Abstract
Background
Adverse cardiovascular effects are associated with both diesel exhaust and road traffic noise, but these exposures are hard to disentangle epidemiologically. We used an experimental setup to evaluate the impact of diesel exhaust particles and traffic noise, alone and combined, on intermediary outcomes related to the autonomic nervous system and increased cardiovascular risk.
Methods
In a controlled chamber 18 healthy adults were exposed to four scenarios in a randomized cross-over fashion. Each exposure scenario consisted of either filtered (clean) air or diesel engine exhaust (particle mass concentrations around 300 µg/m3), and either low (46 dB(A)) or high (75 dB(A)) levels of traffic noise for 3 h at rest. ECG was recorded for 10-min periods before and during each exposure type, and frequency-domain heart rate variability (HRV) computed. Endothelial dysfunction and arterial stiffness were assessed after each exposure using EndoPAT 2000.
Results
Compared to control exposure, HRV in the high frequency band decreased during exposure to diesel exhaust, both alone and combined with noise, but not during noise exposure only. These differences were more pronounced in women. We observed no synergistic effects of combined exposure, and no significant differences between exposure scenarios for other HRV indices, endothelial function or arterial stiffness.
Conclusion
Three-hour exposure to diesel exhaust, but not noise, was associated with decreased HRV in the high frequency band. This indicates activation of irritant receptor-mediated autonomic reflexes, a possible mechanism for the cardiovascular risks of diesel exposure. There was no effect on endothelial dysfunction or arterial stiffness after exposure.
Introduction
Air pollution is the single largest environmental health risk globally, responsible for millions of premature deaths annually, mainly in cardiovascular diseases (GBD Citation2020). One major source is road traffic, and traffic-related air pollution appears to be a specific source associated with cardiovascular risk (Brook et al. Citation2010). Road traffic noise is another ubiquitous environmental exposure, for which cardiovascular health effects have more recently been established (van Kempen et al. Citation2018; van Kamp et al. Citation2020). As road traffic is the main source of both these exposures, they are often highly correlated and difficult to disentangle in epidemiological studies. Knowledge of the specific effects of each exposure as well as of combined effects is needed for correct estimations of health effects and for effective policy decisions.
In experimental setups, different exposures can be isolated and effects on intermediate health outcomes studied to determine the effects of each separately, and possible synergistic effects of combined exposures. The main mechanisms for cardiovascular effects of particulate matter (PM) exposure are assumed to be that inhaled particles cause local pulmonary oxidative stress and inflammation leading to systemic inflammation, direct effects on vasculature and blood of PM translocated into the circulatory system, and effects on the autonomic nervous system (ANS) through particles interacting with pulmonary receptors or nerves (Mills et al. Citation2007; Brook et al. Citation2010). The cardiovascular health effects of noise (e.g. on pulse and blood pressure) are also assumed to be mediated through effects on the ANS and the endocrine system (Hahad et al. Citation2019). Inflammation and the ANS are connected, however, through the cholinergic anti-inflammatory pathway (Borovikova et al. Citation2000; Tracey Citation2007).
Heart rate variability (HRV) and endothelial function are attractive as intermediate outcomes for cardiovascular risk since they react rapidly and can be measured non-invasively with user-independent methods, and both decreased HRV and endothelial dysfunction are known risk factors for cardiovascular morbidity and mortality (Tsuji et al. Citation1996; Patel et al. Citation2017). HRV is a biological signal that mirrors ANS-mediated alterations in cardiovascular reactivity. The outflow from the sympathetic and parasympathetic branches of ANS, together with baroreceptor activity of the vascular system modulates the heart rate and its variability. HRV can be assessed by several different metrics, mainly divided into time-domain and frequency-domain measures. Time-domain measures quantify the amount of HRV observed during monitoring periods and are suitable for sustained recording periods; frequency-domain measures calculate the absolute or relative amount of signal energy within component bands and are considered more reliable for short recording periods (Evrengül et al. Citation2005; Shaffer and Ginsberg Citation2017). The term endothelial function includes several mechanisms of which the hallmark is endothelium-dependent vasodilation, i.e. the blood vessels capacity to dilate in response to ischemia (Sun et al. Citation2019).
Associations between exposure to air pollution and HRV and endothelial function have been shown previously, but results are heterogeneous and sensitive to methodological differences (Buteau and Goldberg Citation2016; Münzel et al. Citation2018; Huang et al. Citation2021). Similarly, while associations between noise exposure and HRV and endothelial function have been shown, the effects are sensitive to the specific setup due to the complexity of noise exposure (Sim et al. Citation2015; Alves et al. Citation2018).
We have used a previously established controlled exposure chamber setup to study the effects of two major traffic-related exposures, diesel exhaust and road traffic noise, separately and combined to investigate possible synergistic effects, on HRV and endothelial dysfunction in healthy adults. The study is a part of the larger project “Health effects of combined exposure to DIesel and NOise” (DINO) that aimed to determine influence of combined exposure to diesel exhaust and traffic noise on human health.
Materials and methods
Subjects and study design
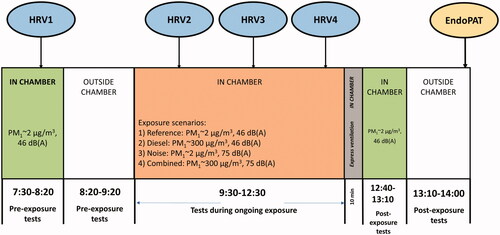
In the experimental chamber study, described previously in more detail (Xu et al. Citation2013; Wierzbicka et al. Citation2014), 18 healthy volunteers, nine females and nine males, aged 40–66 (mean 51 years) participated. All participants were nonsmokers, but six had smoked previously. Four exposure scenarios were used: (1) Reference exposure with filtered clean air (particulate matter with aerodynamic diameter less than 1 μm, PM1 ∼2 μg/m3) and low traffic noise (46 dB(A)); (2) Diesel exposure with high diesel particle concentration (PM1 ∼ 300 µg/m3) and low traffic noise; (3) Noise exposure with filtered air and high traffic noise (75 dB(A)) and (4) Combined exposure with both high diesel particle concentration and high traffic noise. During each exposure scenario, the participants spent 3 h at rest in the exposure chamber. The timeline is described in . Three participants were exposed at each session, and all participants underwent the four exposure scenarios on different days in a randomized sequence, at least one week apart.
The study was conducted according to a protocol where the exposure scenarios were blinded to both the volunteers and to the investigators. The noise levels were easy to distinguish by the participants, however. The study participants were instructed to refrain from beverages containing alcohol and caffeine on the day of attending each exposure scenario, and alcohol intake on the previous day. Prior to the exposure, the volunteers underwent a physical examination including heart and lung status and electrocardiography. Medical and work history was registered according to a structured protocol. Before, during and/or after exposure, various examinations were performed and biological samples collected. In this paper, we present the results for HRV and endothelial function.
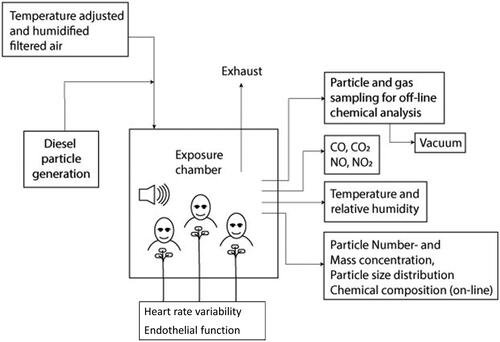
The exposure chamber
The exposure chamber is a 21.6 m3 room supplied with air through a separate conditioning system by which airflow, temperature and humidity could be controlled and adjusted. Details are described in Wierzbicka et al. (Citation2014). In short: all interior surfaces, with the exception of a 0.8 m2 window, are covered with stainless steel. The air entered the chamber at roof level, after passing through an activated carbon filter and an Ultra-Low Penetration Air (ULPA) filter. The air exchange rate in the chamber was kept at 5.0 h−1. The chamber exposure set up is illustrated in .
Diesel exhaust generation system
The laboratory generation system for diesel exhaust is described in detail previously (Wierzbicka et al. Citation2014). Briefly, we used exhaust from an idling Volkswagen Passat TDI (from 1998, emission class EURO 2, without after treatment system, using Swedish Environmental Class 1 diesel fuel with a sulfur content less than 10 ppm) placed just outside the laboratory. The majority of the exhaust was shunted away, while a fraction of the exhaust was diluted in a two-stage system with the filtered air. At first stage of the dilution system, the dilution ratio was controlled by measuring the NOx and CO levels before and after this dilution stage, it served a safety precaution measure to ensure necessary control in case if unexpected increases in CO and NO2 levels would occur. Before entering the exposure chamber the exhaust was further diluted to reach a desired mass concentration of 300 µg/m3, to approximate levels representative of short-term peak street-level exposures, and after each session ended the air in the chamber was rapidly changed to clean filtered air with express ventilation.
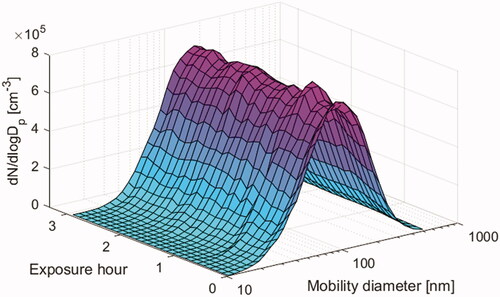
During the exposures, mass concentration of particles in the chamber was monitored with a Tapered Element Oscillating Microbalance (TEOM, Rupprecht & Patashnic Co inc.) equipped with a PM1 cyclone and a nafion drier, and particle number concentration and size distribution (10–500 nm) was monitored by a Scanning Mobility Particle Sizer system (consisting of a CPC 3010, TSI Inc and a long column Hauke DMA). The particle number size distribution and the variation in particle size and number concentration during a typical exposure event are illustrated in . The average particle mass concentration levels during the two exposure scenarios with diesel exhaust (i.e. Diesel and Combined exposure scenarios) were 276 ± 27 µg/m3. Particles were also collected for analysis of elemental and organic carbon (EC and OC) and particle bound polyaromatic hydrocarbons (PAHs). The gas phase of the diesel aerosol was analyzed for NOx, CO, CO2, VOCs and PAHs. A summary of the exposure characteristics can be found in , and more detailed exposure data has been published by Wierzbicka et al. (Citation2014).
Table 1. Summary of exposure characteristics (mean ± standard deviation) of exposures during each exposure scenario.
Traffic noise
Traffic noise recorded at a street crossing in Copenhagen was played from two loudspeakers (Dynaudio Acoustics BM6, reproducing each channel in a stereo recording), set at two different noise levels: 46 dB(A) and 75 dB(A), representing low and high traffic noise. Both loudspeakers were placed on the floor, one in a corner of the room and the other below the window to give an impression of traffic noise from an open window. The noise had continuous natural changes in noise level and included contributions from both light and heavy-duty vehicles, some buses passing by and stopping at the bus stop as well as several neutral phases with more smooth noise but lower levels. The A-weighted background noise in the room was measured to be below 40 dB. The room acoustics in the chamber was similar to a room in an ordinary apartment.
Heart rate variability
HRV was recorded by electrocardiogram (ECG), four times for each exposure scenario (): one time before the exposure started (around 8:00), and three times during the exposure (10:00 after 30 min exposure; 11:00 after 90 min exposure and 12:00 after 150 min exposure). During exposures the volunteers were seated in comfortable chairs in the chamber, and during recording they were asked to maintain a half-lying position with elevated feet on footrest stools. The lights were turned off during preparatory rest (steady state) and registration. After a period of 10 min rest, time series of heartbeats were collected (sampling frequency 200 Hz). All recordings were manually checked for arrhythmias and edited to exclude artifacts (defined as ±25% of median heartbeat interval in ms) and ectopic beats (defined as ±10% of median heartbeat interval in ms).
HRV was computed for different spectral components using the PowerLab system (ADInstruments Pty, Ltd. U-PL/QS-05XB, 2006). In time series of a minimum of 600 heartbeats, power in high frequency (HF) band (0.15–0.4 Hz), low frequency (LF) band (0.04–0.15 Hz) and in total power (TP, the total power of RR interval variability in all four spectral bands) were analyzed, and the ratios of LF to HF power (LF/HF) computed. The physiological origin for HF is the vagal tone, and for LF it is a mix of sympathetic, vagal and baroreflex activity. For LF/HF it is a mix of sympathetic and vagal activity, where a low LF/HF reflects parasympathetic dominance and a high LF/HF indicates sympathetic dominance. TP represents the whole variability in HRV (Laborde et al. Citation2017; Shaffer and Ginsberg Citation2017).
Endothelial function
Endothelial function was evaluated once for each session shortly after the exposure ended outside the chamber using a pulse amplitude tonometry (PAT) device (Endo-PAT 2000, Itamar Medical Ltd., Cesaria, Israel). The EndoPAT uses pneumatic sensors, inflated to a subdiastolic pressure to avoid venous pooling, to measure digital arterial pulse wave amplitudes continuously. The plethysmographic finger probes were placed on the index fingers of both hands. A blood pressure cuff was placed above the elbow of the right arm for hyperemia testing, while the left arm served as an internal control correcting for systemic changes in vascular tone. The test was performed with the participant lying relaxed in a bed, after 15 min of rest, and consisted of three stages: baseline recording (≥5 min), right brachial arterial occlusion induced by inflating the cuff to 60 mm Hg above systolic pressure (exactly 5 min), and a post-occlusion recording of the induced reactive hyperemia response (≥5 min). Data were digitally stored as pulse wave tracings from both hands. The individual recordings were scrutinized manually before analysis according to a pre-determined protocol without knowledge of exposure status. Artifacts due to movements of fingers or due to equipment failure were removed, and recordings considered unreliable due to large pulse break-through or disturbances were excluded from the main analysis. The Reactive Hyperemia Index (RHI), reflecting the blood vessels capacity to dilate in reaction to ischemia, and the Augmentation Index (AI), reflecting arterial stiffness, was then computed using automated algorithms in an operator independent manner by the EndoPAT software package. This algorithm for RHI uses the average amplitude of the PAT signal during the 1-min period beginning 90 s after the cuff deflation divided by the average baseline pulse amplitude of the PAT signal during a 3-min period prior to the cuff inflation. The ratio is adjusted for changes in the control finger and the baseline pulse amplitudes. Additionally, baseline pulse amplitudes in both fingers, reflecting local arterial tone, were calculated as described by Hamburg et al. (Citation2008). AI and AI@75 (the AI normalized to a heart rate of 75 beats per minute to correct for differences in pulse) were calculated based on computerized averaging and analysis of multiple pulse waveforms obtained during the baseline measurements. All tests were performed in a quiet calm environment at a constant temperature of 20.5–22 °C.
Statistics
For HRV, the change in the HF band was used as the primary endpoint since the major part of HRV consists of alterations in vagal dependent parameter. The LF/HF ratio and TP were considered as the secondary endpoints. For each HRV endpoint, within-person percentage changes (Δ%) from baseline were calculated for each individual at each time point during each exposure scenario to reduce the influence of inter-individual variation. Linear mixed models were used to analyze the difference in the percentage changes of HF (ΔHF%), in LF/HF (ΔLF/HF%) and in TP (ΔTP%) during exposure (time-points 2 + 3 + 4) compared to before exposure (time-point 1), between exposure scenarios (). Subject id, the four exposure scenarios, and the time points of measurements were used to indicate the repeated measurements using repeated covariance type as autoregressive (AR1). All models included random factors (i.e. random intercept and slope) using covariance type as scaled identity (ID), and fixed factors (i.e. exposures, time points and exposure sequence). Exposure sequence was included to account for possible adaptation. Interactions between exposure scenarios, time points and sex were also tested in all analyses, and sex-stratified analyses performed. As a sensitivity analysis, the measured levels of HF and LF/HF were analyzed using the same linear mixed model described above with baseline HF or LF/HF as an additional fixed factor, respectively.
For endothelial function, the main analysis was performed on 63 recordings from 16 participants, since a total 9 recordings out of 72 (all four from two subjects and another single recording) were considered unreliable due to large pulse break-through or disturbances. The differences in RHI, AI@75 and log-transformed mean baseline pulse amplitudes between exposure scenarios (e.g. diesel vs. reference) were first assessed using Wilcoxon’s signed ranked test. The analysis was then repeated using a linear mixed model including a random factor (i.e. random intercept), and exposures and exposure sequence as fixed factors. A sensitivity analysis was performed by including all 72 recordings from 18 participants, using the same method as described above. In secondary analyses, results were also calculated stratified by sex, and for log-transformed RHI (LnRHI). All analyses were performed by IBM SPSS 26.0. Statistical significance refers to p < 0.05 (two-tailed).
Ethics
All participants provided informed written consent and the study was approved by the Lund Regional Ethical Review Board (EPN, Lund), Dnr 2009/460.
Results
lists the HRV and endothelial function parameters during each exposure scenario. There was on average a 23% decrease in ΔHF% during the diesel exposure scenario (p = 0.02, ) and a similar but borderline significant average 19% decrease during the combined exposure scenario (p = 0.06, ), compared to the reference exposure of filtered air. There was no significant decrease in ΔHF% during noise exposure alone. For the LF/HF ratio and TP there were no significant differences between the exposure sessions. No interaction was found for exposure scenarios and time points (p > 0.3 for all analyses), indicating that the change of HRV during the exposures followed the same pattern. When not adjusting for the exposure sequence, the results were similar as in the main analysis, but with a marginally statistical tendency of a decrease in ΔHF% after noise exposure (supplemental table s1). In sex-stratified analyses, the decrease in ΔHF% after diesel and combined exposure sessions was significant only in women (supplemental table s2). The average increase in heart rate during exposure compared to baseline was slightly but significantly higher during exposure to both diesel and noise compared to the reference exposure (5–7% compared to 2%, p < 0.05 for the sessions with diesel exposure and p = 0.05 for noise exposure alone).
Table 2. Descriptive statistics (medians and ranges) of HRV and endothelial function parameters for each exposure scenario.
Table 3. Percentage changes in HRV parameters during the different exposure scenarios compared to the reference exposure.
There were no significant differences in RHI, augmentation index (AI@75) or baseline pulse amplitudes after either exposure scenario compared to the reference exposure (). Including also participants excluded prior to analysis did not change the results (supplemental table s3). Results were also similarly unchanged and non-positive for LnRHI and AI not adjusted for heart rate (data not shown), in sex-stratified analyses (supplemental table s4), and in an analysis using the Wilcoxon signed rank test instead of linear regression models (p > 0.2 for all comparisons, data not shown).
Table 4. Reactive Hyperemia Index (RHI), Augmentation Index, and baseline pulse amplitudes after the different exposure scenarios compared to the reference exposure, after exclusions (n = 16).
Discussion
In this experimental chamber study of healthy adults, we observed a decrease in HRV in the high-frequency (HF) domain and an increase in heart rate during exposure to diesel exhaust particles, indicating a decrease in the vagal tone. There were no significant effects of short-term (3 h) exposure to traffic noise alone, and no signs of synergistic effects of the two exposures. For endothelial function and arterial stiffness measured after exposure there was no effect of either exposure, which could indicate that the observed effects on the autonomic nervous system were transient, or that this method was less sensitive than HRV.
The spectral analysis performed in this study was based on short-term recordings over 10 min before and three times during exposure in healthy test subjects and focused on the high frequency part of HRV, known to constitute the major part of the variability. The HF band is sometimes called the respiratory band since it corresponds to the HR variations related to the respiratory cycle, and is often considered to represent the vagal/parasympathetic tone (Malik et al. Citation1996; Grossman and Taylor Citation2007). A lower HF, and deficient vagal inhibition, is correlated with psychological stress and cardiovascular risk factors, and has been associated with increased cardiovascular morbidity (Tsuji et al. Citation1996; Patel et al. Citation2017). For example, a decrease in mean HF has been observed after smoking a cigarette or a period with no physical activity, while increased HF has been observed after quitting smoking and physical training (Thayer et al. Citation2010). Interpreting HRV is not straightforward, though, as HF can be affected by other factors such as respiratory parameters, body position, and external stimuli (Grossman and Taylor Citation2007; Riediker et al. Citation2018). To control for this, we included a period of 10 min with the subjects at silent preparatory rest before recording HRV, and analyzed within-person differences with a mixed model.
Previous epidemiological studies provide some evidence supporting an association between air pollution exposure and reduced HRV, with a number of panel studies showing decreased HF during and after exposure to fine and ultrafine PM (Chan et al. Citation2004; Liao et al. Citation1999, Citation2004; Park et al. Citation2005; Adar et al. Citation2007; Wu et al. Citation2010; Hampel et al. Citation2014). A recent systematic review with meta-analysis reports overall negative associations between fine particles and HRV indices, noting that the effect estimates were higher in studies with older (>40 years old) participants (Niu et al. Citation2020). There are substantial differences between studies in methodology and exposures, however, and an earlier review called for high-quality studies with well-defined study populations, personal exposure assessment and statistical analyses that account for within-subject correlations, i.e. mixed-effects models, to determine the question (Buteau and Goldberg Citation2016). As the HRV signal is very sensitive and reacts with rapid beat-to-beat changes of heart rate to external stimuli, and has a large inter-individual variability, experimental set-ups are crucial to evaluate causal associations.
The evidence from experimental studies such as randomized crossover chamber studies is inconsistent though, with reports of both negative and positive associations as well as null findings (Huang et al. Citation2021). Devlin et al. (Citation2014) note that in a number of studies of controlled exposure to concentrated ambient particles (CAPs) they found negative associations with HRV in older but not younger participants. However, decreases in HF indicating reduced vagal tone, similar to our findings, have been observed in both young and healthy participants and in older or with premorbidities, after exposure to fine and coarse CAPs and ultrafine particles (Brook et al. Citation2014; Devlin et al. Citation2014, Devlin et al. Citation2003; Vora et al. Citation2014). Regarding controlled exposure to diesel exhaust specifically, three previous studies did not find any consistent association with HRV (Peretz et al. Citation2008; Mills et al. Citation2011; Giles et al. Citation2018), and one small study of six participants with GSTM-null genotype (indicating increased susceptibility to oxidative stress) found a decrease in LF but no effect on HF (Tong et al. Citation2014). Methodological differences are a possible explanation for the conflicting results, such as the heterogeneity in length and type of exposure, participant characteristics, and other aspects of study design. Our study used diesel exposure from a light-duty (1.9 L, passenger) vehicle without aftertreatment devices, dominated by elemental carbon, and most other studies used heavy-duty vehicles. Thus, even when the PM1 mass concentration was comparable to other exposure studies (i.e. ∼300 µg/m3), the physical characteristics and chemical composition of the diesel exhaust particles were not the same in all studies. This was discussed in detail in Wierzbicka et al. (Citation2014), which also highlighted the need to report more details regarding the physical and chemical characteristics of particles that study participants are exposed to, to enable comparisons and increase understanding of underlying mechanisms. Additionally, the exposure duration was longer (3 h) in our study than in any of the others (commonly 1 h), our study population was older than the others, except in the subgroup with CVD in Mills et al. (Citation2011), and all participants were at rest in our study while they were physically active during exposure in some other studies.
The more pronounced effects we observed among female participants are not generally reported in other studies of HRV, but in accordance with a study reporting a greater parasympathetic load in females in reproductive age (Abhishekh et al. Citation2013). Interpretation should be with caution though since the numbers of participants were low in our sex-stratified analyses. The meta-analysis of panel studies by Niu et al. (Citation2020) notes that sex differences could not be analyzed since most such studies did not report sex-specific estimates. Given that the epidemiology and expression of cardiovascular disease differ by sex, and some studies report stronger associations between air pollution and cardiovascular diseases in women (Tibuakuu et al. Citation2018), we suggest further studies of effect on HRV should investigate sex-specific effects.
The mechanisms responsible for respiratory modulation of autonomic activity are not fully understood, but HRV synchronized with respiration may have a positive influence on gas exchange in the lung by making the ventilation/perfusion matching efficient (Yasuma and Hayano Citation2004). Inhaled PM may affect HRV through activation of irritant receptor-mediated autonomic reflexes in the lung (Ghelfi et al. Citation2008), and pulmonary nanoparticle (<100 nm) exposure disrupts systemic microvascular nitric oxide signaling (Nurkiewicz et al. Citation2011). Diesel exposure has also been shown to increase pulmonary vascular resistance (Wauters et al. Citation2015), connecting the pulmonary and cardiovascular health effects of PM exposure further. As highlighted in Wierzbicka et al. (Citation2014), reporting only PM mass concentration during human chamber exposures that aim to assess health effects can be misleading. The same PM mass concentration, even for the exposures that originate from the same source e.g. diesel exhaust, can vary to a great extent in terms of particle characteristics such as number concentration, size, surface area or chemical composition as well as co-exposures of gases such as NO2. All these properties are recognized as possible parameters that can influence observed effects of exposure to airborne particles and gases (Giechaskiel et al. Citation2009; Isaxon et al. Citation2013; Hagerman et al. Citation2014). Thus, to understand the possible mechanisms of the effects, a matrix of pollutants’ properties and resulting effects should be created, and this requires detailed characteristics of exposures (both particle and gas phase pollutants) as well as standardized way of their reporting. In this study we have determined detailed particle and gas phase characteristics ( and further details in Wierzbicka et al. (Citation2014), and used PM1 mass (particles with aerodynamic diameter below 1 µm) as the primary exposure metric. Since essentially all diesel exhaust particles are below 0.5 µm, and the mass between 1 and 2.5 µm is insignificant, the PM1 exposures reported here can be approximated as the PM2.5 exposures in other studies of diesel exhaust exposure.
Regarding exposure to road traffic noise our results were overall non-positive, with only a slight non-significant decrease in HF after noise exposure alone, and no indications of any synergistic effects of combined diesel particle and traffic noise exposure. Independent effects of diesel exposure and road traffic noise are consistent with the epidemiological literature overall finding independent effects of each of these exposures and limited confounding (Tétreault et al. Citation2013). Noise is widely regarded as a general stressor though (WHO Citation2011), and our a priori hypothesis was that we would see an effect of noise on HRV and endothelial function indicating a stress response with decreased vagal tone during exposure, and synergistic effects of exposure to diesel and noise.
Studies of combined effects of particles and noise on HRV are rare, but one previous randomized cross-over study of real-life exposure to traffic-related PM (median PM2.5 162 µg/m3 vs 53 µg/m3) and noise found that the reduction in HRV measured as HF and time-domain HRV related to air pollution was amplified at high noise levels (>65.6 dB(A)) in healthy volunteers (Huang et al. Citation2013). A panel study in Toronto also found decreased HRV parameters related to short-term exposures to traffic-related PM and noise, but not related to average daily exposures (Biel et al. Citation2020). A study on airport ground staff exposed to ultrafine particles and noise found a decrease in HF to be associated with noise rather than particles (Lecca et al. Citation2021), and a study of cyclists in traffic in Montreal found no significant effects of either PM or noise on HRV (Buregeya et al. Citation2020). Substantial differences in study design and exposure assessment make direct comparisons difficult though. For exposure to road traffic noise alone, there are studies finding effects of noise on HRV (Kraus et al. Citation2013) and null associations (Sim et al. Citation2015; Alves et al. Citation2018). In a previous study of intensive coronary care patients we observed no effects on HRV in the group exposed to higher noise levels, but higher readmission rates, indicating that other intermediate effects than HRV are important for the cardiovascular effects of noise (Hagerman et al. Citation2005). Short exposure time and lack of statistical power is also a possible explanation for our null findings since there was a non-significant trend in the hypothesized direction in the group exposed to noise alone. It should also be noted that for the noise we compared high versus low levels of road traffic noise (74 vs 46 dB(A)), rather than using no sound at all or other types of sounds as reference exposure.
We found no significant association between any exposure and any of the PAT measurements. There is substantial evidence from in vitro and animal studies showing that exposure to fine particles shifts vasomotor tone toward vasoconstriction through increased levels of endotelin-1 and decreased NO production and bioavailability (Liang et al. Citation2020), and a number of studies show associations between PM exposure and endothelial dysfunction (Münzel et al. Citation2018). There are also studies showing marked endothelial dysfunction by nocturnal exposure to aircraft noise (Schmidt et al. Citation2015, Schmidt et al. Citation2013). The null associations we found between preceding exposure to diesel exhaust or noise and endothelial function and arterial stiffness measured by EndoPAT are, however, in accordance with other chamber studies: in young adults using the same technique after short term exposure to wood smoke (Pope et al. Citation2011; Forchhammer et al. Citation2012), household air pollution (Shahriar et al. Citation2021), in a chamber study of CAP exposure (Brook et al. Citation2014), in a study of young adults after reduction of indoor PM levels (Bräuner, Møller, et al. Citation2008) and in a similar chamber study after 1 h diesel exposure (Törnqvist et al. Citation2007). A study using venous occlusion pletysmography to assess forearm blood flow did find impairment of endothelium-dependent vasodilation after short-term (1 h) exposure to diesel exhaust, however (Mills et al. Citation2005), indicating that arterial effects of experimental diesel exhaust can be detected with more sophisticated methods than EndoPAT. Significant improvement of vascular function as assessed by RH-PAT after reduction of indoor PM levels from traffic (Bräuner, Forchhammer, et al. Citation2008) and wood smoke (Allen et al. Citation2011) has also been found, however. These studies were performed after filtering indoor air for 7 days (Allen et al. Citation2011), and 48 h (Bräuner, Forchhammer, et al. Citation2008), and the latter was performed in elderly participants. As for HRV, this indicates that the effects on the ANS by PM exposure appear primarily in older populations and after longer durations of exposure, and that the short-term exposures of controlled chamber studies do not capture effects that occur during real-life chronic exposure. A crucial difference between PAT and HRV measurements in the current study is that HRV was measured during exposure, while PAT measurements were performed directly after exposure, since the method requires quiet undisturbed surroundings. Startling noises during recording will almost instantaneously decrease the pulse amplitudes for a short period and might affect test results. The findings of associations between exposure and HRV during exposure but null associations for PAT measurements after exposure could either reflect that HRV and PAT measures different aspects of the ANS, indicate that HRV is a more sensitive measurement, or that the effects were transient. A rapid decrease of the effects of air pollution and noise on HRV has been reported previously (Biel et al. Citation2020).
Analyses of other outcomes from the same exposure setup as reported here (DINO project) found that diesel exposure did not cause genotoxicity, oxidative stress or inflammation in peripheral blood mononuclear cells (PBMCs), but suggested possible genotoxic effects of noise (Hemmingsen et al. Citation2015). One publication showed that diesel exhaust induced respiratory symptoms, decreased peak expiratory flow and increased inflammatory markers in blood (Xu et al. Citation2013). In line with the current study no interactions were found between diesel and noise exposure on these respiratory effects. Theoretically, a decrease in HF HRV could increase the inflammatory response, as it is known that the parasympathetic branch of ANS is involved in the modulation of the anti-inflammatory response (Borovikova et al. Citation2000; Tracey Citation2007). There are also some studies reporting correlations between PM-induced systemic inflammation and effects on HRV as well as on vascular function (Sloan et al. Citation2007; Tamagawa et al. Citation2008; Luttmann-Gibson et al. Citation2010). Despite null results for some outcomes, taking into account previously reported outcomes of the DINO project, our overall interpretation of the findings are in line with the evidence of associations between PM exposure and systemic inflammation, indicating that effects on both systemic inflammation and the autonomic nervous system are important intermediate steps for the increased risk of cardiovascular disease.
A strength of this study was the low variation in exposure between sessions that was consistently close to target concentrations (). The experimental setting was strictly controlled, and all HRV recordings were made during steady state conditions (the participants at undisturbed rest before and during recordings), which increases the power to detect a possible effect. The methodology was based on previous controlled chamber studies of well characterized exposures and determination of measurable physiological responses (Isaxon et al. Citation2013; Hagerman et al. Citation2014). The design of the study and the statistical analysis also allowed us to make calculations of within-individual changes as means and standard deviation of both absolute values and relative changes from baseline according to recommendations for short-term experimental settings. This is important for HRV as a biological signal with non-linear properties and values in healthy individuals are characterized by large inter-individual variation (Laborde et al. Citation2017). The levels and composition of the exhaust in the chamber (PM concentrations and numbers, PAH fraction etc) was overall representative for short-term peak exposure at street-level, and for some work environments, but substantially higher than average long-term exposure to ambient air pollution exposure in northern Europe.
Limitations are the relatively small sample size, which limits the power to exclude undetected effects of e.g. noise exposure, and that we did not analyze time-domain parameters for HRV but only frequency domain. However, since we used 10 min short-term recordings, we believe frequency domain are more appropriate measures than time domain parameters, as the latter can be strongly influenced by recording period length, and shorter records are associated with smaller values and poor estimations (Nunan et al. Citation2010). Additionally, the interrelationships between time domain and frequency domain are often very high (Shaffer and Ginsberg Citation2017). For example, Massin et al. (Citation1999) reported correlations above 0.9 for variables dependent on vagal tone, and suggest that they can act as surrogates for each other. Change in HF was a priori chosen as the primary endpoint, as HF represents the major part of HRV with the possibility to detect changes also in a small sample size, but results may have been affected by differences in pre-exposure levels. The size and setup of the study also limited us to comparing high versus low exposure to noise and high versus no exposure to diesel (and combined exposure) rather than several types and levels of exposure. An important inherent limitation of short term (3 h) chamber exposure studies is also that we only analyze short-term effects on intermediate outcomes, while real-life exposure to diesel exhaust is often long-term (chronic). The HRV measurements were timed to assess effects during exposure, and the EndoPAT measurement effects directly after exposure, but we did not assess how long the effects on HRV persisted or were delayed effects. It should also be pointed out that the diesel exhaust source used in this study was of relatively old technology and did not include any after-treatment devices such as particulate filters, oxidation catalyst or De-NOx device. Finally, the participants were relatively healthy, and the results are not necessarily valid for more susceptible populations.
Conclusions
Controlled 3-h exposure to diesel exhaust led to a decrease in the HF band of HRV in healthy individuals, especially women. The HRV reduction was demonstrated in the spectral band that constitutes the major part of the variability and is indicative of vagal tone, and thus may indicate a pathway for biological effect leading to the increased risk of cardiovascular events that has been observed during diesel exposure. Traffic noise did not seem to have any clear effect on HRV, and adding noise did not alter the effect of diesel exhaust on HRV. We found no effects of either diesel exhaust or noise exposure on peripheral endothelial function after exposure.
Supplemental Material
Download MS Word (25.3 KB)Acknowledgments
We thank all the study subjects for participating in the study.
Disclosure statement
All authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Abhishekh HA, Nisarga P, Kisan R, Meghana A, Chandran S, Trichur R, Null, Sathyaprabha TN. 2013. Influence of age and gender on autonomic regulation of heart. J Clin Monit Comput. 27:259–264.
- Adar SD, Gold DR, Coull BA, Schwartz J, Stone PH, Suh H. 2007. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiol Camb Mass. 18:95–103.
- Allen RW, Carlsten C, Karlen B, Leckie S, van Eeden S, Vedal S, Wong I, Brauer M. 2011. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med. 183(9):1222–1230.
- Alves M, Garner DM, Fontes A, Sousa LdA, Valenti VE. 2018. Linear and complex measures of heart rate variability during exposure to traffic noise in healthy women. Complexity. 2018:e2158391–14.
- Biel R, Danieli C, Shekarrizfard M, Minet L, Abrahamowicz M, Baumgartner J, Liu R, Hatzopoulou M, Weichenthal S. 2020. Acute cardiovascular health effects in a panel study of personal exposure to traffic-related air pollutants and noise in Toronto, Canada. Sci Rep. 10(1).
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 405(6785):458–462.
- Bräuner EV, Møller P, Barregard L, Dragsted LO, Glasius M, Wåhlin P, Vinzents P, Raaschou-Nielsen O, Loft S. 2008. Exposure to ambient concentrations of particulate air pollution does not influence vascular function or inflammatory pathways in young healthy individuals. Part Fibre Toxicol. 5:13.
- Bräuner EV, Forchhammer L, Møller P, Barregard L, Gunnarsen L, Afshari A, Wåhlin P, Glasius M, Dragsted LO, Basu S, et al. 2008. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Respir Crit Care Med. 177(4):419–425.
- Brook RD, Bard RL, Morishita M, Dvonch JT, Wang L, Yang H-Y, Spino C, Mukherjee B, Kaplan MJ, Yalavarthi S, et al. 2014. Hemodynamic, autonomic, and vascular effects of exposure to coarse particulate matter air pollution from a rural location. Environ Health Perspect. 122(6):624–630.
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. 2010. Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the Scientific Statement From the American Heart Association. Am Heart Assoc Circ. 121(21):2331–2378.,
- Buregeya JM, Apparicio P, Gelb J. 2020. Short-term impact of traffic-related particulate matter and noise exposure on cardiac function. IJERPH. 17(4):1220.
- Buteau S, Goldberg MS. 2016. A structured review of panel studies used to investigate associations between ambient air pollution and heart rate variability. Environ Res. 148:207–247.
- Chan C-C, Chuang K-J, Shiao G-M, Lin L-Y. 2004. Personal exposure to submicrometer particles and heart rate variability in human subjects. Environ Health Perspect. 112(10):1063–1067.
- Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. 2003. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J Suppl. 40:76s–80s.
- Devlin RB, Smith CB, Schmitt MT, Rappold AG, Hinderliter A, Graff D, Carraway MS. 2014. Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects. Toxicol Sci Off J Soc Toxicol. 140(1):61–72.
- Evrengül H, Tanriverdi H, Dursunoglu D, Kaftan A, Kuru O, Unlu U, Kilic M. 2005. Time and frequency domain analyses of heart rate variability in patients with epilepsy. Epilepsy Res. 63(2–3):131–139.
- Forchhammer L, Møller P, Riddervold IS, Bønløkke J, Massling A, Sigsgaard T, Loft S. 2012. Controlled human wood smoke exposure: oxidative stress, inflammation and microvascular function. Part Fibre Toxicol. 9:7.
- GBD. 2020. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Lond Engl. 396:1223–1249.
- Ghelfi E, Rhoden CR, Wellenius GA, Lawrence J, Gonzalez-Flecha B. 2008. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicol Sci. 102(2):328–336.
- Giechaskiel B, Alföldy B, Drossinos Y. 2009. A metric for health effects studies of diesel exhaust particles. J Aerosol Sci. 40(8):639–651.
- Giles LV, Carlsten C, Koehle MS. 2018. The pulmonary and autonomic effects of high-intensity and low-intensity exercise in diesel exhaust. Environ Health. 17(1):87.
- Grossman P, Taylor EW. 2007. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol. 74(2):263–285.
- Hagerman I, Isaxon C, Gudmundsson A, Wierzbicka A, Dierschke K, Berglund M, Pagels J, Nielsen J, Assarsson E, Andersson UB, et al. 2014. Effects on heart rate variability by artificially generated indoor nano-sized particles in a chamber study. Atmos Environ. 88:165–171.
- Hagerman I, Rasmanis G, Blomkvist V, Ulrich R, Eriksen CA, Theorell T. 2005. Influence of intensive coronary care acoustics on the quality of care and physiological state of patients. Int J Cardiol. 98(2):267–270.
- Hahad O, Kröller-Schön S, Daiber A, Münzel T. 2019. The cardiovascular effects of noise. Dtsch Arzteblatt Int. 116:245–250.
- Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. 2008. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 117(19):2467–2474.
- Hampel R, Rückerl R, Yli-Tuomi T, Breitner S, Lanki T, Kraus U, Cyrys J, Belcredi P, Brüske I, Laitinen TM, et al. 2014. Impact of personally measured pollutants on cardiac function. Int J Hyg Environ Health. 217(4–5):460–464.
- Hemmingsen JG, Møller P, Jantzen K, Jönsson B, Albin M, Wierzbicka A, Gudmundsson A, Loft S, Rissler J. 2015. Controlled exposure to diesel exhaust and traffic noise-effects on oxidative stress and activation in mononuclear blood cells. Mutat Res. 775:66–71.
- Huang F, Zhao Y, Wang P, Wang Y, Zhang L, Luo Y. 2021. Short-term exposure to particulate matter on heart rate variability in humans: a systematic review of crossover and controlled studies. Environ Sci Pollut Res Int. 28(27):35528–35536.
- Huang J, Deng F, Wu S, Lu H, Hao Y, Guo X. 2013. The impacts of short-term exposure to noise and traffic-related air pollution on heart rate variability in young healthy adults. J Expo Sci Environ Epidemiol. 23(5):559–564.
- Isaxon C, Dierschke K, Pagels JH, Wierzbicka A, Gudmundsson A, Löndahl J, Hagerman I, Berglund M, Assarsson E, Andersson UB, et al. 2013. Realistic indoor nano-aerosols for a human exposure facility. J Aerosol Sci. 60:55–66.
- Kraus U, Schneider A, Breitner S, Hampel R, Rückerl R, Pitz M, Geruschkat U, Belcredi P, Radon K, Peters A. 2013. Individual daytime noise exposure during routine activities and heart rate variability in adults: a repeated measures study. Environ Health Perspect. 121(5):607–612.
- Laborde S, Mosley E, Thayer JF. 2017. Heart rate variability and cardiac vagal tone in psychophysiological research - recommendations for experiment planning, data analysis, and data reporting. Front Psychol. 8:213.
- Lecca LI, Marcias G, Uras M, Meloni F, Mucci N, Larese Filon F, Massacci G, Buonanno G, Cocco P, Campagna M. 2021. Response of the cardiac autonomic control to exposure to nanoparticles and noise: a cross-sectional study of airport ground staff. IJERPH. 18(5):2507.
- Liang S, Zhang J, Ning R, Du Z, Liu J, Batibawa JW, Duan J, Sun Z. 2020. The critical role of endothelial function in fine particulate matter-induced atherosclerosis. Part Fibre Toxicol. 17(1):61.
- Liao D, Creason J, Shy C, Williams R, Watts R, Zweidinger R. 1999. Daily variation of particulate air pollution and poor cardiac autonomic control in the elderly. Environ Health Perspect. 107(7):521–525.
- Liao D, Duan Y, Whitsel EA, Zheng Z, Heiss G, Chinchilli VM, Lin H-M. 2004. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population-based study. Am J Epidemiol. 159(8):768–777.
- Luttmann-Gibson H, Suh HH, Coull BA, Dockery DW, Sarnat SE, Schwartz J, Stone PH, Gold DR. 2010. Systemic inflammation, heart rate variability and air pollution in a cohort of senior adults. Occup Environ Med. 67(9):625–630.
- Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ. 1996. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 17(3):354–381.
- Massin MM, Derkenne B, von Bernuth G. 1999. Correlations between indices of heart rate variability in healthy children and children with congenital heart disease. Cardiology. 91(2):109–113.
- Mills NL, Finlayson AE, Gonzalez MC, Törnqvist H, Barath S, Vink E, Goudie C, Langrish JP, Söderberg S, Boon NA, et al. 2011. Diesel exhaust inhalation does not affect heart rhythm or heart rate variability. Heart Br Card Soc. 97(7):544–550.
- Mills NL, Törnqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, et al. 2005. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 112(25):3930–3936.,.
- Mills NL, Törnqvist H, Robinson SD, Gonzalez MC, Söderberg S, Sandström T, Blomberg A, Newby DE, Donaldson K. 2007. Air pollution and atherothrombosis. Inhal Toxicol. 19(Suppl 1):81–89.
- Münzel T, Gori T, Al-Kindi S, Deanfield J, Lelieveld J, Daiber A, Rajagopalan S. 2018. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur Heart J. 39(38):3543–3550.
- Niu Z, Liu F, Li B, Li N, Yu H, Wang Y, Tang H, Chen X, Lu Y, Cheng Z, et al. 2020. Acute effect of ambient fine particulate matter on heart rate variability: an updated systematic review and meta-analysis of panel studies. Environ Health Prev Med. 25(1):77.
- Nunan D, Sandercock G, Brodie DA. 2010. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin Electrophysiol. 33(11):1407–1417.
- Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Moseley AM, Cumpston JL, Goodwill AG, Frisbee SJ, Perrotta PL, Brock RW, et al. 2011. Pulmonary particulate matter and systemic microvascular dysfunction. Res Rep Health Eff Inst. 164:3–48.
- Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J. 2005. Effects of air pollution on heart rate variability: the VA normative aging study. Environ Health Perspect. 113(3):304–309.
- Patel VN, Pierce BR, Bodapati RK, Brown DL, Ives DG, Stein PK. 2017. Association of Holter-derived heart rate variability parameters with the development of congestive heart failure in the cardiovascular health study. JACC Heart Fail. 5(6):423–431.
- Peretz A, Kaufman JD, Trenga CA, Allen J, Carlsten C, Aulet MR, Adar SD, Sullivan JH. 2008. Effects of diesel exhaust inhalation on heart rate variability in human volunteers. Environ Res. 107(2):178–84
- Pope CA, Hansen JC, Kuprov R, Sanders MD, Anderson MN, Eatough DJ. 2011. Vascular function and short-term exposure to fine particulate air pollution. J Air Waste Manag Assoc. 61(8):858–863.
- Riediker M, Franc Y, Bochud M, Meier R, Rousson V. 2018. Exposure to fine particulate matter leads to rapid heart rate variability changes. Front Environ Sci. 6:2.
- Schmidt F, Kolle K, Kreuder K, Schnorbus B, Wild P, Hechtner M, Binder H, Gori T, Münzel T. 2015. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin Res Cardiol. 104(1):23–30.
- Schmidt FP, Basner M, Kröger G, Weck S, Schnorbus B, Muttray A, Sariyar M, Binder H, Gori T, Warnholtz A, et al. 2013. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J. 34(45):3508–3514a.
- Shaffer F, Ginsberg JP. 2017. An overview of heart rate variability metrics and norms. Front Public Health. 5:258.
- Shahriar MH, Chowdhury M, Ahmed S, Eunus M, Kader SB, Begum BA, Islam T, Sarwar G, Al Shams R, Raqib R, et al. 2021. Exposure to household air pollutants and endothelial dysfunction in rural Bangladesh: a cross-sectional study. Environ Epidemiol. 5(2):e132.
- Sim CS, Sung JH, Cheon SH, Lee JM, Lee JW, Lee J. 2015. The effects of different noise types on heart rate variability in men. Yonsei Med J. 56(1):235–243.
- Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. 2007. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 13(3–4):178–184.
- Sun H-J, Wu Z-Y, Nie X-W, Bian J-S. 2019. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front Pharmacol. 10:1568.
- Tamagawa E, Bai N, Morimoto K, Gray C, Mui T, Yatera K, Zhang X, Xing L, Li Y, Laher I, et al. 2008. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am J Physiol Lung Cell Mol Physiol. 295(1):L79–85.
- Tétreault L-F, Perron S, Smargiassi A. 2013. Cardiovascular health, traffic-related air pollution and noise: are associations mutually confounded? A systematic review. Int J Public Health. 58(5):649–666.
- Thayer JF, Yamamoto SS, Brosschot JF. 2010. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 141(2):122–131.
- Tibuakuu M, Michos ED, Navas-Acien A, Jones MR. 2018. Air pollution and cardiovascular disease: a focus on vulnerable populations worldwide. Curr Epidemiol Rep. 5(4):370–378.
- Tong H, Rappold AG, Caughey M, Hinderliter AL, Graff DW, Berntsen JH, Cascio WE, Devlin RB, Samet JM. 2014. Cardiovascular effects caused by increasing concentrations of diesel exhaust in middle-aged healthy GSTM1 null human volunteers. Inhal Toxicol. 26(6):319–326.
- Törnqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Söderberg S, Newby DE, et al. 2007. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 176(4):395–400.
- Tracey KJ. 2007. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 117(2):289–296.
- Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D. 1996. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 94(11):2850–2855.
- van Kamp I, Simon S, Notley H, Baliatsas C, van Kempen E. 2020. Evidence relating to environmental noise exposure and annoyance, sleep disturbance, cardio-vascular and metabolic health outcomes in the context of IGCB (N): a scoping review of new evidence. IJERPH. 17(9):3016.
- van Kempen E, Casas M, Pershagen G, Foraster M. 2018. WHO environmental noise guidelines for the European region: a systematic review on environmental noise and cardiovascular and metabolic effects: a summary. IJERPH. 15(2):379.
- Vora R, Zareba W, Utell MJ, Pietropaoli AP, Chalupa D, Little EL, Oakes D, Bausch J, Wiltshire J, Frampton MW. 2014. Inhalation of ultrafine carbon particles alters heart rate and heart rate variability in people with type 2 diabetes. Part Fibre Toxicol. 11:31.
- Wauters A, Vicenzi M, De Becker B, Riga J-P, Esmaeilzadeh F, Faoro V, Vachiéry J-L, van de Borne P, Argacha J-F. 2015. At high cardiac output, diesel exhaust exposure increases pulmonary vascular resistance and decreases distensibility of pulmonary resistive vessels. Am J Physiol Heart Circ Physiol. 309(12):H2137–E2144.
- WHO. 2011. Burden of disease from environmental noise: quantification of healthy life years lost in Europe. Geneva: WHO. Regional Office for Europe.
- Wierzbicka A, Nilsson PT, Rissler J, Sallsten G, Xu Y, Pagels JH, Albin M, Österberg K, Strandberg B, Eriksson A, et al. 2014. Detailed diesel exhaust characteristics including particle surface area and lung deposited dose for better understanding of health effects in human chamber exposure studies. Atmos Environ. 86:212–219.,
- Wu S, Deng F, Niu J, Huang Q, Liu Y, Guo X. 2010. Association of heart rate variability in taxi drivers with marked changes in particulate air pollution in Beijing in 2008. Environ Health Perspect. 118(1):87–91.
- Xu Y, Barregard L, Nielsen J, Gudmundsson A, Wierzbicka A, Axmon A, Jönsson B, Kåredal M, Albin M. 2013. Effects of diesel exposure on lung function and inflammation biomarkers from airway and peripheral blood of healthy volunteers in a chamber study. Part Fibre Toxicol. 10:1–9.
- Yasuma F, Hayano J-I. 2004. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest. 125(2):683–690.