et al. Citation2012), reveals a significant clearance of ultrafine particles from the lungs in both groups of patients. Even though there is a trend toward larger pulmonary ultrafine particle clearance in IPF subjects, no statistical difference between groups was observed (p = 0.68). Ten days after exposure, the median (IQR) cumulative pulmonary clearance of ultrafine particles was 22.8% (20.8 − 28.5%) and 25.8% (24.2 − 31.0%) for COPD and IPF respectively as compared to 4.3% (mean) in healthy individuals (Klepczyńska-Nyström et al. Citation2012).
Figure 3. Boxplots with the distribution of calculated cumulative pulmonary particle clearance for the group of COPD and IPF patients. Individual values are corrected for activity decay and particle leaching and for comparison interpolated to the same sampling time-points. Whiskers represent the minimum and maximum value of the distribution. Outliers are indicated with the + symbol. Median (interquartile range) cumulative pulmonary clearance at the end of the test period of 10 days were 22.8% (20.8–28.5%) and 25.8% (24.2–31.0%) of inhaled for COPD and IPF, respectively.
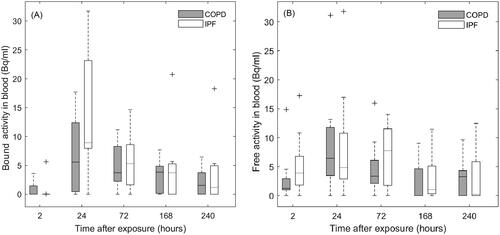
Equal amounts of bound activity were found excreted in urine in both groups of patients ( and ). At group level, the cumulative amount of bound activity in urine 10 days postinhalation, was 1.4% and 0.7% of inhaled activity for COPD and IPF patients respectively. panel A reveals that significative amounts of bound activity crossed the alveolar barrier into the systemic circulation in both groups of patients. Particle translocation was larger in IPF patients as compared to COPD, median (IQR) concentration at peak value of 8.9 Bq/ml (8.0 − 23.8 Bq/ml) and 5.6 Bq/ml (0.4 − 12.4 Bq/ml) respectively, although no statistically significant difference was found (p = 0.15). For both groups of patients, bound activity in bloodstream peaks at 24-h postinhalation followed by an exponential decline, suggesting a particle translocation pattern represented by the combination of a fast and a slow component. panel B reveals the presence of measurable amounts of free activity in blood for both patient groups. Free activity peaked at 24 hours for the COPD group and at 72 hours for the IPF group and corresponded to a median (IQR) of 6.5 Bq/ml (3.5 − 11.8 Bq/ml) and 7.8 Bq/ml (1.7 − 11.5 Bq/ml), respectively (p = 0.84).
Figure 4. Boxplots with the distribution of bound (panel A) and free (panel B) activity concentrations in blood in COPD and IPF patients. For comparison, the distributions are interpolated to the same sampling time-points for all patients. Whiskers represent the minimum and maximum value of the distribution. Outliers are indicated with the + symbol.
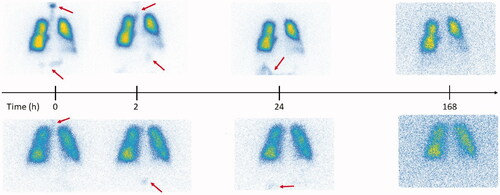
None of the subjects in this study showed accumulation of activity in the liver and spleen throughout the entire follow-up period of 10 days. However, as shown in , gamma camera images taken directly after exposure, revealed the transit of activity through the gastrointestinal tract (GI) from mouth to stomach and intestines for some of the subjects. For all these subjects, activity in the GI was completely cleared during the first 72 h post exposure, which points to oral intake of activity at exposure and not mucociliary transport as the most plausible origin for the activity seen in the GI tract.
Figure 5. Gamma camera images corresponding to the aerosol follow-up in two representative subjects with high (upper row) and low (lower row) aerosol deposition in the digestive tract after aerosol swallowing during exposure. The arrows indicate regions of activity deposition outside of the lungs after exposure.
Discussion
In this study, we have investigated the pulmonary clearance of UFC particles in a group of patients with affected lung function. We have demonstrated that in COPD and IPF patients there is a high degree of pulmonary clearance of UFC particles, as compared to healthy volunteers (Brown et al. Citation2002; Wiebert et al. Citation2006; Klepczyńska-Nyström et al. Citation2012; Miller et al. Citation2017). Interestingly, no statistically significant difference in pulmonary UFC particle clearance between IPF and COPD subjects was observed ( and ). This result is in contradiction to the significantly different washout rate of DTPA aerosol and measured DLCO between groups (). Regarding DTPA clearance, transepithelial alveolar diffusion is the most important transport mechanism (Rinderknecht et al. Citation1980) and is driven by water concentration gradients and osmosis. Instead, UFC particle translocation may capture the alveolar epithelia barrier integrity. The lack of correlation between UFC particle clearance and spirometry parameters reflecting airway dimensions, such as FEV1 and FEV1/FVC ratio is more understandable, as they reflect airways rather than alveolar damage.
The negative health effect of nanoparticles is a continuously evolving research area. However, human studies including patients with impaired lung function are scarce. Preclinical animal studies in this area are more common and have previously shown that nanoparticles translocate from the lung and accumulate in different organs. In accordance with our results, a recent study by Miller at al 2017 using gold-nanoparticles demonstrated pulmonary particle translocation and accumulation in vascular sites with inflammation in both animals and humans. However, it is possible that the mechanisms for pulmonary clearance and extrapulmonary accumulation in our study might not be the same considering the large methodological differences between these two studies with regards to aerosol physical properties and investigated subjects. Further, our study was merely observational and was not designed to explain the possible different mechanisms responsible for pulmonary particle translocation as this involves the use of invasive maneuvers such alveolar lavage and corresponding pathological analyses. Hence, even though the relative importance of monocyte activation and active transport by macrophage to lymphatic vessels on the overall pulmonary clearance of UFC particles in this study could not be studied, there are some other more plausible mechanisms responsible for the large difference in particle clearance compared to healthy individuals (Klepczyńska-Nyström et al. Citation2012). These are passive diffusion through damaged alveolar barrier and mucociliary transport to the gastrointestinal tract. Although not initially deposited in the ciliated airways to any great extent, UFC particles could theoretically be transported by macrophages from the respiratory bronchioles to terminal bronchioles for subsequent clearance of the cilia. The more active and numerous macrophages in both COPD and IPF compared to healthy individuals may support this mechanism. However, this is in contradiction to results by Möller et al Citation2008, which showed a significative higher mucocilliary transport of 100 nm Tc99m-Technegas particles from the airways of healthy individuals as compared to COPD patients (Möller et al. Citation2008). This could be explained by impaired ciliary function in the COPD group. In our previous study of healthy subjects, we reported a faster clearance in the central parts of the lung in relation to peripheral. However, in absolute terms UF particles cleared from the lung by mucociliary transport was merely 2% of inhaled (Klepczyńska-Nyström et al. Citation2012). These results are in line with previous findings on impaired macrophage phagocytosis of ultrafine particles in the peripheral lungs, (Renwick et al. Citation2001; Lundborg et al. Citation2006; Geiser et al. Citation2008), favoring a slow particle translocation into lung tissue and into the vasculature). Extrapolating these results to our current findings, if macrophage phagocytosis of UFC particles is impaired in the P compartment, a constant C/P ratio necessarily implies that the deposition of UFC particles in the tracheobronchial part of the C compartment (where fast mucocilliary transport occurs) is then very limited. Hence altogether these results indicate that in our study mucocillary transport of UFC particles did not play a relevant role, leaving passive transport through the damage alveolar barrier as the most possible major mechanism for UFC particle clearance. Alveolar damage has different pathological origin in COPD subjects as compared to IPF. In COPD, alveolar damage is mainly seen as emphysema by destruction of alveolar septa, partially caused by protease/antiprotease imbalance and inflammation (Demedts et al. Citation2006) and in IPF, fibroblast activation leading to an increase in extracellular matrix (ECM) and thickening of the alveolar membrane (Kuhn and McDonald Citation1991). Interestingly, there are indications that some factors of the pathogenesis are common for both diseases (Chilosi et al. Citation2012). A relationship between increased ECM and higher degree of translocation seems contradictory, but IPF is consistent with extracellular matrix leaking out alveoli compartment (Ahluwalia et al. Citation2014). It would therefore be plausible that decreased integrity could also lead to leakage of UFC particles into the blood. Inflammation in COPD has been established both in the airways and as a general systemic inflammation (Cosio et al. Citation2009). The role of the inflammatory system is debated for IPF, mainly because anti-inflammatory treatment has failed (Raghu et al. Citation2012), but there is evidence that macrophage activation plays a role also in IPF (DI Stefano et al. Citation1998; Wynn and Vannella Citation2016).
and clearly shows an imbalance between the total activity cleared from the lungs and the cumulative activity found in blood and excreted through the urinary system. This suggests that UFC particles cleared from the lungs may accumulate in other organs of the body, typically in liver and spleen (Miller et al. Citation2017). However, none of the subjects in this work concentrated detectable amounts of activity in those organs, suggesting a wider tissue distribution. The low amount of inhaled activity in conjunction to the limited sensitivity of the gamma camera and background radiation, limits the possibility to trace these particles in the body outside the lung region. An approximative value for the minimum detectable activity (MDA) in the liver region can be derived using the Currie formulation (Currie Citation1968). The minimum mean number of counts needed to ensure a false-negative rate of 5% is;
(4)
(4)
where Nbkg is the background count density over the liver region. Assuming photon attenuation in liver with a 5 cm mean photon attenuation pathlength and an effective attenuation coefficient of 0.135 cm−1 for In111, the attenuation corrected minimum mean number of counts is then;
(Brook et al. Citation2018)
Finally, the MDA can be approximated by Nc/(t*ε) where ε is the measured planar imaging sensitivity for In111 for the gamma camera used in this work with medium energy collimators (5.8 cpm/kBq) and ‘t’ the acquisition time. In our study, typical values for Nbkg in the liver region were about 7000 counts for a 10 minutes acquisition time, which results in an MDA of 13.2 kBq. For a subject exposed to 5 MBq the liver MDA corresponds to a UFC accumulation of about 0.3% of inhaled.
As previously mentioned, animal studies have shown different degrees of translocation of particles from the lungs (Takenaka et al. Citation2001; Oberdörster et al., Citation2002, Citation2004; Kreyling et al. Citation2002, Citation2009; Semmler et al. Citation2004; Elder et al. Citation2006; Takenaka et al. Citation2006). However, extrapolation of the results from animal studies to humans ought to be done with caution. Animal studies allows higher particle concentration which in some respects makes the experiment easier, although high particle concentrations can affect the permeability of the lungs (Donaldson et al. Citation2001). There are also well-documented differences between species in how the lung handles particles and that it is seldom possible to use spontaneous breathing which also reduces the value of animal experiments (Geiser and Kreyling Citation2010).
A common complication in this type of studies is the degradation of the isotope-particle complex. Hence, it is essential to check the quality of the label to ensure that labeled particles are measured and not free activity (Brown et al. Citation2002; Wiebert et al. Citation2006; Ghio and Bennett Citation2007). In this regard, translocation of free activity is most likely influencing previously reported rapid clearance of radioactive bound aerosol (Nemmar et al. Citation2002). In the current study, we monitored free activity in samples of whole blood and urine but also in vitro from a sample of inhaled aerosol in saline. Although saline does not provide with the same chemical environment as a lung lining fluid analog, it provides an indicative estimate of activity leaching. In this work, we found that about 2% of the isotope is no longer bound to UF particles in saline 10 days after generation which is much lower than reported for Tc99m-UFC particles (Wiebert et al. Citation2006) and correlates very well with measured free activity in urine shown in and .
In this study, the bound activity found in the urine suggests renal clearance of UFC particles. However, the total amount of bound activity excreted in urine accounted for a small fraction of the total UFC particle cleared from the lungs into the vasculature, suggesting that renal clearance is limited by nanoparticle size. This has already been previously shown in a study by Choi CH et al Citation2011, demonstrating that only glomerular filtration of particles smaller than 10 nm may incite renal clearance (Choi et al. Citation2011). The presence of nanosized carbon particles in the urine has also been demonstrated using an optical technique by Saenen et al Citation2017. In this study, the authors demonstrated higher amounts of carbon particles in urine in children living closer to a main road (Saenen et al. Citation2017).
Study limitations
Compared to earlier results in healthy individuals, a large portion of the inhaled UFC particles are cleared from the lungs in IPF and COPD patients. However, direct quantitative comparisons between studies should be taken with caution due to possible methodological differences affecting aerosol properties. Hence, the lack of a contemporary healthy control group in our study can be considered as a limitation. In the period 2009–2010, Klepczyńska-Nyström et al exposed at our department a cohort of healthy individuals to 111In-UFC aerosol (Klepczyńska-Nyström et al. Citation2012). Aerosol generation, exposure and follow-up was performed with the same equipment and methods as in the current study. Hence, it is plausible to assume that the aerosol in both studies share the same physicochemical properties. Further, this equipment is part of the clinical routine equipment at the Department of nuclear medicine of the Karolinska University Hospital, which is subject to thorough periodic controls to ensure quality and stability in accordance with local and international regulations. Hence, it was not justifiable to expose a new group of healthy individuals in conjunction to the COPD and IPF patients.
In this work we have assumed no differences in filter trapping efficiency between free and particle bound activity. The Teflon filters used throughout this work are known to have about 95% particle collection efficiency for aerosols at nanometer size (Huang et al. Citation2004; Burton et al. Citation2007). Since aerosol sampling was performed with no added pressures, it is most likely that free Indium-111 is caught in the Teflon microfiber structure by diffusion with similar efficiencies as particle bound activity. This assumption is supported by our results of the amount of inhaled free activity of 0.2% (average for the pooled groups, ) which quantitatively is similar to the free activity cleared in the urine 24 hours post administration, 0.1% (average for the pooled groups, and ). Further a fast translocation of free activity from the lungs into the bloodstream was also observed in the first 2 hours post aerosol inhalation ( panel B). The presence of outliers in panels A and B most likely represent the interindividual variability of the data rather than methodological errors and therefore were not rejected from the analysis.
The experimental setup of this study did not allow for determination of the differential regional deposition of the aerosol at different particle sizes. This would have required a different aerosol exposure system and monitoring technique including aerosol sampling at every patient breath with synchronized gamma camera dynamic imaging. Future studies to determine the site of deposition and clearance in the lung at different ultra-fine particles sizes should therefore be performed in different groups of patients and healthy individuals.
Conclusions
The good chemical stability of the aerosol and the constant C/P ratios indicate that the measured fast decline of activity in the lungs of COPD and IPF patients truly reflects particle translocation, most probably through damaged alveolar barrier. Yet this translocated bound activity could not be accounted for by the measured activity in blood and urine nor could it be imaged in liver and spline. The limited gamma camera sensitivity at low levels of activity concentrations suggests that in our study UFC may accumulate into a wide whole-body tissue distribution.
Acknowledgements
The authors would like to give special thanks to the Department of Nuclear Medicine, Clinical Physiology and the Department of Respiratory Medicine and Allergy, Karolinska University Hospital, Stockholm.
Disclosure statement
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript. No potential conflict of interest was reported by the author(s)
Additional information
Funding
References
- Ahluwalia N, Shea BS, Tager AM. 2014. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med. 190(8):867–878.
- Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. 2014. Epidemiological time series studies of PM2. 5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 69(7):660–665.
- Beelen R, Stafoggia M, Raaschou-Nielsen O, Andersen ZJ, Xun WW, Katsouyanni K, Dimakopoulou K, Brunekreef B, Weinmayr G, Hoffmann B, et al. 2014. Long-term exposure to air pollution and cardiovascular mortality: an analysis of 22 European cohorts. Epidemiology. 25(3):368–378.
- Brådvik I, Wollmer P, Evander E, Lárusdóttir H, Blom-Bulow B, Jonson B. 2002. Kinetics of lung clearance of 99mTc-DTPA in smoking patients with sarcoidosis compared to healthy smokers. Respir Med. 96(5):317–321.
- Brook RD, Newby DE, Rajagopalan S. 2018. Air pollution and cardiometabolic disease: an update and call for clinical trials. Am J Hypertension. 31(1):1–10.
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. 2010. Particulate matter air pollution and cardiovascular disease. Circulation. 121(21):2331–2378.
- Brown JS, Zeman KL, Bennett WD. 2002. Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am J Respir Crit Care Med. 166(9):1240–1247.
- Burton NC, Grinshpun SA, Reponen T. 2007. Physical collection efficiency of filter materials for bacteria and viruses. Ann Occup Hyg. 51(2):143–151.
- Chilosi M, Poletti V, Rossi A. 2012. The pathogenesis of COPD and IPF: distinct horns of the same devil? Respir Res. 13(1):3.
- Choi CH, Zuckerman JE, Webster P, Davis ME. 2011. Targeting kidney mesangium by nanoparticles of defined size. Proc Natl Acad Sci USA. 108(16):6656–6661.
- Cosio MG, Saetta M, Agusti A. 2009. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 360(23):2445–2454.
- Currie LA. 1968. Limits for qualitative detection and quantification determination. Anal Chem. 40(3):586–593.
- Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. 2006. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 7:53.
- DI Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, Maestrelli P, Mapp CE, Fabbri LM, Donner CF, Saetta M, et al. 1998. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med. 158(4):1277–1285.
- Donaldson K, Stone V, Clouter A, Renwick L, MacNee W. 2001. Ultrafine particles. Occup Environ Med. 58(3):211–216.
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Ito Y, Finkelstein J, et al. 2006. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 114(8):1172–1178.
- Geiser M, Casaulta M, Kupferschmid B, Schulz H, Semmler-Behnke M, Kreyling W. 2008. The role of macrophages in the clearance of inhaled ultrafine titanium dioxide particles. Am J Respir Cell Mol Biol. 38(3):371–376.
- Geiser M, Kreyling WG. 2010. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 7:2.
- Ghio AJ, Bennett WD. 2007. Metal particles are inappropriate for testing a postulate of extrapulmonary transport. Environ Health Perspect. 115(2):A70.
- Hedenstrom H, Malmberg P, Agarwal K. 1985. Reference values for lung function tests in females. Regression equations with smoking variables. Bull Eur Physiopathol Respir. 21(6):551–557.
- Hedenstrom H, Malmberg P, Fridriksson HV. 1986. Reference values for lung function tests in men: regression equations with smoking variables. Ups J Med Sci. 91(3):299–310.
- Huang HL, Huang YC, Wang DM. 2004. Aerosol filtration efficiency of Teflon fibrous filters. J Aerosol Sci. 35:S973–S974.
- Klepczyńska-Nyström A, Sanchez-Crespo A, Andersson M, Falk R, Lundin A, Larsson B-M, Svartengren M. 2012. The pulmonary deposition and retention of indium-111 labeled ultrafine carbon particles in healthy individuals. Inhal Toxicol. 24(10):645–651.
- Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, Oberdörster G, Ziesenis A. 2002. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A. 65(20):1513–1530.
- Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S, Oberdörster G. 2009. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol. 21(sup1):55–60.
- Kuhn C, McDonald JA. 1991. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 138(5):1257–1265.
- Lundborg M, Dahlén SE, Johard U, Gerde P, Jarstrand C, Camner P, Låstbom L. 2006. Aggregates of ultrafine particles impair phagocytosis of microorganisms by human alveolar macrophages. Environ Res. 100(2):197–204.
- Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP, Wilson S, Vesey AT, Fokkens P, Boere A, et al. 2017. Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano. 11(5):4542–4552.
- Mills IC, Atkinson RW, Kang S, Walton H, Anderson HJBo. 2015. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open. 5(5):e006946–e006946.
- Mills NL, Törnqvist H, Gonzalez MC, Vink E, Robinson SD, Söderberg S, Boon NA, Donaldson K, Sandström T, Blomberg A, et al. 2007. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 357(11):1075–1082.
- Möller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Häussinger K, Kreyling WG. 2008. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med. 177(4):426–432.
- Nemmar A, Hoet PHM, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. 2002. Passage of inhaled particles into the blood circulation in humans. Circulation. 105(4):411–414.
- Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, ESC Heart Failure Association, et al. 2015. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 36(2):83–93.
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. 2004. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 16(6–7):437–445.
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. 2002. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 65(20):1531–1543.
- Raghu G, Anstrom KJ, King TE, Jr., Lasky JA, Martinez FJ. 2012. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 366(21):1968–1977.
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F, American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society, et al. 2018. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 198(5):e44–e68.
- Renwick LC, Donaldson K, Clouter A. 2001. Impairment of alveolar macrophage phagocytosis by ultrafine particles. Toxicol Appl Pharmacol. 172(2):119–127.
- Rinderknecht J, Shapiro L, Krauthammer M, Taplin G, Wasserman K, Uszler JM, Effros RM. 1980. Accelerated clearance of small solutes from the lungs in interstitial lung disease. Am Rev Respir Dis. 121(1):105–117.
- Rosenlund M, Berglind N, Pershagen G, Hallqvist J, Jonson T, Bellander T. 2006. Long-term exposure to urban air pollution and myocardial infarction. Epidemiology. 17(4):383–390.
- Saenen ND, Bové H, Steuwe C, Roeffaers MBJ, Provost EB, Lefebvre W, Vanpoucke C, Ameloot M, Nawrot TS. 2017. Children's urinary environmental carbon load. A novel marker reflecting residential ambient air pollution exposure? Am J Respir Crit Care Med. 196(7):873–881.
- Sanchez-Crespo A, Klepczynska-Nystrom A, Lundin A, Larsson BM, Svartengren M. 2011. 111Indium-labeled ultrafine carbon particles; a novel aerosol for pulmonary deposition and retention studies. Inhal Toxicol. 23(3):121–128.
- Semmler M, Seitz J, Erbe F, Mayer P, Heyder J, Oberdörster G, Kreyling WG. 2004. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung, including transient translocation into secondary organs. Inhal Toxicol. 16(6-7):453–459.
- Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Criner GJ, Frith P, Halpin DMG, Han M, et al. 2019. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 53(5):1900164.
- Takenaka S, Karg E, Kreyling WG, Lentner B, Möller W, Behnke-Semmler M, Jennen L, Walch A, Michalke B, Schramel P, et al. 2006. Distribution pattern of inhaled ultrafine gold particles in the rat lung. Inhal Toxicol. 18(10):733–740.
- Takenaka S, Karg E, Roth C, Schulz H, Ziesenis A, Heinzmann U, Schramel P, Heyder J. 2001. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect. 109(Suppl 4):547–551.
- Wiebert P, Sanchez-Crespo A, Falk R, Philipson K, Lundin A, Larsson S, Möller W, Kreyling WG, Svartengren M. 2006. No significant translocation of inhaled 35-nm carbon particles to the circulation in humans. Inhal Toxicol. 18(10):741–747.
- Wiebert P, Sanchez-Crespo A, Seitz J, Falk R, Philipson K, Kreyling WG, Möller W, Sommerer K, Larsson S, Svartengren M. 2006. Negligible clearance of ultrafine particles retained in healthy and affected human lungs. Eur Respir J. 28(2):286–290.
- Wollmer P, Evander E. 1994. Biphasic pulmonary clearance of 99mTc-DTPA in smokers. Clin Physiol. 14(5):547–559.
- World Health Organization. 2016. Ambient air pollution: a global assessment of exposure and burden of disease. World Health Organization. https://apps.who.int/iris/handle/10665/250141.
- Wynn TA, Vannella KM. 2016. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 44(3):450–462.

